Abstract
The objectives of the current experiments were to evaluate the effect of feeding soybean oil (SO) with different levels of peroxidation on lipid, N, and GE digestibility, gut integrity, oxidative stress, and growth performance in nursery pigs. Treatments consisted diets containing 10% fresh SO (22.5 °C) or thermally processed SO (45 °C for 288 h, 90 °C for 72 h, or 180 °C for 6 h), each with an air infusion of 15 L/min, with postprocessing peroxide values of 7.6, 11.5, 19.1, and 13.4 mEq/kg and p-anisidine values of 1.92, 6.29, 149, and 159, for the 22.5 °C, 45 °C, 90 °C and 180 °C processed SO, respectively. In experiment 1, 64 barrows (7.1 ± 0.9 kg initial BW) were randomly allotted into 2 rooms of 32 pens and individually fed their experimental diets for 21 d, with a fresh fecal sample collected on day 20 for determination of GE and lipid digestibility. In experiment 2, 56 barrows (BW 9.16 ± 1.56 kg) were placed into individual metabolism crates for assessment of GE, lipid, and N digestibility and N retention. Urinary lactulose to mannitol ratio was assessed to evaluate in vivo small intestinal integrity, and urine and plasma were collected to analyze for markers of oxidative stress. Pigs were subsequently euthanized to obtain liver weights and analyze the liver for markers of oxidative stress. In experiment 1, pigs fed the SO thermally processed at 90 °C had reduced ADG (P = 0.01) and ADFI (P = 0.04) compared to pigs fed the other SO treatment groups, with no differences noted among pigs fed the 22.5 °C, 45 °C, and 180 °C SO treatments. No effects of feeding thermally processing SO on dietary GE or lipid digestibility (P > 0.10) were noted in either experiment. In experiment 2, there was no dietary effect of feeding peroxidized SO on the DE:ME ratio, N digestibility, or N retained as a percent of N digested, on the urinary ratio of lactulose to mannitol, on serum, urinary, or liver thiobarbituric acid reactive substances, on plasma protein carbonyls, or on urinary or liver 8-OH-2dG (P > 0.10). In experiment 2, pigs fed the SO thermally processed at 90 °C had the greatest isoprostane concentrations in the serum (P ≤ 0.01) and urine (P ≤ 0.05) compared to pigs fed the unprocessed SO. These results indicate that the change in fatty acid composition and/or the presence of lipid peroxidation products in peroxidized SO may reduce ADG and ADFI in nursery pigs, but appears to have no impact on GE, lipid, or N digestibility, or gut permeability. These data suggest that the presence of lipid peroxidation products may affect certain markers of oxidative stress.
Keywords: digestibility, gastrointestinal integrity, nursery pigs, peroxidized soybean oil
Introduction
Energy is one of the most expensive components of swine feed formulation with lipids providing a concentrated energy source to the diet (Pettigrew and Moser, 1991; Azain, 2001; Lin et al., 2013). Lipids with high concentrations of unsaturated fatty acids such as soybean oil (SO), however, are highly susceptible to lipid peroxidation (Holman, 1954) in the presence of heat, oxygen, light, or transition metals (Labuza, 1971; Lundburg and Jarvi, 1971; Gray, 1978). Lipid peroxidation involves free radical chain reactions with products formed and degraded during the process, including products such as peroxides, lipid hydroperoxides, polar and nonpolar acids, ketones, and aldehydes, and polymers; while antioxidants present in the lipid becoming depleted over time (Gonzalez-Muñoz et al., 1998; Seppanen and Csallany, 2002;Schaich, 2005).
Consumption of peroxidized lipids has been shown to decrease lipid and energy digestibility (Liu et al., 2014c; Lindblom et al., 2018a; Overholt et al., 2018a) and growth performance in growing swine (DeRouchey et al., 2004; Boler et al., 2012; Rosero et al., 2015), but there is limited information as to which components of lipid peroxidation are most detrimental to lipid and energy digestibility, animal performance, and oxidative status. Therefore, the objective of this study was to evaluate the effect of feeding divergently thermally processed SO to nursery pigs on growth performance, digestibility, intestinal integrity, and oxidative stress.
Materials and methods
All animal care and use procedures for this experiment were approved by the Institutional Animal Care and Use Committee at Iowa State University.
Dietary treatments
Each experiment used the same four thermally processed SO added to diets which included either 10% fresh SO (22.5 °C) or SO thermally processed at 45 °C for 288 h, 90 °C for 72 h, or 180 °C for 6 h. Except for the 22.5 °C temperature, each heating process was accompanied with constant air flow (15 L/min) using an air pump and a calibrated air flow controller with air forced into the tank using a 9.5 mm diameter copper pipe. Immersion heaters were used to heat the SO to 45 °C and 90 °C, while a liquid propane heater was used to heat the SO to 180 °C. Oil temperatures were taken at regular intervals to ensure the proper heating temperature was maintained. The stainless steel heating pots were 53 cm in diameter and 61 cm high and were filled two-thirds full during the heating process. Diets for each experiment were mixed with no antioxidant added before or during diet preparation, with each SO and diet sampled at the time of mixing, and stored at 0 °C prior to analysis. Diverse analyses of each SO were conducted as given in Table 1 to characterize the composition and quality of each SO treatment (Table 2). Within each experiment, diets were formulated to be adequate in energy and nutrients relative to the NRC (2012) recommendations, with experiments 1 and 2 diets formulated to contain 1.35% and 1.20% standardized ileal digestible lysine, respectively (Table 3).
Table 1.
Method of analysis for thermally processed soybean oils
| Analyte | Method |
|---|---|
| Aldehydes1 | Wang et al., 2016 |
| p-Anisidine value2 | AOCS Cd 18–90 |
| Fatty acids2 | AOCS Ce 1a–13 |
| Free fatty acids2 | AOCS Ca 5A–40 |
| Free glycerin2 | AOCS Ca 14–56 |
| Insoluble impurities2 | AOCS Ca 3–46 |
| Moisture2 | AOCS Ca 2c–25 |
| Oil stability index2 | AOCS Cd 12b–92 |
| Oxidized fatty acids2 | AOCS G 3–53 |
| Peroxide value2 | AOCS Cd 8b–90 |
| Polymerized triacylglycerides3 | AOAC 993.25 |
| Thiobarbituric acid value2 | AOCS Cd 19–90 |
| Tocopherols2 | AOCS Ce 8–89 |
| Total polar compounds2 | AOCS Cd 20–91 |
| Unsaponifiable matter2 | AOCS Ca 6a–40 |
1Analyzed by the University of Minnesota, St. Paul, MN.
2Analyzed by Barrow-Agee, Memphis, TN; AOCS, 2011.
3Analyzed by the USDA-ARS, Peoria, IL; AOAC, 2007.
Table 2.
Composition and peroxidation analysis of thermally processed soybean oils
| Heating temperature, °C | 22.5 | 45 | 90 | 180 |
|---|---|---|---|---|
| Time heated, h1 | 0 | 288 | 72 | 6 |
| Fatty acids, % of total fat2,3 | ||||
| C14:0, Myristic | 0.08 | ND | 0.08 | 0.08 |
| C16:0, Palmitic | 10.94 | 11.01 | 11.99 | 11.49 |
| C16:1, Palmitoleic | 0.08 | 0.08 | 0.09 | 0.09 |
| C17:0, Margaric | 0.09 | 0.10 | 0.11 | 0.10 |
| C18:0, Stearic | 4.10 | 4.06 | 4.41 | 4.26 |
| C18:1, Oleic | 23.27 | 23.21 | 24.74 | 23.82 |
| C18:2, Linoleic | 53.09 | 53.18 | 50.98 | 51.98 |
| C18:3, Linolenic | 7.38 | 7.42 | 6.42 | 6.85 |
| C20:0, Arachidic | 0.31 | 0.31 | 0.33 | 0.32 |
| C20:1, Gadoleic | 0.17 | 0.18 | 0.19 | 0.28 |
| C22:0, Behenic | 0.33 | 0.32 | 0.37 | 0.36 |
| C24:0, Lignoceric | ND | ND | 0.13 | 0.11 |
| Other FA4 | 0.15 | 0.13 | 0.17 | 0.26 |
| UFA:SFA4 | 5.30 | 5.32 | 4.73 | 4.97 |
| IV5 | 131 | 132 | 127 | 129 |
| Free fatty acids, %2 | 0.03 | 0.03 | 0.20 | 0.08 |
| Free glycerin, %2 | 0.77 | 0.61 | 0.41 | 0.62 |
| Moisture, % | 0.02 | 0.02 | 0.10 | 0.04 |
| Insoluble impurities, % | 0.04 | 0.06 | 0.04 | 0.08 |
| Unsaponifiable matter, % | 0.34 | 0.38 | 0.32 | 0.30 |
| Oxidized FA, %2 | 1.0 | 1.1 | 2.7 | 1.6 |
| OSI at 110 °C, h2,4 | 4.95 | 3.35 | 2.35 | 2.95 |
| p-Anisidine value2,6 | 1.92 | 6.29 | 149 | 159 |
| Peroxide value, mEq/kg2 | 7.6 | 11.5 | 19.1 | 13.4 |
| Polar compounds, %2 | 5.44 | 8.44 | 22.80 | 13.60 |
| PTAG4,7, % | ND | ND | 4.07 | 2.66 |
| TBA value2,6 | 0.05 | 0.07 | 0.06 | 0.07 |
| Aldehydes, mg/kg8 | ||||
| 2,4-decadienal | 0.40 | 4.71 | 835.63 | 679.65 |
| 4-hydroxynonenal | 0.60 | 2.28 | 159.21 | 72.23 |
| Acrolein | 5.85 | 5.97 | 23.23 | 31.59 |
| 2-Decenal | 0.10 | 0.18 | 46.28 | 54.96 |
| 2,4-Heptadienal | 0.15 | 4.16 | 238.13 | 128.12 |
| 2-Heptenal | 1.52 | 3.51 | 220.59 | 74.41 |
| Hexanal | 2.19 | 2.31 | 33.07 | 5.85 |
| 2-Octenal | 0.45 | 1.21 | 166.14 | 40.26 |
| Pentanal | 2.01 | 0.63 | 10.86 | 2.84 |
| 2,4-Undecadienal | 0.03 | 0.15 | 38.97 | 35.31 |
| 2-Undecenal | 0.10 | 0.20 | 43.42 | 66.50 |
| Ratio9 | 0.11 | 0.20 | 0.60 | 0.98 |
| Total tocopherols, mg/kg2 | 870 | 821 | 147 | 595 |
| Alpha | 63 | 49 | 147 | <10 |
| Beta | <10 | <10 | <10 | <10 |
| Delta | 223 | 141 | <10 | 217 |
| Gamma | 584 | 631 | <10 | 378 |
1Thermally processed oils had a constant air flow rate at 15 L/min.
