Abstract
Background
Scleroderma is a connective tissue disease causing fibrosis and commonly affects the skin and internal organs such as the GI tract, lungs, kidney and heart. Most people with scleroderma also have Raynaud's phenomenon (RP). One of the possible treatment options for RP in scleroderma is ketanserin.
Objectives
To assess the effects and toxicity of ketanserin versus placebo for the treatment of Raynaud's phenomenon (RP) in scleroderma.
Search methods
We searched the Cochrane Controlled Trials Register, and Medline up to August 1, 2007 using the Cochrane Collaboration search strategy developed by Dickersin et al.(1994). Key words included: Raynaud's or vasospasm, scleroderma or progressive systemic sclerosis or connective tissue disease or autoimmune disease. Current Contents were searched up to and including August 1, 2007. All bibliographies of articles retrieved were searched and key experts in the area were contacted for additional and unpublished data. The initial search strategy included all languages.
Selection criteria
All randomized controlled trials comparing ketanserin versus placebo were eligible if they reported clinical outcomes of interest. Trials with dropout rates greater than 30% were excluded.
Data collection and analysis
All data were abstracted by two independent and trained reviewers (SH, PT), and verified by a third reviewer (JP). Each trial was assessed independently by the same two reviewers for its quality using a validated quality assessment tool (Altman 2001).
Peto's odds ratios were calculated for all dichotomous outcomes and a weighted mean difference was carried out on all continuous outcomes. Fixed effects and random effects model were used if the data was homogeneous or heterogeneous, respectively.
Main results
Six trials and 146 patients were included. The proportion improved was significantly better in the group on ketanserin with a Peto's odds ratio (OR) of 2.74 (95% CI 1.42, 5.11) (Cadranel 1986; Dormandy 1988; Kirch 1987; Lukac 1985; van de Wal 1987). When comparing ketanserin to placebo, the decrease in frequency of RP attacks favoured placebo and was statistically significant [WMD (fixed) 25.20 (95% CI 22.55,27.85)] (Kirch 1987). Side effects were significantly more common in the group using active treatment [Peto's OR 2.63 (95% CI 1.42, 4.88)] (Cadranel 1986; Kirch 1987; Lukac 1985; Ortonne 1989; van de Wal 1987). Duration of attacks significantly favoured the placebo group over the active treatment [WMD (fixed) 4.10 (95% CI 3.57, 4.63)] (Kirch 1987).
Authors' conclusions
Ketanserin may have some efficacy in the treatment of Raynaud's phenomenon secondary to scleroderma. Overall, ketanserin is not significantly different from placebo for the treatment of Raynaud's phenomenon except for some decrease in the duration of attacks and more subjects improved on ketanserin compared to placebo. However, there were more side effects, and the frequency of attacks actually favored placebo. It can be concluded that ketanserin treatment in Raynaud's phenomenon secondary to scleroderma is not clinically beneficial.
Plain language summary
Ketanserin is a drug that has been studied in the treatment of Raynaud's phenomenon and associated conditions. It is not widely used however.
Raynaud's phenomenon is a disease that causes decreased blood flow and circulation to the extremeties. Symptoms include discolouration, pain, and in some severe cases ulceration of the hands and feet. It is most often triggered by cold, stress, and emotional discomfort. Primary Raynaud's phenomenon has no underlying disease associated with it. Secondary Raynaud's phenomenon is most often associated with scleroderma, but may also be related to systemic lupus erythematosus, mixed connective tissue disease, Sjorgen's syndrome, dermatomyositis, or rheumatoid arthritis. Scleroderma is a connective tissue disease causing hardening and commonly affects the skin and internal organs such as the GI tract, lungs, kidney and heart.
Six trials which investigated the effect of ketanserin on 146 patients with either primary Raynaud's phenomenon or Raynaud's phenomenon secondary to systemic sclerosis were included (Cadranel 1986; Dormandy 1988; Kirch 1987; Lukac 1985; Ortonne 1989; van de Wal 1987). Patients treated with ketanserin experienced a greater improvement in mean functional index scores and more patients improved than those treated with placebo, however they also experienced more side effects and an increase in the frequency and duration of attacks.
This review assessed a limited number of studies and therefore the conclusions reached need to be investigated further.
Background
Scleroderma is a connective tissue disease causing fibrosis and commonly affects the skin and internal organs such as the GI tract, lungs, kidney and heart (Medsger 1985). Most people with scleroderma also have Raynaud's phenomenon (RP). RP is defined as vasospasm of arteries or arterioles causing pallor and at least one other colour change upon reperfusion such as cyanosis or erythema. Primary RP occurs in the absence of causes such as connective tissue diseases. Secondary RP occurs in people with underlying diseases that affect blood vessels especially scleroderma and lupus. The Raynaud's phenomenon that occurs in scleroderma is often more severe in that there is not only vasospasm but also a fixed blood vessel deficit with intimal proliferation and therefore narrowing of the blood vessels. Raynaud's phenomenon may also be accompanied by digital ulcers which are possibly secondary to ischemia.
There have been many randomized controlled trials of both the treatment of idiopathic or primary RP and secondary RP accompanied by scleroderma and other connective tissue diseases. Over the last two decades better drugs have been developed such as calcium channel blockers, prostacyclin analogues and various other medications as opposed to treatment years ago where the choices were ganglion blockers and alpha blockers, both of which had many side effects such as postural hypotension and dry mouth.
