Abstract
Introduction:
Several disease processes trigger prolonged activation of the alternative complement pathway. Crosslinks between complement activation and physiologic changes in platelets and neutrophils have been identified, but how this interplay alters the hemostatic potential in humans remains undefined. We hypothesize that activation of the alternative pathway triggers a hypercoagulable state.
Methods:
C3/C5 convertase Cobra Venom Factor (CVF, 10 Units/mL) was employed to activate the alternative complement pathway in whole blood. Complement inhibition was completed with inhibitors for C3/C3b (Compstatin, 25 and 50 μM), C3a receptor (SB290157, 300 nM, C3aR), and C5a receptor (W54011, 6 nM, C5aR). Coagulation was assessed using native thrombelastography which produces the following: reaction time (R time); angle; maximum amplitude (MA); percent fibrinolysis at 30-minutes post-MA (LY30).
Results:
Inhibition with C3aR and C5aR inhibitors did not alter clot formation (R time, 11.2 vs 11.6 min, p=0.36), clot strength (MA, 52.0 vs 52.3 mm, p=0.43) or fibrinolysis (LY30, 1.6 vs 4.0%, p=0.19). Compstatin did not influence clot formation or clot strength but did induce a dose-dependent increase in fibrinolysis (control LY30 3.0 vs 7.8% and 12.4% for 25 and 50 μM respectively, p=0.0002). CVF increased MA (58.0 vs 62.8 mm, p<0.0001), decreased LY30 (2.3 vs 1.4%, p=0.004), and increased R time (8.4 vs 9.9 min, p=0.008). Compstatin reversed the effects of CVF, while C5a reversed only the change in LY30.
Conclusions:
C3 contributes to fibrinolysis, as inhibition with Compstatin enhanced fibrinolysis, and CVF cleavage of C3 decreased fibrinolysis. CVF also induced a hypercoagulable state with increased clot strength.
Keywords: Coagulation, Trauma, Sepsis, Thrombelastography, Cobra Venom Factor
Introduction:
The relationship between activation of the complement pathway and alterations in coagulation have long been shown.(1-3) Several diseases associated with abnormal complement regulation, such as paroxysmal nocturnal hemoglobinuria, vasculitis, and atypical hemolytic uremic syndrome, produce dramatic, inappropriate complement activation, which can stimulate thrombin generation and dysregulated clot formation.(4-6) This deregulated activation of complement is not limited to chronic disease, but also occurs following trauma, as severe injury triggers robust activation of complement that persists for days after the initial resuscitation is complete.(7-10) In the days following severe injury, a hypercoagulable state frequently develops as well, (11) and consequently, patients face a heightened risk for both venous thromboembolic events and post-injury multiple organ failure.(12-14)
Several studies have explored mechanistic connections between complement and coagulation.(15-17) Complement activation, with production of the anaphylatoxins C3a and C5a, increases monocyte expression of tissue factor, an effective procoagulant.(18, 19) C5a also induces TNF-α production by monocytes, which similarly contribute to a pro-thrombotic state.(20, 21) Likewise, platelets express receptors for both C3a and C5a and are rapidly activated by C3a binding.(22, 23) With insertion of the membrane attack complex (C5b-9) at sub-lytic concentrations, platelets undergo activation and degranulation, and binding of the MAC increases platelet prothrombinase activity.(16, 24) The endothelium also has multiple responses to C5a binding, including increased Von Willebrand factor expression, enhanced prothrombinase activity, and exposure of plasminogen binding sites.(25-27) Similar complement-induced changes in hemostasis have been shown in vivo. As an example, Subramaniam et al. found that C3 knockout mice had defective platelet aggregation, while C5 knockout had reduced fibrin formation.(28)
One limitation of the previously mentioned in vitro studies is the use of simpler plasma-based models. Despite these studies demonstrating mechanistic links between complement and coagulation, how complement contributes to coagulation in whole blood is poorly described. Specifically, the mechanism in which complement-induced activation of cellular components of whole blood alter clot dynamics remains unknown. Several blood-borne cell lines contain complement inhibiting proteins that alter how complement activation changes coagulation. For example, platelets can secrete factor H, the main inhibitor of complement activation, limiting dysregulated activation.(29) Understanding the cellular effects of hemostasis in context of complement activity is necessary to determine the true in vivo effects.(30)
The objective of this study is to evaluate how complement activation affects coagulation in whole blood in vitro, as measured by thrombelastography (TEG), when combined with a sterile activator of the alternative complement pathway, Cobra Venom Factor (CVF). We hypothesize that activation of the alternative complement pathway leads to a hypercoagulable profile with stronger clots, formed more rapidly, and with decreased degradation.
