Abstract
One of the most pernicious characteristics of alcohol use disorder is the compulsion to drink despite negative consequences. The insular cortex controls decision-making under conditions of risk or conflict. Cortical activity is tightly controlled by inhibitory interneurons that are often enclosed by specialized extracellular matrix structures known as perineuronal nets (PNNs), which regulate neuronal excitability and plasticity. The density of PNNs in the insula increases after repeated bouts of binge drinking, suggesting that they may play a role in the transition from social to compulsive, or aversion-resistant, drinking. Here, we investigated whether insular PNNs play a role in aversion-resistant alcohol drinking using a mouse model in which ethanol was adulterated with the bitter tastant quinine. Disrupting PNNs in the insula rendered mice more sensitive to quinine-adulterated ethanol but not ethanol alone. Activation of the insula, as measured by c-fos expression, occurred during aversion-resistant drinking and was further enhanced by elimination of PNNs. These results demonstrate that PNNs control the activation of the insula during aversion-resistant drinking and suggest that proper excitatory/inhibitory balance is important for decision making under conditions of conflict. Disrupting PNNs in the insula or optimizing insula activation may be a novel strategy to reduce aversion-resistant drinking.
Keywords: alcohol, aversion, ethanol, compulsive drinking, extracellular matrix, insula, perineuronal nets
Introduction
Alcohol use disorder (AUD) is a common psychiatric disorder with an estimated lifetime prevalence of 29% (Grant, Goldstein, Saha et al., 2015). AUD is a chronic relapsing condition characterized by loss of control in limiting alcohol intake, a negative emotional state when alcohol use is discontinued, and compulsive drinking. Compulsive drinking is defined as a pathological motivation to consume alcohol despite negative consequences, such as job loss, legal problems, and damage to interpersonal relationships. This type of risky drinking is not addressed by currently approved AUD pharmacotherapies (Kranzler & Soyka, 2018). The identification of the underlying brain mechanisms that promote compulsive drinking is critical for effective development of new interventions to treat AUD.
One brain area that regulates compulsive drinking is the anterior insula (Grodin, Sussman, Sundby et al., 2018; Seif, Chang, Simms et al., 2013), a cortical region located deep within the lateral sulcus (Gogolla, 2017). The importance of the anterior insula in addiction emerged just over a decade ago, when Naqvi et al demonstrated that smokers who had stroke damage in the insula were more likely to quit smoking and reported less craving for cigarettes (Naqvi, Rudrauf, Damasio et al., 2007). More recent studies have demonstrated that alcohol-dependent subjects have decreased anterior insula volume and cortical thinning compared with control subjects and these structural deficits are correlated with higher compulsive drinking measures (Grodin, Cortes, Spagnolo et al., 2017). The anterior insula is also activated during risky-decision making in individuals with AUD, with decreased activation associated with increased risk-taking (Claus & Hutchison, 2012).
Compulsive drinking is modeled in animals by pairing alcohol seeking or intake with an aversive consequence such as an electric foot shock or addition of bitter-tasting quinine to the alcohol solution (Hopf & Lesscher, 2014). Compulsive animals are aversion-resistant, meaning that they willingly seek and consume alcohol despite the threat of shock or aversive taste. Optogenetic inhibition of excitatory inputs from the insula to the nucleus accumbens reduced aversion-resistant alcohol intake in rats (Seif, Chang, Simms et al., 2013), demonstrating a causal role for the insula in regulating compulsive drinking. Analogous behavioral studies in humans showed that heavy drinkers attempted to earn more alcohol drinks than light drinkers under threat of shock and exhibited greater connectivity between the insula and nucleus accumbens, a brain region involved in motivation and reward, when viewing shock-predictive alcohol cues (Grodin, Sussman, Sundby et al., 2018). Higher connectivity between the insula and nucleus accumbens was also associated with compulsive alcohol use scores in these individuals.
Cortical neurons that express the calcium-binding protein parvalbumin are fast-spiking, GABAergic interneurons that tightly regulate the firing of excitatory pyramidal projection neurons (Dehorter, Marichal, Marin et al., 2017; Kvitsiani, Ranade, Hangya et al., 2013). These neurons are critical for effective cognitive functioning and decision-making through their ability to maintain the proper balance between excitation and inhibition in the cortex (Ferguson & Gao, 2018). In the insula, the majority of parvalbumin-expressing interneurons are surrounded by specialized extracellular matrix structures known as perineuronal nets (PNNs), whose primary components are chondroitin sulfate proteoglycans and hyaluronan. PNNs play an important role in regulating the excitability of interneurons by controlling their synaptic inputs (Balmer, 2016; Chu, Abraham, Budhu et al., 2018; Hartig, Brauer, Bigl et al., 1994). PNNs form in an activity-dependent manner during the closure of critical periods of brain development in which plasticity is reduced (Pizzorusso, Medini, Berardi et al., 2002) and are hypothesized to stabilize synapses during memory formation, leading to long-term memory storage (Gogolla, Caroni, Luthi et al., 2009; Thompson, Lensjo, Wigestrand et al., 2018; Tsien, 2013). Recently, PNNs have gained prominence in the addiction research field through studies demonstrating that they regulate drug-associated memories that contribute to relapse (Blacktop, Todd & Sorg, 2017; Lubbers, Matos, Horn et al., 2016; Slaker, Churchill, Todd et al., 2015; Van den Oever, Lubbers, Goriounova et al., 2010; Xue, Xue, Liu et al., 2014).
There are currently very few studies examining the role of PNNs in AUD, although repeated bouts of binge alcohol drinking in mice increased the intensity of PNN fluorescent staining in the insula (Chen, He & Lasek, 2015), suggesting that alcohol induces adaptive changes in the extracellular matrix that could affect the functioning of parvalbumin-expressing neurons in this brain region. Because the insula regulates compulsive drinking, we hypothesized that manipulating insular PNNs in mice would alter alcohol drinking. We tested this by measuring compulsive drinking in mice after digestion of PNNs in the insula using the enzyme chondroitinase ABC. We show that insular PNNs regulate aversion-resistant alcohol consumption and the activity of the insula during quinine-adulterated alcohol drinking. Interfering with PNNs in the insula could be a means to restore control over aversion-resistant drinking.
Materials and Methods
Animals
Male C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) at the age of 8 weeks. Mice were individually housed in a temperature- and humidity-controlled room with a 12-hour reversed light/dark cycle (lights off at 10 am) for 2 weeks prior to beginning experiments. Food and water were available ad libitum. All procedures with mice were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the UIC Animal Care and Use Committee.
Chemicals and antibodies
Biotinylated WFA and Dylight 488-conjugated streptavidin were purchased from Vector Laboratories (Burlingame, CA, USA; catalog #WFAB-1355 and SA-5488). Mouse anti-calcium/calmodulin-dependent protein kinase II alpha (CaMKII-α) was purchased from Millipore Sigma (Burlington, MA, USA; catalog #05–532). Mouse anti-parvalbumin (PV) was purchased from Synaptic Systems (Goettingen, Germany; catalog #195011). Rabbit anti-c-Fos was purchased from Santa Cruz Biotechnology (Dallas, TX, USA; catalog #sc-52). Corresponding Alexa Flour 488- and Alexa Fluor 594-conjugated secondary antibodies were from Jackson ImmunoResearch (West Grove, PA, USA; catalog #715-545-151 and 711-585-152). ChABC (#C3667) and quinine hydrochloride dihydrate (#Q1125) were purchased from Millipore Sigma. Ethanol (95%) was purchased from Decon Labs (King of Prussia, PA, USA).