These newer drugs seem to be effective and in general are better tolerated than the former medications used to treat Raynaud's phenomenon. Ketanserin has been studied to determine if it could improve features of Scleroderma including RP.
We therefore undertook a meta‐analysis to determine the efficacy of Ketanserin for the treatment of RP in scleroderma.
Objectives
The objectives of this review were to determine the effectiveness and toxicity of Ketanserin versus Placebo proposed for the treatment of RP in scleroderma.
The specific hypotheses tested were that Ketanserin can:
1) reduce the frequency of attacks
2) reduce the severity of attacks
3) increase digital skin temperature
4) improve the patient and physician's global assessment of the impact of RP
5) prevent new ulcers and heal existing ulcers
Methods
Criteria for considering studies for this review
Types of studies
We aimed to identify all randomized controlled trials (RCTs) in which ketanserin was compared to placebo for treatment of idiopathic RP and RP resulting from progressive Systemic Sclerosis.
Types of participants
1) The subjects for the trials should have scleroderma as defined by the study authors. It was not necessary that such patients satisfy the Preliminary American College of Rheumatology (ACR) criteria for scleroderma (ACR 1980). Trials could include subjects with any subset of scleroderma and at any stage of disease.
2) In the absence of an accepted definition for RP, all subjects reported to have RP were accepted, but the presence or absence of RP definition provided in each published trial was noted.
Mixed Trials: Some trials included RP patients with a number of different diagnoses. Such trials were included if a subset of patients with scleroderma could be separately identified and their outcome independently assessed.
Design Aspects: Trials must have been truly randomized but, given the nature of some interventions (e.g. infusions), blinding may not have been achieved. Observer or subject blindness was noted. Trials with a dropout rate of greater than 30% were excluded. Both parallel and crossover trials were included.
Types of interventions
Interventions of interest was Ketanserin versus Placebo.
Types of outcome measures
Outcomes were considered for trials of any duration apart from very short duration (<1 week ). They included:
1. Frequency of attacks
2. Severity of attacks
3. Digital skin temperature
4. Patient and physician's global assessment of the impact of RP
5. Onset of new ulcers or healing existing ulcers
Search methods for identification of studies
The aim was to ascertain all trials since 1966 in all languages using the Cochrane search strategy developed by Dickersin 1994. Primary data sources include MEDLINE. Those comparing Ketanserin versus Placebo were selected from all articles retrieved.
Along with the Dickersin 1994 search strategy, the search strategy developed for the Cochrane Musculoskeletal Group was carried out including the following key words:
1. Raynauds or Vasospasm
2 . Scleroderma or Progressive Systemic Sclerosis or Connective Tissue disease or Autoimmune Disease.
Data collection and analysis
All data were abstracted using a standardized form, by two independent and trained reviewers (DF, AT), and verified by a third reviewer (JP). Each trial was assessed independently by the same two reviewers for its quality using a validated quality assessment tool (Jadad 1996).
Abstracts, and articles in languages other than English have been collected and will be translated and reviewed for future updates.
A fixed effects model approach was used to calculate a weighted estimate appropriate for continuous variables and an odds ratio (Peto) for dichotomous variables (Petitti 1994). A random effects model will be used where heterogeneity exists amongst trials. Heterogeneity was tested using a chi square test.
Trials were only included if they were randomized and if the dropout rate did not exceed 30%. The reason for the latter criterion is the fact that the placebo response in RP trials is quite high and therefore results would be biased to the positive if the dropout rate was >30%. Trials that examined subjects with scleroderma and other causes of RP such as idiopathic RP, or other secondary connective tissue diseases were included if the data were separated so that the scleroderma data could be determined. We also decided that if a trial contained at least 80% of patients with scleroderma, and the data were not separated, that the trial would still be acceptable. Patients with both diffuse and limited scleroderma were included in the trials and the diagnosis of scleroderma was usually confirmed by the authors diagnosis and often the American College of Rheumatology preliminary criteria for the diagnosis of scleroderma (ACR 1980) were not mentioned.
Results
Description of studies
Six studies were included in this meta‐analysis with trial quality ranging from 2 to 4. Three were of parallel design (Cadranel 1986; Dormandy 1988; Lukac 1985; Ortonne 1989), two were crossover (Kirch 1987; van de Wal 1987). All studies were placebo controlled, and all but one (Kirch 1987) were double blinded. The doses ranged from 40 to 120 mg per day of oral Ketanserin or an identical placebo. Compared to many RP trials these studies were of long duration. They were, in general, also designed to see if Ketanserin decreased the severity of scleroderma.