Materials and Methods:
Human Subjects
Healthy adult volunteers were enrolled after completion of a questionnaire (supplemental methods) and informed consent. Inclusion criteria were healthy adults (age 18–85) without history of a coagulopathy. Exclusion criteria included prisoners and pregnant subjects or any subject with underlying cardiovascular, liver, or kidney disease, cancer, diabetes, autoimmune or inflammatory disorders, disorders of coagulation, obesity (BMI≥30), infection, recent surgery, or injury. Subjects were also excluded if they were taking non-steroidal anti-inflammatory medications within a week of enrollment or any of the following medications in the month preceding enrollment: aspirin or aspirin-containing drugs, clopidogrel, steroids, heparin, fondaparinux, warfarin, direct thrombin inhibitors, factor Xa inhibitors, abciximab or antifibrinolytics. Subjects were also excluded if they had surgery, traumatic injury, or received a transfusion in the six weeks preceding enrollment, or if they had an active illness. This study was approved by the Colorado Multiple Institutional Review Board (COMIRB #18-0625).
Sample Collection
Blood was collected via venipuncture in the antecubital fossa with collection into 3.2% citrated vacuum tubes. After collection, citrated tubes were incubated for fifteen minutes in a 37°C water bath to allow for equilibration.
Thrombelastography
Citrated native (without activator) thrombelastography (TEG) was performed after recalcification at 370C using the TEG 5000 Thrombelastograph Hemostasis Analyzer (Haemonetics, Niles, IL) according to manufacturer instructions.(31) TEG provides the following measurements: reaction time (R time [minutes]); angle (degrees); maximum amplitude (MA [millimeters]); percent fibrinolysis at 30 minutes post-MA (LY30 [percent]).
Measuring the effects of complement activation on coagulation
To evaluate how activation of the alternative complement pathway alters coagulation, we employed the C3/C5 convertase Cobra Venom Factor (CVF, EMD Millipore Corp, Billerica, MA). CVF was added to 500 μL of whole blood to a final concentration of 10 Units activity/mL. Following the addition of CVF or vehicle control (PBS), to allow for rigorous complement activation, samples were incubated for 30 minutes in 37°C water bath. A trial study was completed comparing 15-minute and 30-minute incubation times (data not shown), and 30-minute incubation was found to induce a stronger signal. Following incubation, citrated native TEG was completed as above.
Inhibition of complement during whole blood coagulation.
Changes in TEG were evaluated following complement inhibition. Two receptor inhibitors were used, one for the C3a receptor (SB 290157, C3aRI, Cayman Chemical Company, Ann Arbor, MI) and another for the C5a receptor (W54011, C5aRI, EMD Millipore, Billerica, MA).(32, 33) The C3aRI and C5aRI were solubilized in dimethyl sulfoxide (DMSO) and further diluted in phosphate-buffered saline (PBS) to obtain the experimental solution per supplier instructions. An equivalent carrier (0.05% v/v DMSO in PBS) was used as a control. The C3aRI and C5aRI inhibitors were added to 500 μL of whole blood (WB) for a final concentration of 300 nM and 6 nM, respectively. These doses are comparable to the inhibitors’ respective IC50 doses of the C3aRI (IC50 of 200 nM) and C5aRI (IC50 of 3.1 nM), respectively. (32, 33) To evaluate inhibition of upstream alternative complement pathway, the C3 and C3b inhibitor Compstatin (Tocris Bioscience, Bristol, UK) was added to whole blood to a final concentration of 25 μM and 50 μM. After the addition of inhibitors, samples were incubated for five minutes in 37°C water bath prior to running TEG using the same methodology as described previously.
Reversal of complement activation and the effects on coagulation.
To block the effects of CVF as a complement activator, Compstatin (50 μM) was added in the presence of CVF (10 U/mL) prior to a 30-minute incubation. A control vehicle of PBS in whole blood was used. All samples were incubated for 30 minutes in a 37°C water bath. Similarly, to reverse the effects of anaphylatoxin release, the C3aRI (2 μM) and C5aRI (500 nM) inhibitors were added in the presence of CVF (10 U/mL) prior to incubation.
Statistical Analysis
Statistical analysis was done using GraphPad Prism version 7.04 for Windows, (GraphPad Software, La Jolla California USA). TEG variables were compared using repeated measures ANOVA or Kruskal-Wallis test depending on whether the variables were normally distributed or skewed. Normality was tested using the Shapiro-Wilk test. Dunnet’s or Dunn’s correction were used to correct for multiple comparisons also depending on whether a normal distribution was present or not.
Results
CVF activation increases clot strength and decreases fibrinolysis.
