Abstract
Objective:
Increased retinal vascular permeability is one of the earliest manifestations of diabetic retinopathy. The aim of this study was to investigate the role of hyperglycemia-induced platelet endothelial cell adhesion molecule −1 (PECAM-1) loss on retinal vascular permeability via the β-catenin pathway.
Methods:
Type I diabetes was induced in male Wistar rats using streptozotocin (STZ) injections, with age-matched non-diabetic rats as controls. Rat retinal microvascular endothelial cells (RRMECs) were grown under normal or high glucose conditions for six days. Small interfering Ribonucleic Acid (siRNA) was used to knock down PECAM-1 in RRMECs for loss-of-function studies. Retinas and RRMECs were subjected to western blot, immunofluorescence labeling, and co-immunoprecipitation (co-IP) analyses to assess protein levels and interactions. A biotinylated-gelatin and fluorescein isothiocyanate (FITC)-avidin assay was used for retinal endothelial cell permeability studies.
Results
β-catenin, β-catenin/PECAM-1 interaction, active Src-homology 2 domain-containing protein tyrosine phosphatase (SHP2), while β-catenin ubiquitination levels and endothelial permeability were significantly increased, in hyperglycemic retinal endothelial cells. Similar results were observed with PECAM-1 partial knockdown, where β-catenin and active SHP2 levels were decreased, while phospho-β-catenin and retinal endothelial cell permeability were increased.
Conclusion:
PECAM-1 loss may contribute to increased retinal endothelial cell permeability by attenuating β-catenin levels under hyperglycemic conditions.
Keywords: Diabetic retinopathy, Permeability, PECAM-1, β-catenin, blood retinal barrier (BRB)
Introduction
A well-developed blood-retinal barrier (BRB) is vital for the normal function of the retina. BRB breakdown and increased retinal vascular permeability have been implicated in various ocular pathologies such as retinopathy of prematurity1, exudative age-related macular degeneration2, and diabetic retinopathy3. Diabetic retinopathy (DR) is a major complication of diabetes, and the leading cause for visual impairment and blindness in working-age adults worldwide4. One of the first observable alterations in the retinal vasculature in DR is BRB breakdown and increased vascular permeability5. The increase in retinal vascular permeability could lead to an increase in fluid accumulation and albumin flux into the retina6, which affects vision and can lead to vision loss. Additionally, studies have indicated a correlation between the degree of permeability and the severity of DR7.
The endothelial cells (ECs) lining the retinal vasculature provide a barrier against plasma component leakage into the underlying tissue, with the help of endothelial cell junctional molecules. Retinal endothelial cells (RECs) have been shown to express multiple junctional proteins, such as occludin, zonula occludens-1 (ZO-1), β-catenin, cadherin-5, vascular endothelial-cadherin (VE-cadherin) and platelet endothelial cell adhesion molecule-1 (PECAM-1)8, 9. PECAM-1 is a cell surface molecule that has been implicated in the normal function and maintenance of endothelial cells, such as EC-EC adhesion, barrier function, survival, cell signaling, leukocyte transmigration, and angiogenesis10–14. Thus, a loss of PECAM-1 can have detrimental effects on RECs, which could contribute to the development of DR. We have previously reported PECAM-1 loss in the diabetic retina15 without a change in retinal capillary density; additionally, other reports have indicated the loss of PECAM-1 from the retinas of db/db mice16, and from endothelial cells grown under hyperglycemic conditions17. However, still to be determined is the effect of PECAM-1 loss on DR development, and how it contributes to the BRB breakdown.
PECAM-1 is a glycoprotein that belongs to the immunoglobulin (Ig) superfamily with an immunoreceptor tyrosine-based inhibitory motif (ITIM), and is expressed heavily on the surface of endothelial cells, with a higher concentration at cell-cell junctions10, 18. PECAM-1 consists of six Ig homology domains, a single-pass transmembrane domain, and a long and complex cytoplasmic tail19 that encompasses two ITIM domains surrounding tyrosine 663 and tyrosine 686 residues20. The phosphorylation of the tyrosine residues at the ITIM domains promotes the recruitment of Src homology-2 (SH2) containing protein phosphatases, such as SHP1 and SHP2, leading to their activation and participation in cell signaling functions21, 22. In addition to the ITIM domains, the cytoplasmic tail of PECAM-1 contains β-catenin and γ-catenin binding sites that sequester them to the plasma membrane23.
β-catenin, which is a member of the Armadillo (ARM) repeat superfamily, is a conserved molecule that is expressed in multiple animal species, and contributes to a number of signaling cascades24. Additionally, β-catenin is a member of the adherens junction25, where it facilitates the interaction of vascular endothelial-cadherin (VE-cadherin) to the actin cytoskeleton26. VE-cadherin associates with the dephosphorylated and active form of β-catenin, where SHP2 dephosphorylates β-catenin rendering it active, to reconstitute and restore the adherens junction27. On the other hand, the phosphorylated form of β-catenin can be targeted for ubiquitination and subsequent degradation by the ubiquitin-proteasome pathway28.
Therefore, the aim of the current study was to test the hypothesis that the hyperglycemia-induced loss of retinal PECAM-1 will attenuate β-catenin levels due to a decrease in its dephosphorylation and activation in retinal endothelial cells, resulting in increased retinal endothelial cell permeability.
Methods
Animal use
Male Wistar rats purchased from Envigo, Indianapolis, IN, (100–120 g), were used in a model of type I diabetes. The Institutional Animal Care and Use Committee of LSUHSC-Shreveport approved the experimental protocols used in this study.