Twenty‐two studies were excluded from this Systematic Review (Arneklo‐Nobin 1988; Arosio 1989; Arosio 1991; Baart 1986; Brouwer 1987; Caputi 1991; Codella 1989; Coffman 1989; Davinroy 1993; Engelhart 1988; Hansteen 1976; Klimiuk 1989; Kunnen 1988; Longstaff 1985; Lukac 1991; Maloney 1987; Marasini 1988; Marasini 1990; Roald 1984; Seibold 1984; Tooke 1990; van de Wal 1985). Nine trials did not contain placebo data (Arneklo‐Nobin 1988; Arosio 1989; Arosio 1991; Baart 1986; Coffman 1989; Kunnen 1988; Longstaff 1985; Marasini 1988; Tooke 1990), four trials were not randomized (Brouwer 1987; Davinroy 1993; Lukac 1991; Seibold 1984), no control group was present for four studies (Codella 1989; Hansteen 1976; Klimiuk 1989; Marasini 1990), there were no useable data for two studies (Maloney 1987; Roald 1984), one study had a dropout rate greater than thirty percent (Engelhart 1988), data was too subjective for one study (Caputi 1991), and one study was another report on the same study by the same author (van de Wal 1985).
Risk of bias in included studies
The quality assessment was carried out independently by two reviewers (SH, PT).
Using a validated quality assessment tool, we assessed quality defined as "the confidence that the trial design, conduct, and analysis have minimized or avoided biases in its treatment comparisons" (Jadad 1996). The scale consists of items pertaining to descriptions of randomization , double blinding, dropouts and withdrawals as described in the report of a RCT. Consensus was reached on all final scores for each trial. Interobserver agreement was measured using kappa values (> 0.60 indicate substantial strength of agreement) (Cohen 1988). Agreement was very high between reviewers.
The quality of the five studies vary:
Cadranel 1986 = 4 Dormandy 1988 = 3 Kirch 1987 = 2 Lukac 1985 = 3 Ortonne 1989 = 3 van de Wal 1987 = 3
Effects of interventions
The study by Cadranel 1986 included twenty‐seven patients in a six month study wherein patients were treated with ketanserin or placebo with a dose of 80 mg per day. Two patients total dropped out, one from each treatment regimen. Outcomes of interest that were measured include number of patients who experienced side effects and the number of patients who improved. The number of patients who experienced side effects while treated with ketanserin was not significantly different from the number of patients treated with placebo [Peto's OR 1.52 (95% CI 0.34, 6.85)]. The number of patients who improved on ketanserin was also not significantly different than those who improved on placebo [Peto's OR 2.20 (95% CI 0.43, 11.12)].
In the study conducted by Dormandy 1988, twenty‐nine patients were entered into a five week long study. The study design consisted of a one week placebo run in period followed by four weeks of treatment with either ketanserin or placebo (20 mg) four times per day for a total daily intake of 80 mg. The number of patients who improved while treated with ketanserin versus placebo was the only outcome of interest measured, and was not significantly different between treatments [Peto's OR 2.68 (95% CI 0.63, 11.46)].
In the single‐blind study conducted by Kirch 1987, ten patients were entered into a fourteen week long study. The study design consisted of a four week placebo washout period followed by four weeks of treatment with either nifedipine (40 mg per day) or ketanserin (40‐80 mg per day), a two week long placebo washout period, and a final four week period during which patients were crossed over to the opposite active treatment. Outcomes of interest that were reported include number of patients who experienced side effects, number of patients who improved, change in frequency, and change in duration. More patients treated with ketanserin experienced side effects than those treated with placebo, but this finding was not statistically significant [Peto's OR 3.50 (95% CI 0.58, 20.99)]. More patients exhibited a general improvement while on placebo than on ketanserin, but was not statistically significant [Peto's OR 0.47 (95% CI 0.04, 5.19)]. Placebo was favoured significantly over ketanserin in the frequency of attacks [WMD (fixed) 25.20 (95% CI 22.55, 27.85)] and in duration [WMD (fixed) 4.10 (95% CI 3.57, 4.63)].
In the study conducted by Lukac 1985, fifteen patients were entered into a three month trial. Seven patients were randomized to receive placebo for this time, while eight were randomized to receive 60 mg per day of ketanserin for the first month followed by 120 mg of ketanserin per day for the following two months. Outcomes of interest that were measured include the number of patients who experienced side effects, and the number of patients who experienced a general improvement. Significantly more patients treated with ketanserin experienced side effects than those treated with placebo [Peto's OR 13.80 (95% CI 1.73, 110.37)], and significantly more patients experienced a general improvement [Peto's OR 17.97 (95% CI 2.19, 147.79)].
In the study conducted by Ortonne 1989, twenty‐four patients were entered into a thirty week long study. The study design consisted of a six week placebo run in period followed by a six month parallel study during which patients were randomized to receive ketanserin or placebo tablets twice per day. The total daily dose of ketanserin was originally 40 mg per day, but increased after two weeks to the full dose of 80 mg per day to avoid side effects. Outcomes of interest measured include side effects and mean functional index. Patients treated with ketanserin experienced significantly more side effects than those treated with placebo [Peto's OR 3.43 (95% CI 0.64, 18.49)], and showed a greater improvement in mean functional index, but this second outcome was not significant [WMD (fixed) ‐4.30 (95% CI ‐34.28, 25.68)].
In the study by van de Wal 1987, forty‐one patients were entered into a sixteen week study. The study design consisted of a four week placebo run in period followed by six weeks of treatment with either placebo or ketanserin, and a following six weeks during which patients were crossed over to the opposite treatment regimen. The doses of ketanserin were up to 40 mg per day. Outcomes of interest measured include side effects and number of patients who improved. More patients treated with ketanserin experienced side effects, but this finding was not statistically significant [Peto's OR 2.04 (95% CI 0.84, 4.94)]. Significantly more patients treated with ketanserin experienced a general improvement than those treated with placebo [Peto's OR 2.69 (95% CI 1.13, 6.44)].