2Analyzed by Barrow-Agee, Memphis, TN.
3No other FA were detected besides those listed.
4ND, not detected; FA, fatty acid; UFA:SFA, unsaturated:saturated fatty acid ratio; TBA, thiobarbituric acid; OSI, oil stability index; TPC, total polar compounds; PTAGS, polymerized tryacylglycerides; IV, iodine value.
5Iodine values were calculated using the FA profile data following the equation proposed by Meadus et al. (2010): IV = (16:1 × 0.95) + (18:1 × 0.86) + (18:2 × 1.732) + (18:3 × 2.616) + (20:1 × 0.795) + (20:2 × 1.57) + (20:3 × 2.38) + (20:4 × 3.19) + (20:5 × 4.01) + 22:4 × 2.93) + (22:5 × 3.68) + (22:6 × 4.64).
6There are no units for p-anisidine value or TBA value.
7Analyzed by the USDA-ARS, Peoria, IL.
8Analyzed by the University of Minnesota, St. Paul, MN.
9Ratio of 2-decenal, 2,4-hydroxynonenal, 2,4-undecadienal, and 2-undecenal as a percent of total aldehydes to acrolein, 2,4-heptadienal, and 2-heptenal as a percent of total aldehydes; Wang et al., 2016.
Table 3.
Ingredient and calculated composition of treatment diets, as-is basis
| Ingredient, % | Experiment 11 | Experiment 21 |
|---|---|---|
| Corn | 45.37 | 54.68 |
| Soybean meal, 46% CP | 16.01 | 16.16 |
| Soybean oil heat treatment2 | 10.00 | 10.00 |
| Dried whey | 13.50 | 9.00 |
| Fish meal | 9.00 | 4.50 |
| Soy protein concentrate | — | 2.25 |
| Porcine plasma | 4.50 | 1.13 |
| Limestone | 0.22 | 0.90 |
| Monocalcium phosphate | — | 0.17 |
| Sodium chloride | 0.36 | 0.36 |
| Vitamin mix3 | 0.23 | 0.23 |
| Trace mineral mix4 | 0.14 | 0.14 |
| l-lysine•HCl | 0.14 | 0.31 |
| l-threonine | — | 0.07 |
| dl-methionine | 0.08 | 0.10 |
| Titanium dioxide | 0.45 | — |
| Total | 100.00 | 100.00 |
| Calculated composition | ||
| ME, kcal/kg | 3,800 | 3,800 |
| CP, % | 22.4 | 18.9 |
| Lys, % | 1.50 | 1.35 |
| SID Lys5, % | 1.35 | 1.20 |
| Ca, % | 0.77 | 0.77 |
| STTD P5, % | 0.53 | 0.36 |
| Analyzed composition6 | ||
| Crude fat, % | 13.54 | 11.73 |
1Pigs were fed for 21 d on each experiment, 7.1 to 16.6 kg BW for experiment 1 and 9.2 to 12.8 kg BW for experiment 2.
2Dietary treatments consisted of 10% refined soybean oil that was either fresh oil, heated for12 d at 45 °C, heated for 72 h at 90 °C, or heated for 6 h at 180 °C. All heated soybean oil groups had a constant compressed air flow rate at 15 L/min.
3Provided the following per kilogram of diet: vitamin A, 7,044 IU; vitamin D3, 805 IU; vitamin E, 57.5 IU; vitamin K, 3.45 mg; vitamin B12, 0.06 mg; riboflavin, 12.65 mg; niacin, 64.4 mg; and pantothenic acid, 31.05 mg.
4Provided the following per kilogram of diet: Cu (as CuSO4), 15.4 mg; Fe (as FeSO4), 154 mg; I (as Ca(IO3)2), 0.28 mg; Mn (as MnSO4), 36.4 mg; Zn (as ZnSO4), 154 mg; and Se (Na2SeO3), 0.28 mg.
5SID, standardized ileal digestible; STTD, standardized total tract digestible.
6Average analysis across all four soybean oil treatments.
Experimental design
Experiment 1
Sixty-four weanling barrows (6.0 × F25 Genetiporc; PIC Inc., Hendersonville, TN) were obtained from a commercial farm at weaning (28 d of age) and transported and housed at the Iowa State University Swine Nutrition Farm (Ames, IA). For 10 d, pigs were group-housed in one large pen and fed a common starter diet to optimize feed intake during the weaning transition period. Pigs were then randomly allotted into two rooms of 32 pens and placed individually into stainless steel pens measuring 0.46 m × 1.22 m (initial BW of 7.1 ± 0.9 kg). Within each room, pigs were randomly allotted to one of four treatments, resulting in 16 replications per treatment across the two rooms. Pigs were individually fed the experimental diets in meal form over the 21 d feeding period and allowed ad libitum access to feed and water. Each room was maintained with 24 h lighting, was mechanically ventilated, and had a pull-plug manure storage system. On day 20, a freshly voided fecal sample was collected from each pig into individual plastic bags and stored at 0 °C until the end of the trial. Pigs and feeders were weighed on day 21 to calculate ADG, ADFI, and G:F. At the end of the trial, diets and feces were dried in a 75 °C forced air oven, weighed, ground through a 1 mm screen, and a subsample was obtained for nutrient analysis.
Experiment 2
Fifty-six weanling barrows (6.0 × F25 Genetiporc; PIC Inc.) were obtained from a commercial farm at weaning (28 d of age) and transported and housed at the Iowa State University Swine Nutrition Farm. For 7 d, pigs were group-housed in four pens of 14 pigs each and fed a common starter diet to optimize feed intake during the weaning transition period. For the next 7 d (day 1 to day 7 on feed), pigs were randomly allotted to one of four dietary treatments and fed their respective experimental diets in groups of 14 pigs to adapt to dietary treatments and to optimize feed intake during the subsequent feeding period. For the next 10 d (day 8 to day 17 on feed) pigs (BW 9.16 ± 1.56 kg) were moved to individual metabolism crates for continued adaptation to diets, metabolism crates, and the twice-daily feeding regimen of 225 g at 0700 h and at 1700 h. After this adaptation period, pigs remained on their respective experimental diets for a 4-d total fecal and urine collection period (day 18 to day 21 on feed). During the collection period, urine was collected twice daily into a plastic bucket containing 15 mL of 6N HCl and stored at 0 °C until the end of the collection period. At the end of the collection period, urine was thawed and weighed, and a subsample was collected and stored at 0 °C until subsequent analysis. Feces were also collected twice daily during the collection period and stored at 0 °C. At the end of the collection period, feces were dried at 75 °C for 48 h, weighed, ground through a 2-mm screen, and a subsample collected for digestibility analysis.
On the evening of day 21 following a 12 h fast, each pig was orally administered a 10 mL deionized water solution containing 7.0 g of lactulose and 0.7 g of mannitol (Spectrum Chemical, Gardena, CA). After administration of the sugar solution, pigs were fed their respective experimental diets, and urine was collected into buckets containing 5 mL chlorohexidine for the next 12 h (overnight), quantified, subsampled, and stored at −20 °C for analysis. Immediately following this collection, urine was collected for an additional 6 h into buckets containing 5 mL chlorohexidine and subsequently analyzed for measures of oxidative stress (18 h since last meal). Following this urine collection, approximately 8 mL of blood (18 h fast) was obtained via venipuncture into heparinized tubes (158 USP units sodium heparin; BD Vacutainer, BD Diagnostics, Franklin Lakes, NJ). Blood samples were centrifuged at 2,500 × g for 15 min at 4 °C and plasma was harvested. Plasma samples were immediately frozen and stored at −80 °C until analysis. At the end of this collection, pigs were weighed (BW 12.77 ± 1.69 kg) and euthanized by captive bolt followed by exsanguination. Livers were excised, weighed, and a sample taken and snap frozen in liquid N, transported on dry ice, and stored at −80 °C until subsequent analyses of oxidative stress markers.
