Abstract
The immunologic and therapeutic effects of intratumoral (IT) delivery of a novel virus-like particle (VLP) as a lymphoma immunotherapy were evaluated in preclinical studies with human cells and a murine model. CMP-001 is a VLP composed of the Qβ bacteriophage capsid protein encapsulating an immunostimulatory CpG-A oligodeoxynucleotide TLR9 agonist. In vitro, CMP-001 induced cytokine production, including IFNα from plasmacytoid DCs, but only in the presence of anti-Qβ antibody. In vivo, IT CMP-001 treatment of murine A20 lymphoma enhanced survival, and reduced growth of both injected and contralateral noninjected tumors in a manner dependent on both the ability of mice to generate anti-Qβ antibody and the presence of T cells. The combination of IT CMP-001 with systemic anti-PD-1 enhanced anti-tumor responses in both injected and noninjected tumors. IT CMP-001 alone or combined with anti-PD-1 augmented T cell infiltration in tumor-draining lymph nodes. We conclude IT CMP-001 induces a robust anti-tumor T cell response in an anti-Qβ antibody-dependent manner and results in systemic anti-tumor T cell effects that are enhanced by anti-PD-1 in a mouse model of B cell lymphoma. Early phase clinical evaluation of CMP-001 and anti-PD1 combination therapy lymphoma will begin shortly based in part on these results.
Keywords: tumor immunity, rodent models of cancer, cancer immunotherapy, in situ immunization, combination immunotherapy, checkpoint blockade, lymphoma
INTRODUCTION
Cancer immunotherapy is creating considerable excitement based in large part on the success of immune checkpoint blockade, such as inhibitors of the PD-1/PD-L1 pathway (1). Despite this excitement, most patients do not respond to PD-1 blockade, especially patients whose tumors lack an interferon (IFN) signature (2). This is leading to evaluation of approaches designed to induce an IFN response such as intratumoral (IT) delivery of agents capable of activating tumor-infiltrating plasmacytoid dendritic cells (pDC), thereby augmenting the tumor-specific T cell response.
Synthetic unmethylated CG-rich oligodeoxynucleotides (CpG ODN) mimic prokaryotic DNA and activate Toll-Like Receptor 9 (TLR9) (3). Structure-activity relationship studies of CpG ODN have defined 3 families with distinct structural and biological characteristics (4–6). CpG-A ODN induce IFNα secretion from pDC, but only weakly stimulate B cells. CpG-B ODN stimulate B cells but induce relatively little IFNα secretion (7). CpG-C ODN are immunologically intermediate between the CpG-A and CpG-B classes (4–6, 8). CpG ODN directly activate innate signaling pathways, and secondarily result in a robust adaptive immune response (9, 10). Several CpG-B and CpG-C TLR9 agonists have been evaluated as cancer immunotherapeutic agents in the laboratory and clinic (11, 12). While TLR9 agonists have been evaluated as immune adjuvants in tumor antigen immunization (13, 14), as systemic therapy alone or in combination with other therapeutics (15–18), and to alter the local tumor microenvironment through direct IT injection (18–22), the effect of IT injection of CpG-A has not been previously reported.
Direct injection of immune stimulatory agents into the tumor (in situ immunization) can be used to activate antigen presenting cells, promote tumor antigen presentation, and stimulate production of a milieu that enhances Th1 cell activation within the tumor microenvironment and draining lymph nodes. Levy and colleagues found that In situ immunization with CpG-B ODN is promising in pre-clinical murine tumor models of lymphoma (19, 23). CD8+ T cells were instrumental in the tumor regression at distant sites. T cell activating antibodies enhanced protection mediated by in situ immunization with TLR9 agonists (24). Preliminary results from a lymphoma clinical trial exploring the combination of local radiation and TLR9 agonist in situ immunization were encouraging as well (21).