PNN digestion using chondroitinase ABC (ChABC)
After acclimating to a reversed light/dark cycle for 2 weeks, mice were anesthetized with ketamine (100 mg/kg) and xylazine (8 mg/kg), placed on a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA), and the skull prepared for intracranial injections. ChABC or PBS was injected using 33 gauge stainless steel hypodermic tubing connected to an infusion pump. Mice were injected bilaterally with 0.5 μl ChABC (50 units/ml) or PBS into the insula or motor cortex at a rate of 0.1 μl/min. Coordinates for bilateral infusion were: insula, anterior/posterior, +1.5 mm from bregma; medial/lateral, ±2.9 mm from midline; and dorsal/ventral −2.6 mm from the top of the skull. Mice recovered for 3 days prior to testing drinking.
Two-bottle choice drinking tests
Water and 15% ethanol in water (E), or water and 15% ethanol with 100 μM quinine in water (EQ) were provided to mice in 10 ml clear polystyrene serological pipets (Fisher Scientific, Waltham, MA, USA) truncated at the end to accommodate connection to a 2.5-inch stainless steel ball-bearing sipper tube (Ancare Corp, Bellmore, NY, USA). For ethanol drinking measured in Figure 1, mice were given continuous access to the 2 tubes in the home cage for 24 hours per day for 4 days, with consumption measured every day. Tube placements were alternated daily to avoid the confound of preference for a particular side. For the measurement of c-fos in Figures 3–5, mice were divided into four groups and given one of the following 2-bottle choice tests: water/water, E/water, quinine (Q)/water, or EQ/water for 2 hours during the dark cycle, 3 hours after lights off. For the sucrose and quinine taste tests in Figure 2, mice were given a choice between water and 100 μM quinine in water, or water and 2% sucrose in water for 24 hours.
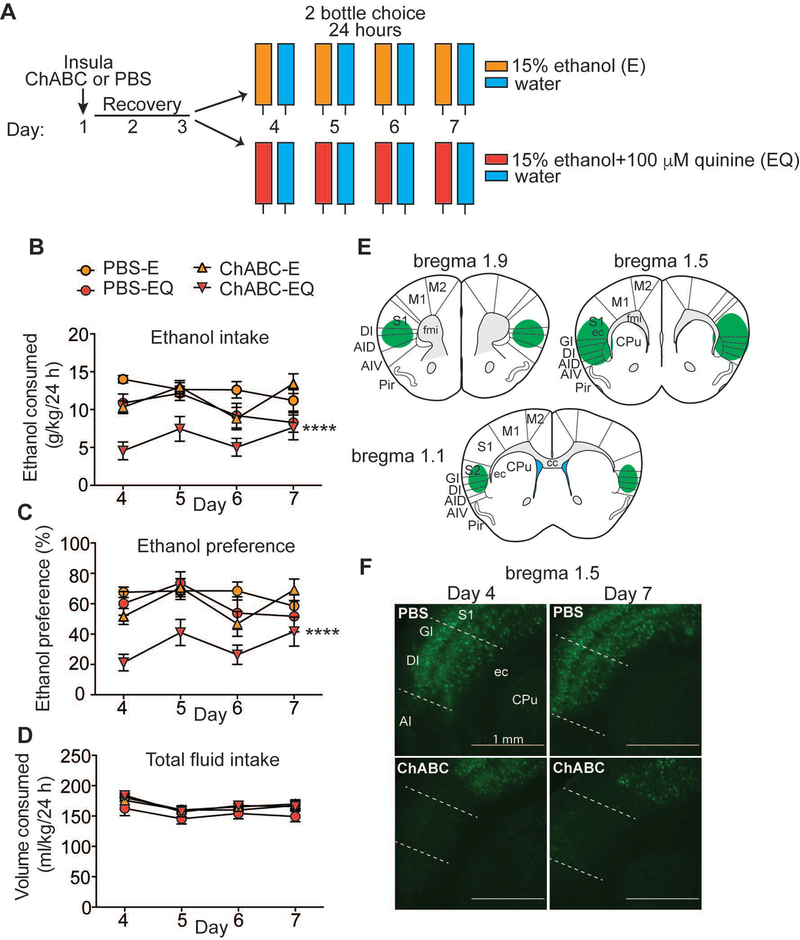
Figure 1.
Digestion of perineuronal nets (PNNs) in the insula renders mice more sensitive to quinine-adulterated ethanol. A, Experimental design. Mice were injected with either chondroitinase ABC (ChABC) or phosphate buffered saline (PBS) in the insula on day 1, recovered for 3 days, and then divided into 2 groups (n = 7–8 per group) and given a two-bottle choice test for consumption of an ethanol solution (E) vs. water or ethanol plus quinine solution (EQ) vs. water for 24 hours over 4 days. B, Daily ethanol consumption in g ethanol per kg body weight. C, Daily ethanol preference (%), calculated as volume of ethanol solution consumed divided by total volume of fluid consumed. D, Daily total fluid intake in ml per kg body weight. E, Representation of injection site at 1.5 mm anterior to bregma and extent of PNN digestion in the insula (green areas) based on a mouse brain atlas. F, Representative images of fluorescent Wisteria floribunda agglutinin binding to PNNs on coronal brain sections containing the insula on days 4 and 7 after injection of ChABC or PBS. Scalebar 1 mm; Abbreviations: CPu, caudate putamen; GI, granular insular cortex; DI, dysgranular insular cortex; AI, agranular insular cortex; S1, primary somatosensory cortex; ec, external capsule. Data are plotted as the mean ± SEM. ****P ≤ 0.0001 for ethanol intake and preference when comparing ChABC-EQ to all other groups. For detailed statistics, see Table 1.
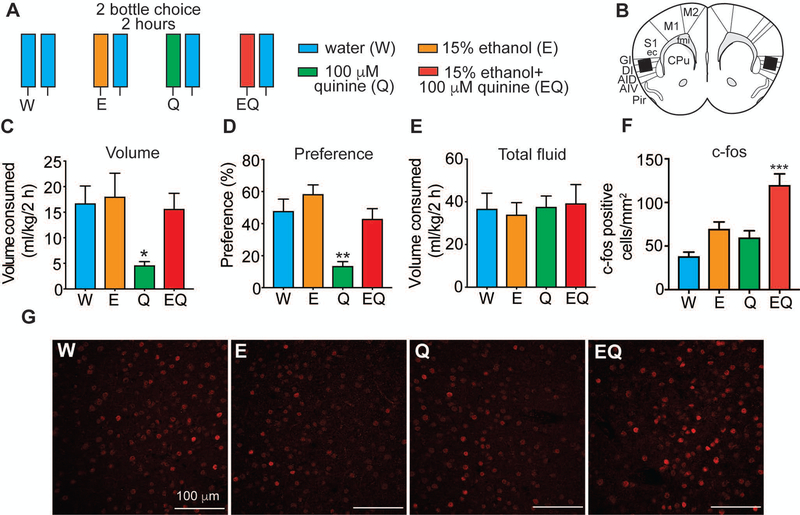
Figure 3.
