Introductory paragraph
Retinal gene therapy is increasingly recognised as a novel molecular intervention that has huge potential in treating common causes of blindness, the majority of which have a genetic aetiology.1–5 Choroideremia is a chronic X-linked retinal degeneration that was first described in 1872.6 It leads to progressive blindness due to deficiency of Rab-escort protein 1 (REP1). We designed an adeno-associated viral vector to express REP1 and assessed it in a gene therapy clinical trial by subretinal injection in 14 patients with choroideremia. The primary endpoint was vision change in treated eyes two years after surgery compared to unoperated fellow eyes. Despite complications in two patients, visual acuity improved in the 14 treated eyes over controls (median 4.5 letter gain, vs 1.5 letter loss, p=0.04), with six treated eyes gaining more than one line of vision (>5 letters). The results suggest that retinal gene therapy can sustain and improve visual acuity in a cohort of predominantly late stage choroideremia patients in whom rapid visual acuity loss would ordinarily be predicted.
Introduction
Choroideremia typically presents with night blindness and progressive visual field restriction in late childhood, leading to profound sight loss in young men beyond the fourth decade.7 The choroideremia gene (CHM) encodes Rab-escort protein-1 (REP1) which facilitates intracellular vesicular trafficking.8,9 Deficiency of REP1 leads to degeneration of the retinal pigment epithelium and photoreceptors in males,10 whereas female carriers generally have a mild disease phenotype due to random X-inactivation.11 The choroid degenerates secondary to loss of the pigmented epithelium, leading to exposure of the underlying white sclera and characteristic retinal appearance. The central cone photoreceptors are usually maintained until late stages, due to the centripetal nature of the degeneration.12 Hence long after visual field loss, there is a terminal period during which central visual acuity begins to decline as the underlying central retinal pigment epithelium becomes dysfunctional.10 There is therefore a potential window of opportunity for improvement in visual acuity if this dysfunction can be reversed by gene replacement therapy before these cells are irreversibly lost.13
The adeno-associated virus serotype 2 (AAV2) vector has been used in a number of clinical trials and is particularly effective at targeting outer retinal layers, but only when injected under the retina correctly.14,15 Hence assessment of retinal gene therapy must include consideration of the surgical technique as well as the biological properties of the investigational medicinal product. We previously reported the early safety data of retinal gene therapy for choroideremia and visual acuity changes in the first 6 patients who received the low dose of an AAV2 vector carrying the human CHM transgene.16,17 Here we report the full results of the trial with all 14 participants in both low and high dose cohorts having reached the 2-year study endpoint.
Results
A total of 14 patients were recruited (Supplementary Table S1), 13 of whom received either ‘low dose’ 1x1010 genome particles (L1-5) or ‘high dose’ 1x1011 gp (C2 and H1-7) of AAV2.REP1 vector (Fig. 1), surgically delivered into the subretinal space via an iatrogenic retinal detachment. At the 2-year trial endpoint, the median visual acuity across all 14 treated eyes had improved by 4.5 Early Treatment Diabetic Retinopathy Study (ETDRS) chart letters (IQR: -2.0 to 8.8) and in the 14 untreated eyes had declined by -1.5 letters (IQR: -4.8 to 0.0), hence favouring the treated eyes overall (two-tailed Wilcoxon test: W=65, p=0.040) (Supplementary Table S2). The trial thus met its primary endpoint of improving vision following gene therapy compared to untreated fellow eyes, despite any potential adverse effects of retinal detachment.
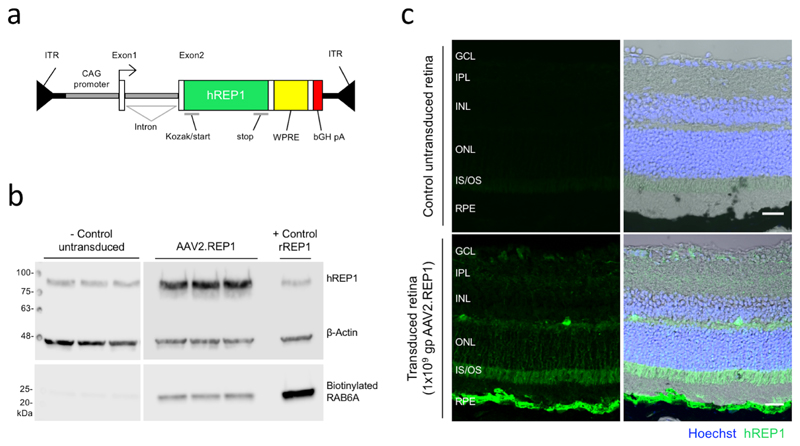
Figure 1. Validation of AAV2.REP1 gene therapy vector.