The current studies were designed to determine whether a virus-like particle (VLP) containing a CpG-A TLR9 agonist can modulate the tumor micro-environment and induce tumor regression. VLPs are non-infectious, self-assembling, highly immunogenic delivery systems (25, 26). CMP-001, formerly known as CYT003 or QβG10, is a VLP comprised of two components: i) purified recombinant Qβ bacteriophage capsid protein, and ii) synthetic G10, a CpG-A ODN (26). CMP-001 was designed to induce high levels of IFNα and a Th1 response through activation of TLR9 in pDCs. Clinical trials (in normal volunteers or subjects with non-cancer diagnoses) demonstrated that CMP-001 therapy has immune stimulatory effects. However, the drug failed to show efficacy in a phase 2 clinical trial of moderate to severe asthma (27), and development of CMP-001 for treatment of allergy and asthma was abandoned. When a tumor antigen, Melan-A, was conjugated to the surface of CMP-001 (MelQβG10), immunized patients showed strong Th1 anti-tumor T-cell responses, but no significant clinical efficacy (26).
Enhancing tumor-specific immune responses by targeting the PD-1/PD-L1 pathway has proven to be of clinical value in a growing number of cancers (1, 28, 29). Anti-tumor CD8+ T cells induced by CpG-based tumor vaccines express high levels of surface PD-1 (30), providing a strong rationale for exploring the combination of TLR9 activation and anti-PD-1 therapy. The present studies were performed to provide a foundation for the further clinical evaluation of CMP-001 alone and in combination with anti-PD1 as a novel approach to immunotherapy.
MATERIALS & METHODS
VLPs containing a TLR9 agonist (CMP-001).
CpG ODN-containing VLPs were provided by Checkmate Pharmaceuticals (Cambridge, MA), and manufactured using the bacteriophage Qβ nanotechnology platform wherein nanoparticles self-assemble upon mixing purified Qβ coat protein with ODN (26, 31). CMP-001, containing nonmethylated CpG-A ODN (G10; 5’-GGG GGG GGG GGA CGA TCG TCG GGG GGG GGG-3’) or its methylCMP-001 version (mCMP-001) were packaged with Qβ at a 4:1 mass ratio (Qβ:CpG). The resulting VLPs were ~30 nm in diameter. “Stressed” versions of CMP-001 were prepared by one of three methods: 1) repeated freeze/thaw cycles, 2) mechanical force of >40g for one month, or 3) storage at 40°C for one month.
Human serum samples and cell culture.
Serum from human subjects was acquired from either the Holden Comprehensive Cancer Center (University of Iowa) or Biostorage Technologies (Indianapolis, IN), in accordance with the Declaration of Helsinki and after approval by an Institutional Review board (IRB) and subject written informed consent. Mononuclear cells (PBMC) were isolated from peripheral blood of healthy subjects over Histopaque-1077 (Sigma-Aldrich). Red blood cells were removed by red cell lysis buffer and pDCs were magnetically purified using a BDCA-4 isolation kit (Miltenyi Biotec). During in vitro culture, cells were suspended in RPMI 1640 (Thermo Fisher Scientific) supplemented with 10% FBS (GE Healthcare), 2nM L-glutamine (Thermo Fisher Scientific), 100 U/mL Penicillin (Thermo Fisher Scientific) and 100 μg/mL Streptomycin (Thermo Fisher Scientific). pDC media was supplemented with 10 ng/mL of IL-3 (R&D Systems).
In vitro cell stimulation and cytokine measurement.