c-fos is induced in the insula of mice drinking quinine-adulterated ethanol. A, Experimental design. Experimentally naïve mice were given a 2-bottle choice test for 2 h followed by perfusion and collection of brain tissue for immunohistochemistry with a c-fos antibody. Mice were divided into 4 groups (n = 6 per group) and given different 2-bottle choice tests: water (W) vs. W, ethanol (E) vs. W, quinine (Q) vs. W, and ethanol plus quinine (EQ) vs. W. B, Diagram of area in which c-fos counts were obtained based on the mouse brain atlas. Black box shows area where counts were taken. C, Volume of one bottle consumption of W, E, Q, and EQ in ml per kg body weight. *p < 0.05 by Tukey’s post-hoc multiple comparisons test when comparing Q to E. D, Percent preference of each solution (W, E, Q, or EQ), calculated as volume of solution consumed divided by total volume consumed. **p < 0.01 by Tukey’s post-hoc multiple comparisons test when comparing Q to all other groups. E, Total fluid consumed in ml per kg body weight. F, Number of c-fos positive cells in the insula of mice drinking W, E, Q, and EQ. ***P < 0.001 when by Tukey’s post-hoc multiple comparisons test when comparing EQ to all other groups. Data are presented as average c-fos counts from 6 mice per group and 4–5 sections per mouse. G, Representative images showing c-fos immunoreactivity in the insula 2 h after consuming W, E, Q, and EQ. Scalebars, 100 μm. Data are plotted as the mean ± SEM.
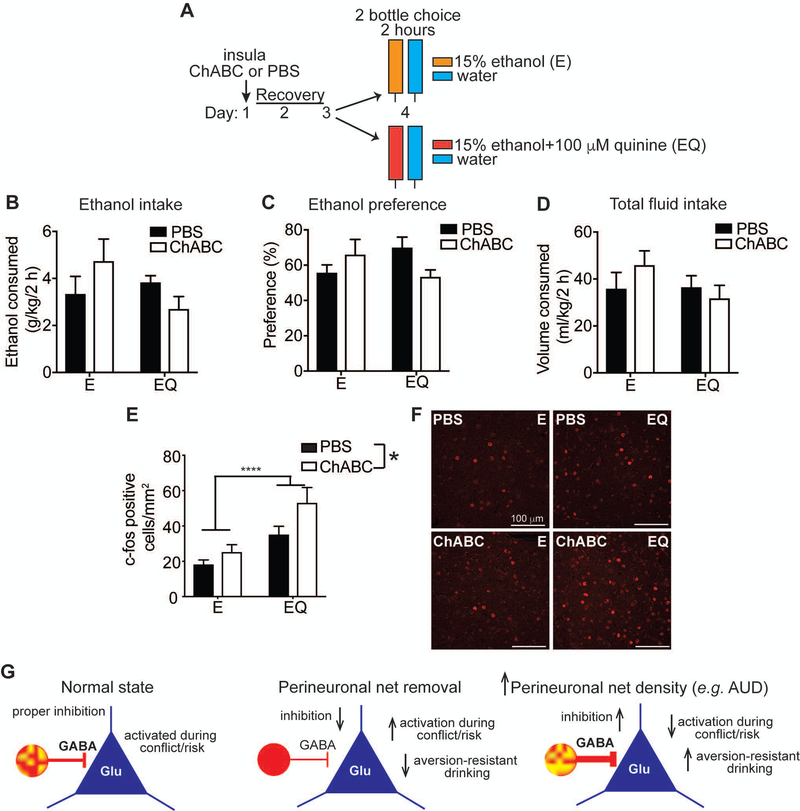
Figure 5.
c-fos induction is elevated during drinking quinine adulterated ethanol in mice lacking perineuronal nets (PNNs) in the insula. A, Experimental Design. Mice were injected with either chondroitinase ABC (ChABC) or phosphate buffered saline (PBS) in the insula on day 1, recovered for 2 days, and then were divided into 2 groups (n = 6 per group) and given a 2-bottle choice for 2 h between ethanol (E) vs. water or ethanol plus quinine (EQ) vs. water followed by perfusion and collection of brain tissue for fluorescent immunohistochemistry with an antibody to c-fos. B, Amount of E or EQ consumed in a 2 h period, expressed as g per kg body weight. C, Percent preference for E or EQ, calculated as volume of E or EQ consumed divided by total fluid consumption. D, Total fluid consumed in 2 h, expressed as ml per kg body weight. E, Number of c-fos positive cells in the insula. ***P < 0.0001, main effect of ethanol solution by two-way ANOVA; *P = 0.026, main effect of ChABC by two-way ANOVA. Data are presented as average c-fos counts from 6 mice per group and 4–5 sections per mouse. F, Representative images of c-fos immunoreactivity in the insula. Scalebars, 100 μm. Data are plotted as the mean ± SEM. G, Model of inhibitory control of pyramidal neurons (blue triangles) by parvalbumin-expressing GABA interneurons (red circles) under normal conditions, after PNN removal by ChABC treatment, and after chronic alcohol drinking or alcohol use disorder (AUD) and hypothesized relationship to aversion-resistant drinking. Density of PNNs is indicated as intensity of yellow staining on red parvalbumin neurons.
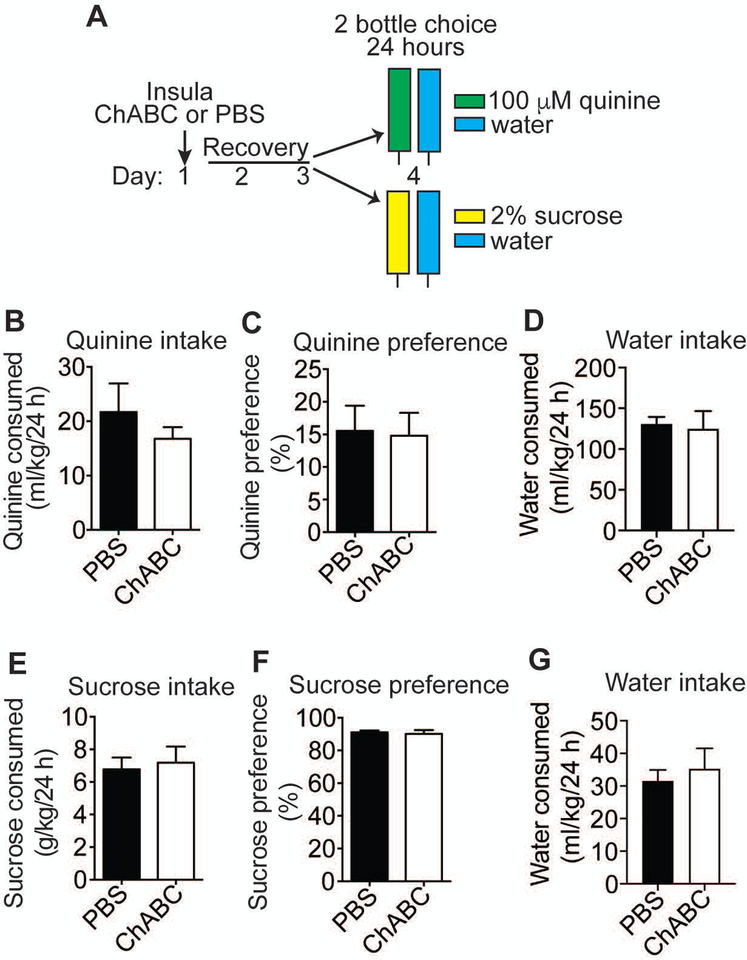
Figure 2.