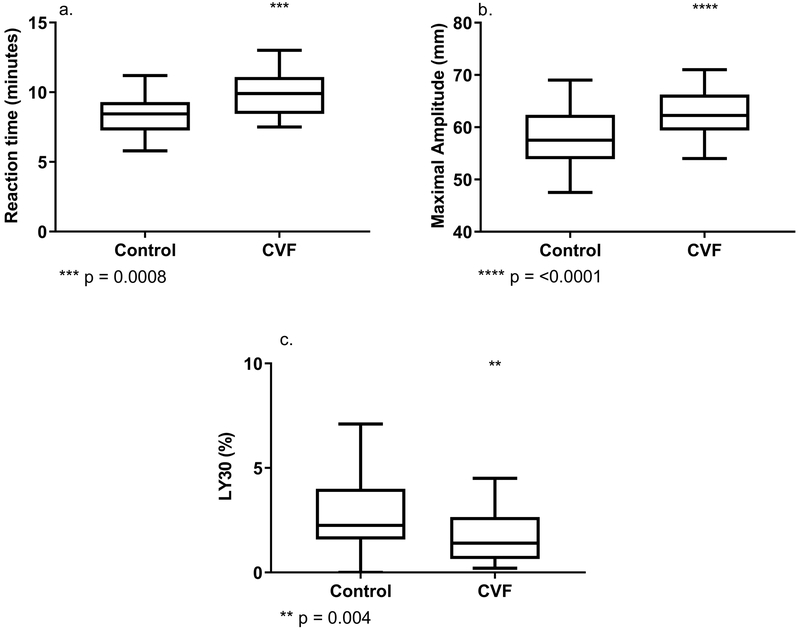
Twenty two subjects provided samples for this experiment. The median age was 31 years (range 23–46) with 54% male. Activation of the alternative complement pathway with CVF induced several effects on coagulation. First, an increase in the R time occurred following incubation with CVF compared to control: median R time 9.9 vs. 8.5 minutes, (P=0.0008, Figure 1A); however, angle was not statistically different (median angle 51.9 vs 50.5 degrees, p=0.90). Secondly, clot strength increased significantly with the addition of CVF: MA 62.3 vs 57.5 mm (p<0.0001, Figure 1B). Lastly, fibrinolysis was diminished with complement activation: median LY30 1.4 vs 2.3% (p= 0.004, Figure 1C).
Figure 1:
a.Complement activation prolongs time to clot initiation, b. increases clot strength, and c. decreases fibrinolysis. (LY30 – lysis 30 minutes after MA, CVF – Cobra Venom Factor)
Inhibition of upstream complement activation, but not the receptor, alters clot dynamics.
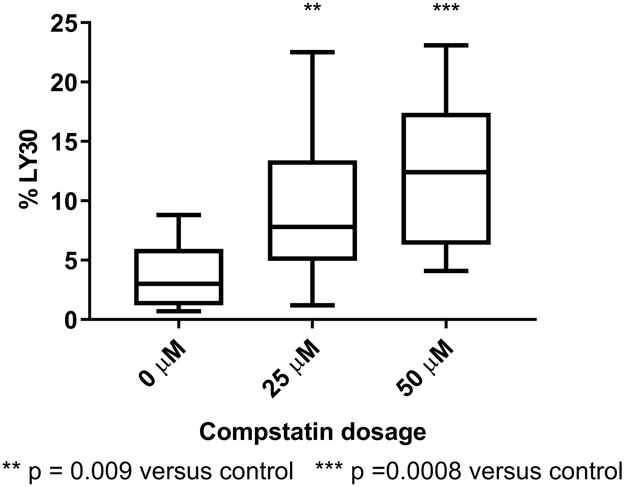
Twelve subjects contributed samples, of whom 8 (67%) were male. The group had a median age of 30 years (range 24-47). The upstream complement inhibitor Compstatin did not alter coagulation, as R time, angle, and MA were unchanged (Table 1). However, clot degradation (fibrinolysis) was significantly increased with the inhibition of C3/C3b by Compstatin (LY30 3.0 vs 7.8%, p=0.0002, Figure 2). Clot dynamics were unchanged with either the C3a or C5a receptor inhibitor (Table 2). Fibrinolysis was higher in the presence of the C3aR and C5aR inhibitors, but this did not reach statistical significance (Table 2).
