Summary
The ability to profile transcription and chromatin binding in a cell type-specific manner is a powerful approach for understanding cell fate specification and cellular function in multicellular organisms. We recently developed Targeted DamID (TaDa) to enable genome-wide, cell-type-specific profiling of DNA- and chromatin-binding proteins in vivo without cell isolation. As a Protocol Extension, this article describes substantial modifications to an existing Protocol and offers additional applications. TaDa builds upon DamID, a technique for detecting genome-wide DNA binding profiles of proteins, by coupling it with the GAL4 system in Drosophila to enable both temporal and spatial resolution. TaDa ensures that Dam-fusion proteins are expressed at very low levels, avoiding toxicity and potential artefacts from over-expression. The modifications to the core DamID technique presented here also increase the speed of sample processing and throughput, and adapt the method to Next-generation Sequencing technology. TaDa is robust, reproducible, and highly sensitive. Compared to other methods for cell-type specific profiling, the technique requires no cell-sorting, crosslinking or antisera, and binding profiles can be generated from as few as 10,000 total induced cells. By profiling the genome-wide binding of RNA polymerase II, TaDa can also identify transcribed genes in a cell type-specific manner. Here we describe a detailed protocol for carrying out TaDa experiments and preparing the material for next generation sequencing. Although we developed TaDa in Drosophila, it should be easily adapted to other organisms with an inducible expression system. Once transgenic animals are obtained, the entire experimental procedure – from collecting tissue samples to generating sequencing libraries – can be accomplished within 5 days.
Keywords: DamID, Targeted DamID, cell type specific, transcriptional profiling, chromatin profiling, RNA polymerase II, RNA Pol II, RNAPII, genome wide, next generation sequencing
Introduction
Understanding what makes cells different from each other, and how their different properties are specified, are key questions for developmental biologists, physiologists and neurobiologists. Profiling gene expression patterns and transcriptional networks in a cell type-specific manner is a powerful method to investigate the mechanisms that specify cell fate and cell properties.
Development of Targeted DamID
We recently developed “TaDa” (Targeted DamID) to profile genome-wide, cell-type-specific protein binding in vivo without cell isolation1. TaDa does not require the purification of cells or nuclei, and the technique is simple, requiring no fixation or immunoprecipitation. TaDa is based on DamID (DNA adenine methyltransferase identification)2,3, an in vivo chromatin profiling technique. For DamID, E. coli DNA adenine methyltransferase (Dam) protein is fused to a DNA- or chromatin-binding protein of interest. When the fusion protein is expressed in vivo, its binding sites are tagged by adenine methylation of the sequence GATC, enabling DNA fragments containing the sites to be isolated after enzymatic digestion. Expression of Dam at high levels is toxic, however and as a result DamID requires the Dam-fusion protein to be expressed at extremely low levels2. A drawback of this approach is that the Dam-fusion protein is expressed constitutively from basal promoters in all cell types. Driving expression of Dam fusions using targeted expression systems, such as the GAL4 system4, results in excessively high levels of the methylase and toxicity1. As a result, it was previously impossible to perform cell type specific DamID in vivo.
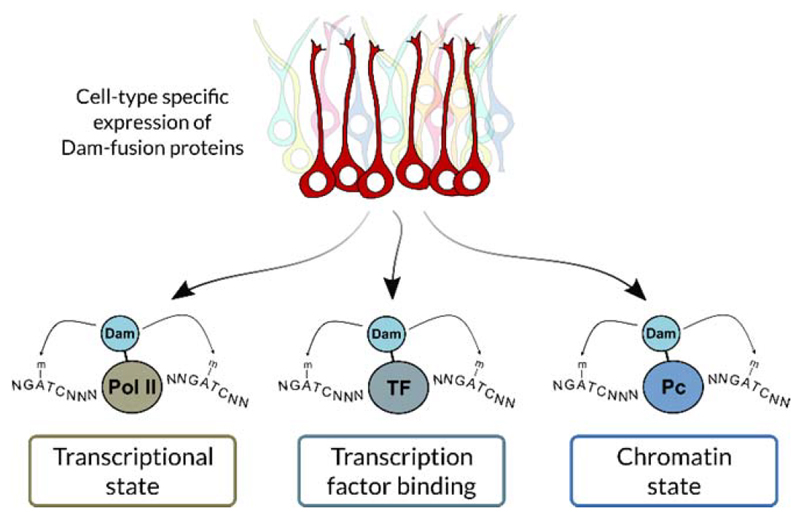
Therefore, we originated TaDa to reduce the level of translation of the Dam-fusion protein and enable expression driven by the GAL4 system or other targeted expression systems. TaDa achieves this by using an unusual feature of eukaryotic ribosome translation, whereby translation of a secondary ORF present on a transcript can be initiated, but at a greatly reduced efficiency. The frequency of translational initiation at the secondary ORF is inversely proportional to the length of the primary ORF, allowing very fine control over protein translation levels.1,5,6 We have shown that TaDa fusion proteins can be driven in the Drosophila CNS throughout development and adult life without toxicity. Importantly, TaDa enables transcription factors to be expressed in a cell-type specific manner at extremely low levels, to allow profiling without altering cell fate (Fig. 1).
Figure 1.
TaDa can profile the interaction of any protein with DNA or chromatin in a cell type-specific manner. For example, RNA polymerase II (Pol II) can be profiled to assess genome-wide transcription. TaDa can also profile transcription factor (TF) binding and assess chromatin states, by profiling chromatin modifying enzyme such as Polycomb (Pc).