Side effects and the number of patients who improved were the only data that were reported in more than one study and were the only meta‐analyzable outcomes. Patients treated with ketanserin experienced significantly more side effects [Peto's OR 2.63 (95% CI 1.42, 4.88)] (Cadranel 1986; Kirch 1987; Lukac 1985; Ortonne 1989; van de Wal 1987), and significantly more patients experienced a general improvement [Peto's OR 2.74 (95% CI 1.47, 5.11)] (Cadranel 1986; Dormandy 1988; Kirch 1987; Lukac 1985; van de Wal 1987) than those treated with placebo.
Discussion
Raynaud's Phenomenon is extremely common in scleroderma and is often severe. The literature search for this meta‐analysis reveals that many different classes of drugs have demonstrated some degree of efficacy in the treatment of RP with respect to decreasing RP frequency and severity, and preventing and healing digital ulcers. Because RP is variable and many patients are entered into studies such as these when they are having frequent and severe attacks, there is a high placebo response because of the variability of RP with respect to temperature and other emotional factors. Also, regression to the mean may occur, explaining improvement in a treatment group. This placebo response should be taken into consideration when other drugs are evaluated in primary and secondary RP.
Twenty eight studies were identified for this meta‐analysis of which only six were included. Several randomized controlled trials were excluded because of a subgroup analysis in the scleroderma patients was not done. When these data in the subgroups become available, the results of the meta‐analysis could change. However, it appears that Ketanserin is only somewhat effective in the treatment of Raynaud's phenomenon in patients with scleroderma. The results are small and do not always favour Ketanserin over placebo. Limitations in these studies include: the dose was variable between 20‐120 mg per day; the trial duration varied; and the duration of study could account for fluctuation in RP severity due to temperature changes over several months. It also appeared in these studies that Ketanserin was not effective in the treatment of skin and other sclerodermatous changes in these patients.
There are several limitations to the meta‐analysis. Our search from MEDLINE and the references of key review articles may only reveal some of the published articles. Therefore, at this point in time some articles could be missing. Additional positive and negative articles may confirm these results but not dramatically change them.
The dose of Ketanserin varied, and efficacy may occur only at higher doses. A dose of 40 mg/d is ineffective in RP secondary to Scleroderma.
We were unable to record temperature as one of our a priori outcome measurement from these trials for two reasons: first, it was not recorded in many of the trials, and second, the digital temperature was recorded quite differently in trials. Therefore, we thought that this was not a clinically helpful outcome measurement compared to the frequency and severity of RP attacks. No analyzable data were obtained with respect to digital ulcers.
This meta‐analysis does not address side effects if a trial had more than 30% of its' participants dropout. One trial was excluded for a dropout rate > 30%. Many of the authors commented that the response in scleroderma with respect to the Raynaud's phenomenon was sub‐optimal compared to those with idiopathic RP. For the various reasons indicated earlier, it makes sense that the scope of this meta‐analysis was not to compare the efficacy of treatment of RP in scleroderma versus other conditions. As a result, this was not addressed in the data presented. With the presentation of changes from baseline and variance or a standard deviation provided for each trial, it will allow future researchers to calculate sample sizes for new drugs that may be used in the treatment of RP in patients with scleroderma.
In conclusion, Ketanserin is not an outstanding drug for the treatment of RP in Scleroderma.
Authors' conclusions
Implications for practice.
Ketanserin has been tested in several clinical trials for Raynaud's phenomenon and appears to lead to small improvement in Raynaud's phenomenon in some patients with scleroderma. However, not all parameters favour active treatment and this drug should not be the usual early choice for Raynaud's phenomenon in this population.
Implications for research.
If other studies are designed using Ketanserin in Raynaud's phenomenon secondary to scleroderma, the past literature reveals that low dose Ketanserin (40 mg/day) is not statistically significantly different in its efficacy from placebo and causes side effects at approximately an equal rate to the higher doses. Therefore this low dose is not clinically effective and shouldn't be used. Also in this meta‐analysis it can be demonstrated that the placebo response in RP trials is high and this will inflate sample size calculations for other randomized controlled trials in secondary Raynaud's phenomenon.
What's new
| Date | Event | Description |
|---|---|---|
| 29 August 2008 | Amended | Converted to new review format. CMSG ID: C047‐R |
Data and analyses
Comparison 1. Ketanserin vs. Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients who improved | 5 | 170 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.74 [1.47, 5.11] |
| 2 side effects | 5 | 166 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.63 [1.42, 4.88] |
| 3 Change in duration of attacks over the course of the study (min) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 4.1 [3.57, 4.63] |
| 4 Change in frequency of attacks over a 2 week period | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | 25.2 [22.55, 27.85] |
| 5 Change in severity (10 point scale) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Mean Functional Index | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐4.30 [‐34.28, 25.68] |
1.1. Analysis.
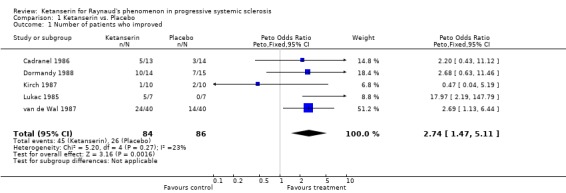
Comparison 1 Ketanserin vs. Placebo, Outcome 1 Number of patients who improved.