(a) Schematic of the AAV2.REP1 vector used for choroideremia gene therapy. Green, human CHM cDNA; yellow, Woodchuck hepatitis virus post-transcriptional regulatory element (WPRE); red, bovine growth hormone polyadenylation signal (bGH pA); ITR, inverted terminal repeat. (b) REP1 protein expression and prenylation activity following transduction of HEK293 cells using the clinical grade vector were tested annually (3 replicates within 1 experiment). Western blot showing increased human REP1 protein expression in comparison to β–actin at day 5 in AAV-transduced HEK293 cells versus untransduced control (both in triplicates). REP1-mediated prenylation activity was assessed through in vitro biotinylation of RAB6A substrate using the cell lysates. Positive control represents untransduced cell lysate supplemented with recombinant fish REP1. Uncropped gel images shown in Supplementary Fig. S1. (c) Confocal stack prepared from histological sections of murine eyes 5 weeks following subretinal inject with research-grade AAV2.REP1 vector at 1x109 gp (representative images from 3 animals). REP1 expression could be seen in the retinal pigment epithelium and photoreceptors. A matched uninjected area of the same eye was used as control. Human REP1 immunostaining (green) and nuclear labelling with Hoechst (blue) were overlaid with the differential interference contrast (DIC) image to demonstrate the retinal layers: GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; IS/OS, inner segment/outer segment junction; RPE, retinal pigment epithelium. Scale bar, 25 μm.
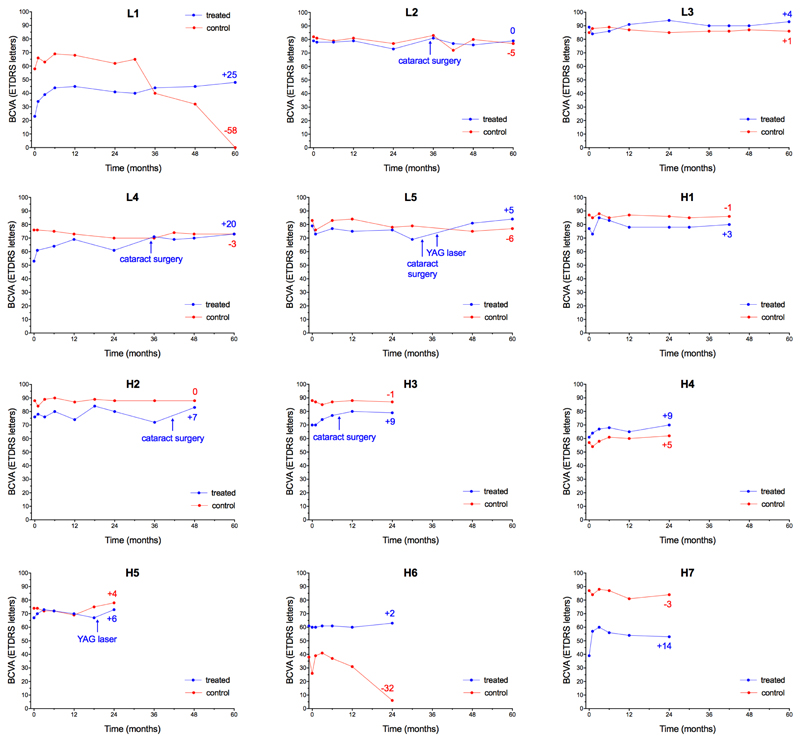
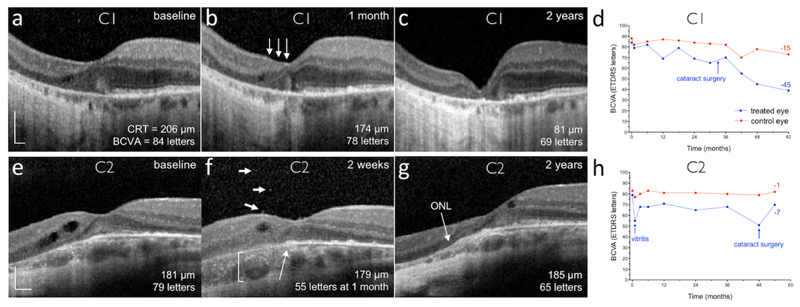
In 12 out of 14 patients, the retinal gene therapy was performed as per protocol, leading to recovery of visual acuity in all eyes and variable degrees of acuity gains which generally occurred within 6 months of treatment and were sustained up to 5 years (Fig. 2 and Supplementary Table S3). Significant adverse events (AEs) relating to vector administration occurred in 2 of the 14 patients: C1 and C2 (Fig. 3). In C1, a surgical complication resulted in retinal thinning and the vector was under-dosed. In C2, there was significant retinal inflammation at 2 weeks post-operatively that was most likely vector-related. The complications in C1 and C2 led to off protocol treatments and the ethics committee approved the recruitment of two further patients, thereby providing 12 patients treated as per protocol with 2-year follow-up. Considering only the 12 treated eyes which had gene therapy surgery as per protocol without complications, visual acuity improved by a median of 5.5 letters (IQR: 2.5 to 9.0) above baseline levels by 24 months (Wilcoxon test: W=60, p=0.016) (Supplementary Fig. S2).