Overview of the procedure
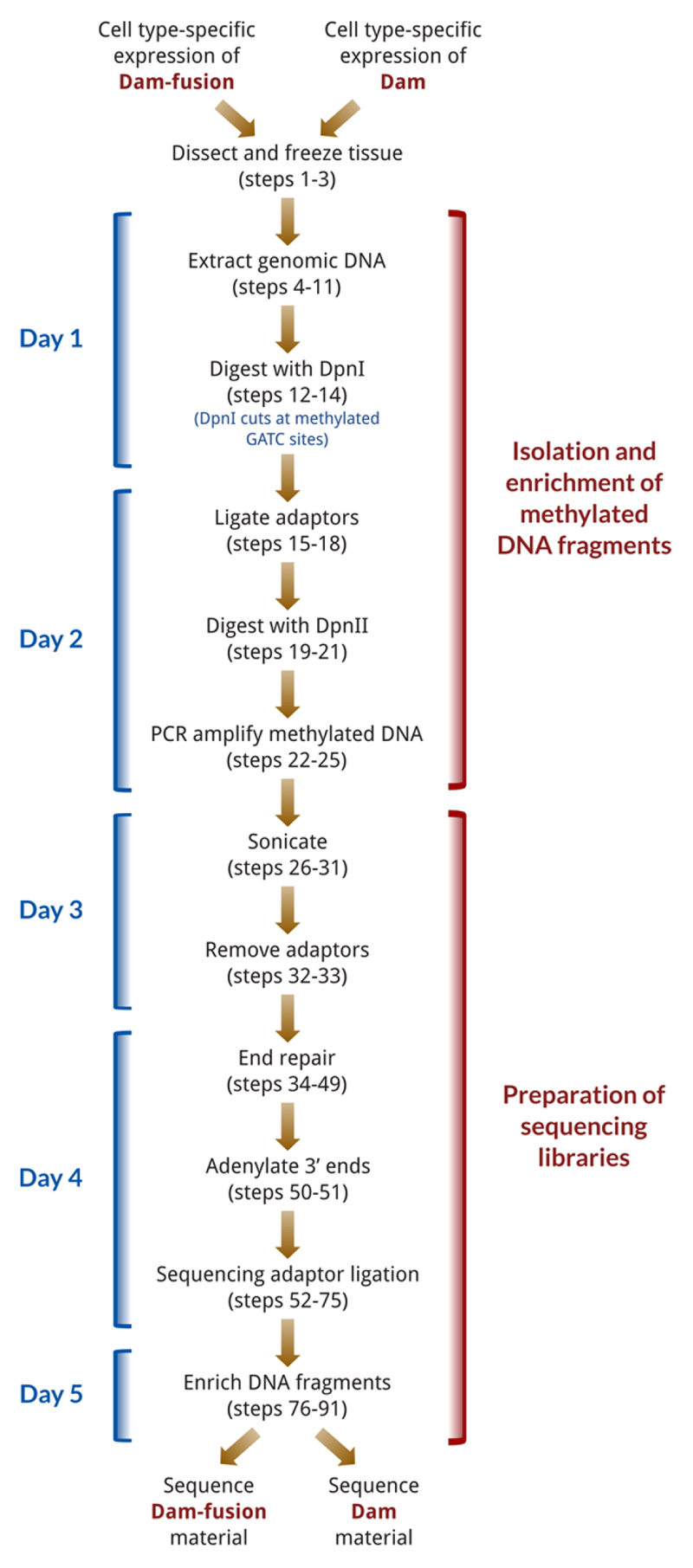
The TaDa procedure involves two main phases (Fig. 2). Briefly, in the first phase tissue is collected and genomic DNA is extracted (steps 1-11) and digested with the restriction enzyme DpnI, which only cuts at adenine-methylated GATC sites. The digested DNA is column purified to exclude un-cut, genomic DNA from uninduced cells (steps 12-14), and adaptors for PCR-amplification are ligated to the DpnI cut fragments (steps 15-18). To prevent unmethylated regions of DNA from being aberrantly amplified, the material is digested with DpnII, which only cuts non-methylated GATC sites (steps 19-21). The resulting DNA is then used as template for a PCR reaction to amplify the methylated fragments (steps 22-25). The product of the PCR reaction will thus be highly enriched for bound GATC fragments. Although based on the original DamID technique3, the procedure described here makes a number of modifications to the method, including avoiding DNA precipitation steps, removing unmethylated genomic material from uninduced cells (via column purification) and increasing the speed of the procedure.
Figure 2.
Flow chart for processing of TaDa material, with protocol steps listed. The entire procedure is completed in under five days.
In the second phase, the DNA is purified and then sonicated in preparation for NGS (steps 26-30), as the some DNA fragments generated by the DamID procedure are too large for sequencing. In order to increase the mapping specificity of reads and overcome problems of low-diversity, DamID adaptors are removed with AlwI (steps 31-33), which removes the DamID adaptors and the initial low-diversity GATC sequence at the start of amplified fragments. (Given the large size of many GATC fragments and the initial low-diversity, NGS adaptors cannot be directly used for the initial enrichment step in Phase 1.) NGS libraries are then constructed using a custom procedure (steps 34-88; a commercial kit can be substituted here if required), in which the number of library PCR cycles are reduced. Following Illumina sequencing, the binding profile of the fusion protein is determined from the sequencing data using an open-source and freely available software pipeline, and either peaks or transcribed genes (in the case of RNA polymerase II) identified. (Box 1).
Box 1. Processing of sequencing data ● TIMING 60 min per sample.
Because the final form of DamID data is a log2 ratio of [Dam-fusion/Dam], care is required to ensure correct normalisation between the fusion-protein and the Dam-only control. Both Dam alone and Dam-fusion proteins will methylate highly accessible regions of the genome when localised to the nucleus, and this background methylation is used as the basis of normalisation.
We have developed damidseq_pipeline7, an automated pipeline for processing samples that is freely available online at (http://owenjm.github.io/damidseq_pipeline/) along with detailed usage instructions. The pipeline handles automatic alignment, extension, normalisation and background reduction of the generated sequencing reads; files for human, mouse and fly genomes are provided on the website, and utilities provided to generate the required files for any other organism. The damidseq_pipeline may be run on files in FASTQ or BAM format as provided by a sequencing facility and generates a final log2 ratio file in bedgraph format.
Following ratio file generation, peaks with an FDR<0.01 can be identified using find_peaks (http://github.com/owenjm/find_peaks). In the specific case of RNA pol II datasets, the transcriptome can be identified using polii.gene.call (http://github.com/owenjm/polii.gene.call), which identifies all genes with an enriched occupancy (implying transcription) and FDR < 0.01.
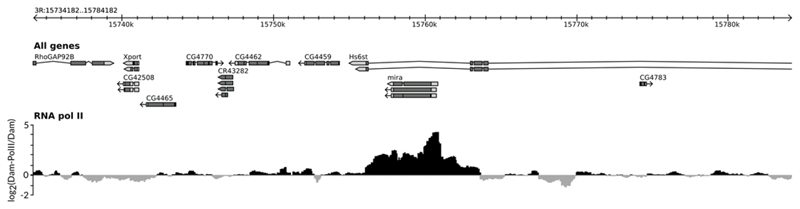
An example of the final output, illustrating the DNA binding profile of RNA pol II in Drosophila larval neural stem cells7, is shown in Fig. 4.
Applications and future uses of the method
We have successfully used TaDa to profile transcriptional activity, transcription factor binding and chromatin factor binding in multiple cell and tissue types in D. melanogaster, including embryonic and larval neural stem cells, and larval and adult neurons (1,7; Brand lab, unpublished). To assess transcription, we fused Dam to RNA polymerase II (Pol II), enabling the identification of transcribed genes in a cell type-specific manner1. Dam-Pol II can be used to compare the transcriptional states of closely related cell types. It can also be used to assess cell type-specific changes in transcription in a mutant background, for example by simultaneously profiling with Dam-Pol II whilst knocking down a gene using RNAi (P. Speder and A.H. Brand, unpublished). Transcription factors are natural candidates for the technique, and their binding sites can also be profiled in a cell-type specific manner (P. Fox, and A.H. Brand, in preparation). Chromatin states can be broadly classified into five different types, which can be identified by profiling five different proteins with DamID8. We have mapped the binding of these five factors by TaDa, thereby enabling cell type-specific profiling of chromatin states in the developing organism (O. Marshall and A.H. Brand, in preparation). The technique is highly robust and the sole limiting factor to cell-type specific profiling is the availability of specific GAL4 driver lines.