1.2. Analysis.
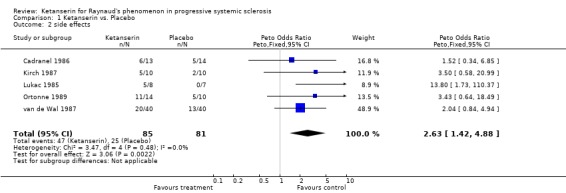
Comparison 1 Ketanserin vs. Placebo, Outcome 2 side effects.
1.3. Analysis.
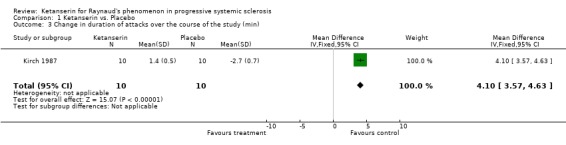
Comparison 1 Ketanserin vs. Placebo, Outcome 3 Change in duration of attacks over the course of the study (min).
1.4. Analysis.
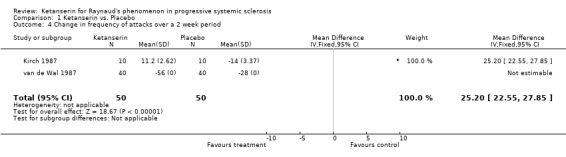
Comparison 1 Ketanserin vs. Placebo, Outcome 4 Change in frequency of attacks over a 2 week period.
1.5. Analysis.
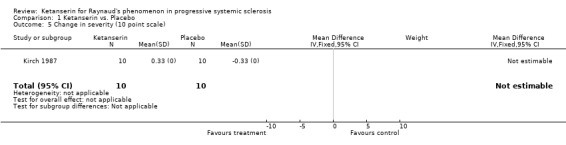
Comparison 1 Ketanserin vs. Placebo, Outcome 5 Change in severity (10 point scale).
1.6. Analysis.
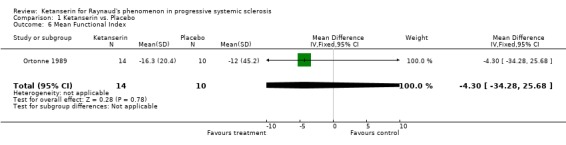
Comparison 1 Ketanserin vs. Placebo, Outcome 6 Mean Functional Index.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cadranel 1986.
| Methods | A randomized double‐blind placebo‐controlled clinical trial Efficacy Parallel design | |
| Participants | N= 27 with PSS, 22 female and 5 male N= 13 patients in the Ketanserin group and N=14 patients in the placebo group Source of population: Community Country: France. No patients dropped out. | |
| Interventions | Ketanserin or placebo for 6 months | |
| Outcomes | Patients reports of arthralgias, dyspnea, and intensity of Raynaud's phenomenon Number of acral ulcers Skin scores Hand mobility Oral aperture Number and duration of RP attacks Total blood viscosity Plasma viscosity Thixotropism | |
| Notes | Quality Score = 4 | |
Dormandy 1988.
| Methods | Double blind Parallel Placebo controlled Randomized | |
| Participants | N=29 patients with typical symptoms and signs of RP for at least 1 year. 10 normal healthy volunteers in addition to 29 afflicted patients. No patients dropped out. | |
| Interventions | 1 week placebo run‐in period. Patients allocated to recieve either placebo or ketanserin for 4 weeks. Before any measurements patients and volunteers rested and avoided drinking and smoking. | |
| Outcomes | At the start of the trial and after 4 weeks of double blind treatment digital blood flows were recorded. In the normal volunteers only one set of measurements was made. At the begining of the study patients were asked to keep a diary of duration and frequency of attacks throughout the trial. Assessment of the occurrence of cold sensation, numbness and pain were recorded. | |
| Notes | Quality Score = 3 | |
Kirch 1987.
| Methods | Single blind Placebo controlled Randomized Crossover design | |
| Participants | N = 10 patients, aged 18‐60 years, 5 male, 5 female Typical RP and related connective tissue disorders. No patients dropped out. | |
| Interventions | Patients recieved no drugs other than ketanserin, nifedipine, or placebo. Initially recieved orally in the washout period placebo for 4 weeks. Then randomly assigned to recieve either nifedipine orally or ketanserin for four weeks. After this a 2 week lasting placebo phase interconnected with a crossover to the final 4 week lasting therapy phase. | |
| Outcomes | Patients recorded possible adverse effects, frequency, duration, and severity of attacks. Severity assessed using a 3 point scale. Patients rated treatment on a 3 point scale. Laboratory parameters including blood cell count, ESR, and a biochemical analysis of renal and hepatic function was performed. Plethysmography, skin temperature recordings, and video microscopy were performed, thereby flowmetry, a standard cold provocation test, and typical morphological changes of skin capillaries were monitored. | |
| Notes | Quality Score = 2 | |
Lukac 1985.