Calculations and methodologies
Diets and feces were analyzed for acid ether extract (AEE, Thermo Scientific Application Note 361; Thermo Fisher Scientific, Salt Lake City, UT) using an accelerated solvent extraction system (model 350; Dionex, Bannockburn, IL) and 100 mL stainless steel extraction cells to accomplish the lipid extraction. Gross energy of the SO, diets, feces, and urine was determined using an isoperibol bomb calorimeter (model 1281; Parr Instrument Co., Moline, IL) using benzoic acid as a standard. Nitrogen was analyzed by thermo-combustion (VarioMAX CNS; Elementar Analysensysteme GmbH, Hanau, Germany) where combustion gases are converted to individual gases and sorted into adsorption columns and are measured using a thermal conductivity detector. In experiment 1, titanium dioxide was used as an indigestible marker to calculate apparent total tract GE and AEE digestibility using the indirect method: [(1 − (Tifeed × Nutrientfeces)/(Tifeces × Nutrientfeed)) × 100]. In experiment 2, digestibility coefficients for AEE, GE, and N were estimated using a time-based collection methodology with ME as a percent of DE calculated by dividing ME intake by DE intake, and N retention as a percent of N digested calculated by dividing N retained by N digested, both reported as a percent. Detailed descriptions of metabolism experimental methods are provided elsewhere (Adeola, 2001; Kerr et al., 2013; Li et al., 2016). Urinary lactulose and mannitol concentrations were measured via HPLC as an in vivo indicator of small intestinal permeability using the method that has been previously described by Kansagra et al. (2003). The ratio of lactulose:mannitol (°) was calculated back to the total amount of urine collected and reported on a recovery basis. For plasma tryptophan (Trp), plasma samples were thawed at 4 °C and diluted with 1:1 with 0.50 M potassium phosphate buffer, pH 6.0, and deproteinized with 2 M trichloroacetic acid. Plasma Trp levels were subsequently determined by separation on a 4-µm spherical silica gel particle column (Superspher 100 RP-18 LiChroCART; Millipore Sigma, Billerica, MA) by an automated HPLC system with a fluorescence detector (Jasco FP-1520; Jasco Analytical Instruments, Easton, MD).
Statistical analysis
In each experiment, data were analyzed as a completely randomized design with an individual pig as the experimental unit, using Proc MIXED procedure of SAS (version 9.4; SAS, 2009) with means reported and separated using LSMEANS. Differences were considered significant at P ≤ 0.05, whereas values of 0.05 ≤ P ≤ 0.10 were considered statistical trends, if present. In addition, relationships between lipid peroxidation measures with growth performance and digestibility variables were evaluated by simple linear correlation (Pearson correlation coefficients) analysis. Correlations were considered significant only if P ≤ 0.05 and r ≥ 0.30.
Results and discussion
Compositional changes of SO due to thermal processing
Lipid peroxidation involves free radical formation and propagation which ultimately bind to PUFA, where the development of lipid peroxidation products is affected by the duration and intensity of thermal processing and presence of oxygen (Holman, 1954; St. Angelo et al., 1996). In general, thermal processing of lipids decreases lipid quality through hydrogenation of double bonds and the formation of peroxides in the initiation phase of lipid peroxidation, which can then be degraded into polar and nonpolar acids, ketones, and aldehydes in the propagation phase, ultimately forming indigestible polymers in the termination phase (Gray, 1978; Gonzalez-Muñoz et al., 1998).
The current experiment induced peroxidation by processing SO at different temperatures and durations prior to being mixed in the diet using the same methods as used in previous experiments (Lindblom et al., 2018a; Overholt et al., 2018a), where SO was either unheated or heated at 45 °C for 288 h, 90 °C SO for 72 h, and 180 °C for 12 h. Prior to feed mixing, each of the four SO was analyzed in detail for FA composition, lipid quality, and various lipid peroxidation products as given in Table 2. The unprocessed SO (i.e., 22.5 °C) had a higher unsaturated to saturated fatty acid ratio (UFA:SFA) than SO processed at 90 °C and 180 °C (5.30 vs. 4.73 and 4.97, respectively), which is due to the slight increases in C16:0 and C18:0 and slight decreases in C18:2 and C18:3 when the SO was processed at 90 °C and 180 °C. Although UFA:SFA is an important measure for energy predictability in swine (Wiseman et al., 1998), it is a fairly crude measure of the degree of unsaturation and thus susceptibility to peroxidation (Holman, 1954). Because of this, iodine value (IV, Meadus et al., 2010) was also calculated as a measure of FA unsaturation. For SO processed at 90 °C and 180 °C there was a slight decrease in IV compared to SO not thermally processed (22.5 °C) or processed at 45 °C (127 and 129 vs. 131 and 133, respectively). The observed changes in both UFA:SFA and IV were expected because as lipid peroxidation progresses, there is general hydrogenation of FA which decreases the number of double bonds available for peroxidation (Yin et al., 2011). The changes in FA composition, UFA:SFA, and IV in the current experiment are similar to changes as reported in previous work by this laboratory (Lindblom et al., 2018a; Overholt et al., 2018a) and by others (DeRouchey et al., 2004; Liu et al., 2014b, Kerr et al., 2015; Rosero et al., 2015; Hanson et al., 2016).
Even though numerous quality factors were measured in an effort to expand the basis of understanding of lipid peroxidation in livestock feeds (Table 2), only a few of the more common factors will be discussed. Peroxide value (PV), a measurement of peroxides and hydroperoxides formed in the initiation phase, was determined to be highest in the 90 °C processed SO followed by the 180 °C and 45 °C processed SO, and lowest in the fresh SO with values of 19.1, 13.4, 11.5, and 7.6 mEq/kg SO, respectively. Oxidized fatty acids (OFA), a measure of lipid hydroperoxides and peroxides and saturated epoxy-, keto-, and hydroxy-acids, did not differ greatly and were low across all SO treatments. Lastly, p-anisidine value (AnV), a measure of high-molecular-weight saturated and unsaturated aldehydes, and total polar compounds (TPC), a measure of monoglycerides, diglycerides, and free fatty acids, were highest in the 90 °C and 180 °C SO compared to the 22.5 °C and 45 °C SO, indicating that the method of thermal processing used generated SO to different degrees of peroxidation. Changes in PV, OFA, AnV, and TPC were expected based on the overall process of lipid peroxidation (Engberg et al., 1996; Kerr et al., 2015). Even though SO was thermally processed in a seemingly identical manner as previously conducted by this laboratory (Lindblom et al., 2018a; Overholt et al., 2018a), it is noteworthy that the PV was very different among the three different experiments. This is most noticeable in the SO thermally processed at 90 °C, with PV of 19.1, 145.3, and 123.6 for the current, Lindblom et al. (2018a), and Overholt et al. (2018a) experiments, respectively. The AnV values also differed among the three similarly conducted experiments, with less variation noted for OFA and TPC. Because of these differences, potential performance effects due to feeding the SO treatments and the subsequent relationships (i.e., correlations) between performance or oxidative stress measures and measures of lipid peroxidation were expected to differ slightly among these three experiments.
Specific aldehydes were also measured because they are commonly measured (i.e., hexanal; Shurson et al., 2015) or are considered highly damaging aldehydes to DNA, proteins, and lipids in vivo (i.e., acrolein; 2,4-decadienal [DDE], 4-hydroxynonenal [HNE]; Esterbauer et al., 1991; Kehrer and Biswal, 2000; Chang et al., 2005; Abraham et al., 2011). In addition, a ratio among two aldehyde clusters which has been shown to be associated with the progression of SO peroxidation (Wang et al., 2016) was measured because very few of these aldehydes or their ratio has been evaluated relative to animal performance or digestive functions. Thermal processing of SO at 45 °C had marginal effects on acrolein, hexanal, DDE, or HNE concentrations, or the aldehyde ratio, compared to the unprocessed SO, Table 2. Thermal processing SO at 90 °C or 180 °C resulted in increases in each of these aldehydes, with the SO thermally processed at 90 °C having greater levels of hexanal, DDE, and HNE compared to the SO thermally processes at 180 °C, Table 2. These data are similar to those previously reported by this laboratory (Lindblom et al., 2018a; Overholt et al., 2018a) albeit some variations were noted for each of these aldehydes among the three experiments. Total tocopherols (TOC) were also measured because they are natural antioxidants found in SO and aid in protecting SO from peroxidation (Kamal-Eldin, 2006). In the current study, the 90 °C processed SO had the lowest TOC concentration with 147 mg/kg oil followed by 180 °C, 45 °C, and fresh oil with 595, 821, and 870 mg/kg, respectively. These data are similar to the work of Miyagawa et al. (1991) who evaluated a blend of SO and rapeseed oil and with the work of Lindblom et al. (2018a) and Overholt et al. (2018a) using SO who reported that thermally processing these oils would result in a degradation of TOCs.