Animal model of type I diabetes in rats
Blood glucose levels were tested before the induction of diabetes and in control rats. Streptozotocin (STZ, diabetic, Sigma-Aldrich, St. Louis, MO), 30 mg/kg/day, or vehicle (sodium citrate buffer, control) were intraperitoneally injected in age-matched male Wistar rats for three consecutive days. Blood glucose and weight measurements were tested at one week, five weeks, and eight weeks post STZ injections to verify consistent hyperglycemia. Body weight and glucose levels were documented, and hyperglycemia defined as having non-fasting glucose values of >300 mg/dl, with non-diabetic rats having glucose values of ~150 mg/dl. At the eight-week post-STZ injection time point, rats were anesthetized with ketamine/pentobarbital (100 and 50 mg/kg, respectively), and the eyes were enucleated and retinas collected for analysis.
Cell lines
Primary rat retinal microvascular endothelial cells (RRMECs, Cell Biologics, Chicago, IL) were used to establish an in vitro model of diabetic retinopathy. RRMECs were cultured in Dulbecco’s modified eagle media (DMEM) containing 10% heat inactivated fetal bovine serum (FBS) under either normal glucose conditions (NG, 5 mM), or high glucose conditions (HG, 25 mM), for six days. RRMECs were used at the 7th passage as cells beyond 10 passages are not viable, while passage 7 provides consistent results. RMECSs were grown to 100% confluency, and cultured in a humidified atmosphere at 37 °C with 5% CO2.
Partial knockdown of PECAM-1 using siRNA
ON-TARGETplus Rat PECAM-1, siGLO cyclophilin B control, and non-targeting siRNAs were synthesized by Dharmacon (Lafayette, CO). RRMECs were plated at a cell density of 80% confluency. siRNA and Dharmafect transfection reagent (Dharmacon) were diluted in Opti-MEM and added to the cells. For controls, RRMECs were treated with Dharmafect only (Sham), siGLO cyclophilin B positive transfection control, and non-targeting siRNA negative control. After 48 hours of transfection, cells were subjected to immunofluorescence staining, permeability assay, or collected for western blot analyses. siRNA sequences and concentrations were the following: On-TARGETplus SMARTpool PECAM-1 siRNA, CGUGCAAAGUGGAAUCGAA, UCUCCAUCCUGUCGGGUAA, GGACAAAUCAUAGGUAUCA, GGGCAGACCCUUCCACGAA (10 nM), siGLO Cyclophilin B control siRNA, GGAAAGACUGUUCCAAAAA (5 nM, ON-TARGETplus Non-targeting siRNA, UGGUUUACAUGUCGACUAA (5 nM).
Western blotting (WB)
Retinas obtained from non-diabetic or diabetic rats, or RRMECs grown under NG or HG conditions, were collected in ice-cold radio immunoprecipitation assay (RIPA, Sigma-Aldrich, St. Louis, MO) buffer with protease inhibitors (Sigma-Aldrich, St. Louis, MO) added. Retinas and RRMECs were homogenized, and homogenates were centrifuged at 10,000 g for 5 minutes. Supernatants were subjected to the bicinchoninic acid assay (BCA, Thermo Fisher Scientific, Waltham, MA) for protein concentrations, and equal amounts of protein were loaded on 8–12% SDS-polyacrylamide gels under reducing and denaturing conditions. After the protein transfer into nitrocellulose membranes (Bio-Rad, Hercules, CA), membrane blots were blocked using protein-free block (Thermo Fisher Scientific, Waltham, MA), and primary antibodies were added for overnight incubation at 4 °C (PECAM-1; Santa Cruz Biotechnology, Dallas, TX, β-catenin, and Non-phospho (Active) β-catenin at serine 45 (Ser45), Phospho-β-Catenin at serine 33/37 and threonine 41 (Ser33/37/Thr41); Cell Signaling, Danvers, MA; SHP2 (Tyr477), SHP2 (phospho Tyr580); abcam, Cambridge, MA). Secondary antibodies conjugated to HRP (Jackson ImmunoResearch, West Grove, PA) were added for one hour at room temperature. To ensure equal loading and proper transfer, an internal house-keeping protein was used (β-actin, Sigma-Aldrich). Blots were imaged using the ChemiDoc XRS gel imaging system (Bio-Rad, Hercules, CA) for detection of specific bands, and the bands were quantified by densitometry using ImageJ software (National Institutes of Health (NIH), Bethesda, MD, USA)29.
Immunofluorescence (IF) labeling
For immunofluorescence labeling of RRMECs, cells were fixed using ice-cold methanol for 5 minutes on ice, followed by cell membrane permeabilization using 0.1% Triton-X100 in phosphate buffered saline (PBS), followed by blocking using 5% FBS, and 5% BSA for one hour at room temperature. Primary antibodies were added for overnight incubation at 4 °C, followed by fluorescently labeled secondary antibodies for one hour at room temperature. A mounting medium containing DAPI (Vector Labs, Burlingame, CA, USA) was added to the cells, and images were taken using a NIKON E600FN fluorescent microscope, and analyzed using ImageJ (NIH). Secondary antibody staining without a primary antibody was used to account for non-specific staining and to ensure specificity.
Co-immunoprecipitation (Co-IP)
RRMECs and retinas were lysed in cold IP lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1 mM EDTA, pH 7.6) containing protease inhibitors (Sigma). Primary antibodies for proteins of interest for immunoprecipitation (PECAM-1, β-catenin) were incubated with SureBeads magnetic beads (Bio-rad) for 10 minutes at room temperature. Equal amounts of retinal or cell lysates were added to the antibody-beads complex, and incubated for 1 hour at RT. To elute the pulled down protein, 1X Laemmli buffer was added to the beads, heated to 75 °C for 15 minutes, and samples were subjected to SDS-PAGE western blot analysis. Primary antibodies against the proteins being investigated for interaction (PECAM-1, β-catenin, SHP2, Ubiquitin (Enzo-life sciences, Farmingdale, NY)) were added to the membranes for incubation at 4 °C overnight, and HRP-conjugated secondary antibodies were added for one hour at room temperature. Specific bands were imaged using the ChemiDoc XRS gel imaging system, and analyzed using densitometry analysis (ImageJ).
