Diabetes is a chronic, progressive disease characterised by elevated levels of blood glucose. Diabetes has reached epidemic proportions.1 2 The prevalence of diagnosed diabetes in the USA in 2016 is 23 million people, of which 91% were type 2 diabetes.1
The strategy of screening for and treating pre-diabetes for diabetes prevention is endorsed by the US Preventive Services Task Force (USPSTF), the American Diabetes Association (ADA), Diabetes UK, the National Health Service in the UK and Diabetes Canada. Detection and treatment of pre-diabetes is a fundamental strategy in diabetes prevention.3–8 Although there may be some slight differences between countries in the definition of pre-diabetes, in the USA, both the USPSTF and the ADA suggest screening for pre-diabetes among middle-aged adults (USPSTF—individuals 40–70 who are overweight or obese; ADA—individuals 45 and older) at the HbA1c level of 5.7%–6.4%.3 4 Without treatment or lifestyle modifications, 15%–30% of people with pre-diabetes will develop type 2 diabetes within 5 years. As for pre-diabetes, it is estimated that 86 million American adults have pre-diabetes (or 37% of adults) and approximately 88% of them do not know they have it.9
Treatment of pre-diabetes is associated with delayed onset of diabetes and thus has economic benefits of preventing any downstream costs of diabetes care. The National Diabetes Prevention Programme (DPP), a clinical research study to evaluate prevention of diabetes, found that lifestyle alterations were quite effective at preventing pre-diabetes from progressing into diabetes and thus, minimising the incidence of diabetes in people at high risk. Further, studies have found the original DPP to be cost-effective.10 Unfortunately, even when laboratory results are consistent with pre-diabetes, primary care physicians’ treatment recommendations are not optimal for diabetes prevention.11
In addition to the morbidity and mortality associated with diabetes, the cost of diabetes care in the USA is substantial accounting for one in four healthcare dollars.12 13 On average, patients with diagnosed diabetes have medical expenditures 2.3 times higher than patients without diabetes.
Illustration of the value of diabetes prevention
We undertook an analysis to determine the impact of not screening and treating pre-diabetes versus recommended strategies for screening and treating pre-diabetes as diabetes prevention. We used as a starting point the US population who would be recommended for screening and treatment by the US Preventive Services Task Force, individuals with undiagnosed pre-diabetes aged 40–70 years who are overweight or obese. The estimate comes from data from the National Health and Nutrition Examination Survey 2015–2016. HbA1c values between 5.7% and 6.4% were used as an indicator of pre-diabetes.
We included the cost of HbA1c test and physician office visit for both Medicare and privately insured population to estimate the total screening cost. We assumed that each person will have two level-3 physician visits and receive one HbA1c test per visit. All persons after positive screening test will be assumed to be receiving a follow-up test to confirm the diagnosis (the USPSTF recommends a follow-up with the same test). These assumptions are guideline consistent. The cost of test and physician visits were estimated using the physician fee schedule from Centers for Medicare and Medicaid Services (CMS) for study population of ages 66 and above and a conversion factor (229%) was used to estimate the screening cost for privately insured population below 66 years of age. This conversion factor used to estimate the screening cost under private health plans was taken from a study conducted by Rand Corporation.14
We looked at the downstream cost of preventing transition to diabetes (cost of the screening test and the prevention treatment) versus transition to diabetes. We used the data from the DPP Outcomes Study to estimate how many will progress to diabetes if they got preventive treatment versus left untreated.15 We predicted the incidence of diabetes in the next 20 years for our study population under three scenarios: (1) no intervention, (2) lifestyle intervention and (3) metformin intervention. We used the probability estimates from a previously conducted randomised control trial to predict the number of new cases and cumulative cases of diabetes in our 20 years prediction model for all three scenarios.10 According to this study, the yearly transition probabilities from pre-diabetes to diabetes were 5.3% for lifestyle intervention, 6.4% for metformin and 7.8% for no intervention. We estimated the 20-year cost of both lifestyle and metformin interventions using the results from the same trial which also reported the yearly cost of each intervention (ie, lifestyle and metformin).