DamID has been used successfully in yeast9, plants10, C. elegans11, and human tissue culture cells12,13. Therefore, TaDa should be amenable for use in other eukaryotic organisms when coupled with appropriate cell-type specific control of induction. Our preliminary data suggest that TaDa works successfully in mammalian cells (S. Cheetham, W. Gruhn, A. Surani and A.H. Brand, unpublished).
Comparisons with other methods
A number of techniques currently exist for performing cell-type specific profiling, which take one of two approaches. The first approach focuses on specifically labelling and isolating the subset of cells of interest before undertaking conventional ChIP profiling. Examples include BITS-ChIP, where ells14 or fixed nuclei15,16 are isolated by fluorescent activated cell sorting (FACS), and the INTACT method, were nuclei are isolate by biotin labelling and pulldown17–19. These methods have the advantage that they allow a large range of factors to be profiled via a single transgenic animal. However, they have the disadvantage of requiring very large amounts of material (typically in excess of 106 cells for ChIP), can be technically challenging, and can potentially incorporate unlabelled cells or nuclei.
The second approach is to label specific factors in a cell-type specific manner, precluding the need for cell-type purification. For example, in the CAST-ChIP method20 GFP-tagged fusion proteins are expressed under the control of the GAL4 system and then, using an anti-GFP antibody, profiled by ChIP. This method requires no cell-type specific purification but involves expressing the protein of interest at high levels, which may in turn influence cell fate or result in aberrant fusion-protein binding. Cell-type specific transcriptional profiling can also be performed in this manner21, via the targeted expression of a tagged RNA-binding protein22, a ribosomal protein23, or an RNA modifying enzyme24. These methods often require large amounts of starting material and immunoprecipitation.
In contrast, TaDa requires neither large numbers of dissociated or fixed cells, nor highly induced protein expression levels. TaDa profiles protein binding in vivo without potential artefacts that may arise from fixation, and does so with very low levels of protein translation (so low that TaDa fusion proteins are undetectable via western blot1), thus minimising the impact of protein over-expression. Furthermore, the number of induced cells required for TaDa profiling is extremely low: we have successfully transcriptionally profiled ~100 neurons in whole Drosophila heads (>200,000 cells per head, thus a 1:2000 ratio of methylated DNA to total DNA), using only 100 heads and thus ~10,000 cells in total (T. D. Southall and A. H. Brand, unpublished).
Limitations of TaDa
The resolution of TaDa depends on the frequency of GATC sites in the genome (present in the Drosophila genome at a median spacing of ~190bp). Therefore it cannot always provide the same resolution as ChIP-seq experiments. TaDa profiling of Pol II occupancy does not distinguish between all alternatively spliced transcripts and cannot provide information on the direction of transcription. TaDa profiling of Pol II occupancy will provide qualitative differences in transcription between cell types or conditions; however, it does not provide quantitative levels of mRNA produced. Although TaDa cannot directly profile histone modifications, it can profile the proteins that generate, remove or recognise these modifications.
Experimental design
Dam-fusion protein expression vector
In order to obtain inducible expression coupled with low-level translation, DNA-binding proteins of interest should be cloned into the pUAST-attB-LT3-NDam vector1 (see Supplementary Fig. 1; full vector sequence available from Genbank acc. no KU728166; vector available upon request to AHB). The vector uses mCherry as the primary ORF in the bicistronic transcript, allowing easy confirmation of GAL4 induction. Coding sequences cloned in frame into the MCS of the vector will create a fusion protein with Dam at the N-terminus of the protein, followed by a myc tag and a short linker separating Dam and the protein of interest. It is important to note that following cloning or re-transformation, the Dam coding sequence should always be sequenced. We have occasionally observed spontaneous mutations arising in the Dam sequence after passage through bacteria, possibly due to the role of adenine methylation in mismatch repair in E. coli25. Bacteria carrying mutated Dam appear to have a growth advantage, therefore we tend to select the smaller colonies following transformation of the plasmid.
Cell-type specific expression
To profile protein-DNA interactions in cells of interest the choice of GAL4 driver line is important. TaDa is highly sensitive and, therefore, it is important that the GAL4 line is specific to the cells of interest. Drivers generated by homologous recombination26 or by conversion of MiMIC lines27 are usually optimal. The split-GAL4 system28 is also an option for targeting specific groups of cells that are not delimited by the expression of a single gene.
Temporal control of expression
To achieve temporal control of TaDa, a temperature sensitive repressor of GAL4, GAL80ts, can be utilised29,30. GAL80ts inactivates GAL4 at 18°C but not at 29°C. With GAL80ts in the genetic background, TaDa profiling can be initiated at any point during development or adult life by rearing the organism at 18°C and shifting to 29°C as required. Constitutively expressed GAL80ts (tub-GAL80ts) can be introduced into the background of either the GAL4 driver line or the UAS-Dam-fusion line. We have successfully expressed Dam-fusion proteins in time windows ranging from 6hrs to ~72hrs (1,7; D. Doupé and A.H. Brand, unpublished). Time windows may need to optimised, depending upon the fusion protein and cell-type being profiled: 16-24 hours works well for many tissues and GAL4 drivers.1,7 Another option for temporal control of the GAL4 system is the GeneSwitch, which involves feeding Drosophila RU486 to activate gene expression.31
Controls
To control for non-specific methylation of DNA by the Dam methylase, DamID and TaDa experiments require a Dam-only experiment to be performed in parallel with the Dam-fusion experiment (the Dam-only fly line is available upon request to AHB).2,7 The decision to use the whole organism or to dissect the tissue depends on the specificity of the driver and how many cells are being profiled relative to the total number of cells in the sample, although some degree of dissection is generally recommended when working with larvae or adult flies.
Sequencing
Libraries can be prepared using any NGS kit, modifying the amplification step as appropriate to prevent concatamer formation. The software pipeline is optimised to work with single-end reads (we recommend 50nt read length), although BAM files generated from paired-end reads are compatible. For the Drosophila genome, samples should be sequenced so as to obtain 20M mapable reads.
Data Analysis
The damidseq_pipeline software should generate accurate binding profiles with default settings for almost all Dam-fusion proteins analysed. An exception to the default settings may arise when profiling proteins that are completely sequestered from open chromatin – in these circumstances, using the optional readcount (RPM) normalisation procedure may generate more appropriate binding profiles.
Materials
Reagents
Dam-fusion (user-generated) and Dam-only (available from AHB on request) flies crossed to an appropriate GAL4 driver line (available from Drosophila stock centres e.g. Bloomington)
Autoclaved dH2O
EDTA (Sigma-Aldrich, cat. no. E6758)
QIAamp DNA micro kit (Qiagen, cat. no. 56304)
Ethanol (100%) (VWR, cat. no. 20821.330)
DpnI and CutSmart buffer (NEB, cat. no. R0176S)
PCR purification kit (Qiagen, cat. no. 28104)
dsADR adaptors (0.05 μmole, DST purity from, e.g. Sigma); see Table 1 for oligo sequence and Reagent Setup for preparation.