Table 1:
Compstatin did not alter R time, angle, or MA.
| Control (Median [IQR]) |
Compstatin | P value |
||
|---|---|---|---|---|
| 25 uM (Median [IQR]) |
50 uM (Median [IQR]) |
|||
| R time (minutes) | 9.8 (7.0 - 13.9) | 14.1 (9.2 - 16.1) | 10.8 (9.2 - 13.9) | 0.30 |
| Angle (degrees) | 42.8 (32.3 - 54.9) | 47.2 (30.5 - 53.3) | 42.0 (34.0 - 51.8) | 0.93 |
| MA (mm) | 51.8 (47.1 - 56.4) | 48.5 (42.9 - 52.4) | 45.3 (42.6 - 52.1) | 0.76 |
| LY30 (%) | 3.0 (1.2 - 6.0) | 7.8 (5.0 - 13.4) | 12.4 (6.3 - 17.4) | < 0.01 |
R – reaction time, MA – Maximal Amplitude, LY30 – Lysis 30 minutes after MA, IQR – interquartile range
Figure 2:
Inhibition of complement C3 with Compstatin increases clot degradation. (LY30 – lysis 30 minutes after MA)
Table 2:
TEG Changes Following Inhibition of the C3a and C5a receptor.
| Control Median (IQR) |
C3aR Inhibitor Median (IQR) |
C5aR Inhibitor Median (IQR) |
P value | |
|---|---|---|---|---|
| Reaction time (minutes) | 11.2 (9.2 – 11.5) | 11.6 (10.5 – 12.7) | 11.4 (9.0 – 13.7) | 0.36 |
| Angle (degrees) | 42.3 (36.6 – 49.6) | 45.5 (39.9 – 51.9) | 45.7 (40.6 – 51.5) | 0.18 |
| MA (mm) | 52.0 (48.1 – 59.0) | 52.3 (50.1 – 55.6) | 54.3 (50.0 – 58.0) | 0.43 |
| LY30 (%) | 1.6 (1.2 – 3.9) | 4.0 (0.9 – 9.2) | 5.0 (1.5 – 7.7) | 0.19 |
R – reaction time, MA – Maximal Amplitude, LY30 – Lysis 30 minutes after MA, IQR – interquartile range. C3aR – C3a receptor, C5aR – C5a receptor.
Reversing complement activation with Compstatin blocks alterations in coagulation.
Fifteen subjects provided samples, of whom 9 (60%) were male. The group had a median age of 31 years (range 24–46). Compstatin inhibited each of CVF’s procoagulant effects. Comparing the combination of Compstatin and CVF to a control, no difference was seen between R time, angle, MA, or LY30 (Table 3).
Table 3:
Comparison of Compstatin and CVF to control
| Control Median (IQR) |
CVF + Compstatin Median (IQR) |
P value | |
|---|---|---|---|
| Reaction time (min) | 8.2 (7.1 – 8.6) | 8.8 (7.9 – 10.7) | 0.30 |
| Angle (degrees) | 51.0 (49.4 – 58.9) | 51.6 (46.2 – 58.2) | 0.49 |
| MA (mm) | 62 (55 – 63.5) | 60 (53 – 65) | 0.95 |
| LY30 (%) | 2.1 (1.5 – 2.8) | 1.7 (0.7 – 4.5) | 0.99 |
R – reaction time, MA – Maximal Amplitude, LY30 – Lysis 30 minutes after MA, IQR – interquartile range. CVF – Cobra Venom Factor
Reversing complement activation with C5aRI blocks alterations of fibrinolysis.
This experiment consisted of a cohort of fourteen subjects, of whom 8 (57%) were male. The median age of this cohort was 31 years (range 24–33). The receptor inhibitors for C3a and C5a failed to abrogate several of the effects of CVF. The C3aR and C5aR inhibitor did not prevent CVF’s effects on the R time or MA (Table 4). However, the C5aR inhibitor did reverse CVF’s effects on fibrinolysis (2.2 vs 1.2%, p=0.58), while the C3aRI did not. Also, while CVF and the C3aRI alone had no effect on angle in the previous experiments, the combination of C3aRI and CVF decreased the angle compared to controls (45.8 vs 51.2°, p=0.008).