Biotinylated gelatin permeability assay
Cell culture dishes were coated with biotinylated gelatin. Briefly, porcine skin gelatin (10 mg/ml, Sigma) was added to 0.1 mol/L NaHCO3 (pH 8.3) and heated at 70 °C until fully dissolved. Ez-link-biotin (Thermo scientific) was added to the gelatin mix (0.57 mg/ml) and incubated at room temperature for one hour. Poly-L-Lysine was added to culture dishes, and incubated at room temperature for 20 minutes, followed by 0.5% glutaraldehyde for 15 minutes at room temperature. Biotinylated gelatin was added (0.25 mg/ml in NaHCO3 buffer), and incubated overnight at 4 °C. Residual glutaraldehyde was quenched by adding DMEM with 10% FBS for 30 minutes at room temperature.
RRMECs were plated and grown under either NG or HG conditions for 6 days, or with the addition of PECAM-1 small interfering Ribonucleic Acid (siRNA), until fully confluent. FITC-avidin (Thermo scientific) was added to the cell media (0.025 mg/ml) and incubated for 3 minutes. Cells were fixed with 4% paraformaldehyde, and pictures were taken using a NIKON E600FN fluorescent microscope. The images were quantified using ImageJ software.
Statistical analysis
Student t-tests or one-way analysis of variance (ANOVA) were conducted to compare the means of the groups, followed by Student-Newman-Keuls post-hoc correction, using GraphPad prism software (La Jolla, CA). P < 0.05 was considered statistically significant, and the data presented express the mean ± standard error.
Results
Retinal β-catenin levels are decreased in hyperglycemia
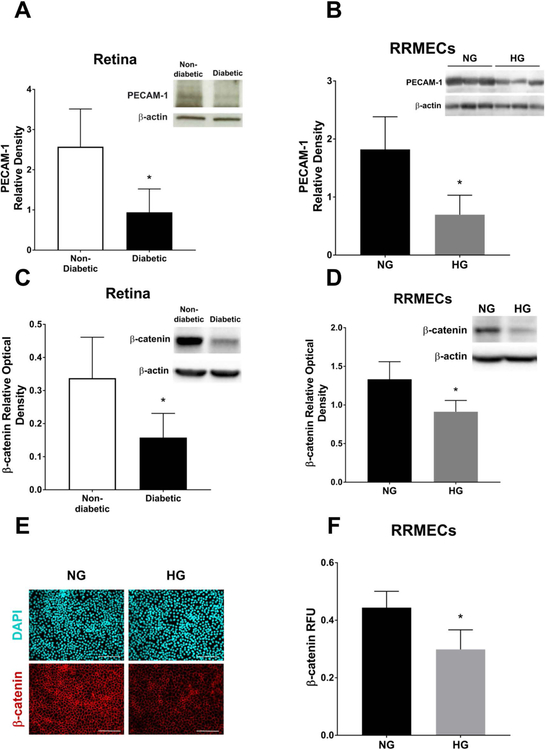
PECAM-1 levels in retinas collected from diabetic rats, and from RRMECs grown under HG conditions were significantly decreased (Figure 1A and 1B, 63% and 61% decrease, respectively, p<0.05), in agreement with our previously published data15. In addition to PECAM-1 loss, β-catenin levels were significantly decreased in the diabetic retinas (Figure 1C, 56% decrease, p<0.05) and in hyperglycemic RRMECs as measured with western blotting (32%, p<0.05, Figure 1D) and immunofluorescence (32%, p<0.05, Figure E, F) analyses.
Figure 1. Hyperglycemia induced PECAM-1 loss in retinal endothelial cells was accompanied by a decrease in β-catenin levels.
PECAM-1 levels were significantly decreased in retinas collected from diabetic rats (A) and RRMECs grown under HG conditions (B) when compared to controls. C) β-catenin levels in the diabetic retinas were significantly decreased when compared to non-diabetic retinas. D) β-catenin levels were significantly decreased in RRMECs grown under hyperglycemic conditions. E) Representative images showing fluorescently labeled β-catenin in NG and HG RRMECs. F) Image analysis of β-catenin fluorescence intensity showing a significant decrease in RRMECs grown under HG conditions. Western blotting data is a comparison of the relative PECAM-1 /β-actin and β-catenin /β-actin band densities. Relative fluorescence unit (RFU) is a comparison between β-catenin fluorescence intensity and the total number of nuclei. Scale bar = 50 μm.*p<0.05, N=7–9 per group in vivo and N=3 per group in vitro.
Retinal β-catenin/PECAM-1 binding is decreased in hyperglycemia
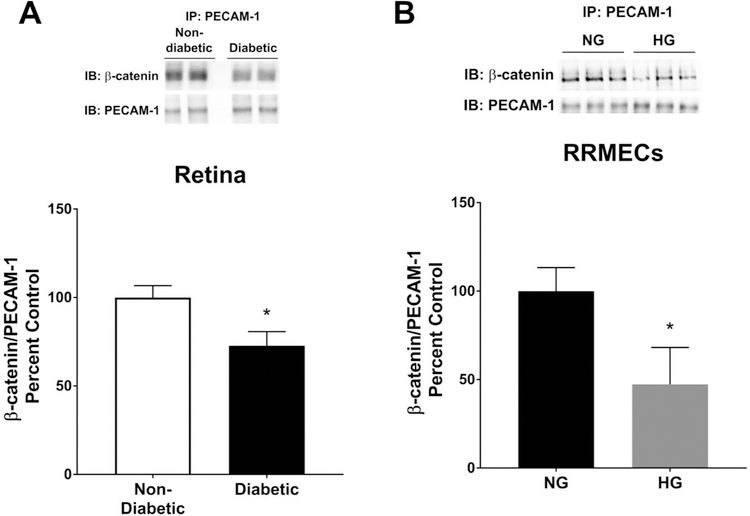
To assess the levels of PECAM-1 bound β-catenin, PECAM-1 was purified from the retinas collected from non-diabetic and diabetic rats, or from RRMECs grown under NG and HG conditions, using immunoprecipitation followed by blotting for β-catenin. Under hyperglycemic conditions, PECAM-1 bound β-catenin was significantly decreased in the diabetic retinas (27% decrease, p<0.05, Figure 2A), and in RRMECs grown under hyperglycemic conditions (52% decrease, p<0.05, Figure 2B).