Digestion of perineuronal nets (PNNs) in the insula does not affect sucrose or quinine drinking. A, Experimental design. Mice were injected with either chondroitinase ABC (ChABC) or phosphate buffered saline (PBS) in the insula on day 1, recovered for 2 days, and then were divided into 2 groups (n=6 per group) and given a two-bottle choice for 24 hours between a 2% sucrose solution vs. water or a 100 μM quinine solution vs. water. B, Quinine solution consumed, in ml per kg body weight. C, Percent quinine preference, calculated as volume of quinine solution consumed divided by volume of total fluid consumed. D, Water consumed, in ml per kg body weight during the quinine test. E, Sucrose solution consumed, in g sucrose per kg body weight. F, Percent sucrose preference, calculated as volume of sucrose solution consumed divided by volume of total fluid consumed. G, Water consumed (ml per kg body weight) during the sucrose test. Data are plotted as the mean ± SEM.
Immunohistochemistry
Brains were processed for immunohistochemistry as described in Chen et al (Chen, He & Lasek, 2015), with additional details in Supplementary Methods. Images were captured using a Zeiss LSM710 confocal microscope (Carl Zeiss) for c-fos analysis. An Evos FL microscope (Thermo Fisher Scientific) was used for visualization of ChABC digestion. C-fos counts were obtained from 6 mice per group and 4–5 sections per mouse, with numbers of c-fos positive cells averaged across all sections. Cells were analyzed in cortical layers II/III of the granular and dysgranular insula, where PNNs predominate. Cell counting was performed manually using ImageJ software (National Institutes of Health) and a threshold was applied during image analysis to normalize background staining.
Statistical analysis
Data are presented as the mean ± SEM. Statistical testing was performed using Prism software (version 7, GraphPad). For the ethanol drinking tests in Figure 1, a three-way ANOVA was first performed for variables of day, treatment, and ethanol solution. There were significant main effects of day, treatment, and ethanol solution; treatment by ethanol solution and treatment by day interactions, but no significant three-way interaction. Therefore, data was collapsed across days and a two-way ANOVA was performed for effects of treatment and ethanol solution, followed by post-hoc Tukey’s multiple comparisons tests as appropriate. A one-way ANOVA was performed for the data in Figure 3 followed by Tukey’s post hoc multiple comparisons tests. For Figure 5, a two-way ANOVA was performed for effects of treatment and ethanol solution. A P value of less than 0.05 was accepted as statistically significant.
Results
Digestion of PNNs in the insula renders mice more sensitive to quinine-adulterated ethanol
Disruption of PNN structure was achieved by using the enzyme chondroitinase ABC (ChABC), which digests the chondroitin sulfate chains on the proteoglycan constituents of the extracellular matrix including PNNs, which are enriched in chondroitin sulfate proteoglycans (Moon, Asher, Rhodes et al., 2001). To disrupt PNNs in the insula and test the behavioral consequences, ChABC or phosphate-buffered saline (PBS) was microinjected directly into the anterior insula and mice were allowed to recover for 3 days prior to testing them in a two-bottle choice test of ethanol consumption for 24 hours over the course of 4 days (Figure 1A). The inclusion of bitter-tasting quinine in the ethanol solution effectively models aversion-resistant drinking in rodents (Hopf & Lesscher, 2014), therefore mice were divided into two groups: one group received a choice between 15% ethanol vs. water to measure ethanol drinking and the other group received a choice between 15% ethanol containing 100 μM quinine (EQ) vs. water to measure aversion-resistant ethanol drinking. PBS-treated mice drank 20% less EQ than E over the 4 day period, indicating that they were mildly sensitive to this quinine concentration (Figure 1B, ANOVA results in Table 1), while mice treated with ChABC drank 45% less EQ than E and 46% less EQ than PBS-treated mice drinking EQ. Importantly, ChABC digestion of PNNs in the insula had no significant effect on ethanol drinking when quinine was not included in the ethanol solution, indicating a specific role for insular PNNs in aversion-resistant drinking. Mice treated with ChABC also had less preference for the EQ solution (33% preference) compared with all of the other groups (60–65% preference), as measured by the ratio of the volume of EQ over total volume of fluid consumed (Figure 1C). There was a small, but significant, decrease (~8–9%) in total fluid intake in PBS-treated mice drinking EQ compared with the other three groups; however, ChABC treatment did not decrease total fluid consumption (Figure 1D), demonstrating that digestion of PNNs did not adversely affect overall drinking behavior.
Table 1.
ANOVA Results for Figure 1
| Measure | Ethanol solution | Treatment | Interaction | P, PBS-E vs. PBS-EQ | P, PBS-EQ vs. ChABC-EQ | P, ChABC-E vs. ChABC-EQ |
|---|---|---|---|---|---|---|
| Ethanol intake (Figure 1B) | F1, 124 = 36.34, P < 0.0001 | F1, 124 = 16.26, P < 0.0001 | F1, 124 = 5.00, P = 0.027 | 0.04 | 0.0001 | <0.0001 |
| Ethanol preference (Figure 1C) | F1, 124 = 19.13, P < 0.0001 | F1, 124 = 19.83, P < 0.0001 | F1, 124 = 7.86, P = 0.0059 | 0.68 | <0.0001 | <0.0001 |
| Total fluid intake (Figure 1D) | F1, 124 = 3.55, P = 0.0618 | F1, 124 = 5.38, P = 0.022 | F1, 124 = 4.41, P = 0.038 | 0.03 | 0.01 | 0.99 |
Immediately after the drinking test, we confirmed PNN digestion in each mouse by staining PNNs in coronal brain sections throughout the insula using fluorescently-labeled Wisteria floribunda agglutinin (WFA), a plant protein that binds to chondroitin sulfate glycosaminoglycans in PNNs (Hartig, Brauer & Bruckner, 1992). PNNs were eliminated throughout the anterior portion of the insula on day 7 after behavioral testing (Figure 1E, F). We also verified in a separate group of mice that PNNs were digested on day 4 when the drinking test began (Figure 1F). These results indicate that PNNs in the insula are important for aversion-resistant drinking and that disrupting these structures can render mice more sensitive to quinine-adulterated ethanol.