Growth performance
In experiment 1, pigs fed the 90 °C SO had reduced ADG by approximately 15% (P = 0.01) and ADFI by approximately 12% (P = 0.04) compared to pigs fed the other SO treatment groups, with no differences noted among pigs fed the 22.5 °C, 45 °C, and 180 °C SO treatments, Table 4. The reduction in ADG in pigs fed the 90 °C SO is in agreement with previous work from this laboratory using growing (Lindblom et al., 2018a) and finishing pigs (Overholt et al., 2018a), and with others who have reported decreased ADG in pigs fed lipids with increased concentrations of lipid peroxidation products compared to pigs fed lipids with low levels of lipid peroxidation products (DeRouchey et al., 2004; Boler et al., 2012; Liu et al., 2014a; Rosero et al., 2015; Hanson et al., 2016). The reduction in ADG by 15% due to processing SO at 90 °C in the current experiment is also similar to the 16% reduction in ADG compared to pigs fed fresh lipid sources in a review of the literature by Hung et al. (2017). Consumption of lipid peroxidation compounds has also been shown to reduce feed intake (Boler et al., 2012; Liu et al., 2014a, Rosero et al., 2015). In the current experiment, the reduction in ADFI by approximately 13% is similar to the review published by Hung et al. (2017), but is in contrast to Lindblom et al. (2018a) and Overholt et al. (2018a) who did not report any effect of feeding similarly produced peroxidized SO on ADFI, as well as by Hanson et al. (2016) who fed graded levels of peroxidized corn oil to nursery pigs. The lack of an effect of feeding peroxidized SO on G:F in the current experiment is supported by DeRouchey et al. (2004) who fed peroxidized choice white grease to nursery pigs, Boler et al. (2012) who fed peroxidized corn oil to finisher pigs, and by Rosero et al. (2015) who fed peroxidized SO to nursery pigs. In contrast, similarly peroxidized SO at 90 °C resulted in a reduced G:F in growing and finishing pigs (Lindblom et al., 2018a and Overholt et al., 2018a, respectively) and as indicated in the review of the literature reported by Hung et al. (2017).
Table 4.
Growth performance and apparent total tract digestibility of energy and fat in pigs fed soybean oil with differing peroxidation levels, experiment 1
| Processed soybean oil1,2 | Statistics | |||||
|---|---|---|---|---|---|---|
| Parameter | 22.5 | 45 | 90 | 180 | SEM | P-value |
| ADG, kg | 0.478a | 0.465a | 0.406b | 0.455a | 0.016 | 0.01 |
| ADFI, kg | 0.629a | 0.609a | 0.548b | 0.615a | 0.021 | 0.04 |
| G:F | 0.762 | 0.764 | 0.745 | 0.739 | 0.012 | 0.37 |
| ATTD GE, %3 | 88.48 | 87.61 | 87.54 | 87.50 | 0.41 | 0.28 |
| ATTD AEE, %3 | 85.71 | 84.28 | 84.97 | 87.38 | 0.55 | 0.21 |
1Data are least-square mean of 14 observations for 22.5 and 15 each for 45, 90, and 180. Processing temperatures: 22.5 = fresh oil; 45 = oil heated for12 d at 45 °C with constant compressed air flow rate at 15 L/min; 90 = oil heated for 72 h at 90 °C with constant compressed air flow rate at 15 L/min; 180 = oil heated for 6 h at 180 °C with constant compressed air flow rate at 15 L/min. Performance data were collected over 21 d with an initial BW of 7.1 ± 0.9 kg (P = 0.54) and a final BW of 16.6 ± 1.8 kg (P = 0.10).
2Superscripts reflect peroxidized soybean oil treatment differences (ab, P < 0.05).
3ATTD, apparent total tract digestibility; AEE, acid hydrolyzed ether extract.
The different thermal processing temperatures and times were selected to generate different concentrations and patterns of lipid peroxidation products to increase our understanding as to which lipid peroxidation product or products are most detrimental to pig performance. Even though thermally processing SO at 45 °C or 180 °C generated different concentrations and patterns of lipid peroxidation products (Table 2), inclusion of these oils at 10% into the diet did not affect pig performance. This is similar to that reported by Lindblom et al. (2018a) and Overholt et al. (2018a) and supported by Rosero et al. (2015) and Hanson et al. (2016) who have reported that consumption of lipids with low levels of lipid peroxidation products has little to no measurable effects on pig performance.
Energy, lipid and N digestibility, and N balance
In experiments 1 and 2 there were no effects of feeding thermally processing SO on apparent total tract digestibility of dietary GE or AEE (Tables 4 and 5, respectively). In addition, there was no effect of dietary treatment on the DE:ME ratio, N digestibility, or N retained as a percent of N digested in experiment 2 (Table 5). While these results are supported by Liu et al. (2014c) who reported no changes in DE as a percentage of GE or ME as a percentage of DE when pigs were fed various peroxidized lipids, they are in contrast with DeRouchey et al. (2004), Rosero et al. (2015), Lindblom et al. (2018a), and Overholt et al. (2018a) who reported reductions in energy and lipid digestibility when pigs were fed peroxidized lipids relative to unperoxidized lipids. The lack of an effect of lipid peroxidation on N digestibility or N retained as a percent of N digested is supported, however, by DeRouchey et al. (2004) who did not observe any differences among N digestibility in nursery pigs fed thermally processed choice white grease, Liu et al. (2014c) who did not observe any differences in N digestibility or N retention due to feeding various peroxidized lipids in nursery pigs, and Overholt et al. (2018a) who did not observe any differences in N digestibility or N retention due to feeding thermally processed SO in finishing pigs. In contrast, Lindblom et al. (2018a) reported a slight decrease in N digestibility and N retention in growing pigs fed SO thermally processes at 90 °C for 72 h. The varied responses in GE, AEE, and N digestibility and retention suggest that further research is needed to delineate what components of digestion on in vivo metabolism are being affected by the consumption of peroxidized SO in swine.
Table 5.
Energy and lipid digestibility, nitrogen balance, and intestinal permeability in finishing pigs fed various levels of peroxidized soybean oil, experiment 2
| Processed soybean oil1 | Statistics | |||||
|---|---|---|---|---|---|---|
| Parameter | 22.5 | 45 | 90 | 180 | SEM | P-value2 |
| DE, % of GE | 90.09 | 90.53 | 89.61 | 90.75 | 0.46 | 0.35 |
| ME, % of DE | 98.10 | 98.22 | 97.99 | 98.08 | 0.09 | 0.35 |
| AEE digestibility, % | 83.62 | 86.15 | 84.64 | 85.06 | 0.87 | 0.23 |
| Nitrogen digested, % | 88.77 | 88.33 | 87.16 | 89.08 | 0.55 | 0.12 |
| Nitrogen retained, %2 | 85.92 | 87.12 | 86.14 | 85.07 | 0.09 | 0.40 |
| Urinary L:M ratio3 | 0.13 | 0.12 | 0.12 | 0.08 | 0.02 | 0.35 |
1Data are least-square mean of 14 observations for 22.5 and 90; and 13 observations for 45 and 180. Processing temperatures: 22.5 = fresh oil; 45 = oil heated for12 d at 45 °C with constant compressed air flow rate at 15 L/min; 90 = oil heated for 72 h at 90 °C with constant compressed air flow rate at 15 L/min; 180 = oil heated for 6 h at 180 °C with constant compressed air flow rate at 15 L/min. Pigs were fed diets for 21 d with an initial BW of 9.16 ± 1.56 kg (P = 0.48) and a final BW of 12.77 ± 1.69 kg (P = 0.36).
2Nitrogen retained as a percent of N digested.
3Urinary lactulose:mannitol ratio.
Intestinal barrier function
Intestinal permeability has been shown to increase when animals consume a diet high in SFA (Laugerette et al., 2012; Mani et al., 2012; Liu et al., 2014d). In addition, changes in intestinal integrity have been associated with changes in the absorption of nutrients and to the resistance of pathogens (Wijtten et al., 2011). In contrast, little data are available on the effects of feeding peroxidized lipids on intestinal permeability. One of the most common in vivo indicators of small intestinal paracellular permeability is the ratio of lactulose to mannitol in the urine (Kansagra et al., 2003; Wijtten et al., 2011). In the current study, there were no differences in the urinary L:M ratio noted among pigs fed the different SO treatments. While an increase in intestinal permeability in pigs fed 90 °C and 180 °C SO might have been expected because of the changes in lipid saturation, this was not the case, but not surprising given the small change in UFA:SFA due to peroxidation (Table 2). These data suggest that lipid peroxidation products have a minor effect on intestinal permeability, at least relative to the degree of lipid peroxidation achieved in the current study. The current findings are in agreement with others (Liu et al., 2014d; Lindblom et al., 2018a; Overholt et al., 2018a) who reported no significant differences in urinary L:M ratios among pigs fed lipids with different levels of lipid peroxidation.