Figure 2. β-catenin binding to PECAM-1 in retinal endothelial cells was significantly decreased under hyperglycemic conditions.
To study the effects of hyperglycemia on β-catenin binding to PECAM-1, co-IP experiments were conducted, and a significant decrease in PECAM-1/β-catenin interaction (relative to PECAM-1) in the diabetic retinas (A) and RRMECs grown under HG conditions (B) were observed when compared to controls. *p<0.05, N=7–8 per group in vivo and N=3 per group in vitro.
Decrease in active SHP2 levels and active β-catenin in hyperglycemia
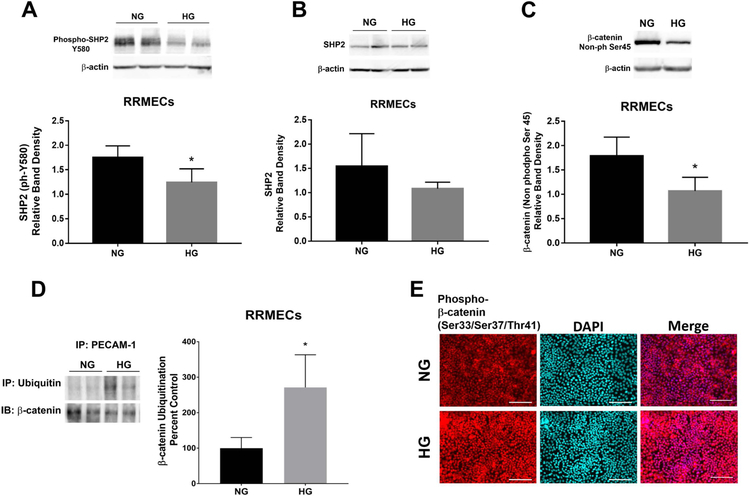
We hypothesized that the decrease in retinal PECAM-1 may affect the activation of SHP2, which in turn may affect the levels of dephosphorylated β-catenin. Active (phosphorylated) SHP2 levels were significantly decreased in RRMECs grown under hyperglycemic conditions (26% decrease, p<0.05, Figure 3A), without a statistically significant change in total SHP2 (Figure 3B). Additionally, the non-phosphorylated (Ser45) levels of β-catenin were significantly decreased in RRMECs under HG conditions (41% decrease, p<0.05, Figure 3C). Moreover, ubiquitinated levels of β-catenin were significantly increased (170% increase, p<0.05, Figure 3D) in RRMECs grown under hyperglycemic conditions. Interestingly, there was an increase in phosphorylated β-catenin nuclear translocation in hyperglycemic RRMECs shown in Figure 3E with IF staining of RRMECs under HG vs NG conditions.
Figure 3. The ubiquitination of β-catenin under hyperglycemic conditions was significantly increased in retinal endothelial cells.
A) Active SHP2 levels were significantly decreased in RRMECs grown under HG conditions. B) Total SHP2 levels in RRMECs grown under NG and HG conditions. C) The non-phosphorylated, active form of β-catenin was significantly decreased in hyperglycemic RRMECs. D) Ubiquitinated β-catenin levels were significantly increased in RRMECs grown under HG conditions. E) Representative images showing the increase in the phosphorylated β-catenin nuclear localization under hyperglycemic conditions in RRMECs. Western blotting data is a comparison of the relative SHP2 /β-actin, ph-SHP2 /β-actin, and active β-catenin/β-actin band densities. Scale bar = 50 μm. *p<0.05, N=3 per group.
The decrease in PECAM-1, either due to hyperglycemia or siRNA knockdown, may contribute to an increase in REC permeability
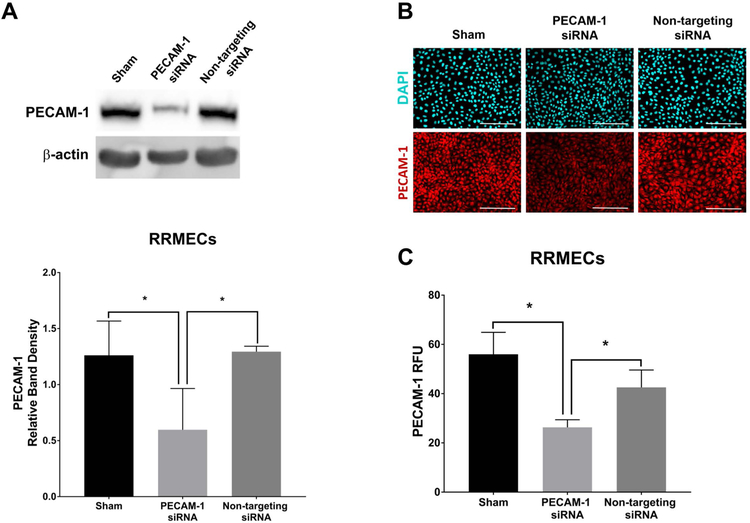
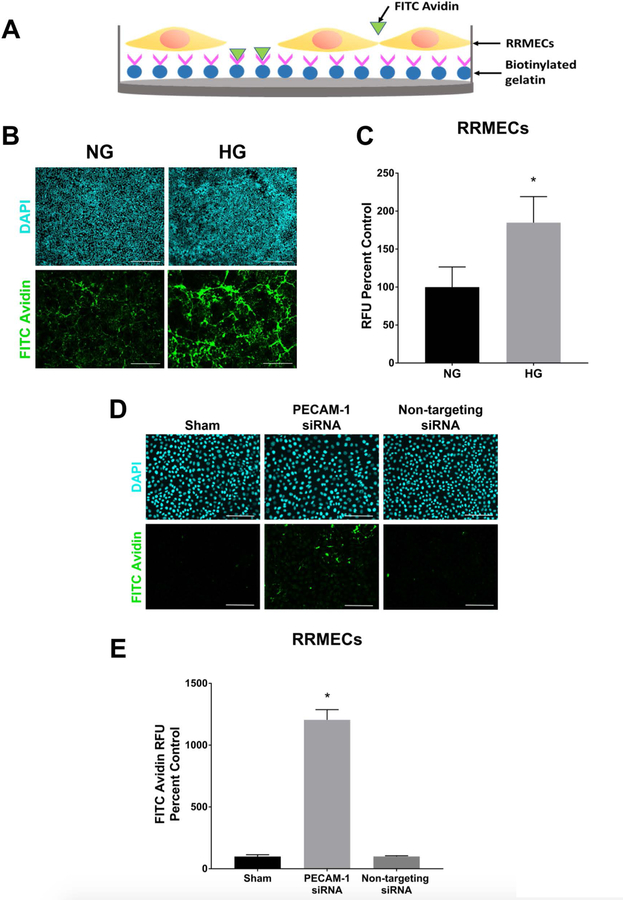
To delineate the role of retinal PECAM-1 loss on increased vascular permeability, we performed a loss-of-function experiment with PECAM-1 knockdown in RRMECs using transcriptional gene silencing siRNA against rat PECAM-1 (40–54% decrease, p<0.05, Figure 4). RRMEC permeability was significantly increased under HG conditions (84% increase, p<0.05), as well as in RRMECs with PECAM-1 partial knockdown when compared to RRMECs with non-targeting siRNA (10-fold increase, p<0.05, Figure 5).