We estimated the yearly cost of diabetes in our 20-year model using results of a recently published study, which reported the economic burden of diabetes if it is not prevented.16 According to the study, the average annual burden per case for diagnosed diabetes is US$13 240. We multiplied the annual number of individuals who will transition to diabetes in the USA by the average annual healthcare expenditure per case for diagnosed diabetes, that is, US$13 240 to estimate the yearly cost of diabetes under all three scenarios: lifestyle intervention, metformin intervention and no intervention. Cost-savings were estimated by comparing lifestyle and metformin interventions with no intervention. Figures 1 and 2 show the number of cases of diabetes and the cost of diabetes for the US population under those different strategies. Not surprisingly, our example showed that for the population, costs for screening and treatment are higher for prevention strategies than doing nothing at the beginning but costs decrease over time compared with the do nothing group.
Figure 1.
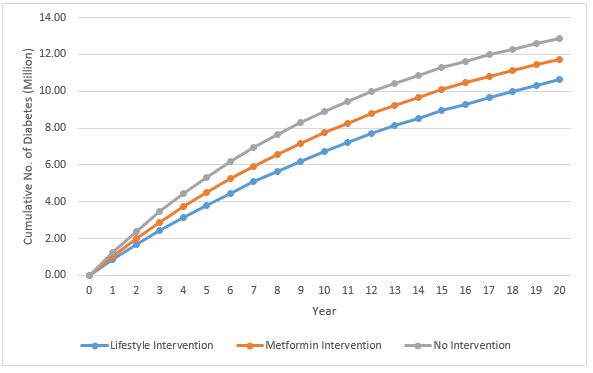
The 20-year prediction model of cumulative diabetes cases under three scenarios: lifestyle intervention, metformin intervention and no intervention.
Figure 2.
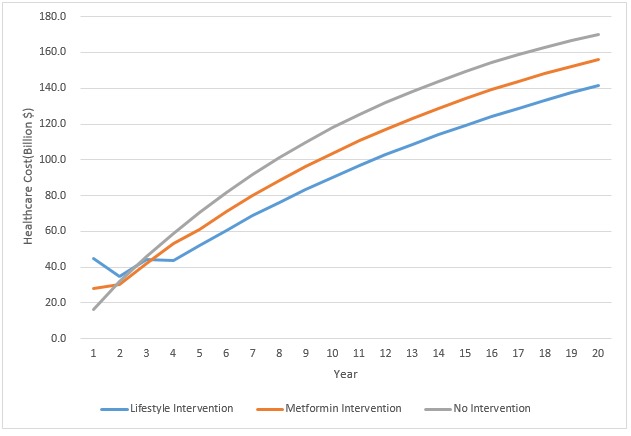
The 20-year cost prediction model under three scenarios: lifestyle intervention, metformin intervention and no intervention.
Diabetes prevention works and decreases costs, so why is it not embraced?
On a population level, this treatment of expenditures as cost may make sense.13 However, in a fee-for service world, health systems are incentivised to see this expenditure not as cost but rather as revenue. Paying for a DPP without a means to bill and collect money from payers is not in the financial interest of the health system. Although some insurance companies do cover insured members who participate in a DPP, coverage among insured patients is by no means universal. As cynical and counterintuitive for the provision of healthcare as it may sound, keeping the health system’s patients from developing diabetes is not a way to ensure a steady revenue stream. Managing diabetes once patients have developed it provides revenue to the health system through laboratory tests, physician visits, procedures and hospitalisations. When thinking of value-based reimbursement, currently, the healthcare system and quality measures are so entrenched towards diabetes management that major quality indicators for diabetes do not include prevention. Both the Medicare Merit-based Incentive Payment System in CMS’s Quality Payment Programme and Healthcare Effectiveness Data and Information Set from the National Committee for Quality Assurance have quality measures for diabetes but do not include diabetes prevention in their activities, only management of diabetes postdiagnosis.17 In other words, it costs money for most health systems that have a fee-for-service case mix to prevent diabetes but managing diabetes has substantial revenue through extra utilisation. Thus, it is misleading to classify all expenditures for diabetes as ‘costs’ since it is revenue for many stakeholders.