Table 1. Oligos required for TaDa (DST purity).
| Oligo name | Sequence (5’ to 3’) | Purpose |
|---|---|---|
| AdRt oligo (unmodified) | CTAATACGACTCACTATAGGGCAGCGTGGTCGCGGCCGA GGA | DamID adaptor (top strand) for amplification |
| AdRb oligo (unmodified) | TCCTCGGCCG | DamID adaptor (bottom strand) for amplification |
| DamID_PCR oligo (unmodified) | GGTCGCGGCCGAGGATC | Primer for amplifying dsAdR adaptor-ligated DNA |
| NGS_PCR1 (* = Phosphorothioa te linkage) | AATGATACGGCGACCACCGA*G | Primer for amplifying sequencing adaptor-ligated DNA. Phosphorothioa te linkages decrease degradation of the final library. |
| NGS_PCR2 (* = Phosphorothioa te linkage | CAAGCAGAAGACGGCATACGA*G | Primer for amplifying sequencing adaptor-ligated DNA. Phosphorothioa te linkages decrease degradation of the final library. |
NGS Primer mix see Table 1 for oligo sequences and Reagent Setup for preparation
Resuspension buffer: 10mM Tris pH 8.0, 0.1mM EDTA
Sequencing adaptors (0.05 μmole, DST purity from, e.g. Sigma); see Supplementary Table 1 for oligo sequence and Reagent Setup for preparation.
DpnII and DpnII buffer (NEB, cat. no. R0543S)
Advantage 2 cDNA polymerase (Clontech, cat. no. 639201)
(An alternative polymerase is MyTaq HS DNA Polymerase (Bioline, cat. no. BIO-21112); S. S. de Vries and B. van Steensel, personal communication.)
▲ CRITICAL STEP A polymerase with hotstart is required to prevent primer dimer formation during PCR
AlwI (NEB, cat. no. R0513S)
RNase A (DNase-free) (Roche, cat. no. 11119915001) diluted to 12.5μg/ml
Qubit assay tubes (Invitrogen, cat. no. Q32856)
Qubit dsDNA HS assay kit, (Invitrogen, cat. no. Q32851)
Agilent reagents for TapeStation: Genomic DNA ScreenTape(5067-5365) and reagents (5067-5366)
Agilent reagents for Bioanalyzer:Agilent DNA 1000 Kit (5067-1504)
Agencourt AMPure XP Beads (Beckman Coulter, cat. no. A63880)
(Seramag SpeedBeads, 3 EDAC/PA5 (Fisher Scientific, cat. no. 12326433) prepared using the method of Rohland and Reich, 201232 with 20%(w/v) PEG-8000 are a cost-effective alternative to AMPure XP beads)
Quick ligase (NEB, cat. no. M2200S)
T4 DNA ligase (400,000U/ml) and 10x buffer (NEB, cat. no. M0202S)
T4 DNA polymerase (NEB, cat. no. M0203S)
Klenow Fragment (NEB, cat. no. M0210S)
Klenow 3' to 5' exo- (NEB, cat. no. M0541S)
T4 polynucleotide kinase (NEB, cat. no. M0201S)
NEBNext High-Fidelity 2X PCR Master Mix (NEB, cat. no. M0541S)
dNTPs (NEB, cat. no. N0446S)
Equipment
Temperature controlled heat block capable of heating to 95°C.
Benchtop centrifuge capable of spinning at 20,000 x g.
PCR machine (no specific manufacturer or requirements)
Sonicator (for example, Diagenode Bioruptor cat. no. B01020001)
Magnetic rack (for example, DynaMag-96 side magnet (Invitrogen cat. no. 12331D))
DNA fluorometer (for example, Qiagen Qubit 3.0 cat. no. Q33216)
DNA analyser for sizing and quality control of DNA samples (for example, Agilent 2200 TapeStation cat. no. G2965AA)
Reagent Setup
Generation of dsAdR adaptors
Mix together 50 μl AdRt (100 μM) and 50 μl AdRb (100 μM) in a 1.5 ml tube and incubate in removable metal heating block at 95°C for 2 mins. Remove heating block and allow to cool to room temperature (should take > 45mins – slow cooling allows efficient annealing of the oligos). Annealed adaptors can be stored at -20°C for >6 months.
Generation of NGS adaptors
Resuspend adaptor oligos at 100μM in TE + 50mM NaCl. Mix 50μl Universal adaptor with 50μl of relevant index adaptor in a 1.5ml tube and incubate in removable metal heating block at 95°C for 2 mins. Remove heating block and allow to cool to room temperature (should take > 45mins – slow cooling allows efficient annealing of the oligos). Annealed oligos can be stored at -20°C for >6 months.
NGS Primer Mix
Mix 50μl NGS_PCR1 (50μM) and 50μl NGS_PCR2 (50μM). Can be stored at -20°C for >6 months.
10mM dNTPs
Mix 25μl each of 100mM dNTPs (dATP, dTTP, dCTP and dTTP) with 150μl dH2O. Can be stored at -20°C for >6 months.
Adaptor ligation buffer
Prepare as tabulated below. The buffers may be stored as aliquots at -20°C for up to one year (multiple freeze/thaw cycles of buffer aliquots should be avoided if possible).
| Component | Volume (x1) | Master mix x100 |
|---|---|---|
| 10x T4 DNA ligase buffer | 2 μl | 200 μl |
| 0.8μL dsAdR | 0.8 μl | 80 μl |
| dH2O | 1.2 μl | 120 μl |
TaDa DpnII digestion buffer
Prepare as tabulated below. The buffers may be stored as aliquots at -20°C for up to one year (multiple freeze/thaw cycles of buffer aliquots should be avoided if possible).
| Component | Volume (x1) | Master mix x100 |
|---|---|---|
| 10x Dpn II Buffer | 4 μl | 400 μl |
| dH2O | 15 μl | 1500 μl |
DamID PCR buffer
Prepare as tabulated below. The buffers may be stored as aliquots at -20°C for up to one year (multiple freeze/thaw cycles of buffer aliquots should be avoided if possible).
| Component | Volume (x1) | Master mix x50 |
|---|---|---|
| 10X cDNA PCR reaction buffer | 16 μl | 800 μl |
| DamID_PCR primer (50 μM) | 2.5 μl | 125 μl |
| 10mM dNTPs | 3.2 μl | 160 μl |
| dH2O | 96.3 μl | 4815 μl |
End Repair Buffer
Prepare as tabulated below. The buffers may be stored as aliquots at -20°C for up to one year (multiple freeze/thaw cycles of buffer aliquots should be avoided if possible).
| Component | Volume (x1) | Master mix x50 |
|---|---|---|
| 10x T4 DNA ligase buffer | 3.0 μl | 150 μl |
| 10mM dNTPs | 1.2 μl | 60 μl |
| dH2O | 3.3 μl | 165 μl |
End Repair Enzyme mix
May be stored at -20°C for up to one year; it does not need to be stored as aliquots because the enzyme mix will not freeze when stored at -20°C.
| Component | Volume (x1) | Master mix x50 |
|---|---|---|
| T4 DNA polymerase (3U/μL) | 1.14 μl | 56.82 μl |
| Klenow Fragment (5 U/μL) | 0.23 μl | 11.36 μl |
| T4 polynucleotide kinase (10 U/μL) | 1.14 μl | 56.82 μl |
Procedure
Dissection of tissue (if required) ● TIMING ~30 min per condition
-
1 |
Induce GAL4 activation of the TaDa cassette 16-24 hours prior to collection
CRITICAL STEP: A Dam-only control, driven under the same experimental conditions as the Dam-fusion protein, is always required to be processed in parallel.