Figure 4. PECAM-1 partial knockdown in RRMECs using PECAM-1 siRNA.
A) The partial knockdown of PECAM-1 using PECAM-1 siRNA showing significantly reduced PECAM-1 levels in RRMECs. B) Representative images of PECAM-1 immunofluorescence labeling of RRMECs with PECAM-1 partial knockdown. C) Image analysis of PECAM-1 fluorescence intensity in RRMECs showing a significant decrease in PECAM-1 levels with PECAM-1 partial knockdown. Western blotting data is a comparison of the relative PECAM-1 /β-actin band densities. PECAM-1 RFU is the relative analysis of PECAM-1 fluorescence intensity to total number of nuclei. Scale bar = 50 μm. *p<0.05, N=3 per group.
Figure 5. The increase in RRMEC permeability under HG conditions and with PECAM-1 partial knockdown.
A) Experimental design for the biotinylated gelatin and FITC-avidin in vitro permeability assay. B) Representative images of RRMEC permeability assay under NG and HG conditions. C) Image analysis of RRMEC permeability assay showing a significant increase under HG conditions. D) Representative images of the RRMEC permeability assay with PECAM-1 partial knockdown. E) Image analysis of the permeability assay showing a significant increase in RRMECs with PECAM-1 partial knockdown. The relative fluorescence unit (RFU) is the fluorescence intensity of FITC-avidin as percent of control (NG). Scale bar = 50 μm (B), 100 μm (D). *p<0.05, N=3 per group.
PECAM-1 Knockdown in RRMECs attenuated active SHP2 levels and increased phospho-β-catenin levels
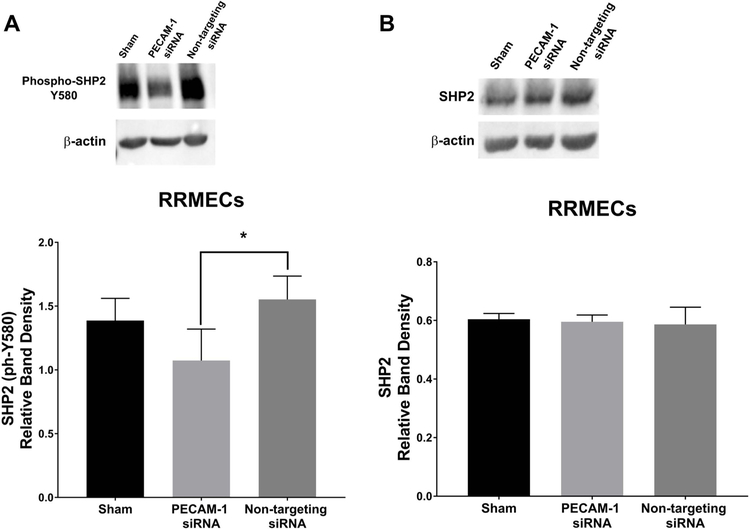
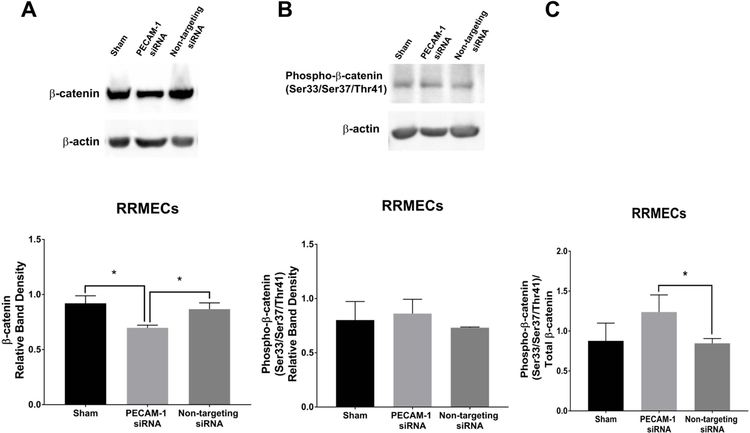
Since PECAM-1 participates in activating SHP2 via its recruitment to the ITIM domain in the PECAM-1 cytoplasmic tail, we hypothesized that PECAM-1 loss can affect the activity of SHP2. In RRMECs with PECAM-1 knockdown, phosphorylated (active) SHP2 levels were significantly decreased compared to non-targeting siRNA (35% decrease, p<0.05, Figure 6A) with no change in total SHP2 (Figure 6B). Additionally, compared to RRMECs administered non-targeting siRNA, cells with PECAM-1 knockdown had decreased total β-catenin (20% decrease, p<0.05, Figure 7A), no change in phosphorylated β-catenin (Figure 7B), but an increase in the ratio of phosphorylated to total β-catenin (45% increase, p<0.05, Figure 7C).