Figure 2.
Visual acuity changes in the 12 patients who received retinal gene therapy for choroideremia without complications as per protocol. Individual plots of best-corrected visual acuity (BCVA) measured as number of letters read (out of 100) on the ETDRS chart at 4 m in the treated (blue) and control (red) eyes (dots represent each follow-up visit). Participants L1-5 received low dose (1x1010 gp) of the vector, while H1-7 received high-dose (1x1011 gp) of vector. Positive or negative numeric values at the end of each line indicate change from baseline visual acuity at the last follow-up (number of letters). Four patients (L2, L4, L5 and H2) had cataract surgery in the treated eye after the 2-year trial endpoint. One patient (H3) had cataract surgery during the trial period, but the visual acuity gain had already occurred by that point. H5 received gene therapy in a pseudophakic eye. L5 and H5 also received subsequent YAG laser capsulotomy for opacification of the posterior lens capsule, which is common after cataract surgery. In H6, the low visual acuity in the worse eye led to fluctuations in ETDRS readings. Following discussion with the patient, it was decided to treat the eye with better BCVA (60 letters) instead. Note that this made H6 complementary to L1, as both had asymmetric visual acuities: in L1 the worse eye was treated, leading to a sustained visual acuity gain that eventually overtook the formerly better eye; whereas in H6 gene therapy was applied to the better eye, stabilizing the visual acuity whilst the untreated eye declined further. The pattern of a significant early acuity gain which is then sustained is also seen in L4 and H7. Each plot represents multiple test points to reduce variability – up to ten times over 5 years in the first 5 patients.
Figure 3. Retinal structural and visual acuity changes in participants C1 and C2.
Subretinal delivery of gene therapy vector in participant C1 (a-d) was complicated by retinal stretch, resulting in a reduced vector dose: spectral-domain optical coherence tomography (OCT) cross-section through the fovea of the treated (left) eye at (a) baseline, (b) 1 month and (c) 2 years. One month after surgery, a reduction in outer nuclear layer (ONL) thickness was noted nasal to the fovea (arrows), although the temporal half of the fovea and the maximal retinal thickness remain similar to baseline. By 2 years, retinal thinning had stabilised, but the best-corrected visual acuity (BCVA, number of ETDRS letters) has reduced consistent with the foveal collapse (d). CRT, central retinal thickness. (e-h) OCT cross-section through the fovea of the treated (left) eye at (e) baseline, (f) 2 weeks (note visual acuity is from the 1 month visit) and (g) 2 years in participant C2, who experienced significant intraocular inflammation after gene therapy. This patient did not have a clear ellipsoid zone layer before surgery and subtle cystic degenerative changes can be seen nasally. Two weeks after gene therapy, vitreous cells (f: short arrows), outer retinal opacities (f: long arrow) and choroidal thickening (f: bracket) could be seen around the fovea. This was associated with an acute drop in BCVA, which recovered partially after a prolonged course of oral corticosteroid (h). The clear laminated appearance of the ONL appears to have improved by 2 years (g). Scale bars represent 200 μm.
Microperimetry is a modified visual field test that assesses retinal sensitivity by determining the minimum threshold of a light stimulus that can be seen at various points across the macula. Hence in contrast to visual acuity which measures just one point (usually the fovea), microperimetry provides a mean value of retinal function across a larger area, although with greater test-retest variability.15 The mean retinal sensitivity of treated eyes was 4.0±0.7 dB at baseline and 3.3±0.6 at 2 years, representing a small non-statistically significant decline of -0.7 dB (paired t-test: n=12, t=1.98, df=11, p=0.07). In contrast, the untreated eyes fell slightly more from 4.8±0.8 dB at baseline to 3.3±0.7 dB, equivalent to a -1.5 dB loss at 2 years (paired t-test: n=12, t=3.62, df=11, p=0.004). Although this represented a relative gain favouring the treated eyes over the untreated eyes of 0.8±0.53 dB (95% CI: -0.3 to 1.8 dB) at the 2-year study endpoint, the difference was not statistically significant (paired t-test: n=12, t=1.49, df=11, p=0.17) (Supplementary Fig. S3).