Table 4:
Comparison of CVF and Receptor Inhibitors to Controls
| Control median (IQR) |
CVF + C3aRI median (IQR) |
CVF + C5aRI median (IQR) |
|
|---|---|---|---|
| Reaction time (min) | 8.3 (6.9 - 8.7) | 9.7 (8.3 - 11.1)* | 8.5 (7.9 - 9.8)* |
| Angle (degrees) | 51.2 (48.1 - 59.2) | 45.8 (40.6 - 53.8)** | 53.0 (44.7 - 55.8) |
| MA (mm) | 58.8 (54.3 - 63.5) | 61.8 (57.9 - 66.3)** | 63.8 (60.8 - 68.6)** |
| LY30 (%) | 2.2 (1.7 - 3.0) | 0.7 (0.5 - 1.5)** | 1.2 (0.8 - 5.1) |
R – reaction time, MA – Maximal Amplitude, LY30 – Lysis 30 minutes after MA, IQR – interquartile range. CVF – Cobra Venom Factor
p<0.05 compared to controls
p<0.005 compared to controls
Discussion:
Using a whole blood assay, this study demonstrates several ways in which complement contributes to clot formation and degradation. First, the alternative pathway (AP) of complement alters fibrinolysis, with activation of this pathway decreasing fibrinolysis and inhibition of C3 increasing fibrinolysis. Secondly, activation of the AP increases clot strength. In contrast, a surprising prolongation of the time to clot formation occurred with incubation with CVF. Compstatin, an inhibitor of C3, effectively reversed each of the effects of CVF, while the C5aR inhibitor reversed the effect of CVF on fibrinolysis alone.
Activation of the AP primarily drives changes in coagulation via an increase in platelet activation, presenting as increased clot strength. This AP-induced activation is most likely explained by interactions of the terminal complement complex with the platelet membrane, as the anaphylatoxins C3a and C5a appear to play a minimal role in platelet activity. This supports the findings by Wiedmer et al. who found that platelet activation increased in the presence of the membrane attack complex.(24) However, Subramaniam et al. reported that platelet activation by complement occurred independent of C5b-9 activation.(28) Our data suggest that C5b-9 is the primary driver of platelet activation in response to complement activation, as C5a and C3a receptor inhibition failed to reverse the effects of complement activation.
Previous studies evaluating alterations of fibrinolysis by AP activation primarily implicated C5a via activation of macrophages and granulocytes, priming for the release of antifibrinolytic proteins.(15, 34) Other in vitro studies have shown C5a-induced expression of tissue factor by neutrophils, providing the activating ingredient for the extrinsic coagulation pathway.(35) C5a has been shown to stimulate PAI-1 release by human macrophages and basophils, thus inhibiting tPA mediated fibrinolysis.(15, 34) This is supported by our finding that a C5aR inhibitor reverses inhibition of fibrinolysis by activation of the AP. Alternatively, depletion or activation of C3 may alter fibrinolysis by changing C3 binding to the fibrin clot itself.(36, 37) This may explain our finding that the addition of Compstatin to whole blood increases fibrinolysis via Compstatin blocking C3 from interacting with the fibrin clot.
Several in vivo studies have implicated complement in coagulation, but not via the C5b-9.(38) For instance, knockout rodent studies have implicated both C3 and C5 in clot development and stability.(28, 39) However, the data presented here challenges several of these previous assertions. First, C5a does not appear to affect clot strength in whole blood. Secondly, C3 does not appear to contribute to clot initiation or clot strength, as has been suggested in rodent knockout models.(28) Instead, it appears the terminal complement complex (C5b-9) is the primary driver of complement activation of coagulation.(16) Importantly, no whole blood assays of coagulation have been completed in concordance with in vivo studies to measure the hemostatic potential following complement activation in vivo.
An unexpected finding is the prolongation of the R time with incubation with CVF. This prolongation may reflect low-level prothrombinase activity, a purported result of complement activation on platelets.(40) While the citrated samples did not undergo clot formation prior to recalcification, a degree of prothrombinase activity was likely occurring in the presence of CVF, leading to consumption of clotting factors and thus resulting in a prolongation in time to clot initiation on TEG.
This finding has important implications in trauma and sepsis patients, as it may explain the development of coagulopathy with prolonged complement activation. As complement activation persists, an increasing degree of coagulation factor consumption occurs. This consumptive coagulopathy is similar to the coagulopathy seen with disseminated intravascular coagulation with excessive clot formation and diminished clotting potential occurring concurrently with persistent inflammatory activation. Additionally, the mechanism underlying the synergistic effect of CVF and the C3aR inhibitor against clot formation, as demonstrated by the reduced TEG angle, remains unclear. This may reflect off target effects of the C3aR inhibitor that, during activated complement states, leads to consumption of fibrinogen or factor XIII.
One explanation for the inconsistency of studies exploring how complement activation alters coagulation is the existence of interspecies differences in expression of membrane-bound complement receptor proteins. For instance, studies have demonstrated that platelets in guinea pigs express the C3a and C5a receptor, (41-43) but studies in mice failed to identify these receptors on platelets.(44) Meanwhile, platelets in humans express receptors for both anaphylatoxins, and concentrations of these receptors are increased during inflammatory processes.(22) Membrane-bound complement-regulating proteins also differ significantly between species. Humans rely on the complement-regulating proteins Decay Accelerating Factor (DAF) and Membrane Cofactor Protein (MCP) to prevent inadvertent complement activation,(45) and these proteins are expressed on human platelets.(46, 47) In rodents, while DAF is widely distributed, it appears MCP is only expressed in the murine testis.(45) Rodent species also utilize an additional regulatory protein that is not present in humans, the complement receptor 1-related gene/protein Y (Crry).(48) Ultimately, how these differences in expression of complement proteins lead to differences in the coagulation-complement crosstalk is unknown.