Figure 6. Phosphorylated SHP2 levels were significantly decreased in RRMECs with PECAM-1 partial knockdown.
A) Phosphorylated (Y580), active SHP2 levels in RREMCs were significantly decreased with PECAM-1 partial knockdown. B) Total SHP2 levels in RRMECs with PECAM-1 partial knockdown were not altered. Western blotting data is a comparison of the relative SHP2 /β-actin band densities. *p<0.05, N=3 per group.
Figure 7. The decrease in β-catenin levels with PECAM-1 partial knockdown.
A) Total β-catenin levels in RRMECs were significantly decreased with PECAM-1 partial knockdown. B) Phosphorylated β-catenin levels in RRMECs with PECAM-1 partial knockdown. C) The ratio of phosphorylated β-catenin to total β-catenin in RRMECs was significantly increased with PECAM-1 partial knockdown. Western blotting data is a comparison of the relative β-catenin/β-actin band densities. *p<0.05, N=3 per group.
Discussion and conclusion
The BRB can be divided into two barriers, the inner blood-retinal barrier (iBRB) and the outer blood-retinal barrier (oBRB). The iBRB is formed by the endothelial cells lining the blood vessels supplying oxygen and nutrients to the inner retina, while the oBRB is formed by the tight junctions between the retinal pigmented epithelium30. The breakdown of iBRB is considered a vital process in the pathogenesis of diabetic macular edema31. Most current treatments for DR are targeted to the later stages of DR when visual impairment has already been established. Thus, the investigation and study of the early molecular mechanisms leading to iBRB breakdown is vital to limit the progression of and to identify therapeutic targets for the treatment of DR at its early stages.
Retinal vascular endothelial cells create the barrier needed to limit plasma leakage into the retinal neuronal tissue. However, in pathophysiological conditions, such as the inflammatory response during DR32, the intercellular junction may break down as a response to cytokines, such as TNF-α or histamine, leading to the extravasation of inflammatory cells, proteins, and fluid into the extravascular space33. Under normal circumstances, this process is reversible due to the ability of the intercellular junction proteins, such as VE-cadherin, and its adaptor protein β-catenin, to restore the barrier function34. However, in DR, the barrier function is not fully maintained or restored, leading to BRB breakdown.
Several studies have indicated the important role of PECAM-1 in the maintenance of vascular barrier function, which extends beyond it simply being an endothelial cell marker. PECAM-1 has been shown to have an integral role in the restoration of the vascular barrier after its perturbation due to histamine35. Moreover, PECAM-1 loss led to increased brain microvascular permeability in mice with experimental autoimmune encephalitis11. Furthermore, PECAM-1 deficient mice had an increased vascular permeability during LPS-induced endotoxemia36, 37. Recently, our group reported the loss of PECAM-1 in the diabetic retina15, with a possible role of matrix metalloproteinases (MMPs) and/or inflammation in this loss. The mechanisms of hyperglycemia-induced loss of PECAM-1 are still under investigation. Additionally, the consequences of this loss are important to determine, given the multiple roles of PECAM-1 in endothelial function. Thus, we investigated the potential role of PECAM-1 loss in the increased retinal vascular permeability in hyperglycemia. In this study, we found a significant increase in RRMEC permeability under hyperglycemic conditions, and in RRMECs with PECAM-1 knockdown. These findings further support our hypothesis of a role for PECAM-1 loss in increasing endothelial cell permeability in the diabetic retina.
PECAM-1 acts as a scaffold for multiple adaptor proteins, such as β-catenin23, which binds to a domain in the cytoplasmic tail of PECAM-1 that is encoded by axon 1538. Thus, PECAM-1 can affect β-catenin localization to the cell membrane, limiting its cytoplasmic pool, and controlling its translocation to the nucleus where it can promote the transcription of its target genes39, 40. Interestingly, we found a significant decrease in total β-catenin levels in the diabetic retina, and in RRMECs grown under hyperglycemic conditions. In addition, β-catenin levels were significantly decreased in RRMECs with PECAM-1 knockdown when compared to controls. Furthermore, β-catenin/PECAM-1 interaction was significantly decreased in the diabetic retina and in hyperglycemic RRMECs. These findings indicate a role for retinal PECAM-1 loss in attenuating β-catenin levels in retinal endothelial cells.
β-catenin levels in the cell are regulated by its phosphorylation/dephosphorylation process, which also controls its activity and function. The dephosphorylation of β-catenin renders it active, and able to associate with VE-cadherin at the endothelial junctions. On the other hand, phosphorylated β-catenin is targeted for ubiquitination, and subsequent degradation by the ubiquitin-proteasome pathway. PECAM-1 can participate in a wide range of cellular events, such as cell signaling, via its two ITIM domains present on its cytoplasmic tail. The ITIM domains can recruit src homology 2 (SH2) containing molecules, such as src homology phosphatase 2 (SHP2), which upon its phosphorylation and activation, can dephosphorylate β-catenin. Therefore, we speculated that the hyperglycemia-induced decrease in β-catenin levels in RECs is due to its degradation as a result of the increase in phospho-β-catenin ratio. Thus, we assessed β-catenin ubiquitination in RRMECs, and found a significant increase under hyperglycemic conditions when compared to controls. Moreover, active (pY580) SHP2 levels were significantly decreased in RRMECs grown under hyperglycemic conditions, as well as in RRMECs with PECAM-1 knockdown.