| Methods | A randomized double‐blind placebo‐controlled clinical trial Parallel design | |
| Participants | N=15, 13 female and 2 male N= 8 in the Ketanserin group and N=7 in the placebo group Mean age = 51.1 yrs Source of Population: Community Country: Czechoslovakia. 1 patient dropped out. | |
| Interventions | Oral Ketanserin or placebo 20mg TID for 1st month Oral Ketanserin or placebo 40mg TID for 2nd and 3rd months | |
| Outcomes | Infrared radiometry for circulatiory damage U/S doppler for circulatory damage Capillary microscopy for circulatory damage Hand temperature Circulatory defects by ring thermographic index in std conditions and after cold stress Velocity of blood flow Number and extent of acral ulcerations BP Heart frequency Body weight Patient complaints ESR, CBC, plasma electrophoresis, AST, ALT, ANA, C3, latex fixation test Measurements taken at baseline and 3 months Duration: 3 months | |
| Notes | Qualtiy Score = 3 | |
Ortonne 1989.
| Methods | A randomized controlled trial Double blind Efficacy Parallel design | |
| Participants | Twenty four patients with systemic sclerosis Source of population: community Country: France Mean age: 53.7. All patients completed the trial. | |
| Interventions | Ketanserin or identical placebo | |
| Outcomes | Functional signs (VAS) Raynaud's phenomenon (i.e. severity, frequency of attacks, duration of pallor phase, Duration of cyanotic phase) Clinical signs (oral aperture, height, width, finger circumstance, SBP, DPB, Heart rate) Global assessments (mean functional index, global physician assessment) | |
| Notes | Quality Score = 3 | |
van de Wal 1987.
| Methods | Randomized Double blind Placebo controlled Crossover design | |
| Participants | N=41 patients with primary RP. 26 female, 15 male. Mean age = 46, ranging from 15 to 74. All patients experienced vasospastic attacks induced by cold or emotion. Mean duration of symptoms was 7 years. 21 patients were smokers. 1 patient dropped out. | |
| Interventions | Additional vasoactive drugs prohibited for the trial period, other medications continued. 16 weeks long, divided into patients introduction placebo runin period of 4 weeks, and the 12 week study with crossover after 6 weeks. For the next 4 weeks patients in the active group recieved Ketanserin BID. | |
| Outcomes | Patients asked to keep a diary of duration and frequency of attacks. Existence of edema was examined and checked by body weight. Patients were asked about side effects. Diary contained details of cold sensation, numbness, parasthesia, pain, and smoking habits. Non‐invasive vascular measurements included a study of digital skin temperature, digital cystolic blood pressure, and doppler spectral analysis. All measurements performed on each visit to the vascular laboratory before and after instant provocation on the left hand. | |
| Notes | Quality Score = 3 | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arneklo‐Nobin 1988 | Data not presented per subgroup; unable to include statistical data. |
| Arosio 1989 | Data not presented per subgroup; unable to include statistical data. |
| Arosio 1991 | Data not present per subgroup; unable to include statistical data. |
| Baart 1986 | Data not present per subgroup; unable to include statistical data. |
| Brouwer 1987 | Not randomized |
| Caputi 1991 | Data too subjective, no statistical outcomes report |
| Codella 1989 | No placebo |
| Coffman 1989 | Data not presented per subgroup; unable to include statistical data. |
| Davinroy 1993 | Not randomized |
| Engelhart 1988 | Dropout greater than 30 percent, does not meet inclusion criteria |
| Hansteen 1976 | No control group |
| Klimiuk 1989 | No control group |
| Kunnen 1988 | Data not presented per subgroup; unable to include statistical data. |
| Longstaff 1985 | Data not presented per subgroup; unable to include statistical data. |
| Lukac 1991 | Not randomized |
| Maloney 1987 | No useable data |
| Marasini 1988 | Data not presented per subgroup; unable to include statistical data. |
| Marasini 1990 | No placebo |
| Roald 1984 | Did not meet inclusion criteria, no outcomes of interest |
| Seibold 1984 | Not randomized |
| Tooke 1990 | Data not presented per subgroup; unable to include statistical data. |
| van de Wal 1985 | Same study as van de Wal 1987 |
Contributions of authors
Pope J ‐ Guarantor, Coordinator, Conceiving the review, Designing search strategies, Screening search results, Obtaining and screening data on unpublished studies, Providing general advice on the review, Securing funding for the review
Tingey P ‐ Data collection, Designing the review, Undertaking searches, Organized retrieval of papers, Screening retrieved papers against inclusion criteria, Appraising quality of papers, Extracting data from papers, Obtaining and screening data on unpublished studies, Data management, Entering data into Revman, Analysis of data, Interpretation of data, Writing the review
Harding S ‐ Data collection, Designing the review, Undertaking searches, Organized retrieval of papers, Screening retrieved papers against inclusion criteria, Appraising quality of papers, Extracting data from papers, Obtaining and screening data on unpublished studies, Data management, Entering data into Revman, Analysis of data, Interpretation of data, Writing the review
Sources of support
Internal sources
University of Western Ontario, London, Canada.