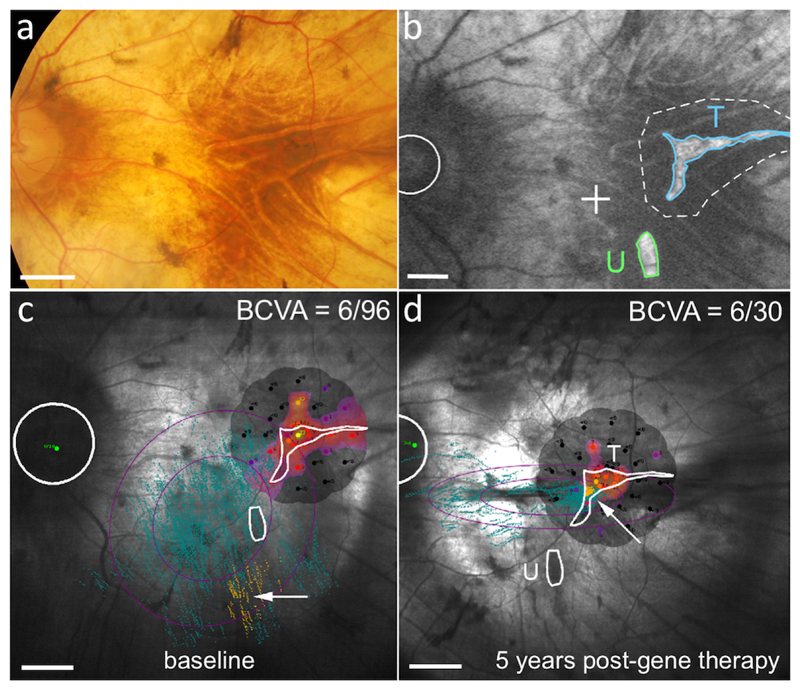
Microperimetry however provides more useful information about fixation, that is, the retinal locus that has maximal sensitivity (usually the fovea). All patients except L1 still retained some degree of foveal or parafoveal fixation, consistent with the centripetal nature of visual field loss in this disease. In L1 however the fovea had already degenerated at baseline, leaving two peripheral islands of surviving retina; one of which was targeted with gene therapy (Fig. 4). It was previously noted that L1 changed his fixation (or preferred retinal locus) to use this treated island, whilst bypassing the untreated island of retina.16 A change in fixation provides good independent evidence of the therapeutic effects of retinal gene therapy and was maintained up to 5 years in this patient, consistent with the sustained improvement in visual acuity (Fig. 2).18
Figure 4. Preferred retinal locus shift is maintained in the treated area after 5 years.
(a) Colour retinal photograph of the treated (left) eye of participant L1. (b) Blue-light autofluorescence image of the retina at baseline in L1. There are two retinal islands remaining, one of which (T, outlined in blue) was treated with the vector bleb (dotted line) whilst the other (U, outlined in green) remained untreated. The position of the degenerate fovea is marked as a cross. The optic disc is indicated by a circle. (c) Microperimetry fixation chart at baseline before gene therapy shows a vague preferential retinal locus (yellow dots – fixation points during testing) in the inferior macula area (white arrow). (d) The fixation shift to the region treated (T) by gene therapy (white arrow) is maintained after 5 years, with no fixation on the untreated area of retina (U) below. This was associated with an improvement in best-corrected visual acuity (BCVA) from 6/96 to 6/30 Snellen equivalent. Scale bars represent 1.0 mm.
Anatomical assessments included optical coherence tomography (OCT), which gives a cross-sectional view and measurement of retinal thickness,19 and blue-light autofluorescence, which generates a map that can be used to estimate the surviving retinal area.20,21 It should however be noted that the eyes were not selected for anatomical symmetry and several eyes had residual retinal areas that had degenerated too much to be accurately measured. In choroideremia, the fovea is thickened early in the disease process before the onset of degeneration and it has been proposed that this is the result of glial cell activation resulting from retinal stress.22,23 A small reduction in retinal thickness, if associated with improved retinal function, might therefore be considered a therapeutic effect - as occurs in diabetic maculopathy.24 We have previously shown that retinal structural and functional recovery occurred in the 5 eyes (H3-H7) that received subretinal vector injection using the automated injection system by 1 month25. This would suggest that optimally performed surgery does not damage the retina significantly. Over the 2-year period and across the whole of 12 patients, the mean retinal thickness at the central point of fixation reduced by 17.1±4.0 μm in the treated eyes and by 6.3±2.2 μm in the untreated eyes (paired t-test: t=2.40, df=11, p=0.04). The clinical significance of this marginal difference is unknown. Full plots of retinal thickness changes over time in individual participants are shown in Supplemental Fig. S4.
The area of retinal autofluorescence is correlated to the area of surviving photoreceptors calculated from multiple slices through the ellipsoid zone.19,26 Across the whole group of 12 patients who received gene therapy per protocol, similar areas of autofluorescence were preserved in treated and untreated eyes at two years (80.7±3.0% and 80.8±2.1%, respectively) (Supplementary Fig. S5 and Table S4). It should be noted however that shrinkage only occurs from the most peripheral retinal cells located at the leading edge of the degeneration, and 2 years may be an insufficient period of time to assess the long-term effects of retinal gene therapy on the healthier central zones which correspond to the retinal loci responsible for increased visual acuity.
As part of the gene therapy safety assessment, vector shedding through body fluids and anti-AAV2 neutralising antibody assays were also performed, which did not detect any signs of viral replication or systemic immune response (Supplementary Table S5 and S6).