The phosphorylation of β-catenin at Ser45 by casein kinase 1α (CK1α)41, primes β-catenin to be phosphorylated by glycogen synthase kinase 3 β (GSK3β) at Ser33/Ser37/Thr4142, which allows β-catenin to become ubiquitinated and recognized for degradation by proteasomes43, 44. The non-phospho-Ser45 (active) β-catenin levels were significantly decreased in hyperglycemic conditions. Moreover, the increase in phospho-β-catenin (Ser33/Ser37/Thr41) nuclear translocation in hyperglycemic RRMECs indicates a possible role in the induction of target genes, which needs to be further examined. Additionally, phospho-β-catenin (Ser33/Ser37/Thr41) levels were significantly increased in RRMECs with PECAM-1 knockdown, which could be due to its decreased dephosphorylation by active SHP2.
PECAM-1 could also affect β-catenin phosphorylation, by the modulation of GSK3β activity. GSK3β is constitutively active in cells45, and its activity can be inhibited by its phosphorylation by AKT46. Interestingly, PECAM-1 can recruit and activate phosphoinositide 3-kinases (PI3Ks) via PECAM-1 ITIM domains14, which in turn activates AKT. Thus, the hyperglycemia-induced loss of retinal PECAM-1 can indirectly affect the activity of GSK3β, which in turn can affect β-catenin phosphorylation, ubiquitination, and degradation. This pathway needs to be further examined to gain a better understanding of the multifactorial role of PECAM-1 in modulating β-catenin levels in RECs under hyperglycemic conditions.
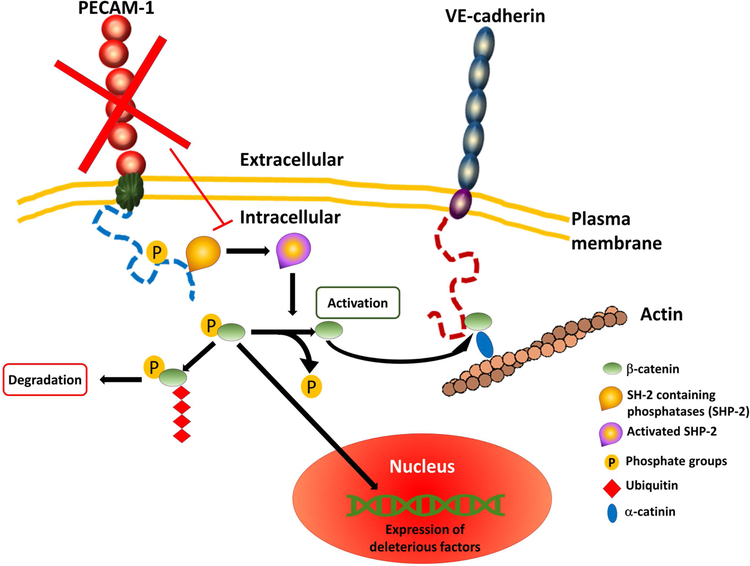
In conclusion, β-catenin levels were significantly decreased in the diabetic retina, in RRMECs grown under hyperglycemic conditions, as well as in RRMECs with PECAM-1 partial knockdown. These results indicate a novel role for the hyperglycemia-induced PECAM-1 loss in the increased vascular permeability in DR via the attenuation of β-catenin levels, where PECAM-1 loss promotes an increase in β-catenin phosphorylation, ubiquitination, and degradation via the ubiquitin-proteasome pathway (Figure 8). Therefore, these findings may provide a therapeutic target for limiting the advancement of DR and limiting the damage in the retina due to increased vascular permeability. Additionally, the findings could complement studies involving retinal endothelial junctional proteins, to which possible interactions with PECAM-1 could be investigated in hyperglycemia to fully understand the deleterious effects on BRB integrity.
Figure 8. A graphical representation of the hypothesized mechanism.
The role of PECAM-1 loss in retinal endothelial cells on the increase of permeability via attenuating β-catenin levels under hyperglycemic conditions.
Perspective:
Retinal PECAM-1 loss may play a role in increasing retinal vascular permeability under hyperglycemic conditions, possibly by attenuating β-catenin levels. These findings broaden the understanding of the molecular mechanisms in DR pathogenesis, and provide a therapeutic target for limiting the progression of DR.
Acknowledgements
We would like to thank Dr. Pattillo and his lab members for the usage and guidance of the ChemiDoc XRS gel imaging system.
Grant support:
This work was supported by funding from the National Institute of Health (NIH) EY025632 and an American Heart Association (AHA) predoctoral fellowship.
Contributor Information
Randa S. Eshaq, Louisiana State University Health Sciences Center-Shreveport, LA, Department of Molecular and Cellular Physiology
Norman R. Harris, Louisiana State University Health Sciences Center-Shreveport, LA, Department of Molecular and Cellular Physiology.