-
2 |
Dissect tissue in PBS. Collect enough material to include at least 15,000 labelled cells; include more labelled cells if expression of the fusion-protein was induced for less than 16 hours.
-
3 |
When enough cells have been collected, remove excess PBS and store until required.
PAUSE POINT: samples can be stored at -20°C or -80°C until required
Extraction of genomic DNA (using Qiagen DNA Micro Kit) ● TIMING 1 h 30 min to 2 h
-
4 |
This step can be performed using Option A or Option B, depending on whether whole organisms or dissected tissues are used.
▲CRITICAL STEP From this point until the completion of DpnI digestion, do not vortex or pipette vigorously as this can result in shearing of the DNA. Mix samples by either very gentle flicking or gentle pipetting using a P1000.
-
A)
Whole embryos or other tissues requiring mechanical disruption (e.g. adult heads) TIMING 15 min
Take samples from freezer and add 175 μl 1X PBS to eppendorf tube containing sample. If processing samples containing gut tissue, we recommend the addition of EDTA in order to prevent fragmentation of genomic DNA by nucleases.
Add 20 μl RNase (12.5μg/ml) and pipette mix.
Use sterile pestle (washed in 100% ethanol) to homogenise sample in PBS.
Add 20 μl Proteinase K (Qiagen DNA Micro Kit) and mix gently; leave for 1 min at room temperature (RT; 22°C).
Add 200 μl Buffer AL (Qiagen DNA Micro Kit) and mix gently; incubate at 56°C for 10 mins.
Cool to room temperature, add 200 μl 100% ethanol and mix gently.
-
B)
Small volumes (<10μl total volume) of dissected tissue TIMING 1 h – 6 h
Take cut larvae or dissected tissue from freezer. If processing samples containing gut tissue, we recommend the addition of EDTA in order to prevent fragmentation of genomic DNA by nucleases.
Add 180μL ATL buffer (Qiagen DNA Micro Kit)
Add 20μL Proteinase K (Qiagen DNA Micro Kit) and mix gently.
-
Incubate at 56°C on heat block until digested; flick gently to mix occasionally.
▲CRITICAL STEP Digestion will typically take one hour for most tissues, but may require longer if larger volumes of material are used.
Cool to room temperature and add 20 μL RNase (12.5μg/ml)
Incubate at room temperature for 2 mins
Mix 200μL Buffer AL (Qiagen DNA Micro Kit) and 200μL 100% EtOH together (per sample) in a separate tube; mix well by vortexing
Add 400μL of Buffer AL/EtOH mix to each sample; mix well by inversion.
-
A)
-
5 |
Add all solution (from step 4A(vii) or 4B(viii)) to a spin column (Qiagen DNA Micro Kit).
-
6 |
Spin at >6000 x g for 1 min, discard flow-through and collecting tube.
-
7 |
Add 500 μl AW1 solution (Qiagen DNA Micro kit) and spin at >6000 x g for 1 min, discard flow-through and collecting tube.
-
8 |
Add 500 μl AW2 (Qiagen DNA Micro kit) solution and spin at >6000 x g for 1 min, discard flow-through and collecting tube.
-
9 |
Spin at 20,000 x g for 3 mins to dry the column.
-
10 |
Transfer spin column to a new 1.5 ml tube, add 50 μl AE buffer (Qiagen DNA Micro kit) and leave at RT for at least 30 min. Spin at >6000 x g for 1 min and keep the flow-through.
-
11 |
Run 1 μl on a 0.8% agarose gel to check quality. The genomic DNA should be visible as a single band at the top of the gel and not a smear. (TROUBLESHOOTING)
Digestion of methylated DNA ● TIMING ~ 16 hrs
-
12 |
Transfer 43.5μl to a new 1.5ml tube.
-
13 |
Add 5μl NEB CutSmart Buffer and 1.5 μl DpnI enzyme, mix very gently with either flicking or a P1000 and digest overnight (at least 12 hours) at 37°C.
▲CRITICAL STEP Do not vortex the DpnI solution as this can result in shearing of the genomic DNA.
-
14 |
Clean-up DpnI digested DNA with Qiagen PCR Purification kit according to the manufacturer’s instructions; elute in 32 μl H2O.
■ PAUSE POINT DNA can be stored for up to 6 months at 20°C.
Ligation of DamID adaptors to DpnI digested DNA ● TIMING 2.5 hrs
-
15 |
Measure DNA concentration on Qubit or Nanodrop and use either 15 μl of undiluted sample (if less than a total of 750 ng) or dilute with H2O to a total of 750 ng in 15 μl for the next step (the remaining DNA can be stored at -20°C). Depending on the starting material and the proportion of induced cells, very low to undetectable DNA concentrations at this step are completely normal.
-
16 |
Transfer 15 μl of sample to 0.2 ml PCR tubes and add 4 μl of pre-made Adaptor Ligation Buffer (see REAGENT SETUP).
-
17 |
Add 1μl (400U) T4 DNA ligase enzyme and mix well.
-
18 |
Incubate ligation reaction for 2 hours at 16°C in a PCR machine, followed by 10 mins at 65°C to inactivate the ligase.
DpnII digestion of DNA ● TIMING 2 hrs
-
19 |
Add 19μl of pre-made TaDa DpnII Digestion Buffer (see REAGENT SETUP).
-
20 |
Add 1μl DpnII enzyme and mix well.
-
21 |
Digest at 37°C (use incubator or PCR machine) for at least 2 hours (a longer digestion is acceptable).
PCR amplification ● TIMING 2.5 hrs
-
22 |
Add 118μl of pre-made DamID PCR Buffer (see REAGENT SETUP)
-
23 |
Add 2μl Advantage cDNA polymerase enzyme and mix well.
-
24 |
Split the reaction into 4 x 40 μl reactions in 0.2 ml PCR tubes.
▲CRITICAL STEP For an efficient PCR amplification, the PCR reaction volume should be kept low (≤ 40 μl). Larger reaction volumes can result in inefficient amplification of large DNA fragments.
-
25 |Perform PCR using the following program:
Cycle number Denature Anneal Extend 0 68°C 10 min 1 94°C 30 sec 65°C 5 min 68°C 15 min 2-4 94°C 30 sec 65°C 1 min 68°C 10 min 5-21 94°C 30 sec 65°C 1 min 68°C 2 min 22 68°C 5 min ■ PAUSE POINT PCR amplified DNA can be stored overnight at 4°C or for up to 6 months at 20°C.
Sonication and removal of PCR adaptors ● TIMING ~16 hrs
-
26 |
Run 3 μl of the PCR product on a 0.8% agarose gel to check quality. A successful amplification should show a smear concentrated between 400bp and 2kb; some unique bands may also be present depending on the particular binding characteristics of the Dam-fusion protein. See Figure 3 for an example.