External sources
No sources of support supplied
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Cadranel 1986 {published data only}
- Cadranel J, Bletry O, Guillevin L, Lacombe C, Fraitag B, Duloroy J, Mouthon JM, Godeau P. [Treatment of systemic scleroderma with ketanserin. Randomized, double‐blind 6‐months study of 27 cases]. [French] [Traitment de la sclerodermie systemique par la ketanserine]. Annales de Medecine Interne 1986;137(3):260‐3. [PubMed] [Google Scholar]
Dormandy 1988 {published data only}
Kirch 1987 {published data only}
- Kirch W, Linder HR, Hutt HJ, Ohnhaus EE, Mahler F. Ketanserin versus nifedipine in secondary Raynaud's phenomenon. VASA 1987;16(1):77‐80. [PubMed] [Google Scholar]
Lukac 1985 {published data only}
- Lukac J, Rovensky J. Tauchmannova H.Zitnan D. Effect of ketanserin on Raynaud's phenomenon in progressive systemic sclerosis: a double‐blind trial. Drugs Under Experimental & Clinical Research 1985;11(9):659‐63. [PubMed] [Google Scholar]
Ortonne 1989 {published data only}
- Ortonne JP, Torzuoli C. Dujardin P.Fraitag B. Ketanserin in the treatment of systemic sclerosis: a double‐blind controlled trial. British Journal of Dermatology 1989;120(2):261‐6. [DOI] [PubMed] [Google Scholar]
van de Wal 1987 {published data only}
- Wal HJCM, Wijn PFF, Leer HJJ, Skotnicki SH. The effectiveness of ketanserin in patients with primary Raynaud's phenomenon. A randomized, double blind, placebo controlled study. International Angiology 1987;6(3):313‐22. [PubMed] [Google Scholar]
References to studies excluded from this review
Arneklo‐Nobin 1988 {published data only}
- Arneklo‐Nobin B, Elmer O, Akesson A. Effect of long‐term ketanserin treatment on 5‐HT levels, platelet aggregation and peripheral circulation in patients with Raynaud's phenomenon. A double‐blind, placebo‐controlled cross‐over study. International Angiology 1988;7(1):19‐25. [PubMed] [Google Scholar]
Arosio 1989 {published data only}
- Arosio E, Montesi G, Zannoni M, Paluani F, Lechi A. Comparative efficacy of ketanserin and pentoxiphylline in treatment of Raynaud's phenomenon. Angiology 1989;40(7):633‐8. [DOI] [PubMed] [Google Scholar]
Arosio 1991 {published data only}
- Arosio E, Montesi G, Zannoni M, Perbellini L, Paluani F, Lechi A. Efficacy of ketanserin in the therapy of Raynaud's phenomenon: thermometric data. Angiology ‐ The Journal of Vascular Diseases 1991;42(5):408‐13. [DOI] [PubMed] [Google Scholar]
Baart 1986 {published data only}
- Baart de la Faille H, Weelden H, Banga JD, Kesteren RG. Cold‐induced Raynaud's phenomenon ameliorated by intravenous administration of ketanserin: a double‐blind cross‐over study. Archives of Dermatological Research 1986;279(1):3‐7. [DOI] [PubMed] [Google Scholar]
Brouwer 1987 {published data only}
- Brouwer RML, Wenting GJ, Man in't Veld AJ, Schalekamp MADH. Role of alpha‐adrenergic blockade in the cardiovascular actions of ketanserin: studies in patients with essential hypertension, autonomic insufficiency, and Raynaud's disease. Journal of Cardiovascular Pharmacology 1987;10(Supplement 3):S26‐31. [PubMed] [Google Scholar]
Caputi 1991 {published data only}
- Caputi CA, Carolis G, Tomasetti C. Regional intravenous ketanserin and guanethidine therapy in Raynaud's phenomenon. Angiology 1991;42(6):473‐80. [DOI] [PubMed] [Google Scholar]
Codella 1989 {published data only}
- Codella O, Caramaschi P, Olivieri O, Perbellini L, Perbellini A, Bambara LM, Corrocher R, Sandre G. Controlled comparison of ketanserin and nifedipine in Raynaud's phenomenon. Angiology 1989;40(2):114‐21. [DOI] [PubMed] [Google Scholar]
Coffman 1989 {published data only}
- Coffman JD, Clement DL, Creager MA, Dormandy JA, Janssens MM, McKendry RJ, Murray GD, Nielsen SL. International study of ketanserin in Raynaud's phenomenon. American Journal of Medicine 1989;87(3):264‐8. [DOI] [PubMed] [Google Scholar]
Davinroy 1993 {published data only}
- Davinroy M, Mosnier M. Double‐blind clinical evaluation of naftidrofuryl in Raynaud's phenomenon. Semaine des Hopitaux 1993;69(36):1322‐6. [Google Scholar]
Engelhart 1988 {published data only}
- Engelhart M. Ketanserin in the treatment of Raynaud's phenomenon associated with generalized scleroderma. British Journal of Dermatology 1988;119(6):751‐4. [DOI] [PubMed] [Google Scholar]
Hansteen 1976 {published data only}
- Hansteen V. Medical treatment in Raynaud's disease. Acta Chirurgica Scandinavica. Supplementum 1976;465:87‐91. [PubMed] [Google Scholar]
Klimiuk 1989 {published data only}