How do we move to diabetes prevention?
We need to align incentives for diabetes to keep patients well rather than using a business model based on waiting for patients to get sick and then treating them. We need to move to a population health orientation where disease prevention has financial benefits to the providers. The goal should be the health of the population and keeping them well. This can and is done in health plans that are capitated, and providers realise that downstream expenditures cost them money rather than making money. Experiments on bundling of payments are a first step but incentivising keeping patients well rather than paying for care once they are sick would seem to be the way to address not only the diabetes epidemic but other chronic diseases as well.
Footnotes
Contributors: All authors participated in the conception, design and writing of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bullard KM, Cowie CC, Lessem SE, et al. . Prevalence of diagnosed diabetes in adults by diabetes type - United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:359–61. 10.15585/mmwr.mm6712a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menke A, Casagrande S, Geiss L, et al. . Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–9. 10.1001/jama.2015.10029 [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care 2019;42:S13–28. 10.2337/dc19-S002 [DOI] [PubMed] [Google Scholar]
- 4.United States Preventive Services Task Force (USPSTF) Final recommendation: abnormal blood glucose and type 2 diabetes mellitus: screening, 2015. Available: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes [Accessed 8 Jul 2019].
- 5.Diabetes UK Early identification of people with, and at high risk of type 2 diabetes and interventions for those at high risk. London, UK, 2015. Available: https://www.diabetes.org.uk/Professionals/Position-statements-reports/Type-2-diabetes-prevention-early-identification/Early-identification-of-people-with-Type-2-diabetes/ [Accessed 8 May 2019].
- 6.National Health Service NHS health check best practice guidance. London, UK, 2017. Available: http://www.healthcheck.nhs.uk/ [Accessed 8 May 2019].
- 7.Canadian Diabetes Association Clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2013;37:S1–216. [DOI] [PubMed] [Google Scholar]
- 8.Tabák AG, Herder C, Rathmann W, et al. . Prediabetes: a high-risk state for diabetes development. Lancet 2012;379:2279–90. 10.1016/S0140-6736(12)60283-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopalan A, Lorincz IS, Wirtalla C, et al. . Awareness of prediabetes and engagement in diabetes risk–reducing behaviors. Am J Prev Med 2015;49:512–9. 10.1016/j.amepre.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 10.Diabetes Prevention Program Research Group The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care 2012;35:723–30. 10.2337/dc11-1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mainous AG, Tanner RJ, Baker R. Prediabetes diagnosis and treatment in primary care. J Am Board Fam Med 2016;29:283–5. 10.3122/jabfm.2016.02.150252 [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46. 10.2337/dc12-2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–28. 10.2337/dci18-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White C, Whaley C. Prices paid to hospitals by private health plans are high relative to Medicare and vary widely: findings from an Employer-Led transparency initiative. Santa Monica, CA: Rand Corporation, 2019. [PMC free article] [PubMed] [Google Scholar]
- 15.Knowler WC, Fowler SE, Hamman RF, et al. . 10-Year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet 2009;374:1677–86. 10.1016/S0140-6736(09)61457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dall TM, Yang W, Gillespie K, et al. . The economic burden of elevated blood glucose levels in 2017: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care 2019;42:1661–8. 10.2337/dc18-1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mainous AG, Schatz DA. Is it time to prioritize diabetes prevention in practice? J Am Board Fam Med 2019;32:457–9. 10.3122/jabfm.2019.04.190114 [DOI] [PubMed] [Google Scholar]