-
27 |
Use Qiagen PCR purification kit according to the manufacturer’s instructions to purify the rest of PCR product, use 32 μl H2O to elute (leave water on column for at least 5 min before final spin).
-
28 |
Measure concentration of purified DNA using a Qubit or Nanodrop and dilute 2 μg in 90 μl H2O in a 1.5ml tube. (TROUBLESHOOTING)
-
29 |
Add 10μl NEB CutSmart Buffer and mix well.
-
30 |
Sonicate DNA. Sonication time and conditions are highly dependent upon the equipment used, and should be optimised to generate an average DNA fragment size of ~300 bp. (With a Bioruptor Plus, we use 5 min, high power, 30 sec on / 30 sec off.)
-
31 |
Check sonicated DNA on an Agilent Tapestation or Bioanalyzer. The fragments should have a mean size of ~300bp (see Fig. 3 for an example).
-
32 |
Remove DamID adaptors from sonicated DNA by adding 1μl of AlwI (mix well). Incubate overnight at 37°C. (TROUBLESHOOTING)
-
33 |
Transfer 70μl of each sample to PCR strips for library preparation.
■ PAUSE POINT Sonicated DNA can be stored for up to 6 months at 20°C.
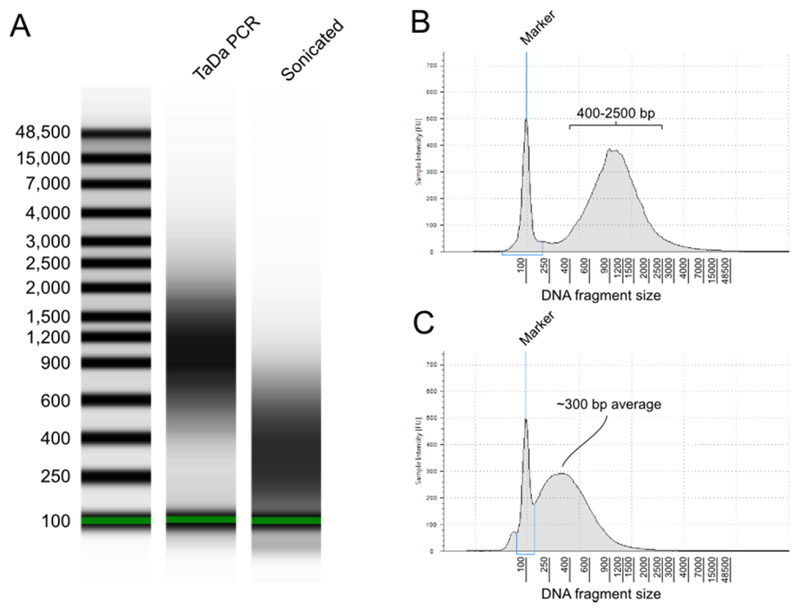
Figure 3.
Sample Tapestation plots showing the expected fragment size distribution following TaDa. (A) Gel image of samples following the TaDa PCR step (step 29) and sonication (step 35). (B) Electropherogram of the TaDa PCR sample; (C) electropherogram of the sample post-sonication. The sample illustrated was prepared by driving the DAM-only construct in adult neurons for 24 hours, with DNA isolated from whole adult heads.
Sequencing library preparation: DNA clean-up ● TIMING 40 min
-
34 |
Add 105 μl of AMPure XP beads to 70 μl sample and mix well.
▲CRITICAL STEP Ensure that the bead stock is fully mixed before pipetting (use vortex)
-
35 |
Incubate at RT for 10 min.
-
36 |
Place on magnetic stand for 10 min (or until clear) and keep tubes on magnetic stand for the following steps 37-39.
-
37 |
Remove and discard supernatant
▲CRITICAL STEP Do not touch the bead pellet with the pipette tip
-
38 |
Wash the beads twice in 190 μl 80% EtOH (leave beads on magnet; 30 secs wash time).
-
39 |
Remove as much liquid as possible and leave for 5 min to air dry.
-
40 |
Remove PCR strip from magnetic stand and resuspend beads in 25 μl Resuspension Buffer.
-
41 |
Incubate for 2 min at RT.
-
42 |
Place on magnetic stand for 5 min (or until clear).
-
43 |
Put 22.5 μl of the supernatant into a new, clean tube for the next step.
■ PAUSE POINT Purified DNA can be stored for up to 6 months at 20°C.
Sequencing library preparation: Adjust concentrations ● TIMING 20 min
-
44 |
Use 1μl to measure sample concentration on a Qubit or similar fluorimeter.
-
45 |
Dilute sample to (no more than) 500ng of DNA in 20μl Resuspension Buffer (remainder can be discarded).
Sequencing library preparation: End repair ● TIMING 55 min
-
46 |
Add 7.5μl End Repair Buffer
-
47 |
Add 2.5 μl End Repair Enzyme Mix and mix well.
-
48 |
Incubate for 30 min at 30°C.
-
49 |
Heat inactivate enzymes for 20 mins at 75°C
■ PAUSE POINT End repaired DNA can be stored for up to 6 months at -20°C.
Sequencing library preparation: Adenylate 3’ Ends ● TIMING 35 min
-
50 |
Add 0.75 μl Klenow 3' – 5' exo- enzyme.
-
51 |
Incubate at 37°C for 30 min.
Sequencing library preparation: Sequencing adaptor ligation ● TIMING 35 min
-
52 |
Add 2.5μl of relevant sequencing adaptor.
▲CRITICAL STEP If multiplexing four or fewer libraries, selecting adaptors with barcodes that are too similar may result in a reduced number of reads passing filter. In these case, the preferred indices to use (in order) are: 4, 7, 6 and 8.
-
53 |
Add 2.5 μl NEB Quick ligase enzyme
-
54 |
Incubate at 30°C for 10 min.
-
55 |
Add 5μl 0.5M EDTA to stop the ligation reaction.
■ PAUSE POINT Ligated DNA can be stored for up to 6 months at -20°C.
Sequencing library preparation: DNA clean-up ● TIMING 60 min
▲CRITICAL Two rounds of bead clean-up (steps 56-65 and 66-75) are required to ensure the complete removal of adaptor dimers
-
56 |
Add 41 μl AMPure beads and mix well.
-
57 |
Incubate at RT for 10 min.
-
58 |
Place on magnetic stand for 5 min (or until clear).
-
59 |
Remove 80 μl of supernatant.
-
60 |
Wash twice in 200μl 80% EtOH (leave beads on magnet; 30 secs wash time).
-
61 |
Leave for 5 min to air dry.
-
62 |
Remove PCR strip from magnetic stand and resuspend the beads in 52.5 μl Resuspension Buffer.
-
63 |
Incubate for 2 min at RT.
-
64 |
Place on magnetic stand for 5 min (or until clear).
-
65 |
Transfer 50 μl of the supernatant to a new, clean tube for the next round of clean-up (steps 66-75).
■ PAUSE POINT Purified DNA can be stored for up to 6 months at -20°C.
-
66 |
Add 50 μl AMPure beads.
-
67 |
Incubate at RT for 10 min.