References
- 1.Nakamura S, Imai S, Ogishima H, Tsuruma K, Shimazawa M, Hara H. Morphological and functional changes in the retina after chronic oxygen-induced retinopathy. PLoS One 2012;7:e32167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishikawa M, Jin D, Sawada Y, Abe S, Yoshitomi T. Future therapies of wet age-related macular degeneration. J Ophthalmol 2015;2015:138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poulaki V Retinal Vascular Permeability in Health and Disease. Frontiers in Diabetes 2010;20:20–41. [Google Scholar]
- 4.Eshaq RS, Aldalati AMZ, Alexander JS, Harris NR. Diabetic retinopathy: Breaking the barrier. Pathophysiology 2017;24:229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol 1999;14:240–248. [DOI] [PubMed] [Google Scholar]
- 6.Antonetti DA, VanGuilder HD, Mao-Lin C. Vascular Permeability in Diabetic Retinopathy In: Duh EJ (ed), Diabetic Retinopathy. Totowa, NJ: Humana Press; 2008:333–352. [Google Scholar]
- 7.Krogsaa B, Lund-Andersen H, Mehlsen J, Sestoft L, Larsen J. THE BLOOD-RETINAL BARRIER PERMEABILITY IN DIABETIC PATIENTS. Acta Ophthalmologica 1981;59:689–694. [DOI] [PubMed] [Google Scholar]
- 8.Russ PK, Davidson MK, Hoffman LH, Haselton FR. Partial characterization of the human retinal endothelial cell tight and adherens junction complexes. Invest Ophthalmol Vis Sci 1998;39:2479–2485. [PubMed] [Google Scholar]
- 9.Su X, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Mol Vis 2003;9:171–178. [PubMed] [Google Scholar]
- 10.Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol 1991;114:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graesser D, Solowiej A, Bruckner M, et al. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest 2002;109:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol 2002;158:773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao C, Sun W, Christofidou-Solomidou M, et al. PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood 2003;102:169–179. [DOI] [PubMed] [Google Scholar]
- 14.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol 2003;23:953–964. [DOI] [PubMed] [Google Scholar]
- 15.Eshaq RS, Harris NR. Loss of Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) in the Diabetic Retina: Role of Matrix Metalloproteinases. Invest Ophthalmol Vis Sci 2019;60:748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung AK, Fung MK, Lo AC, et al. Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes 2005;54:3119–3125. [DOI] [PubMed] [Google Scholar]
- 17.Cao Y, Feng B, Chen S, Chu Y, Chakrabarti S. Mechanisms of endothelial to mesenchymal transition in the retina in diabetes. Invest Ophthalmol Vis Sci 2014;55:7321–7331. [DOI] [PubMed] [Google Scholar]
- 18.Jackson DE. The unfolding tale of PECAM-1. FEBS Lett 2003;540:7–14. [DOI] [PubMed] [Google Scholar]
- 19.Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol 2007;27:2514–2523. [DOI] [PubMed] [Google Scholar]
- 20.Paddock C, Lytle BL, Peterson FC, et al. Residues within a lipid-associated segment of the PECAM-1 cytoplasmic domain are susceptible to inducible, sequential phosphorylation. Blood 2011;117:6012–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson DE, Ward CM, Wang R, Newman PJ. The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation. Evidence for a mechanistic link between PECAM-1- and integrin-mediated cellular signaling. J Biol Chem 1997;272:6986–6993. [DOI] [PubMed] [Google Scholar]
- 22.Pumphrey NJ, Taylor V, Freeman S, et al. Differential association of cytoplasmic signalling molecules SHP-1, SHP-2, SHIP and phospholipase C-gamma1 with PECAM-1/CD31. FEBS Lett 1999;450:77–83. [DOI] [PubMed] [Google Scholar]
- 23.Ilan N, Cheung L, Pinter E, Madri JA. Platelet-endothelial cell adhesion molecule-1 (CD31), a scaffolding molecule for selected catenin family members whose binding is mediated by different tyrosine and serine/threonine phosphorylation. J Biol Chem 2000;275:21435–21443. [DOI] [PubMed] [Google Scholar]
- 24.Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. EMBO J 2012;31:2714–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dejana E, Orsenigo F. Endothelial adherens junctions at a glance. J Cell Sci 2013;126:2545–2549. [DOI] [PubMed] [Google Scholar]
- 26.Stepniak E, Radice GL, Vasioukhin V. Adhesive and signaling functions of cadherins and catenins in vertebrate development. Cold Spring Harb Perspect Biol 2009;1:a002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hordijk PL, Anthony E, Mul FP, Rientsma R, Oomen LC, Roos D. Vascular-endothelial-cadherin modulates endothelial monolayer permeability. J Cell Sci 1999;112 ( Pt 12):1915–1923. [DOI] [PubMed] [Google Scholar]
- 28.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 1997;16:3797–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasban WS. ImageJ. Bethesda, Maryland, USA: U. S. National Institutes of Health; 1997–2012. [Google Scholar]
- 30.Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. Eur J Ophthalmol 2011;21 Suppl 6:S3–9. [DOI] [PubMed] [Google Scholar]
- 31.Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res 2013;34:19–48. [DOI] [PubMed] [Google Scholar]
- 32.Rübsam A, Parikh S, Fort PE. Role of Inflammation in Diabetic Retinopathy. Int J Mol Sci 2018;19. [DOI] [PMC free article] [PubMed]
- 33.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 2007;7:803–815. [DOI] [PubMed] [Google Scholar]
- 34.Dejana E Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol 2004;5:261–270. [DOI] [PubMed] [Google Scholar]
- 35.Biswas P, Canosa S, Schoenfeld D, et al. PECAM-1 affects GSK-3beta-mediated beta-catenin phosphorylation and degradation. Am J Pathol 2006;169:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am J Pathol 2005;166:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maas M, Stapleton M, Bergom C, Mattson DL, Newman DK, Newman PJ. Endothelial cell PECAM-1 confers protection against endotoxic shock. Am J Physiol Heart Circ Physiol 2005;288:H159–164. [DOI] [PubMed] [Google Scholar]
- 38.Biswas P, Zhang J, Schoenfeld JD, et al. Identification of the regions of PECAM-1 involved in beta- and gamma-catenin associations. Biochem Biophys Res Commun 2005;329:1225–1233. [DOI] [PubMed] [Google Scholar]
- 39.Sharma M, Jamieson C, Johnson M, Molloy MP, Henderson BR. Specific armadillo repeat sequences facilitate β-catenin nuclear transport in live cells via direct binding to nucleoporins Nup62, Nup153, and RanBP2/Nup358. J Biol Chem 2012;287:819–831. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Henderson BR, Fagotto F. The ins and outs of APC and beta-catenin nuclear transport. EMBO Rep 2002;3:834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amit S, Hatzubai A, Birman Y, et al. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev 2002;16:1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J 1998;17:1371–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tauriello DV, Maurice MM. The various roles of ubiquitin in Wnt pathway regulation. Cell Cycle 2010;9:3700–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu C, Li Y, Semenov M, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002;108:837–847. [DOI] [PubMed] [Google Scholar]
- 45.Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther 2015;148:114–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang X, Yu SX, Lu Y, Bast RC, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci U S A 2000;97:11960–11965. [DOI] [PMC free article] [PubMed] [Google Scholar]