Serum tryptophan
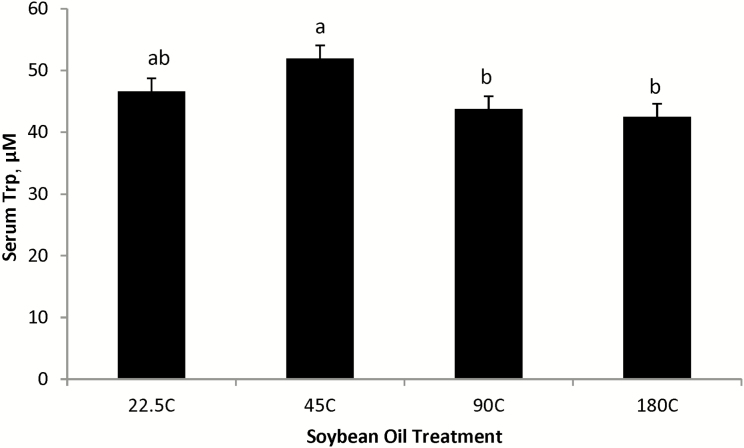
Serum Trp affects brain function and is an immediate precursor for serotonin synthesis, thereby affecting the regulation of many behavioral and physiological processes, including feed intake (Baranyiova, 1991; Seve, 1999). As shown in Figure 1, pigs in experiment 2 fed the thermally processed SO at 90 °C and 180 °C had reduced serum Trp compared to pigs fed the SO processed at 45 °C, with pigs fed the unprocessed SO (22.5 °C) being intermediate. This coincides with the reduction in ADFI observed in pigs fed the 90 °C SO diet in experiment 1 and is supported by Lindblom et al. (2018a) and Overholt et al. (2018a) who also observed a reduction in serum Trp in pigs fed the thermally SO processed at 90 °C and 180 °C. This reduction in serum Trp may be a consequence of the activation of the Trp-NAD+ pathway whose metabolites are cofactors or substrates involved in detoxification of reactive aldehydes through aldehyde dehydrogenase (Wang et al., 2018), an area of research that needs further elucidation.
Figure 1.
Effect of thermally peroxidized soybean oil on serum Trp concentrations in nursery pigs, experiment 2. 22.5C = fresh oil; 45C = oil heated for12 d at 45 °C; 90C = oil heated for 72 h at 90 °C; 180C = oil heated for 6 h at 180 °C. Except for soybean oil at 22.5 °C, each processed soybean oil had a constant compressed air flow rate of 15 L/min. Peroxidation effect P = 0.01 with superscripts reflecting peroxidized soybean oil treatment differences (ab, P ≤ 0.05).
Oxidative stress
Oxidative stress results when free radical production overwhelms antioxidant compounds leading to modifications of lipids, DNA, and proteins (Betteridge, 2000). There are multiple measures to characterize oxidative status including thiobarbituric acid reactive substances (TBARS) and F2-isoprostane (ISP) concentrations as markers of lipid damage (Montuschi et al., 2004; Dalle-Donne et al., 2006), 8-hydroxy-2′-deoxyguanosine (8-OH-2dG) as a marker of DNA damage (Wu et al., 2004; Mateos and Bravo, 2007), and protein carbonyl (PC) as a marker of protein damage (Dalle-Donne et al., 2003).
In experiment 2, there was no effect (P > 0.10) of feeding peroxidized SO on serum, urinary, or liver TBARS, Table 6. The lack of an effect on serum or liver TBARS due to feeding peroxidized SO is similar to previous work from this laboratory in growing and finishing pigs (Lindblom et al., 2018b and Overholt et al., 2018a, respectively). In contrast, the lack of an impact of SO peroxidation on urinary TBARS in the current experiment is not supported by previous research at this laboratory, where pigs fed the peroxidized SO, specifically the SO thermally processed at 90 °C, had greater urinary TBARS compared to pigs fed the unprocessed SO (Lindblom et al., 2018b; Overholt et al., 2018a). Others (Boler et al., 2012; Varady et al., 2012; Liu et al., 2014c; Lu et al., 2014) have shown that consumption of peroxidized oils often induces oxidative stress as measured by TBARS in either blood or tissues. Because TBARS are a nonspecific metric of lipid peroxidation, ISP was measured because it is a specific product of the oxidation of arachidonic acid and is often a preferred measure of lipid damage (Morrow et al., 1995; Dalle-Donne et al., 2006). In the current experiment, pigs fed the SO thermally processed at 90 °C had the greatest ISP concentrations in the serum (P ≤ 0.01) and urine (P ≤ 0.05) compared to pigs fed the unprocessed SO, Table 6. This is similar to previous reports from this laboratory (Lindblom et al., 2018b; Overholt et al., 2018a) and suggests that lipid damage did occur in response to the consumption of lipid peroxidation products.
Table 6.
Oxidative status in serum, urine, and liver of pigs fed soybean oil with differing peroxidation levels, experiment 2
| Processed soybean oil1 | Statistics | |||||
|---|---|---|---|---|---|---|
| Parameter2 | 22.5 | 45 | 90 | 180 | SEM | P-value |
| Serum3 | ||||||
| TBARS, μM/mL | 13.5 | 14.9 | 12.0 | 14.6 | 1.2 | 0.26 |
| ISP, pg/mL | 20.4c | 31.9bc | 67.1a | 41.6b | 6.0 | 0.01 |
| PC, nmol/mL | 39.7 | 44.4 | 46.8 | 40.7 | 5.8 | 0.79 |
| Urine4 | ||||||
| TBARS, μM | 13.6 | 11.1 | 17.5 | 12.9 | 2.0 | 0.15 |
| ISP, pg | 6,538b | 10,699ab | 15,816a | 9,639b | 2,128 | 0.04 |
| 8-OH-2dG, μg | 381 | 209 | 123 | 248 | 136 | 0.48 |
| Liver5 | ||||||
| TBARS, μM | 58.7 | 61.6 | 50.6 | 57.7 | 3.7 | 0.21 |
| 8-OH-2dG, pg | 155 | 201 | 357 | 236 | 55 | 0.15 |
1Processing temperatures: 22.5, fresh oil; 45, SO heated for 288 h at 45 °C; 90, SO heated for 72 h at 90 °C; 180, SO heated for 6 h at 180 °C. All processed oil treatments were heated with a constant compressed air flow rate at 15 L/min. Superscripts reflect peroxidized soybean oil treatment differences (abc, P ≤ 0.05; xyz, P ≤ 0.10).
2TBARS, thiobarbituric acid reactive substances; PC, protein carbonyls; GPx, glutathione peroxidase activity; ISP, F2-isoprostanes; 8-OH-2dG, 8-hydroxy-2′-deoxyguanosine.
3Serum obtained after a 17 h fast.
4Urine collected and quantitated for 5 h following a 12 h fast.
5Liver obtained on day 49 from pigs in a fed state.
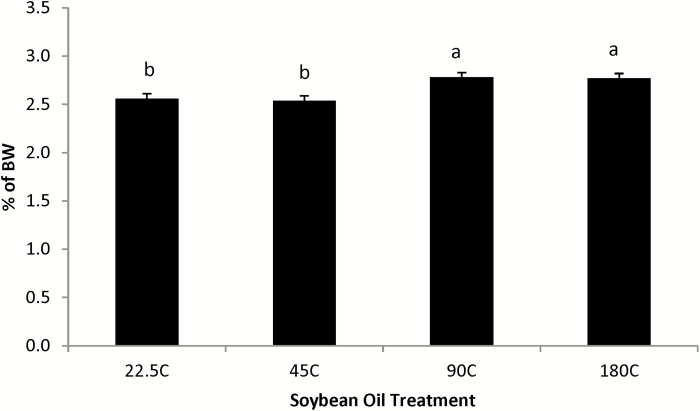
Proteins and amino acids can also be damaged by oxidation in vivo, with carbonyls being an indicator of this damage (Beal, 2002). In experiment 2, there was no impact of feeding peroxidized SO to nursery pigs on serum PC, Table 6, which is similar to no change in plasma or liver PC due to feeding peroxidized SO to finishing pigs as reported by Overholt et al. (2018a). In contrast, Lindblom et al. (2018b) reported an increase in serum PC, but not liver PC, in pigs fed peroxidized SO. DNA is also sensitive to oxidative damage in vivo, of which guanine is the most readily oxidized nucleic acid (Wu et al., 2004; Mateos and Bravo, 2007). Feeding pigs the thermally processed SO in experiment 2 did not affect urinary or liver 8-OH-2dG (P > 0.10), which is similar to that reported by Lindblom et al. (2018b) for urinary 8-OH-2dG excretion, but not for liver 8-OH-2dG, and for both urinary and liver 8-OH-2dG as reported by Overholt et al. (2018a). The lack of an apparent liver oxidative status change is interesting given that pigs fed the 90 °C and 180 °C processed SO had greater (P ≤ 0.01) liver weights compared to pigs fed the unprocessed or 45 °C processed SO (Figure 2). Increased liver weights due to feeding peroxidized SO have been reported previously (Eder, 1999; Anjum et al., 2004; Liu et al., 2014a; Lu et al., 2014; Lindblom et al., 2018b; Overholt et al., 2018b).
Figure 2.
Effect of thermally peroxidized soybean oil on liver weight as a percentage of BW in nursery pigs, experiment 2. 22.5C = fresh oil; 45C = soybean oil heated for 288 h at 45 °C; 90C = soybean oil heated for 72 h at 90 °C; 180C = soybean oil heated for 6 h at 180 °C. Except for soybean oil at 22.5 °C, each processed soybean oil had a constant compressed air flow rate of 15 L/min. Average final BW of 12.77 ± 1.69 kg (P = 0.36). Peroxidation effect P = 0.01, with superscripts reflecting peroxidized soybean oil treatment differences (ab, P ≤ 0.05).