-
68 |
Place on magnetic stand for 5 min (or until clear).
-
69 |
Remove 95 μl of supernatant.
-
70 |
Wash twice in 200μl 80% EtOH (leave beads on magnet; 30 secs wash time).
-
71 |
Leave for 5 min to air dry.
-
72 |
Remove PCR strip from magnetic stand and resuspend the beads in 22.5 μl Resuspension Buffer.
-
73 |
Incubate for 2 min at RT.
-
74 |
Place on magnetic stand for 5 min (or until clear).
-
75 |
Transfer 20 μl of the supernatant to a new, clean tube for the next step.
■ PAUSE POINT Purified DNA can be stored for up to 6 months at 20°C.
Sequencing library preparation: Enrich DNA fragments ● TIMING 25 - 40 min
-
76 |
Add 5 μl PCR Primer Mix.
-
77 |
Add 25 μl PCR Master Mix.
-
78 |Perform PCR cycle:
Cycle number Denature Anneal Extend 0 98°C 30 sec 1-6 98°C 10 sec 60°C 30 sec 72°C 30 sec 7 72°C 5 min
▲CRITICAL STEP Greater than 6 cycles may need to be performed if amount of starting material is less than recommended. However, it is important not to use too many cycles as this can result in adaptor concatemers (see Troubleshooting).
Sequencing library preparation: DNA clean-up ● TIMING 30 min
-
79 |
Add 50 μl AMPure beads to the 50 μl PCR sample.
-
80 |
Incubate at RT for 10 min.
-
81 |
Place on magnetic stand for 5 min (or until clear).
-
82 |
Remove 95 μl.
-
83 |
Wash twice in 200 μl 80% EtOH (leave beads on magnet; 30 secs wash time).
-
84 |
Leave for 5 min to air dry.
-
85 |
Remove PCR strip from magnetic stand and resuspend the beads in 32.5 μl Resuspension Buffer.
-
86 |
Incubate for 2 min at RT.
-
87 |
Place on magnetic stand for 5 min (or until clear).
-
88 |
Transfer 30μl of the supernatant to a new, clean tube for the next step.
Sequencing library quality control ● TIMING 60 min
-
89 |
Check DNA on Agilent Tapestation or Bioanalyzer (see Anticipated Results) and note average fragment size.
-
90 |
Measure DNA concentration with Qubit fluorometer (if starting with 500ng of material, final concentration should be between 15-30 ng/μl).
-
91 |
Pool libraries to final recommended concentration for Illumina sequencing. (DNA molarity =~ (1500 / (fragment size in bp)) x (concentration in ng/μl). Ensure that all libraries have the same final concentration when pooled.
-
92 |
Sequence single-ended 50 nt (SE50) reads on an Illumina sequencer. For Drosophila samples, aim for at least 20 million reads per library.
▲CRITICAL STEP Other organisms may require significantly more reads per library, depending upon genome size.
-
93 |
Process Illumina sequencing data as outlined in Box 1.
Timing
A schematic of the Procedure and timeline can be found in Figure 2.
Day 1: Steps 1-13.
Day 2: Steps 14-25.
Day 3: Steps 26-32.
Day 4: Steps 33-75
Day 5: Steps 76-91
Troubleshooting
Troubleshooting advice can be found in Table 3.
Table 3. Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 11 | Insufficient genomic DNA | Insufficient starting material | Use more starting material (step 2) Although not optimal, as little as 1 μg of genomic DNA can be used. |
| Sample not homogenised enough | Perform more vigorous homogenisation (step 4) | ||
| 28 | Insufficient PCR product | Small number of cells expressing the Dam-fusion protein, relative to the rest of the tissue | Increase starting material at step 2 or the length of GAL4 induction time at step 1. |
| 32 | DNA fragment sizes too large | Insufficient sonication | Re-optimise sonication time (step 30) |
| 87 | Secondary peak in sequencing library | Exhaustion of PCR in sequence library preparation, resulting in adaptor concatemers | Reduce amplification cycles (step 78) or reduce input DNA quantity (step 45). It is not advised to perform fewer than 6 cycles. |
| 87 | No DNA after sequencing library preparation | Problem with a reagent | Use fresh 80% ethanol when washing bead preps Use new AmpureXP beads Use fresh enzymes for end repair, tailing, ligation and PCR |
| 91 | Low numbers of reads, or a low percentage of reads mapped back to genome | Contamination Failure to remove initial adaptors with AlwI | Use fresh AlwI enzyme (step 32) and check that the correct buffer is being used (step 29)– failure to remove the original adaptors will cause problems with clustering on the Illumina sequencer and greatly reduce the number of reads passing filter. |
| Adaptor dimers or adaptor concatemers present in sequencing library | Adaptor dimers or concatamers will be visible in at step 87. Concatamers are prevented by reducing PCR cycles or reducing template DNA (see above). Dimers should be removed by bead purification steps 54-73. Repeat using fresh beads and check pipette accuracy. | ||
Anticipated results
Amplification of Dam-methylated DNA (Steps 1-25)
The PCR amplification should produce DNA fragments ranging from approximately 400 bp to 2500 bp (see Figure 3A,B). The total yield may range from 2 to 20 μg, depending on the number of labelled cells and the factor being profiled.
Sonication and removal of adaptors (Steps 26-33)
Sonication of the amplified DNA should provide fragments with an average size of 300 bp (± 50 bp). See Figure 3A,C for an example. DamID adaptors are subsequently removed with AlwI digestion.
Sequencing library preparation (Steps 34-90)
Final library concentration should be in excess of 50nM, with fragments spread around a mean size of 450bp (note that the apparent size on the TapeStation will be larger, typically 500-550bp).
We assess overall quality through the percent of mapped reads, which ideally should be higher than 90% in Drosophila. Lower percentages of mapped reads may indicate primer-dimers or library contamination. The final log2 ratio profile from a DamID-seq experiment should show strong signal for bound regions with low background (see Figure 4 for an example profile using RNA pol II.) If profiling a transcription factor, peaks should be detectable through software (see Box 1); if profiling transcription via RNA pol II, genes with an FDR < 0.01 should be detectable following software analysis (see Box 1).
Figure 4.
Example log2 ratio profile of RNA pol II binding in Drosophila neural stem cells. DNA was isolated from 50 Drosophila melanogaster larvae for both Dam-polII and Dam-only samples; larvae were torn in half and the anterior halves (which contain the brain) retained for DNA isolation and processing. Expression of the TaDa proteins was driven using a neural stem cell-specific driver (worniu-GAL4) in the presence of tub-GAL80ts and induced for 16 hrs at 29°C from 72 hrs after larval hatching. The gene miranda (mira) illustrated here is required for the correct segregation of cell fate determinants during the division of Drosophila neural stem cells.
Supplementary Material
Editorial summary.
Targeted DamID (TaDa) extends DamID to enable cell-type specific profiling of genome-wide protein binding. Transcription factor binding sites, RNA Pol II occupancy and chromatin states can be studied to provide insights into cell fate specification.