- Klimiuk PS, Kay EA, Mitchell WS, Taylor L, Gush R, Gould S, Jayson MIV. Ketanserin: An Effective Treatment Regimen for Digital Ischaemia in Systemic Sclerosis. Scandinavian Journal of Rheumatology 1989;18:107‐11. [DOI] [PubMed] [Google Scholar]
Kunnen 1988 {published data only}
- Kunnen JJ, Dahler HP, Doorenspleet JG, Oene JC. Effects of intra‐arterial ketanserin in Raynaud's phenomenon assessed by 99MTc‐pertechnetate scintigraphy. European Journal of Clinical Pharmacology 1988;34(3):267‐71. [DOI] [PubMed] [Google Scholar]
Longstaff 1985 {published data only}
- Longstaff J, Gush R, Williams EH, Jayson MI. Effects of ketanserin on peripheral blood flow, haemorheology, and platelet function in patients with Raynaud's phenomenon. Journal of Cardiovascular Pharmacology 1985;7 Suppl 7:S99‐101. [DOI] [PubMed] [Google Scholar]
Lukac 1991 {published data only}
- Lukac J, Rovensky J, Tauchmannova H, Zitnan D. Long‐term ketanserin treatment in patients with systemic sclerosis in Raynaud's phenomenon. Current Therapeutic Research 1991;50(6):869‐77. [Google Scholar]
Maloney 1987 {published data only}
Marasini 1988 {published data only}
- Marasini B, Biondi ML, Bianchi E, Dell'Orto P, Agostoni A. Ketanserin treatment and serotonin in patients with primary and secondary Raynaud's phenomenon. European Journal of Clinical Pharmacology 1988;35(4):419‐21. [DOI] [PubMed] [Google Scholar]
Marasini 1990 {published data only}
- Marasini B, Bassani C. Digital blood flow and 5‐hydroxytryptamine receptor blockade after ketanserin in patients with Raynaud's phenomenon. British Journal of Clinical Pharmacology 1990;30:847‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roald 1984 {published data only}
- Roald OK, Seem E. Treatment of Raynaud's phenomenon with ketanserin in patients with connective tissue disorders. British Medical Journal Clinical Research Ed 1984;289(6445):577‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seibold 1984 {published data only}
- Seibold JR, Jageneau AHM. Treatment of Raynaud's Phenomenon with Ketanserin, a Selective Antagonist of the Serotonin2 (5‐HT2) Receptor. Arthritis and Rheumatism 1984;27(2):139‐46. [DOI] [PubMed] [Google Scholar]
Tooke 1990 {published data only}
- Tooke JE, Williams SA, Rawlinson DW, Black C. Ketanserin and capillary flow in Raynaud's phenomenon. International Journal of Microcirculation: Clinical & Experimental 1990;9(3):249‐55. [PubMed] [Google Scholar]
van de Wal 1985 {published data only}
- Wal HJCM, Wijn PFF, Leer HJJ, Skotnicki SH. Quantitative study of the effects of ketanserin in patients with primary Raynaud's phenomenon. Microcirculation, Endothelium, and Lymphatics 1985;2:657‐85. [PubMed] [Google Scholar]
Additional references
ACR criteria
- Masi AT, Rodnan GP, Medsger TA Jr, et al. ACR Preliminary criteria for the classification of systemic sclerosis (scleroderma): Special article. Arthritis and Rheumatism 1980;23:581‐90. [DOI] [PubMed] [Google Scholar]
Altman 2001
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbournse D, Gotzsche PC, Lang T. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Annals of Internal Medicine 2001;134:663‐94. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillside, New Jersey: Lawrence Erlbaum Associates, Inc, 1988:21‐34. [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freedman 1984
- Freedman RR, Ianni P, Wenig P. Behavioral treatment of Raynaud's phenomenon in scleroderma. Journal of Behavioral Medicine 1984;7(4):343‐53. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad A, Moore A, Carrol D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Kahaleh 1994
- Kahaleh MB. Raynaud's phenomenon and vascular disease in scleroderma. Current Opinion in Rheumatology 1994;6:621‐7. [DOI] [PubMed] [Google Scholar]
LeRoy 1992
- LeRoy EC, Medsger TA. Raynaud's Phenomenon: A Prosposal for Classification. Clinical and Experimental Rheumatology 1992;10:485‐8. [PubMed] [Google Scholar]
Medsger 1985
- Medsger TA JR. Systemic sclerosis (scleroderma), eosinophilic fasciitis, and calcinosis. In: McCarty DJ editor(s). Arthritis and Allied Conditions. 10. Philadelphia: Lea & Febiger. [Google Scholar]
Petitti 1994
- Petitti D. Meta‐analysis, decision analysis, and cost‐effectiveness analysis: methods for quantitative synthesis in medicine. New York: Oxford University Press, 1994:90‐114. [Google Scholar]
Sapira 1972
- Sapira JD, Rodnan GP, Scheib ET, Klaniecki T, Rizk M. Studies of Endogenous Catecholamines in Patients with Raynaud's Phenomenon Secondary to Progressive Systemic Sclerosis (Scleroderma). The American Journal of Medicine 1972;52:330‐7. [DOI] [PubMed] [Google Scholar]