We performed additional control experiments to verify that the effects of PNN digestion in the insula specifically affected aversion-resistant alcohol drinking. Because the insula contains the gustatory cortex, a region that is critical for taste processing (Maffei, Haley & Fontanini, 2012), we tested whether the effect of ChABC digestion on drinking EQ was simply due to increased sensitivity to bitter taste. Mice were injected in the insula with ChABC or PBS and tested for two-bottle choice consumption of a 100 μM quinine solution vs. water for 24 hours (Figure 2A). Mice were clearly able to distinguish quinine vs. water and exhibited an aversion to the quinine solution as demonstrated by 15% preference for this solution compared with water (Figure 2C). Digestion of PNNs in the insula with ChABC had no effect on quinine drinking or preference relative to water (Figure 2B, C), indicating that the effect of disrupting PNNs on aversion-resistant alcohol drinking is not simply due to increased sensitivity to the bitter taste of quinine. PNN digestion in the insula also did not affect consumption of a sweet solution (2% sucrose, Figure 2E, F), further demonstrating that digesting PNNs in the insula likely does not affect taste perception.
The motor cortex is functionally distinct from the insula but contains a similar density and distribution of PNNs as the insula (Chen, He & Lasek, 2015). As an anatomical control, mice were injected with ChABC or PBS in the motor cortex and tested for ethanol and EQ drinking. Digestion of PNNs in the motor cortex did not alter ethanol or EQ drinking, preference, or total fluid consumption (Figure S1). These results suggest that PNNs in the insula play a specific role in aversion-resistant alcohol drinking.
C-fos expression is induced in the insula of mice drinking quinine-adulterated ethanol
We hypothesized that during the initial stages of drinking a quinine-adulterated ethanol solution that the insula would become engaged because of its role in decision-making under conditions of conflict or risk (Gogolla, 2017). To test this, naïve mice were given a two-bottle choice test for 2 hours to measure expression of c-fos, a proxy for neural activation. Drinking was conducted for 2 hours instead of 24 hours in order to capture peak c-fos expression during the drinking session. Mice were divided into four groups: control mice received water in both bottles, while the other three groups received either E, quinine, or EQ in one of the bottles (Figure 3A). During the 2-hour drinking session, mice that received E or EQ drank comparable volumes of either ethanol or the EQ solution as the water drinking controls, whereas mice that received the quinine solution consumed significantly less of this solution (Figure 3C; F3, 20 = 3.63, P = 0.031; P = 0.038, 0.068, and 0.11 when comparing quinine to E, water, and EQ groups). Mice drank on average 2.8 g/kg and 2.2 g/kg ethanol from the E or EQ bottle, respectively, during the 2-hour period. Mice also had similar preferences for water, E, or EQ, but showed decreased preference for the quinine solution (Figure 3D; F3, 20 = 10.72, P = 0.0002; P < 0.01 when comparing quinine to each of the other groups), demonstrating that the quinine-only solution was clearly aversive. Total fluid consumption was equivalent in all four groups, however, indicating that the mice given quinine in one bottle were not merely consuming less overall fluid during the 2-hour session (Figure 3E). Immediately after the 2-hour drinking session, brains were processed for immunohistochemistry using a c-fos antibody. Strikingly, the number of c-fos positive cells was significantly greater in mice that drank the EQ solution compared with all of the other groups (Figure 3F; F3, 104 = 13.37, P < 0.0001; P < 0.0001 when comparing EQ to water and quinine-only groups, and P = 0.001 when comparing EQ to E). In addition, the number of c-fos positive cells in the insula positively correlated with the amount of ethanol consumed in the EQ, but not the E group (Figure S2, EQ: R2 = 0.798, P = 0.016; E: R2= 0.014, P = 0.821). Expression of c-fos did not differ between mice drinking either E- or quinine-only solutions compared with water (P = 0.144 for ethanol vs. water and P = 0.51 for quinine vs. water). These results suggest that the insula is not significantly engaged during consumption of ethanol or bitter tastant alone, but instead is activated during drinking of quinine-adulterated ethanol, further supporting its role in the decision to continue drinking under conflict.
c-fos is induced in glutamatergic neurons in the insula of mice drinking quinine-adulterated ethanol
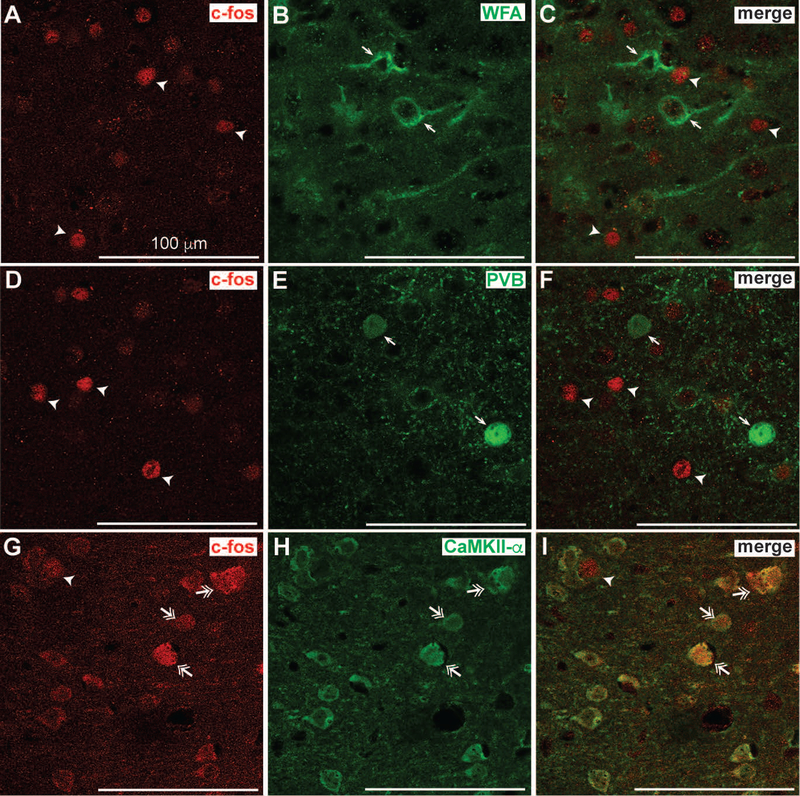
In the insula, PNNs primarily surround parvalbumin-expressing GABAergic interneurons and regulate their excitability (Balmer, 2016; Chu, Abraham, Budhu et al., 2018). We speculated that c-fos might be induced in these neurons during EQ drinking because digestion of PNNs in the insula alters consumption of EQ. We therefore examined which type(s) of neurons in the insula express c-fos during EQ drinking by performing dual fluorescent immunohistochemistry on brain sections containing the insula from mice that drank EQ for 2 hours. Brain sections were fluorescently labeled with antibodies to c-fos and WFA (for PNN-enclosed cells), parvalbumin, or CaMKII-α (for glutamatergic neurons). Unexpectedly, c-fos expression was almost entirely localized to CaMKII-α-expressing neurons (Figure 4), not parvalbumin-expressing neurons or neurons with PNNs. Quantification of co-localization indicated that ~95% of c-fos positive cells were colocalized with CaMKII-α, whereas only ~2–4% of c-fos positive cells were colocalized with PV and WFA (data not shown). These results indicate that the insular neurons activated during EQ drinking are primarily glutamateric.