Human PBMCs were treated with saline, CMP-001 or mCMP-001 (10 μg/mL final) and naïve or immune patient serum (obtained before and after CMP-001 treatment; 1.25–2.5% final concentration) or recombinant anti-Qβ IgG (Cytos Biotechnology; 10 μg/mL final concentration). For FcR-blocking experiments, titrated concentrations of anti-human CD32 or control antibody (R & D Systems) were pre-incubated with cells for 15 minutes prior to the addition of CMP-001 and recombinant anti-Qβ. Murine splenocytes, isolated from dissociated spleens (gentleMACS™ Dissociator, Miltenyi Biotec), were treated with CMP-001 and naïve or anti-Qβ immune serum (obtained from mice previously treated with CMP-001; 2% final concentration). After 2 days, supernatants were harvested and tested for cytokine levels with a VeriKine™ Human IFNα ELISA kit (PBL Assay Science), Life Technologies Human magnetic 25-Plex kit (Thermo Fisher Scientific), Mouse 26-Plex ProcartaPlex® Immunoassay (Thermo Fisher Scientific).
Murine tumor cell growth conditions, authentication and sterility testing.
A20 cells were grown in RPMI-1640 (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (Atlanta Biologicals), 1mM sodium pyruvate (Thermo Fisher Scientific), 10 mM HEPES (Thermo Fisher Scientific), 0.05 mM 2-mercaptoethanol (Sigma-Aldrich), and 50 μg/mL gentamycin sulfate (Mediatech, Inc). A20 cell banks were confirmed to be Mycoplasma-negative and underwent CellCheck authentication and Cell Line Sterility Testing (IDEXX BioResearch) within the year prior to use and went through less than 5 passages before use.
Pre-clinical studies.
Mouse studies were approved by and performed according to guidelines established by the University of Iowa Institutional Animal Care and Use Committee. Inbred 6–8 week old female Balb/c mice were obtained from Jackson Laboratories. Inbred 6–8 week old female Jh−/− on a Balb/c background and control wild-type Balb/c were obtained from Taconic Biosciences. All mice were maintained in filtered cages.
Select mice were injected subcutaneously (SC) one week prior to tumor implantation with CMP-001 in order to initiate the development of anti-Qβ antibodies (“primed”). For SC tumor implantation, mice were anesthetized by intraperitoneal injection of a ketamine/xylazine mix [80–100 mg/kg of ketamine (Phoenix Pharmaceutical, Inc) and 10–13 mg/kg of xylazine (provided by the Office of Animal Resources, University of Iowa)]. Jh−/− and Balb/c mice were implanted SC on one or both flanks with 4×106 A20 tumor cells (ATCC) delivered in saline as reported earlier (17, 19, 23, 24).
After tumor implantation, CMP-001, G10 CpG ODN, or saline was administered IT. Mice bearing tumors on both flanks received IT treatment in only one tumor. In select experiments, 175 μg of either anti-mouse PD-1 (rat IgG2a, clone RMP1–14) or the 2A3 rat IgG2a isotype control (BioXCell) was administered intraperitoneally. To deplete T cells, 200 μg of antibodies against CD4 (rat IgG2b, clone GK1.5), CD8α (rat IgG2b, clone 2.43) or rat IgG2b isotype control (BioXCell) were administered intraperitoneally. To deplete NK cells, 25 μg of antibody against asialo-GM1 (rabbit polyclonal, Thermo Fisher Scientific) was administered intraperitoneally. All depleting antibodies were administered starting 2 days prior to the first IT/IP treatment and continued per treatment schedules explained in figures. T and NK cell depletion were verified in peripheral blood by staining with antibodies against CD3e (Biolegend), CD4 and CD8α (BD Biosciences) or CD335 (Biolegend). Samples were acquired on an LSR Violet Flow Cytometer (BD Biosciences) and analyzed using FlowJo® v10.2 (FlowJo®, LLC).
Tumor size was measured twice weekly by calipers and mice were sacrificed when tumor diameter became greater than 20mm in any dimension. Cytokines were measured by a mouse 7-Plex ProcartaPlex® Immunoassay (Thermo Fisher Scientific) in serum samples collected 24 hours after IT administration of either saline or CMP-001.
Detection of anti-Qβ Ig by ELISA.