Discussion
Here we report the final outcomes of a 2-year study assessing retinal gene therapy for choroideremia in a Phase I/II clinical trial. Across the whole cohort of 14 participants, including the two patients in whom complications occurred, visual acuity in treated eyes improved relative to untreated eyes over the 2-year trial period. The clinical trial thus met its primary endpoint. Furthermore, three of the study eyes gained three lines or more of vision 12 months after gene therapy. Longer term follow-up with a mean of 3.6 years for the 12 protocol-treated participants confirmed that visual acuity gains were sustained.
The visual acuity gains appeared similar in both low and high dose cohorts, but this was a small study group with only 5 patients treated at the lower (1x1010 gp) dose and generally the patients had only small fraction of the macula remaining, for which a lower dose of vector might be adequate. Both doses are however within the ranges shown previously to be therapeutic in RPE65-related Leber congenital amaurosis. Moreover, the inclusion of the translational enhancer, Woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) could potentially increase transgene expression when compared with other AAV constructs.27,28 Since the retinal pigment cells primarily affected in choroideremia have a key role in supporting the visual cycle of the overlying photoreceptors, their dysfunction prior to cell death is likely to have an adverse effect on visual acuity. Hence some improvement in visual acuity is a likely consequence of improving surviving cone function at the fovea following AAV2.REP1 gene therapy. Alongside visual acuity gains in some participants within 6 months, subjective improvements in colour perception were also described, however, colour vision assessment using the Farnsworth-Munsell 100 hue test was unreliable due to the constricted visual field (Supplementary Fig. S6).
With regard to anatomical changes, it should be noted that advancement of this very slow degeneration measured over the 2-year timeframe was only at the peripheral retina, whereas visual acuity gains arose mainly centrally. The retina in choroideremia patients is difficult to detach peripherally and the biconvex shape of the subretinal space following detachment means that the height of the bleb is greatest centrally. The centripetal nature of fluid reabsorption may provide the central retina with several additional hours of exposure to the vector compared with more peripheral areas of the detachment.
Whilst visual acuity was maintained or increased in all protocol-treated eyes throughout the duration of the study, this was not seen with retinal sensitivity. Although microperimetry is generally more variable,15 the tests do have subtle differences in what they measure. Microperimetry assesses retinal function from many points averaged over the central retina, whereas visual acuity is a measurement taken from a single point (usually the fovea) with maximal sensitivity.29 Maintaining retinal sensitivity therefore requires successful transduction of the entire area being measured, right up to the edges of the surviving tissue and may also be reduced due to the development of cataract in treated eyes, which is known to impact on microperimetry more than visual acuity.30
Among the adverse events (AE) encountered in the trial (Supplementary Table S7), one incident of retinal stretch (C1) was clearly related to surgery. The other significant adverse event of inflammation (C2) might equally be related to surgery if significant vector reflux into the vitreous cavity occurred, which is known to trigger inflammation (Supplementary Fig. S7). Following the protocol change midway through the trial, we developed an automated system for subretinal injection, which was further facilitated by intra-operative retinal scanning using OCT, which helped to identify the correct plane of the subretinal space in some of the more advanced patients.
A recent Canadian Phase I gene therapy trial also showed a significant visual acuity gain in one of 6 patients using the same batch of vector. This was corroborated by an improvement in cone thresholds together with preservation of outer retinal structures measured with OCT in the same patient.31 Conversely one of their 6 patients had a surgical complication of subretinal air and haemorrhage during vector injection and developed inflammation at a later stage. Although their results were mixed, it should be noted that they did not use intraoperative OCT, which should improve the precision of subretinal vector delivery in future studies. Robot-assisted infusion of vector is also being developed to improve surgical consistency and safety.32
Nevertheless, the results of this Phase I/II clinical trial show that gene therapy for choroideremia is generally safe. Small but sustained visual acuity gains were seen over a period of several years in end-stage eyes in which rapid visual acuity loss would ordinarily be expected, with several patients experiencing gains of three lines or more, an improvement widely accepted to be clinically significant.