While endogenous antioxidant enzyme activities such as glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) assist in protecting the body from reactive oxygen species and free radicals (Lykkesfeldt and Svendsen, 2007; Kalyanaraman, 2013; Royer et al., 2016), they were not assessed in the current experiment because in previous experiments conducted by this laboratory, only liver CAT, but not liver SOD or GPx activity, was affected by consumption of peroxidized SO (Lindblom et al., 2018b), and Overholt et al. (2018a) reported no effect of consumption of peroxidized SO on liver CAT or GPx.
Correlations
It is well known that simple correlations do not represent a cause and effect relationship (Steel and Torrie1980; Aldrich, 1995) and lipid peroxidation measures are often correlated to each other (Liu et al., 2014b). However, because there is a lack of comprehensive measures of lipid peroxidation products and their relationship to pig performance and to different measures of oxidative stress, a correlation analysis may be of value to provide guidance on future research involving lipid peroxidation on animal production and oxidative status. To minimize the confusion and focus on key components from which to conduct future research, a correlation r-value of ≥0.30 in combination with a correlation P-value ≤0.05 was selected as cutoff points for reporting in the current experiment. Likewise, because the differences in FA profile, FFA, UFA:SFA, IV, and TBA values were considered to be insignificant in the lipids reported in Table 2, these relationships were not reported or discussed.
For experiment 1, a correlation analysis between pig performance and various measures of lipid peroxidation products indicated that all but acrolein and the aldehyde ratio provided correlations to ADG, and all but AnV, OSI, acrolein, DDE, and the aldehyde ratio were correlated to ADFI, Table 7. No correlations were noted between G:F, DE as a percent of GE, or AEE digestibility and any SO quality indices. For experiment 2, there were numerous correlations between SO quality indices and percent liver weight, plasma Trp, plasma ISP, urinary ISP, and liver DNA damage (r ≥ 0.30 and P ≤ 0.05), Table 8. Lindblom et al. (2018a) also reported numerous correlations between SO quality indices and ADG as well as to G:F, but few correlations to ADFI; while Overholt et al. (2018a) reported a few correlations between SO quality indices and ADG and G:F, but not with ADFI. Even though PV was one SO quality parameter that was correlated to pig performance among each of these three similarly conducted experiments (the current trial, Lindblom et al., 2018a, and Overholt et al., 2018a), these data are biased because in most cases the SO thermally processed at 90 °C resulted in the only treatment being significantly different than the other treatments. While the current data are supported by the negative relationship between dietary PV and ADG in swine reported by Hung et al. (2017), it is important to recognize that of the 65 data points comparing growth and peroxidized lipids in swine and poultry in their review, all 65 reported PV and only 22 reported TBARS, but a mere 7 reported AnV. Thus, if any performance parameter was related to lipid peroxidation as reviewed by Hung et al. (2017), the only relationship could only have been with PV because other measures of lipid peroxidation were not measured.
Table 7.
Pearson correlation coefficients among SO composition and peroxidation products with performance, digestibility, and gut integrity responses, experiment 11
| SO quality indices2 | ADG | ADFI |
|---|---|---|
| PV | −0.41 (0.01) | −0.35 (0.01) |
| AnV | −0.30 (0.02) | — |
| OFA | −0.42 (0.01) | −0.35 (0.01) |
| TPC | −0.42 (0.01) | −0.35 (0.01) |
| PTAGS | −0.38 (0.01) | −0.30 (0.02) |
| OSI | 0.35 (0.01) | — |
| Hexanal | −0.41 (0.01) | −0.37 (0.01) |
| Acrolein | — | — |
| DDE | −0.35 (0.01) | — |
| HNE | −0.41 (0.01) | −0.34 (0.01) |
| Ratio | — | — |
| TOC | 0.42 (0.01) | 0.35 (0.01) |
1Correlation is the r value and correlation significance (P-value) is in parentheses. If no value is given, it was not found to be significant at P ≤ 0.05 and has a correlation of ≥0.30. Feed efficiency (G:F), DE as a percent of GE, and AEE digestibility were not correlated to any oil composition or peroxidation parameter.
2PV, peroxide value; AnV, p-anisidine value; OFA, oxidized fatty acids; TPC, total polar compounds; PTAGS, polymerized triacylglycerides; OSI, oxygen stability index; DDE, 2,4-decadienal; HNE, 4-hydroxynonenal; Ratio, ratio of aldehydes as described by Wang et al. (2016); TOC, total tocopherols.
Table 8.
Pearson correlation coefficients among SO composition and peroxidation products with performance, digestibility, and gut integrity responses, experiment 21
| SO quality indices2 | Liver | Plasma Trp | Plasma ISP | Urinary ISP | Liver DNA |
|---|---|---|---|---|---|
| PV | 0.43 (0.01) | — | 0.65 (0.01) | 0.42 (0.01) | 0.45 (0.02) |
| AnV | 0.52 (0.01) | −0.46 (0.01) | 0.51 (0.01) | — | 0.33 (0.01) |
| OFA | 0.44 (0.01) | −0.35 (0.03) | 0.65 (0.01) | 0.39 (0.01) | 0.44 (0.02) |
| TPC | 0.46 (0.01) | −0.34 (0.03) | 0.65 (0.01) | 0.40 (0.01) | 0.45 (0.02) |
| PTAGS | 0.50 (0.01) | −0.44 (0.01) | 0.61 (0.01) | 0.33 (0.02) | 0.41 (0.04) |
| OSI | −0.39 (0.01) | — | −0.58 (0.01) | −0.38 (0.01) | −0.39 (0.05) |
| Hexanal | 0.37 (0.01) | — | 0.61 (0.01) | 0.39 (0.01) | 0.42 (0.03) |
| Acrolein | 0.48 (0.01) | −0.45 (0.01) | 0.41 (0.01) | — | — |
| DDE | 0.52 (0.01) | −0.46 (0.01) | 0.57 (0.01) | — | 0.38 (0.05) |
| HNE | 0.47 (0.01) | −0.40 (0.01) | 0.63 (0.01) | 0.37 (0.02) | 0.43 (0.02) |
| Ratio | 0.45 (0.01) | −0.41 (0.01) | 0.37 (0.01) | — | — |
| TOC | −0.45 (0.01) | 0.35 (0.03) | −0.64 (0.01) | −0.39 (0.01) | −0.44 (0.02) |
1Correlation is the r value and correlation significance (P-value) is in parentheses. If no value is given, it was not found to be significant at P ≤ 0.05 and has a correlation of ≥0.30. No correlations were noted for AEE digestibility, GE digestibility, DE:ME, ND, NR, LM, plasma TBARS, plasma PC, urinary TBARS, urinary DNA, or liver TBARS with any oil composition or peroxidation parameter.
2PV, peroxide value; AnV, p-anisidine value; OFA, oxidized fatty acids; TPC, total polar compounds; PTAGS, polymerized triacylglycerides; OSI, oxygen stability index; DDE, 2,4-decadienal; HNE, 4-hydroxynonenal; Ratio, ratio of aldehydes as described by Wang et al. (2016); TOC, total tocopherols.
While the correlation analysis as conducted herein and by Lindblom et al. (2018a,b) and Overholt et al. (2018a) provides some guidance into specific SO quality indices that may need to be evaluated in future research, it must be reiterated that correlations do not necessitate a cause and effect relationship and that correlations assume a linear relationship between the two variables of interest; whereupon it is unknown whether the relationship between lipid peroxidation and growth, and for oxidative stress, is or is not a linear relationship. What is needed to delineate the cause and effect relationship between lipid peroxidation and pig performance (or oxidative stress) is having a lipid with known level of peroxidation that clearly depresses pig performance (or increases oxidative stress) from which to conduct a dilution study to apply a regression approach to data analysis and avoid inappropriately implying a linear correlation.
Conclusions
Thermal processing of SO, especially at 90 °C for 72 h, reduced UFA:SFA and IV, but increased numerous lipid peroxidation compounds including PV, AnV, polar compounds, and several aldehydes in comparison to other SO treatment groups. This processing time and temperature combination subsequently resulted in reduced ADG and ADFI, and increased oxidative stress in the pig as measured by serum ISP concentration and urinary ISP excretion. Thermal processing of SO at any time and temperature combination had no effect on energy, lipid, or N digestibility, or intestinal permeability as measured by urinary L:M. While there were some common indices of lipid peroxidation products correlated with growth and oxidative stress, the experimental design did not allow for delineating a cause and effect relationship between lipid peroxidation and pig performance (or oxidative stress).
Acknowledgments
Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the USDA, Iowa State University, or the University of Illinois and does not imply approval to the exclusion of other products that may be suitable. The USDA is an equal opportunity provider and employer.