A limitation of these findings is the absence of the endothelial component of the cellular based model of hemostasis, as the vascular endothelium broadly responds to complement in several ways. For example, receptors for C5a are expressed in several tissue beds including vascular endothelium, and these tissue beds respond to exposure in several ways.(49) C5a exposure to endothelium leads to expression of tissue factor, a powerful procoagulant.(50) These studies should be extended to include models that incorporate the contributions of the endothelium. Additionally, the precise mechanism of how AP activation promotes a procoagulant state is not fully elucidated. Another limitation is that these experiments were not done concurrently and utilized separate cohorts of healthy volunteers. This raises the potential for the changes seen with each subsequent experiment to be due to an unknown confounder rather than the addition of an experimental variable such as a complement inhibitor. Future studies will focus on the formation of the terminal complement complex, and how the formation of the terminal component of complement activation contributes to the findings described here. Complement inhibitors may offer an alternative to anticoagulant medications by removing the underlying insult initiating thrombotic states such as that seen in the post-injury phase following severe trauma.
Supplementary Material
Acknowledgements:
Funding: Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health (T32 GM008315 and P50 GM049222) and Department of Defense (USAMRAA, W81XWH-12-2-0028). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other sponsors of the project.
Footnotes
Presentation history: Portions of this data were presented at the 64th Annual International Society on Thrombosis and Haemostasis (ISTH) Scientific and Standardization Committee meeting Dublin, Ireland, July 18–21, 2018.
Financial Disclosures: The authors appreciate research support from Haemonetics with shared intellectual property.
References:
- 1.Karpman D, Stahl AL, Arvidsson I, Johansson K, Loos S, Tati R, Bekassy Z, Kristoffersson AC, Mossberg M and Kahn R: Complement Interactions with Blood Cells, Endothelial Cells and Microvesicles in Thrombotic and Inflammatory Conditions. Adv Exp Med Biol 865:19–42, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE and Lambris JD: Complement and coagulation: strangers or partners in crime? Trends Immunol 28(4):184–92, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Foley JH: Examining coagulation-complement crosstalk: complement activation and thrombosis. Thromb Res 141 Suppl 2:S50–4, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Logue GL, Rosse WF and Adams JP: Mechanisms of immune lysis of red blood cells in vitro. I. Paroxysmal nocturnal hemoglobinuria cells. J Clin Invest 52(5):1129–37, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron JS and Vick R: Letter: Plasma-C3 in haemolytic-uraemic syndrome and thrombotic thrombocytopenic purpura. Lancet 2(7835):975, 1973. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Jayne DRW and Zhao MH: Complement in ANCA-associated vasculitis: mechanisms and implications for management. Nat Rev Nephrol 13(6):359–367, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Kalbitz M, Karbach M, Braumueller S, Kellermann P, Gebhard F, Huber-Lang M and Perl M: Role of Complement C5 in Experimental Blunt Chest Trauma-Induced Septic Acute Lung Injury (ALI). PLoS One 11(7):e0159417, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hecke F, Schmidt U, Kola A, Bautsch W, Klos A and Kohl J: Circulating complement proteins in multiple trauma patients--correlation with injury severity, development of sepsis, and outcome. Crit Care Med 25(12):2015–24, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Ganter MT, Brohi K, Cohen MJ, Shaffer LA, Walsh MC, Stahl GL and Pittet JF: Role of the alternative pathway in the early complement activation following major trauma. Shock 28(1):29–34, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Satyam A, Andreo K, Lapchak PH, Dalle Lucca JJ, Davis RB, Tsokos MG, Shapiro NI and Tsokos GC: Complement Deposition on the Surface of RBC After Trauma Serves A Biomarker of Moderate Trauma Severity: A Prospective Study. Shock, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selby R, Geerts W, Ofosu FA, Craven S, Dewar L, Phillips A and Szalai JP: Hypercoagulability after trauma: hemostatic changes and relationship to venous thromboembolism. Thromb Res 124(3):281–7, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Dewar D, Moore FA, Moore EE and Balogh Z: Postinjury multiple organ failure. Injury 40(9):912–8, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Brill JB, Badiee J, Zander AL, Wallace JD, Lewis PR, Sise MJ, Bansal V and Shackford SR: The rate of deep vein thrombosis doubles in trauma patients with hypercoagulable thromboelastography. J Trauma Acute Care Surg 83(3):413–419, 2017. [DOI] [PubMed] [Google Scholar]