Online Methods
Gene therapy vector design
The AAV serotype 2 vector comprised a chicken beta-actin (CBA) promoter with a cytomegalovirus (CMV) enhancer flanking a rabbit β-globulin intron/exon splice site - collectively termed CAG promoter - driving the cDNA of the human CHM gene, which encodes the REP1 protein (Fig. 1a).9 The vector also included WPRE to enhance gene expression and a bovine polyA signal.28
Validation of the AAV2.REP1 vector
During the trial period, the clinical-grade gene therapy vector was tested annually to confirm REP1 protein expression and prenylation activity using in vitro assays.33 Briefly, HEK293 cells were transduced with clinical-grade AAV2.REP1 vector at a multiplicity of infection (MOI) of 10,000 genome particles (gp) per cell (in triplicates). Untransduced control cells (in triplicate) were processed in parallel. Cells were harvested at 5 days post-transduction and prenylation reactions were prepared with 20 μg of total protein extract. The positive control consisted of untransduced cell lysate supplemented with recombinant fish REP1 protein (25 nM). Western blot was used to detect any increase in incorporation of biotinylated prenyl groups into a RAB6A substrate, which would be proportional to the amount of vector-derived REP1 prenylation activity. The immunostaining of mouse retina shown was performed 5 weeks after subretinal injection of the AAV2.REP1 vector at 1x109 gp to confirm correct localisation of the REP1 protein. The animal work under the UK Home Office approved project licence (33/3363) complied with local and national regulations on the use of animals in scientific research (see Life Sciences Reporting Summary). After fixation in 4% paraformaldehyde, retinal sections were blocked and incubated overnight at 4°C with rabbit anti-human REP1 primary antibody (HPA003231, Sigma-Aldrich, Gillingham, UK) 1:1,000, and then for 1 hr at room temperature with donkey anti-rabbit Alexa Fluor 568 secondary antibody (A10042, Thermo-Fisher Scientific, Loughborough, UK) 1:500. All sections were counterstained with Hoechst 33342 and mounted with ProLong Gold for imaging.
Gene therapy clinical trial design and summary
In this unmasked, non-randomised, prospective interventional gene therapy clinical trial, 14 participants were recruited with informed consent and underwent gene therapy treatment to one eye using the AAV2.REP1 vector (ClinicalTrials.gov ref. NCT01461213).16 The clinical trial protocols were approved by the UK National Research Ethics Committee (London – West London; ref. GTAC171) and the study adhered to the Declaration of Helsinki 2013. Since choroideremia affects both eyes fairly symmetrically over the longer term,15 the primary endpoint was defined in terms of vision change in the treated eyes compared to the untreated eyes in each patient. Eight of the 14 treated eyes had visual acuities greater than 70 letters (6/12) at baseline. In these eyes significant visual acuity gains would not be expected since a three-line (15 letter) gain would require them to surpass 85 letters (6/6) after surgery. All participants were male ranging from 25 to 73 years of age with confirmed null mutations in the CHM gene (Supplementary Table S1). The primary objective of the trial was to assess safety in relation to maintaining vision by two years after surgery. Initially 12 patients were to be recruited into two dose cohorts of six patients, each of whom would be monitored for 24 months. Complications in two patients however led to a 24-month delay midway through the trial and a change in protocol relating to improved surgical technique and immune suppression regimen. The ethics committee approved an extension of the trial together with the recruitment of two further patients so that 12 patients in total received the gene therapy treatment as per the protocol without complications. Consequently, all 14 patients have now reached the 2-year follow-up point that signifies the formal end of the trial. In addition, longer term data up to 5 years are also available for the patients recruited prior to the mid-way protocol amendment.
Subretinal administration of AAV vector
Surgical delivery of the AAV2.REP1 vector into the subretinal space has previously been described in detail.16,34,35 In the first cohort of 6 patients, a subretinal injection of up to 1x1010 gp assayed using a supercoiled plasmid reference was performed as a two-step procedure. This comprised an initial detachment of the retina with balanced salt solution delivered through a 41 gauge Teflon cannula (DORC BV, Zuidland, Netherlands) and secondary injection of the AAV2.REP1 vector into the newly created subretinal space. In patient C1, difficulties in detaching the retina and stretching of the papillomacular bundle resulted in a reduced gene therapy dose of ≤6×109 gp and subsequent retinal thinning, but all other patients received either the full low dose of 1x1010 gp (L1-5) or high dose of 1x1011 gp (C2 and H1-7), as per protocol. Initially oral prednisolone was administered at 1 mg/kg for 3 days before and 7 days after gene therapy, but following the development of visually significant vitritis and retinitis in C2 two weeks post-operatively, the protocol was amended so that H1-7 received an extension of the prednisolone regime: 0.5 mg/kg (days 8-14), 0.25 mg/kg (days 15-16), then 0.125 mg/kg (days 17-18). Further details of the surgery and visual function tests can be found in the Supplementary methods.
Statistical analysis
Due to the ceiling effects of including eyes with near maximal visual acuity in the study, the letter scores were found to be skewed (Shapiro-Wilk normality test at 0.05 alpha) and are therefore presented as median values with interquartile ranges (IQR).36 Changes between treated and control eyes were compared using two-tailed Wilcoxon signed-rank test. Microperimetry data and anatomical assessments were found to be normally distributed. These data are therefore presented as mean ± standard error of mean (SEM) and compared using two-tailed paired t-test.
Supplementary Material
Acknowledgements
We thank all trial participants for their commitment to attending extensive follow-up visits, KM Jasani for helping with collection of the OCT and adverse events data, M Hassall for helping with analysis of the AF data, and staff members of the Eye Research Group Oxford (ERGO) for their support throughout the study. This work was supported primarily by grant (HICF-1009-006) from the Health Innovation Challenge Fund, a funding partnership between the UK Department of Health and the Wellcome Trust. Additional funding support from the Health Foundation; Fight for Sight; the Lanvern Foundation; the Special Trustees of Moorfields Eye Hospital; the Royal College of Surgeons of Edinburgh and the National Institute for Health Research (NIHR) Biomedical Research Centres (BRC) at the Oxford University Hospitals NHS Foundation Trust (which includes the University of Oxford) and Moorfields Eye Hospital NHS Foundation Trust (which includes the University College London Institute of Ophthalmology).