Acknowledgements
This work was funded by a Wellcome Trust Senior Investigator Award (103792), Wellcome Trust Programme Grant (092545) and BBSRC Project Grant (BB/L00786X/1) to A.H.B. A.H.B acknowledges core funding to the Gurdon Institute from the Wellcome Trust (092096) and CRUK (C6946/A14492).
Footnotes
Author contributions
OJM, TDS and AHB designed the research and analysed the data; TDS and OJM developed the protocols; OJM, TDS and AHB wrote the paper.
Competing financial interests
The authors declare that they have no competing financial interests.
References
- 1.Southall TD, et al. Cell-Type-Specific Profiling of Gene Expression and Chromatin Binding without Cell Isolation: Assaying RNA Pol II Occupancy in Neural Stem Cells. Dev Cell. 2013;26:101–12. doi: 10.1016/j.devcel.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Steensel B, Henikoff S. Identification of in vivo DNA targets of chromatin proteins using tethered dam methyltransferase. Nat Biotechnol. 2000;18:424–8. doi: 10.1038/74487. [DOI] [PubMed] [Google Scholar]
- 3.Vogel MJ, Peric-Hupkes D, van Steensel B. Detection of in vivo protein-DNA interactions using DamID in mammalian cells. Nat Protoc. 2007;2:1467–1478. doi: 10.1038/nprot.2007.148. [DOI] [PubMed] [Google Scholar]
- 4.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 5.Luukkonen BG, Tan W, Schwartz S. Efficiency of reinitiation of translation on human immunodeficiency virus type 1 mRNAs is determined by the length of the upstream open reading frame and by intercistronic distance. J Virol. 1995;69:4086–4094. doi: 10.1128/jvi.69.7.4086-4094.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Blokland HJM, Hoeksema F, Siep M, Otte AP, Verhees JA. Methods to create a stringent selection system for mammalian cell lines. Cytotechnology. 2011;63:371–384. doi: 10.1007/s10616-011-9354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall OJ, Brand AH. damidseq_pipeline: an automated pipeline for processing DamID sequencing datasets. Bioinformatics. 2015;31:3371–3. doi: 10.1093/bioinformatics/btv386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filion GJ, et al. Systematic protein location mapping reveals five principal chromatin types in Drosophila cells. Cell. 2010;143:212–24. doi: 10.1016/j.cell.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woolcock KJ, Gaidatzis D, Punga T, Bühler M. Dicer associates with chromatin to repress genome activity in Schizosaccharomyces pombe. Nat Struct Mol Biol. 2011;18:94–99. doi: 10.1038/nsmb.1935. [DOI] [PubMed] [Google Scholar]
- 10.Germann S, Juul-Jensen T, Letarnec B, Gaudin V. DamID, a new tool for studying plant chromatin profiling in vivo, and its use to identify putative LHP1 target loci. Plant J. 2006;48:153–163. doi: 10.1111/j.1365-313X.2006.02859.x. [DOI] [PubMed] [Google Scholar]
- 11.Schuster E, et al. DamID in C. elegans reveals longevity-associated targets of DAF-16/FoxO. Mol Syst Biol. 2010;6:399. doi: 10.1038/msb.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guelen L, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–51. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- 13.Vogel MJ, et al. Human heterochromatin proteins form large domains containing KRAB-ZNF genes. Genome Res. 2006;16:1493–1504. doi: 10.1101/gr.5391806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryant Z, et al. Characterization of differentially expressed genes in purified Drosophila follicle cells: Toward a general strategy for cell type-specific developmental analysis. Proc Natl Acad Sci. 1999;96:5559–5564. doi: 10.1073/pnas.96.10.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonn S, et al. Tissue-specific analysis of chromatin state identifies temporal signatures of enhancer activity during embryonic development. Nat Genet. 2012;44:148–156. doi: 10.1038/ng.1064. [DOI] [PubMed] [Google Scholar]
- 16.Bonn S, et al. Cell type-specific chromatin immunoprecipitation from multicellular complex samples using BiTS-ChIP. Nat Protoc. 2012;7:978–94. doi: 10.1038/nprot.2012.049. [DOI] [PubMed] [Google Scholar]
- 17.Deal RB, Henikoff S. A simple method for gene expression and chromatin profiling of individual cell types within a tissue. Dev Cell. 2010;18:1030–1040. doi: 10.1016/j.devcel.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry GL, Davis FP, Picard S, Eddy SR. Cell type-specific genomics of Drosophila neurons. Nucleic Acids Res. 2012;40:9691–9704. doi: 10.1093/nar/gks671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steiner FA, Talbert PB, Kasinathan S, Deal RB, Henikoff S. Cell-type-specific nuclei purification from whole animals for genome-wide expression and chromatin profiling. Genome Res. 2012;22:766–777. doi: 10.1101/gr.131748.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schauer T, et al. CAST-ChIP maps cell-type-specific chromatin states in the Drosophila central nervous system. Cell Rep. 2013;5:271–82. doi: 10.1016/j.celrep.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otsuki L, Cheetham SW, Brand AH. Freedom of expression: Cell-type-specific gene profiling. Wiley Interdisciplinary Reviews: Developmental Biology. 2014;3:429–443. doi: 10.1002/wdev.149. [DOI] [PubMed] [Google Scholar]
- 22.Roy PJ, Stuart JM, Lund J, Kim SK. Chromosomal clustering of muscle-expressed genes in Caenorhabditis elegans. Nature. 2002;418:975–979. doi: 10.1038/nature01012. [DOI] [PubMed] [Google Scholar]
- 23.Thomas A, et al. A Versatile Method for Cell-Specific Profiling of Translated mRNAs in Drosophila. PLoS One. 2012;7:e40276. doi: 10.1371/journal.pone.0040276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller MR, Robinson KJ, Cleary MD, Doe CQ. TU-tagging: cell type-specific RNA isolation from intact complex tissues. Nat Methods. 2009;6:439–441. doi: 10.1038/nmeth.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modrich P, Lahue R. Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Annu Rev Biochem. 1996;65:101–133. doi: 10.1146/annurev.bi.65.070196.000533. [DOI] [PubMed] [Google Scholar]
- 26.Baena-Lopez LA, Alexandre C, Mitchell A, Pasakarnis L, Vincent J-P. Accelerated homologous recombination and subsequent genome modification in Drosophila. Development. 2013;140:4818–25. doi: 10.1242/dev.100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venken KJT, et al. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat Methods. 2011;8:737–743. doi: 10.1038/nmeth.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luan H, Peabody NC, Vinson C, White BH. Refined Spatial Manipulation of Neuronal Function by Combinatorial Restriction of Transgene Expression. Neuron. 2006;52:425–436. doi: 10.1016/j.neuron.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto K, Toh-e A, Oshima Y. Genetic control of galactokinase synthesis in Saccharomyces cerevisiae: evidence for constitutive expression of the positive regulatory gene gal4. J Bacteriol. 1978;134:446–457. doi: 10.1128/jb.134.2.446-457.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 31.Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohland N, Reich D. Cost-effective, high-throughput DNA sequencing libraries for multiplexed target capture. Genome Res. 2012;22:939–46. doi: 10.1101/gr.128124.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.