- 14.Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, Banerjee A and Sauaia A: Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg 77(6):811–7; discussion 817, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wojta J, Kaun C, Zorn G, Ghannadan M, Hauswirth AW, Sperr WR, Fritsch G, Printz D, Binder BR, Schatzl G, et al. : C5a stimulates production of plasminogen activator inhibitor-1 in human mast cells and basophils. Blood 100(2):517–23, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Sims PJ and Wiedmer T: The response of human platelets to activated components of the complement system. Immunol Today 12(9):338–42, 1991. [DOI] [PubMed] [Google Scholar]
- 17.Morigi M, Galbusera M, Gastoldi S, Locatelli M, Buelli S, Pezzotta A, Pagani C, Noris M, Gobbi M, Stravalaci M, et al. : Alternative pathway activation of complement by Shiga toxin promotes exuberant C3a formation that triggers microvascular thrombosis. J Immunol 187(1):172–80, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Landsem A, Fure H, Christiansen D, Nielsen EW, Osterud B, Mollnes TE and Brekke OL: The key roles of complement and tissue factor in Escherichia coli-induced coagulation in human whole blood. Clin Exp Immunol 182(1):81–9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kambas K, Markiewski MM, Pneumatikos IA, Rafail SS, Theodorou V, Konstantonis D, Kourtzelis I, Doumas MN, Magotti P, Deangelis RA, et al. : C5a and TNF-alpha up-regulate the expression of tissue factor in intra-alveolar neutrophils of patients with the acute respiratory distress syndrome. J Immunol 180(11):7368–75, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaeffer V, Cuschieri J, Garcia I, Knoll M, Billgren J, Jelacic S, Bulger E and Maier R: The priming effect of C5a on monocytes is predominantly mediated by the p38 MAPK pathway. Shock 27(6):623–30, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Poll T, Jansen PM, Van Zee KJ, Welborn MB 3rd, , de Jong I, Hack CE, Loetscher H, Lesslauer W, Lowry SF and Moldawer LL: Tumor necrosis factor-alpha induces activation of coagulation and fibrinolysis in baboons through an exclusive effect on the p55 receptor. Blood 88(3):922–7, 1996. [PubMed] [Google Scholar]
- 22.Patzelt J, Mueller KA, Breuning S, Karathanos A, Schleicher R, Seizer P, Gawaz M, Langer HF and Geisler T: Expression of anaphylatoxin receptors on platelets in patients with coronary heart disease. Atherosclerosis 238(2):289–95, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Polley MJ and Nachman RL: Human platelet activation by C3a and C3a des-arg. J Exp Med 158(2):603–15, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiedmer T, Esmon CT and Sims PJ: Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood 68(4):875–80, 1986. [PubMed] [Google Scholar]
- 25.Hattori R, Hamilton KK, McEver RP and Sims PJ: Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J Biol Chem 264(15):9053–60, 1989. [PubMed] [Google Scholar]
- 26.Hamilton KK, Hattori R, Esmon CT and Sims PJ: Complement proteins C5b-9 induce vesiculation of the endothelial plasma membrane and expose catalytic surface for assembly of the prothrombinase enzyme complex. J Biol Chem 265(7):3809–14, 1990. [PubMed] [Google Scholar]
- 27.Christiansen VJ, Sims PJ and Hamilton KK: Complement C5b-9 increases plasminogen binding and activation on human endothelial cells. Arterioscler Thromb Vasc Biol 17(1):164–71, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Subramaniam S, Jurk K, Hobohm L, Jackel S, Saffarzadeh M, Schwierczek K, Wenzel P, Langer F, Reinhardt C and Ruf W: Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood 129(16):2291–2302, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devine DV and Rosse WF: Regulation of the activity of platelet-bound C3 convertase of the alternative pathway of complement by platelet factor H. Proc Natl Acad Sci U S A 84(16):5873–7, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffman M and Monroe DM 3rd, : A cell-based model of hemostasis. Thromb Haemost 85(6):958–65, 2001. [PubMed] [Google Scholar]
- 31.Haemonetics: TEG 5000 System User Manual. P/N 06–510-US, Manual revision: AC. Haemonetics Corporation, Haemoscope Division, Niles, IL, 2010. [Google Scholar]