Figure 4.
c-fos expression is induced in glutamatergic neurons in the insula of mice drinking quinine-adulterated ethanol. Experimentally naïve mice (n = 6 per group) were given a 2-bottle choice test for ethanol plus quinine vs. water for 2 h followed by perfusion and collection of brain tissue for dual fluorescent immunohistochemistry with antibodies to c-fos and parvalbumin (PVB), calcium/calmodulin dependent protein kinase II alpha (CaMKII-α), or fluorescently-labeled Wisteria floribunda agglutinin (WFA). Shown are representative single-channel fluorescent images of c-fos (A, D, and G, in red) and B, WFA, E, PVB and H, CaMKII-α (in green) followed by the merged images in C, F, and I. Arrowheads indicate cells that are positive for c-fos only, while single arrows indicate cells that are positive for WFA, B, or PVB, E, only. Double arrowheads indicate cells that are positive for both c-fos and CaMKII-α. Note the co-localization of c-fos with CaMKII-α but not PVB- or WFA-positive cells. Scalebar, 100 μm.
c-fos induction is elevated during drinking of quinine-adulterated ethanol in mice lacking PNNs in the insula
We next reasoned that although c-fos was induced in glutamatergic neurons in the insula during EQ drinking, digestion of PNNs might alter activation of these neurons during drinking of quinine-adulterated ethanol because of the ability of PNNs to regulate the excitability of fast-spiking interneurons which tightly control the firing of cortical pyramidal neurons (Dehorter, Marichal, Marin et al., 2017). Mice were injected with ChABC or PBS in the insula, recovered for 3 days, and then underwent a 2-hour drinking session (again, to capture peak c-fos expression) for either E or EQ before measuring c-fos immunoreactivity in the insula (Figure 5A). Ethanol intake (g/kg), preference, and total fluid consumed were not significantly different between groups after 2 hours of drinking, although there was a slight decrease in EQ drinking and preference in the ChABC-treated group (Figure 5B–C; treatment by ethanol solution interaction, g/kg: F1, 20 = 3.5, P = 0.076; treatment by ethanol solution interaction, preference: F1, 20 = 4.91, P = 0.039; no significant differences by post-hoc multiple comparisons tests). This was not entirely unexpected even though ChABC-treatment resulted in decreased EQ drinking during a 24-hour session, because of the fairly short length of the drinking session. However, the number of c-fos positive cells in the insula was significantly higher in mice drinking EQ (Figure 5E–F; ethanol solution, F1, 88 = 16.65, P < 0.0001) and in mice treated with ChABC (treatment, F1, 88 = 5.14, P = 0.026; interaction, F1, 88 = 0.995, P = 0.321). We also quantified co-localization of c-fos with CaMKII-α, PV, and WFA in insula sections from mice treated with ChABC. The distribution of c-fos positive cells did not change with ChABC treatment and were 95% CaMKII-α-positive and only 2–4% PV positive, similar to what was observed in the untreated group. These results suggest that manipulating PNNs in the insula leads to greater activation of this region during the initial drinking of quinine-adulterated ethanol.
Discussion
The insula is an integrator of sensory, emotional, motivational, and cognitive functions and has emerged as a key brain region involved in addiction to several drugs of abuse. Here, we provide evidence that degradation of PNNs in the insula decreases quinine-adulterated ethanol drinking, suggesting these extracellular matrix structures are important in promoting aversion-resistant alcohol consumption. Our results also indicate that PNNs control the activity of the insula early during drinking of quinine-adulterated alcohol when animals must decide whether to continue drinking alcohol despite the addition of a bitter tastant. The insula is activated during drinking of quinine-adulterated ethanol and this activation is augmented when PNNs are disrupted. Subsequently, mice drink less quinine-adulterated ethanol. This may appear counterintituitive, since greater insula activation might be expected to promote compulsive drinking. However, a study in human subjects with AUD found that greater insula activation is correlated with decreased risk taking (Claus & Hutchison, 2012). This might suggest that a threshold of insula activation is required in order for proper decision-making under conflict or risk.
Strikingly, we did not observe significant induction of c-fos when mice were drinking solutions containing only ethanol or quinine, nor did PNN digestion affect drinking of the ethanol- or quinine-only solutions. The combination of ethanol and quinine contains both rewarding and aversive elements, thus deciding whether to drink EQ likely presents a conflict to the animal. This implies that when there is no conflict, the insula is not activated and therefore does not play a significant role in alcohol drinking under risk-free circumstances. Insula activation, as measured by c-fos, is also observed in alcohol-preferring P rats during alcohol seeking in a punishment context after extended abstinence (Campbell, Flanagan, Walker et al., 2019).
The concentration of quinine (100 μM) that we used here was clearly aversive in the absence of ethanol, since mice drinking water adulterated with quinine drank much less of and showed decreased preference for this solution. In addition, control mice injected with PBS in the insula drank 20% less EQ than E, suggesting that they were only slightly aversion-sensitive. A previous study in ethanol naïve C57BL/6J male mice indicated a much greater degree of EQ sensitivity (50% less EQ than ethanol-only drinking) (Lei, Wegner, Yu et al., 2016) than what we found here. In contrast, and similar to our findings, a study by Lesscher et al (Lesscher, van Kerkhof & Vanderschuren, 2010), showed that ethanol naïve C57BL6/J mice were resistant to 100 μM quinine in ethanol and that 250 μM quinine was required to render them significantly aversion-sensitive. Interestingly, Lei et al found that a single ethanol drinking session was sufficient to induce subsequent aversion-resistant ethanol consumption with 100 μM quinine adulteration (Lei, Wegner, Yu et al., 2016), whereas others have found that even after 20 days of ethanol pre-exposure, male C57BL/6J mice were still sensitive to 100 μM quinine in ethanol (Fulenwider, Nennig, Price et al., 2019), suggesting that this behavior is variable depending on environmental conditions and ethanol access and pre-exposure schedules. The relatively low level of sensitivity to EQ in our mice might reflect differences in housing conditions, or alternatively could be due to the stress of surgery 3 days prior to measuring EQ drinking. Nonetheless, PNN digestion in the insula was able to increase aversion-sensitivity. Future experiments should examine if PNN digestion in the insula after an ethanol pre-exposure (when mice are fully aversion-resistant) has the same effect as in ethanol naïve mice.
One interpretation of our data is that greater insula activity facilitates better decision-making under conditions of conflict or risk. As described above, a study in human subjects with AUD found that greater anterior insula activation is correlated with decreased risk-taking (Claus & Hutchison, 2012). Brain imaging studies have also shown that the size of the anterior insula is decreased in alcohol-dependent individuals compared with healthy controls (Grodin, Cortes, Spagnolo et al., 2017; Senatorov, Damadzic, Mann et al., 2015) and that a smaller anterior insula predicts increased compulsivity (Grodin, Cortes, Spagnolo et al., 2017). The deficits in insula volume could be caused by chronic alcohol exposure, or could be a predisposing factor for AUD. In rats, chemogenetic silencing of the anterior insula increases alcohol self-administration (Jaramillo, Randall, Stewart et al., 2018). Together, these studies imply that individuals with AUD are unable to appropriately engage the insula during risky decision-making because of structural deficits in the insula.