Glossary
Abbreviations
- AEE
acid ether extract
- AnV
p-anisidine value
- ATTD
apparent total tract digestibility
- CAT
catalase
- DDE
2,4-decadienal
- GPx
glutathione peroxidase
- HNE
4-hydroxynonenal
- ISP
isoprostane
- IV
iodine value
- OFA
oxidized fatty acids
- PC
protein carbonyl
- PV
peroxide value
- SO
soybean oil
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid reactive substances
- TOC
tocopherols
- TPC
total polar compounds
- Trp
tryptophan
- 8-OH-2dG
8-hydroxy-2′-deoxyguanosine
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature cited
- Abraham K., Andres S., Palavinskas R., Berg K., Appel K. E., and Lampen A.. . 2011. Toxicology and risk assessment of acrolein in food. Mol. Nutr. Food Res. 55:1277–1290. doi: 10.1002/mnfr.201100481 [DOI] [PubMed] [Google Scholar]
- Adeola O. 2001. Digestion and balance techniques in pigs. In: Lewis A. J. and Southern L. L., editors. Swine nutrition. 2nd ed. New York (NY): CRC Press; p. 903–916. [Google Scholar]
- Aldrich J. 1995. Correlations genuine and spurious in Pearson and Yule. Stat. Sci. 10:364–376. doi: 10.1214/ss/1177009870 [DOI] [Google Scholar]
- American Oil Chemists’ Society (AOCS). 2011. Official methods and recommended practices of the AOCS. 6th ed. (2nd printing). Urbana (IL). [Google Scholar]
- Anjum M. I., Mirza I. H., Khan A. G., and Azim A.. . 2004. Effect of fresh versus oxidized soybean oil on growth performance, organs weights and meat quality of broiler chicks. Pakistan Vet. J. 24:173–178. [Google Scholar]
- AOAC Int. 2007. Official methods of analysis. 18th ed. Rev. 2. Howitz W., and Latimer G. W. Jr., editors. Gaithersburg (MD): AOAC Int. [Google Scholar]
- Azain M. J. 2001. Fat in swine nutrition. In: Lewis A. J., and Southern L.L., editors. Swine nutrition. Boca Raton: CRC Press; p. 95–106. [Google Scholar]
- Baranyiova E. 1991. Effect of serotonin on food intake by piglets during the early postnatal period. Acta Vet. (Brno) 60:127–136. doi: 10.2754/avb199160020127 [DOI] [Google Scholar]
- Beal F. 2002. Serial review: oxidatively modified proteins in aging and disease. Free Radic. Biol. Med. 32:797–803. doi: 10.1016/S0891-5849(02)00780-3 [DOI] [PubMed] [Google Scholar]
- Betteridge D. J. 2000. What is oxidative stress? Metabolism 49(Suppl. 1):3–8. doi: 10.1016/S0026-0495(00)80077-3 [DOI] [PubMed] [Google Scholar]
- Boler D. D., Fernández-Dueñas D. M., Kutzler L. W., Zhao J., Harrell R. J., Campion D. R., McKeith F. K., Killefer J., and Dilger A. C.. . 2012. Effects of oxidized corn oil and synthetic antioxidant blend on animal performance in finishing barrows. J. Anim. Sci. 90:5159–5169. doi: 10.2527/jas.2012-5266 [DOI] [PubMed] [Google Scholar]
- Chang L. W., Lo W. S., and Lin P.. . 2005. Trans, trans-2,4-decadienal, a product found in cooking oil fumes, induces cell proliferation and cytokine production due to reactive oxygen species in human bronchial epithelial cells. Toxicol. Sci. 87:337–343. doi: 10.1093/toxsci/kfi258 [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I., Rossi R., Colombo R., Giustarini D., and Milzani A.. . 2006. Biomarkers of oxidative damage in human disease. Clin. Chem. 52:601–623. doi: 10.1373/clinchem.2005.061408 [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I., Rossi R., Giustarini D., Milzani A., and Colombo R.. . 2003. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta. 329:23–38. doi: 10.1016/s0009-8981(03)00003-2 [DOI] [PubMed] [Google Scholar]
- DeRouchey J. M., Hancock J. D., Hines R. H., Maloney C. A., Lee D. J., Cao H., Dean D. W., and Park J. S.. . 2004. Effects of rancidity and free fatty acids in choice white grease on growth performance and nutrient digestibility in weanling pigs. J. Anim. Sci. 82:2937–2944. doi: 10.2527/2004.82102937x [DOI] [PubMed] [Google Scholar]
- Eder K. 1999. The effects of a dietary oxidized oil on lipid metabolism in rats. Lipids 34:717–725. doi: 10.1007/s11745-999-0418-0 [DOI] [PubMed] [Google Scholar]
- Engberg R. M., Lauridsen C., Jensen S. K., and Jakobsen K.. . 1996. Inclusion of oxidized vegetable oil in broiler diets. Its influence on nutrient balance and on the antioxidative status of broilers. Poult. Sci. 75:1003–1011. doi: 10.3382/ps.0751003 [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Schaur R. J., and Zollner H.. . 1991. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 11:81–128. doi: 10.1016/0891-5849(91)90192-6 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Muñoz M. J., Bastida S., and Sanchez-Muniz F. J.. . 1998. Short-term in vivo digestibility of triglyceride polymers, dimers, and monomers of thermoxidized palm olein used in deep-frying. J. Agric. Food Chem. 46:5188–5193. doi: 10.1021/jf980598i [DOI] [Google Scholar]
- Gray J. I. 1978. Measurement of lipid oxidation: a review. J. Am. Oil Chem. Soc. 55:539–546. doi: 10.1007/BF02668066 [DOI] [Google Scholar]
- Hanson A. R., Urriola P. E., Wang L., Johnston L. J., Chen C., and Shurson G. C.. . 2016. Dietary peroxidized maize oil affects the growth performance and antioxidant status of nursery pigs. Anim. Feed Sci. Tech. 216:251–261. doi: 10.1016/j.anifeedsci.2016.03.027 [DOI] [Google Scholar]
- Holman R. 1954. Autoxidation of fats and related substances. Prog. Chem. Fats Other Lipids. 2:51–98. doi: 10.1016/0079-6832(54)90004-X [DOI] [Google Scholar]
- Hung Y. T., Hanson A. R., Shurson G. C., and Urriola P. E.. . 2017. Peroxidized lipids reduce growth performance of poultry and swine: a meta-analysis. Anim. Sci. Feed Tech. 231:47–58. doi: 10.1016/j.anifeedsci.2017.06.013 [DOI] [Google Scholar]
- Kalyanaraman B. 2013. Teaching the basics of redox biology to medical and graduate students: oxidants, antioxidants and disease mechanisms. Redox Biol. 1:244–257. doi: 10.1016/j.redox.2013.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal-Eldin A. 2006. Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. Eur. J. Lipid Sci. Technol. 108:1051–1061. doi: 10.1002/ejlt.200600090 [DOI] [Google Scholar]
- Kansagra K., Stoll B., Rognerud C., Niinikoski H., Ou C. N., Harvey R., and Burrin D.. . 2003. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 285:G1162–G1170. doi: 10.1152/ajpgi.00243.2003 [DOI] [PubMed] [Google Scholar]
- Kehrer J. P., and Biswal S. S.. . 2000. The molecular effects of acrolein. Toxicol. Sci. 57:6–15. doi: 10.1093/toxsci/57.1.6 [DOI] [PubMed] [Google Scholar]
- Kerr B. J., Dozier W. A. 3rd, and Shurson G. C.. . 2013. Effects of reduced-oil corn distillers dried grains with solubles composition on digestible and metabolizable energy value and prediction in growing pigs. J. Anim. Sci. 91:3231–3243. doi: 10.2527/jas.2013-6252 [DOI] [PubMed] [Google Scholar]
- Kerr B. J., Kellner T. A., and Shurson G. C.. . 2015. Characteristics of lipids and their feeding value in swine diets. J. Anim. Sci. Biotechnol. 6:30. doi: 10.1186/s40104-015-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuza T. P. 1971. Kinetics of lipid oxidation in foods. Crit. Rev. Food Tech. 2:355–405. doi: 10.1080/10408397109527127 [DOI] [Google Scholar]
- Laugerette F., Furet J. P., Debard C., Daira P., Loizon E., Géloën A., Soulage C. O., Simonet C., Lefils-Lacourtablaise J., Bernoud-Hubac N., . et al. 2012. Oil composition of high-fat diet affects metabolic inflammation differently in connection with endotoxin receptors in mice. Am. J. Physiol. Endocrinol. Metab. 302:E374–E386. doi: 10.1152/ajpendo.00314.2011 [DOI] [PubMed] [Google Scholar]
- Li Y. S., Tran H., Bundy J. W., Burkey T. E., Kerr B. J., Nielsen M. K., and Miller P. S.. . 2016. Evaluation of collection method and diet effects on apparent digestibility and energy values of swine diets. J. Anim. Sci. 94:2415–2424. doi: 10.2527/jas.2016-0275 [DOI] [PubMed] [Google Scholar]
- Lin X., Azain M., and Odle J.. . 2013. Lipids and lipid utilization in swine. In: Chiba L., editor. Sustainable swine nutrition. Oxford (UK): Blackwell Publishing Ltd; p. 59–79. [Google Scholar]