- 32.Ames RS, Lee D, Foley JJ, Jurewicz AJ, Tornetta MA, Bautsch W, Settmacher B, Klos A, Erhard KF, Cousins RD, et al. : Identification of a selective nonpeptide antagonist of the anaphylatoxin C3a receptor that demonstrates antiinflammatory activity in animal models. J Immunol 166(10):6341–8, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Sumichika H, Sakata K, Sato N, Takeshita S, Ishibuchi S, Nakamura M, Kamahori T, Ehara S, Itoh K, Ohtsuka T, et al. : Identification of a potent and orally active non-peptide C5a receptor antagonist. J Biol Chem 277(51):49403–7, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Kastl SP, Speidl WS, Kaun C, Rega G, Assadian A, Weiss TW, Valent P, Hagmueller GW, Maurer G, Huber K, et al. : The complement component C5a induces the expression of plasminogen activator inhibitor-1 in human macrophages via NF-kappaB activation. J Thromb Haemost 4(8):1790–7, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Lupu F, Keshari RS, Lambris JD and Coggeshall KM: Crosstalk between the coagulation and complement systems in sepsis. Thromb Res 133 Suppl 1:S28–31, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howes JM, Richardson VR, Smith KA, Schroeder V, Somani R, Shore A, Hess K, Ajjan R, Pease RJ, Keen JN, et al. : Complement C3 is a novel plasma clot component with anti-fibrinolytic properties. Diab Vasc Dis Res 9(3):216–25, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Richardson VR, Schroeder V, Grant PJ, Standeven KF and Carter AM: Complement C3 is a substrate for activated factor XIII that is cross-linked to fibrin during clot formation. Br J Haematol 160(1):116–9, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Kondo C, Mizuno M, Nishikawa K, Yuzawa Y, Hotta N and Matsuo S: The role of C5a in the development of thrombotic glomerulonephritis in rats. Clin Exp Immunol 124(2):323–9, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gushiken FC, Han H, Li J, Rumbaut RE and Afshar-Kharghan V: Abnormal platelet function in C3-deficient mice. J Thromb Haemost 7(5):865–70, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sims PJ, Rollins SA and Wiedmer T: Regulatory control of complement on blood platelets. Modulation of platelet procoagulant responses by a membrane inhibitor of the C5b-9 complex. J Biol Chem 264(32):19228–35, 1989. [PubMed] [Google Scholar]
- 41.Fukuoka Y and Hugli TE: Demonstration of a specific C3a receptor on guinea pig platelets. J Immunol 140(10):3496–501, 1988. [PubMed] [Google Scholar]
- 42.Becker S, Hadding U, Schorlemmer HU and Bitter-Suermann D: Demonstration of high-affinity binding sites for C3a anaphylatoxin on guinea-pig platelets. Scand J Immunol 8(6):551–5, 1978. [DOI] [PubMed] [Google Scholar]
- 43.Kretzschmar T, Kahl K, Rech K, Bautsch W, Kohl J and Bitter-Suermann D: Characterization of the C5a receptor on guinea pig platelets. Immunobiology 183(5):418–32, 1991. [DOI] [PubMed] [Google Scholar]
- 44.Kim DD, Miwa T, Kimura Y, Schwendener RA, van Lookeren Campagne M and Song WC: Deficiency of decay-accelerating factor and complement receptor 1-related gene/protein y on murine platelets leads to complement-dependent clearance by the macrophage phagocytic receptor CRIg. Blood 112(4):1109–19, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miwa T and Song WC: Membrane complement regulatory proteins: insight from animal studies and relevance to human diseases. Int Immunopharmacol 1(3):445–59, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Nicholson-Weller A, March JP, Rosen CE, Spicer DB and Austen KF: Surface membrane expression by human blood leukocytes and platelets of decay-accelerating factor, a regulatory protein of the complement system. Blood 65(5):1237–44, 1985. [PubMed] [Google Scholar]
- 47.Liszewski MK, Post TW and Atkinson JP: Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol 9:431–55, 1991. [DOI] [PubMed] [Google Scholar]
- 48.Paul MS, Aegerter M, Cepek K, Miller MD and Weis JH: The murine complement receptor gene family. III. The genomic and transcriptional complexity of the Crry and Crry-ps genes. J Immunol 144(5):1988–96, 1990. [PubMed] [Google Scholar]
- 49.Wetsel RA: Expression of the complement C5a anaphylatoxin receptor (C5aR) on non-myeloid cells. Immunol Lett 44(2–3):183–7, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Ikeda K, Nagasawa K, Horiuchi T, Tsuru T, Nishizaka H and Niho Y: C5a induces tissue factor activity on endothelial cells. Thromb Haemost 77(2):394–8, 1997. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.