Notably, intoxicating doses of alcohol also reduce the activity of the insula, increase risk-taking behavior (Gilman, Smith, Ramchandani et al., 2012; Tsurugizawa, Uematsu, Uneyama et al., 2010), and dampen the response of the anterior insula to notification of a negative outcome after risk-taking (Gilman, Smith, Ramchandani et al., 2012). Decreased insula activity in response to alcohol intoxication is blocked by a GABAA receptor antagonist, demonstrating that inhibitory neurotransmission is responsible for dampening insula activity during intoxication (Tsurugizawa, Uematsu, Uneyama et al., 2010). Silencing the insula also potentiates the interoceptive effects of alcohol (Jaramillo, Agan, Makhijani et al., 2017). We propose that a threshold of activation of the anterior insula during risky alcohol drinking promotes a decision to cease drinking. Further, this suggests that individuals with AUD drink compulsively because they are unable to appropriately engage the insula while consuming alcohol and thus continue to drink despite risk. This hypothesis is consistent with decreased anterior insula volume observed in heavy drinkers, which would prohibit optimal activation of the insula while drinking when negative consequences are likely. Compulsive drinkers might also be more sensitive to alcohol-induced inactivation of the insula.
However, these results are in apparent contradiction with other studies demonstrating that heavy drinkers show greater connectivity between the anterior insula and nucleus accumbens than light drinkers when viewing alcohol cues that predict electric shock and attempt to earn more alcohol during the high-threat conditions (Grodin, Sussman, Sundby et al., 2018). Similarly, Seif et al showed in rats that inhibition of insular inputs to the nucleus accumbens decreases aversion-resistant drinking, using an ethanol and quinine drinking protocol similar to the one used in this study, and Pushparaj and Foll found that pharmacological silencing of the posterior granular insula in rats decreases alcohol self-administration (Pushparaj & Le Foll, 2015; Seif, Chang, Simms et al., 2013). In addition, inactivation of the anterior insula using the GABA receptor agonists muscimol and baclofen reduced reinstatement to alcohol seeking in a punishment context in P rats (Campbell, Flanagan, Walker et al., 2019). These discrepancies with our findings might be reconciled by differences in the activation of specific insular circuits. The insula sends projections to brain regions involved in both reward and aversion (Allen, Saper, Hurley et al., 1991), including the amygdala, and activation of different circuits during decision-making likely differentially guides behavior. Indeed, chemogenetic silencing of insula to nucleus accumbens afferents decreases ethanol self-administration, yet silencing of insula itself increases ethanol self-administration (Jaramillo, Randall, Stewart et al., 2018), implicating different circuits in mediating these opposing behavioral responses. We measured c-fos in cortical layers II/III of the rostral granular and dysgranular insular cortex, where PNNs are abundant, but did not measure the contribution of individual circuits when examining c-fos. Tracing studies in rats indicate that these regions of the insula send efferents to limbic regions such as the amygdala and lateral hypothalamus (Allen, Saper, Hurley et al., 1991) and to the agranular insular cortex (Shi & Cassell, 1998), whereas the anterior agranular insular cortex projects to the nucleus accumbens (Reynolds & Zahm, 2005) . More studies are needed to dissect the role of specific insular subregions and circuits in guiding aversion-resistant versus aversion-sensitive alcohol drinking.
Cortical PNNs primarily surround fast-spiking parvalbumin-positive interneurons that are critical for the maintenance of a proper excitatory/inhibitory balance. The increased activation of glutamatergic neurons after PNN removal in the insula during quinine-adulterated ethanol drinking suggests that PNN digestion disinhibits pyramidal projection neurons, resulting in increased cortical excitability (Figure 5G). In support of this hypothesis, several studies have found that digestion of PNNs with ChABC decreases firing of inhibitory interneurons and their GABA neurotransmission onto pyramidal cells (Balmer, 2016; Chu, Abraham, Budhu et al., 2018; Slaker, Churchill, Todd et al., 2015; Tewari, Chaunsali, Campbell et al., 2018). In vivo recordings of cortical neurons after ChABC digestion also showed decreased inhibitory activity and a disruption in the excitatory/inhibitory balance (Lensjo, Lepperod, Dick et al., 2017). Our results suggest that strong inhibitory control over insular principal neurons promotes aversion-resistant ethanol drinking.
Our previous work showed that the density of PNNs around neurons in the insula increased after repeated bouts of binge drinking in male mice (Chen, He & Lasek, 2015). Increased thickness of PNNs is predicted to facilitate rapid firing of parvalbumin neurons, thus increasing inhibitory control over excitatory projection neurons (Figure 5G). As a result, it may be more difficult to activate the insula after chronic alcohol exposure when PNNs are more pronounced. Together with the results presented here, these data suggest that increased PNNs and the resulting GABAergic inhibition in the insula after repeated bouts of binge drinking could play a role in the transition from social, non-compulsive drinking to compulsive alcohol abuse. One limitation of this study is that we only used male mice. It will be important to determine if manipulation of PNNs in the insula of female mice increases sensitivity to quinine-adulterated ethanol and whether chronic ethanol consumption increases the thickness of PNNs in the insula of females. Female mice appear to be more aversion-resistant than males because higher concentrations of quinine are necessary to reduce ethanol intake (Fulenwider, Nennig, Price et al., 2019). The number and intensity of PNNs in the insula of females could conceivably differ from males. Indeed, sex differences in PNN development in the hippocampus have been observed (Griffiths, Madden, Edwards et al., 2019).
Disrupting PNNs in the insula and restoring normal excitatory/inhibitory balance in the insula may be a novel therapeutic approach to treating compulsive drinking. Injection of an extracellular matrix-degrading enzyme such as ChABC into the insula is not a viable therapeutic option, given the invasive and temporary nature of this manipulation, but a pharmacotherapeutic aimed at reducing PNNs might be possible. The extracellular matrix has gained prominence in recent years as yet another mechanism involved in the regulation of synaptic plasticity and neuronal activity. Our results provide further evidence that PNNs are important for addiction but play a specific role in the insula in controlling aversion-resistant drinking. Degrading PNNs, decreasing interneuron excitability, or increasing excitatory neurotransmission in the insular cortex may restore healthy insula function in individuals with AUD, leading to better decision-making under conditions of risk or conflict.