Footnotes
Author contributions
K.X., J.K.J., A.R.B., A.R., A.P.S., M.I.P. and T.L.E. collected the data and performed data analysis. K.X. and M.G. assisted surgery. T.T., A.R.B., M.I.P. and H.O.O. tested the vector. G.C.B., A.R.W., A.J.L., S.M.D. and R.E.M. were clinical trial investigators, who designed the trial protocol, managed patient recruitment and interpreted the data. G.E.H. performed electrophysiology, data analysis and helped with trial design. R.E.M. and M.C.S. obtained funding and designed the study. R.E.M. and K.X. wrote the manuscript. All authors provided scientific input and read and approve the manuscript.
Competing interests
R.E.M.: scientific co-founder of Nightstar Therapeutics Inc. – a gene therapy company established by the University of Oxford and originally funded by the Wellcome Trust through Syncona Partners Ltd. A.R.B., G.C.B., A.J.L., G.C.B. and M.C.S.: consulting or on advisory board for Nightstar Therapeutics Inc. M.I.P., M.C.S. and R.E.M.: named inventors on patents relating to choroideremia gene therapy owned by the University of Oxford and Nightstar Therapeutics Inc. R.E.M., A.J.L., G.C.B.: scientific advisory board to Spark Therapeutics Inc. The companies had no role in the conduct of this University sponsored clinical trial, nor in the interpretation of the data nor in the writing up of the results. The views expressed are those of the authors and not necessarily those of the Wellcome Trust, the National Health Service, the NIHR, or the UK Department of Health.
Data availability
The authors declare that all of the data supporting the findings of this study are available within the paper and the supplementary appendix and are available from the corresponding author upon reasonable request.
References
- 1.Boye SE, Boye SL, Lewin AS, Hauswirth WW. A comprehensive review of retinal gene therapy. Mol Ther. 2013;21:509–19. doi: 10.1038/mt.2012.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahel JA, Marazova K, Audo I. Clinical characteristics and current therapies for inherited retinal degenerations. Cold Spring Harb Perspect Med. 2014;5 doi: 10.1101/cshperspect.a017111. a017111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandenberghe LH. What Is next for retinal gene therapy? Cold Spring Harb Perspect Med. 2015;5 doi: 10.1101/cshperspect.a017442. pii: a017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghazi NG, Abboud EB, Nowilaty SR, et al. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: results of a phase I trial. Hum Genet. 2016;135:327–43. doi: 10.1007/s00439-016-1637-y. [DOI] [PubMed] [Google Scholar]
- 5.Bennett J. Taking Stock of Retinal Gene Therapy: Looking back and moving forward. Mol Ther. 2017;25:1076–1094. doi: 10.1016/j.ymthe.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mauthner L. Ein Fall von Choroideremia. Berl Natur-med Ver Innsbruck. 1872;2:191. [Google Scholar]
- 7.Barnard AR, Groppe M, MacLaren RE. Gene therapy for choroideremia using an adeno-associated viral (AAV) vector. Cold Spring Harb Perspect Med. 2014;5 doi: 10.1101/cshperspect.a017293. a017293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cremers FP, van de Pol DJ, van Kerkhoff LP, Wieringa B, Ropers HH. Cloning of a gene that is rearranged in patients with choroideraemia. Nature. 1990;347:674–7. doi: 10.1038/347674a0. [DOI] [PubMed] [Google Scholar]
- 9.Seabra MC, Brown MS, Goldstein JL. Retinal degeneration in choroideremia: deficiency of rab geranylgeranyl transferase. Science. 1993;259:377–81. doi: 10.1126/science.8380507. [DOI] [PubMed] [Google Scholar]
- 10.Aleman TS, Han G, Serrano LW, et al. Natural history of the central structural abnormalities in choroideremia: a prospective cross-sectional study. Ophthalmology. 2017;124:359–373. doi: 10.1016/j.ophtha.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards TL, Groppe M, Jolly JK, Downes SM, MacLaren RE. Correlation of retinal structure and function in choroideremia carriers. Ophthalmology. 2015;122:1274–6. doi: 10.1016/j.ophtha.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 12.Morgan JI, Han G, Klinman E, et al. High-resolution adaptive optics retinal imaging of cellular structure in choroideremia. Invest Ophthalmol Vis Sci. 2014;55:6381–97. doi: 10.1167/iovs.13-13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jolly JK, Xue K, Edwards TL, Groppe M, MacLaren RE. Characterizing the natural History of visual function in choroideremia using microperimetry and multimodal retinal imaging. Invest Ophthalmol Vis Sci. 2017;58:5575–5583. doi: 10.1167/iovs.17-22486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandenberghe LH, Bell P, Maguire AM, et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002103. 88ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacLaren RE, Groppe M, Barnard AR, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–37. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards TL, Jolly JK, Groppe M, et al. Visual acuity after retinal gene therapy for choroideremia. N Engl J Med. 2016;374:1996–8. doi: 10.1056/NEJMc1509501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cideciyan AV, Hauswirth WW, Aleman TS, et al. Vision 1 year after gene therapy for Leber's congenital amaurosis. N Engl J Med. 2009;361:725–7. doi: 10.1056/NEJMc0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue K, Oldani M, Jolly JK, et al. Correlation of optical coherence tomography and autofluorescence in the outer retina and choroid of patients with choroideremia. Invest Ophthalmol Vis Sci. 2016;57:3674–84. doi: 10.1167/iovs.15-18364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jolly JK, Edwards TL, Moules J, Groppe M, Downes SM, MacLaren RE. A qualitative and quantitative assessment of fundus autofluorescence patterns in patients with choroideremia. Invest Ophthalmol Vis Sci. 2016;57:4498–4503. doi: 10.1167/iovs.15-18362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aylward JW, Xue K, Patrício MI, et al. Retinal degeneration in choroideremia follows an exponential decay function. Ophthalmology. 2018 doi: 10.1016/j.ophtha.2018.02.004. pii: S0161-6420(18)30245-8. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson SG, Cideciyan AV, Sumaroka A, et al. Remodeling of the human retina in choroideremia: rab escort protein 1 (REP-1) mutations. Invest Ophthalmol Vis Sci. 2006;47:4113–20. doi: 10.1167/iovs.06-0424. [DOI] [PubMed] [Google Scholar]
- 23.Heon E, Alabduljalil T, McGuigan DB, III, et al. Visual function and central retinal structure in choroideremia. Invest Ophthalmol Vis Sci. 2016;57:377–87. doi: 10.1167/iovs.15-18421. [DOI] [PubMed] [Google Scholar]
- 24.Diabetic Retinopathy Clinical Research Network. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simunovic MP, Xue K, Jolly JK, MacLaren RE. Structural and functional recovery following limited iatrogenic macular detachment for retinal gene therapy. JAMA Ophthalmol. 2017;135:234–241. doi: 10.1001/jamaophthalmol.2016.5630. [DOI] [PubMed] [Google Scholar]
- 26.Hariri AH, Velaga SB, Girach A, et al. Natural History of the Progression of Choroideremia (NIGHT) Study Group Measurement and reproducibility of preserved ellipsoid zone area and preserved retinal pigment epithelium area in eyes with choroideremia. Am J Ophthalmol. 2017;179:110–117. doi: 10.1016/j.ajo.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patrício MI, Barnard AR, Orlans HO, McClements ME, MacLaren RE. Inclusion of the woodchuck hepatitis virus posttranscriptional regulatory element enhances AAV2-driven transduction of mouse and human retina. Mol Ther Nucleic Acids. 2017;6:198–208. doi: 10.1016/j.omtn.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duncan JL, Aleman TS, Gardner LM, et al. Macular pigment and lutein supplementation in choroideremia. Exp Eye Res. 2002;74:371–81. doi: 10.1006/exer.2001.1126. [DOI] [PubMed] [Google Scholar]
- 30.Richter-Mueksch S, Sacu S, Weingessel B, Vécsei-Marlovits VP, Schmidt-Erfurth U. The influence of cortical, nuclear, subcortical posterior, and mixed cataract on the results of microperimetry. Eye (Lond) 2011;25:1317–21. doi: 10.1038/eye.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimopoulos IS, Hoang SC, Radziwon A, et al. Two-year results after AAV2-mediated gene therapy for choroideremia: the Alberta experience. Am J Ophthalmol. 2018 Jun 22; doi: 10.1016/j.ajo.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Edwards TL, Xue K, Meenink HCM, et al. First-in-human study of the safety and viability of intraocular robotic surgery. Nature Biomed Eng. 2018 doi: 10.1038/s41551-018-0248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patrício MI, Barnard AR, Cox CI, Blue C, MacLaren RE. Biological activity of AAV vectors for choroideremia gene therapy can be measured by in vitro prenylation of RAB6A. Molecular Therapy Methods & Clinical Development. 2018;9:288–295. doi: 10.1016/j.omtm.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer MD, Hickey DG, Singh MS, MacLaren RE. Evaluation of an optimized injection system for retinal gene therapy in human patients. Hum Gene Ther Methods. 2016;27:150–8. doi: 10.1089/hgtb.2016.086. [DOI] [PubMed] [Google Scholar]
- 35.Xue K, Groppe M, Salvetti AP, MacLaren RE. Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye (Lond) 2017;31:1308–1316. doi: 10.1038/eye.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro SS, Wilk MB. Analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.