- Lindblom S. C., Gabler N. K., and Kerr B. J.. . 2018a. Influence of feeding thermally peroxidized soybean oil on growth performance, digestibility, and gut integrity in growing pigs. J. Anim. Sci. 96:558–569. doi: 10.1093/jas/sky004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom S. C., Gabler N. K., Dilger R. N., Olson Z. F., Loving C. L., and Kerr B. J.. . 2018b. Influence of feeding thermally peroxidized soybean oil on oxidative status in growing pigs. J. Anim. Sci. 96:545–557. doi: 10.1093/jas/sky005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Chen C., Kerr B. J., Weber T. E., Johnston L. J., and Shurson G. C.. . 2014a. Influence of thermally oxidized vegetable oils and animal fats on growth performance, liver gene expression, and liver and serum cholesterol and triglycerides in young pigs. J. Anim. Sci. 92:2960–2970. doi: 10.2527/jas.2012-5709 [DOI] [PubMed] [Google Scholar]
- Liu P., Pieper R., Tedin L., Martin L., Meyer W., Rieger J., Plendl J., Vahjen W., and Zentek J.. . 2014b. Effect of dietary zinc oxide on jejunal morphological and immunological characteristics in weaned piglets. J. Anim. Sci. 92:5009–5018. doi: 10.2527/jas.2013-6690 [DOI] [PubMed] [Google Scholar]
- Liu P., Kerr B. J., Chen C., Weber T. E., Johnston L. J., and Shurson G. C.. . 2014c. Influence of thermally oxidized vegetable oils and animal fats on energy and nutrient digestibility in young pigs. J. Anim. Sci. 92:2980–2986. doi: 10.2527/jas.2012-5711 [DOI] [PubMed] [Google Scholar]
- Liu P., Kerr B. J., Weber T. E., Chen C., Johnston L. J., and Shurson G. C.. . 2014d. Influence of thermally oxidized vegetable oils and animal fats on intestinal barrier function and immune variables in young pigs. J. Anim. Sci. 92:2971–2979. doi: 10.2527/jas.2012-5710 [DOI] [PubMed] [Google Scholar]
- Lu T., Harper A. F., Dibner J. J., Scheffler J. M., Corl B. A., Estienne M. J., Zhao J., and Dalloul R. A.. . 2014. Supplementing antioxidants to pigs fed diets high in oxidants: II. Effects on carcass characteristics, meat quality, and fatty acid profile. J. Anim. Sci. 92:5464–5475. doi: 10.2527/jas.2013-7112 [DOI] [PubMed] [Google Scholar]
- Lundburg W. O., and Jarvi P.. . 1971. Peroxidation of polyunsaturated fatty compounds. Prog. Chem. Fats Lipids. 9:377–406. doi: 10.1016/0079-6832(71)90032-2 [DOI] [Google Scholar]
- Lykkesfeldt J., and Svendsen O.. . 2007. Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet. J. 173:502–511. doi: 10.1016/j.tvjl.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Mani V., Weber T. E., Baumgard L. H., and Gabler N. K.. . 2012. Growth and development symposium: endotoxin, inflammation, and intestinal function in livestock. J. Anim. Sci. 90:1452–1465. doi: 10.2527/jas.2011-4627 [DOI] [PubMed] [Google Scholar]
- Mateos R., and Bravo L.. . 2007. Chromatographic and electrophoretic methods for the analysis of biomarkers of oxidative damage to macromolecules (DNA, lipids, and proteins). J. Sep. Sci. 30:175–191. doi: 10.1002/jssc.200600314 [DOI] [PubMed] [Google Scholar]
- Meadus W. J., Duff P., Uttaro B., Aalhus J. L., Rolland D. C., Gibson L. L., and Dugan M. E.. . 2010. Production of docosahexaenoic acid (DHA) enriched bacon. J. Agric. Food Chem. 58:465–472. doi: 10.1021/jf9028078 [DOI] [PubMed] [Google Scholar]
- Miyagawa K, Hirai K., and Takezoe R.. . 1991. Tocopherol and fluorescence levels in deep frying oil and their measurement for oil assessment. J. Am. Oil Chem. Soc. 68:163–166. doi: 10.1007/BF02657761 [DOI] [Google Scholar]
- Montuschi P., Barnes P. J., and Roberts L. J. 2nd. 2004. Isoprostanes: markers and mediators of oxidative stress. FASEB J. 18:1791–1800. doi: 10.1096/fj.04-2330rev [DOI] [PubMed] [Google Scholar]
- Morrow J. D., Frei B., Longmire A. W., Gaziano J. M., Lynch S. M., Shyr Y., Strauss W. E., Oates J. A., and Roberts L. J. 2nd. 1995. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N. Engl. J. Med. 332:1198–1203. doi: 10.1056/NEJM199505043321804 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th ed. Washington, DC: Natl. Acad. Press. [Google Scholar]
- Overholt M. F., Dilger A. C., Boler D. D., and Kerr B. J.. . 2018a. Influence of feeding thermally peroxidized soybean oil on growth performance, digestibility, and gut integrity in finishing pigs. J. Anim. Sci. 96:2789–2803. doi: 10.1093/jas/sky091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholt M. F., Kim G. D., Boler D. D., Kerr B. J., and Dilger A. C.. . 2018b. Influence of feeding thermally peroxidized soybean oil to finishing pigs on carcass characteristics, loin quality, and shelf life of loin chops. J. Anim. Sci. 96:2710–2722. doi: 10.1093/jas/sky176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew J. E., and Moser R. L.. . 1991. Fat in swine nutrition. In: Miller E. R., Ullery D. E., and Lewis A. J., editors. Swine nutrition. Stoneham (MA): Butterworth-Heinemann; p. 133–145. [Google Scholar]
- Rosero D. S., Odle J., Moeser A. J., Boyd R. D., and van Heugten E.. . 2015. Peroxidised dietary lipids impair intestinal function and morphology of the small intestine villi of nursery pigs in a dose-dependent manner. Br. J. Nutr. 114:1985–1992. doi: 10.1017/S000711451500392X [DOI] [PubMed] [Google Scholar]
- Royer E., Barbé F., Guillou D., Rousselière Y., and Chevaux E.. . 2016. Development of oxidative stress model in weaned pigs highlighting plasma biomarkers’ specificity to stress inducers. J. Anim. Sci. 94:48–53. doi: 10.2527/jas2015-9857 [DOI] [Google Scholar]
- Schaich K. M. 2005. Lipid oxidation: theoretical aspects. In: Bailey’s industrial oil and fat products. Edible oil and fat products: chemistry, properties, and health effects. vol. 1 Hoboken (NJ): John Wiley and Sons, Inc.; p. 269–355. [Google Scholar]
- Seppanen C. M., and Csallany A. S.. . 2002. Formation of 4-hydroxynonenal, a toxic aldehyde, in soybean oil at frying temperature. J. Am. Oil Chem. Soc. 79:1033–1038. doi: 10.1007/s11746-002-0598-z [DOI] [Google Scholar]
- Seve B. 1999. Physiological roles of tryptophan in pig nutrition. In: Huether G., Kochen W., Simat Th. J., and Steinhart H., editors. Tryptophan, serotonin, and melatonin: basic aspects and applications. New York (NY): Kluwer Academic/Plenum Publishers; p. 729–741. [Google Scholar]
- Shurson G. C., Kerr B. J., and Hanson A. R.. . 2015. Evaluating the quality of feed fats and oils and their effects on pig growth performance. J. Anim. Sci. Biotechnol. 6:10. doi: 10.1186/s40104-015-0005-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Angelo A. J., Vercellotti J., Jacks T., and Legendre M.. . 1996. Lipid oxidation in foods. Food Sci. Nutr. 36:175–224. doi: 10.1080/10408399609527723 [DOI] [PubMed] [Google Scholar]
- Steel R.G.D., and Torrie J. H.. . 1980. Principles and procedures of statistics. A biometrical approach. 2nd ed. New York, USA: McGraw-Hill. [Google Scholar]
- Varady J., Gessner D. K., Most E., Eder K., and Ringseis R.. . 2012. Dietary moderately oxidized oil activates the Nrf2 signaling pathway in the liver of pigs. Lipids Health Dis. 11:21. doi: 10.1186/1476-511x-11-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Csallany A. S., Kerr B. J., Shurson G. C., and Chen C.. . 2016. Kinetics of forming aldehydes in frying oils and their distribution in French Fries revealed by LC-MS-based chemometrics. J. Agric. Food Chem. 64:3881–3889. doi: 10.1021/acs.jafc.6b01127 [DOI] [PubMed] [Google Scholar]
- Wang L., Yao D., Urriola P. E., Hanson A. R., Saqui-Salces M., Kerr B. J., Shurson G. C., and Chen C.. . 2018. Identification of activation of tryptophan–NAD+ pathway as a prominent metabolic response to thermally oxidized oil through metabolomics-guided biochemical analysis. J. Nutr. Biochem. 57:255–267. doi: 10.1016/j.jnutbio.2018.04.009 [DOI] [PubMed] [Google Scholar]
- Wijtten P. J., van der Meulen J., and Verstegen M. W.. . 2011. Intestinal barrier function and absorption in pigs after weaning: a review. Br. J. Nutr. 105:967–981. doi: 10.1017/S0007114510005660 [DOI] [PubMed] [Google Scholar]
- Wiseman J., Powles J., and Salvador F.. . 1998. Comparison between pigs and poultry in the prediction of the dietary energy value of fats. Anim. Feed Sci. Technol. 71:1–9. doi: 10.1016/S0377-8401(97)00142-9 [DOI] [Google Scholar]
- Wu L. L., Chiou C. C., Chang P. Y., and Wu J. T.. . 2004. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta. 339:1–9. doi: 10.1016/j.cccn.2003.09.010 [DOI] [PubMed] [Google Scholar]
- Yin H., Xu L., and Porter N. A.. . 2011. Free radical lipid peroxidation: mechanisms and analysis. Chem. Rev. 111:5944–5972. doi: 10.1021/cr200084z [DOI] [PubMed] [Google Scholar]