Supplementary Material
Acknowledgements
We would like to thank Dr. Elizabeth Glover and Dr. Amynah Pradhan for helpful comments on this manuscript. The authors declare no financial or potential conflicts of interest. This work was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health (INIA consortium grant U01 AA020912 and the Center for Alcohol Research in Epigenetics grant P50 AA022538). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Allen GV, Saper CB, Hurley KM, Cechetto DF (1991) Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol 311:1–16. [DOI] [PubMed] [Google Scholar]
- Balmer TS (2016) Perineuronal Nets Enhance the Excitability of Fast-Spiking Neurons. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop JM, Todd RP, Sorg BA (2017) Role of perineuronal nets in the anterior dorsal lateral hypothalamic area in the acquisition of cocaine-induced conditioned place preference and self-administration. Neuropharmacology 118:124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EJ, Flanagan JPM, Walker LC, Hill M, Marchant NJ, Lawrence AJ (2019) Anterior Insular Cortex is Critical for the Propensity to Relapse Following Punishment-Imposed Abstinence of Alcohol Seeking. J Neurosci 39:1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, He D, Lasek AW (2015) Repeated Binge Drinking Increases Perineuronal Nets in the Insular Cortex. Alcoholism, clinical and experimental research 39:1930–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu P, Abraham R, Budhu K, Khan U, De Marco Garcia N, Brumberg JC (2018) The Impact of Perineuronal Net Digestion Using Chondroitinase ABC on the Intrinsic Physiology of Cortical Neurons. Neuroscience 388:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Hutchison KE (2012) Neural mechanisms of risk taking and relationships with hazardous drinking. Alcoholism, clinical and experimental research 36:932–940. [DOI] [PubMed] [Google Scholar]
- Dehorter N, Marichal N, Marin O, Berninger B (2017) Tuning neural circuits by turning the interneuron knob. Curr Opin Neurobiol 42:144–151. [DOI] [PubMed] [Google Scholar]
- Ferguson BR, Gao WJ (2018) PV Interneurons: Critical Regulators of E/I Balance for Prefrontal Cortex-Dependent Behavior and Psychiatric Disorders. Front Neural Circuits 12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulenwider HD, Nennig SE, Price ME, Hafeez H, Schank JR (2019) Sex Differences in Aversion-Resistant Ethanol Intake in Mice. Alcohol Alcohol. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Smith AR, Ramchandani VA, Momenan R, Hommer DW (2012) The effect of intravenous alcohol on the neural correlates of risky decision making in healthy social drinkers. Addiction biology 17:465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N (2017) The insular cortex. Curr Biol 27:R580–R586. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Luthi A, Herry C (2009) Perineuronal nets protect fear memories from erasure. Science 325:1258–1261. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS (2015) Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths BB, Madden AMK, Edwards KA, Zup SL, Stary CM (2019) Age-dependent sexual dimorphism in hippocampal cornu ammonis-1 perineuronal net expression in rats. Brain Behav 9:e01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Cortes CR, Spagnolo PA, Momenan R (2017) Structural deficits in salience network regions are associated with increased impulsivity and compulsivity in alcohol dependence. Drug Alcohol Depend 179:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Sussman L, Sundby K, Brennan GM, Diazgranados N, Heilig M, Momenan R (2018) Neural Correlates of Compulsive Alcohol Seeking in Heavy Drinkers. Biol Psychiatry Cogn Neurosci Neuroimaging. [DOI] [PubMed] [Google Scholar]
- Hartig W, Brauer K, Bigl V, Bruckner G (1994) Chondroitin sulfate proteoglycan-immunoreactivity of lectin-labeled perineuronal nets around parvalbumin-containing neurons. Brain research 635:307–311. [DOI] [PubMed] [Google Scholar]
- Hartig W, Brauer K, Bruckner G (1992) Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport 3:869–872. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Lesscher HM (2014) Rodent models for compulsive alcohol intake. Alcohol (Fayetteville, NY) 48:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo AA, Agan VE, Makhijani VH, Pedroza S, McElligott ZA, Besheer J (2017) Functional role for suppression of the insular-striatal circuit in modulating interoceptive effects of alcohol. Addiction biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo AA, Randall PA, Stewart S, Fortino B, Van Voorhies K, Besheer J (2018) Functional role for cortical-striatal circuitry in modulating alcohol self-administration. Neuropharmacology 130:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Soyka M (2018) Diagnosis and Pharmacotherapy of Alcohol Use Disorder: A Review. JAMA 320:815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, Kepecs A (2013) Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature 498:363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Wegner SA, Yu JH, Simms JA, Hopf FW (2016) A single alcohol drinking session is sufficient to enable subsequent aversion-resistant consumption in mice. Alcohol (Fayetteville, NY) 55:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensjo KK, Lepperod ME, Dick G, Hafting T, Fyhn M (2017) Removal of Perineuronal Nets Unlocks Juvenile Plasticity Through Network Mechanisms of Decreased Inhibition and Increased Gamma Activity. The Journal of neuroscience : the official journal of the Society for Neuroscience 37:1269–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, van Kerkhof LW, Vanderschuren LJ (2010) Inflexible and indifferent alcohol drinking in male mice. Alcoholism, clinical and experimental research 34:1219–1225. [DOI] [PubMed] [Google Scholar]
- Lubbers BR, Matos MR, Horn A, Visser E, Van der Loo RC, Gouwenberg Y, Meerhoff GF, Frischknecht R, Seidenbecher CI, Smit AB, Spijker S, van den Oever MC (2016) The Extracellular Matrix Protein Brevican Limits Time-Dependent Enhancement of Cocaine Conditioned Place Preference. Neuropsychopharmacology 41:1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Haley M, Fontanini A (2012) Neural processing of gustatory information in insular circuits. Curr Opin Neurobiol 22:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon LD, Asher RA, Rhodes KE, Fawcett JW (2001) Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nature neuroscience 4:465–466. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007) Damage to the insula disrupts addiction to cigarette smoking. Science 315:531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L (2002) Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298:1248–1251. [DOI] [PubMed] [Google Scholar]
- Pushparaj A, Le Foll B (2015) Involvement of the caudal granular insular cortex in alcohol self-administration in rats. Behav Brain Res 293:203–207. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS (2005) Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci 25:11757–11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW (2013) Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nature neuroscience 16:1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatorov VV, Damadzic R, Mann CL, Schwandt ML, George DT, Hommer DW, Heilig M, Momenan R (2015) Reduced anterior insula, enlarged amygdala in alcoholism and associated depleted von Economo neurons. Brain 138:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD (1998) Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol 399:440–468. [DOI] [PubMed] [Google Scholar]
- Slaker M, Churchill L, Todd RP, Blacktop JM, Zuloaga DG, Raber J, Darling RA, Brown TE, Sorg BA (2015) Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory. The Journal of neuroscience : the official journal of the Society for Neuroscience 35:4190–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari BP, Chaunsali L, Campbell SL, Patel DC, Goode AE, Sontheimer H (2018) Perineuronal nets decrease membrane capacitance of peritumoral fast spiking interneurons in a model of epilepsy. Nat Commun 9:4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EH, Lensjo KK, Wigestrand MB, Malthe-Sorenssen A, Hafting T, Fyhn M (2018) Removal of perineuronal nets disrupts recall of a remote fear memory. Proc Natl Acad Sci U S A 115:607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY (2013) Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc Natl Acad Sci U S A 110:12456–12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurugizawa T, Uematsu A, Uneyama H, Torii K (2010) The role of the GABAergic and dopaminergic systems in the brain response to an intragastric load of alcohol in conscious rats. Neuroscience 171:451–460. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Lubbers BR, Goriounova NA, Li KW, Van der Schors RC, Loos M, Riga D, Wiskerke J, Binnekade R, Stegeman M, Schoffelmeer AN, Mansvelder HD, Smit AB, De Vries TJ, Spijker S (2010) Extracellular matrix plasticity and GABAergic inhibition of prefrontal cortex pyramidal cells facilitates relapse to heroin seeking. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 35:2120–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue YX, Xue LF, Liu JF, He J, Deng JH, Sun SC, Han HB, Luo YX, Xu LZ, Wu P, Lu L (2014) Depletion of perineuronal nets in the amygdala to enhance the erasure of drug memories. The Journal of neuroscience : the official journal of the Society for Neuroscience 34:6647–6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.