96-well Costar ELISA plates (Thermo Fisher Scientific) were coated with CMP-001 at a concentration of 10 μg/mL in PBS. Following coating, the plates were washed with PBS-Tween 20 0.05% (PBS-T), blocked with 5% dry milk in PBS-T, washed again and then incubated with titrated dilutions of murine or human serum. After washing, HRP-conjugated goat anti-mouse Ig (Southern Biotech) or goat anti-human Ig (Southern Biotech) detection antibodies diluted in PBS-T were added. The plate was developed with the addition of TMB substrate (Sigma-Aldrich), followed by 2N H2SO4 stop solution. Color development was read on a plate reader at an absorbance of 450nm. Murine serum samples were obtained from mice that had previously been injected with CMP-001, or from control naïve mice. Human serum samples were obtained from clinical trial patients pre- and post-treatment with CMP-001.
Flow cytometry.
A20 tumors and draining inguinal lymph nodes (DLN) were harvested and dissociated (gentleMACS™ Dissociator, Miltenyi Biotec) to yield single cell suspensions. Blood was lysed with red blood cell lysis buffer to yield peripheral blood leukocytes. Cells were stained with Zombie Aqua™ Fixable Viability dye (Biolegend) and antibodies against the following surface markers: CD3e, CD4, CD8α, CD11b, CD11c, CD19, CD45, CD335, F4/80, Ly6G, Ly6C, and MHC II (Biolegend). To distinguish tumor cells from infiltrating leukocytes, tumor samples were also stained with an antibody against the A20 surface idiotype (produced in our laboratory). Anti-mouse CD16/32 (eBioscience) was included to block Fc receptors. For Foxp3 staining, cells were fixed and permeabilized with the eBioscience Foxp3 Staining Buffer Set (Thermo Fisher Scientific) followed by staining with an antibody against Foxp3 (Biolegend). CountBright Absolute Counting Beads (ThermoFisher) were added to samples for quantification of absolute cell number. A20 cells cultured directly with CMP-001 −/+ immune serum were stained with Zombie Aqua™ and an antibody against PD-L1 (Biolegend). All samples were acquired on an LSR Violet Flow Cytometer (BD Biosciences) and data analyzed using FlowJo® v10.2 (FlowJo®, LLC). For tumor and DLN samples, gating on live CD45+Idiotype- cells, followed by gating on specific immune cell subset surface markers was performed to calculate number or frequency. Positive staining gates were determined with FMO controls.
Microscopy.
Tumor tissues frozen in Tissue-Tek® O.C.T. (Sakura Finetek) were sectioned and stained by IHC with an antibody against murine CD3e. In total, 4 saline injected tumors and 4 CMP-001 injected tumors from 8 individual mice were sectioned and examined using a FLoid™ Imaging Station (Thermo Fisher Scientific). For confocal imaging, pDCs were isolated from human PBMC with the Plasmacytoid Dendritic Cell Isolation Kit II (Miltenyi Biotec) and incubated with A647-labeled CMP-001 alone or with recombinant anti-Qβ for 2hrs. Cells were then stained with a primary antibody against CD303 (BDCA-2; Miltenyi Biotec), followed by a secondary goat anti-mouse (Invitrogen). After staining, cells were fixed and cytospin processed and lastly were mounted with Vectashield® containing DAPI (Vector Laboratories). Imaging was performed on a Zeiss 710 confocal microscope and images were reconstructed using Imaris v9.5 software (Oxford Instruments).
Statistical analysis.
Cytokine data were analyzed by either: 1) unpaired Student’s t-test, 2) one-way ANOVA with Tukey’s multiple comparisons test, or 2) two-way ANOVA with Sidak’s multiple comparisons test. Survival data were analyzed using the Logrank test. Immune cell quantification by flow cytometry data were analyzed by one-way or two-way ANOVA with either Tukey’s or Dunnett’s multiple comparisons test. All analyses were performed using GraphPad Prism version 7.00.
RESULTS
CMP-001 induces secretion of IFNα by pDCs and IFN-inducible chemokines by PBMCs in an anti-Qβ antibody dependent manner.
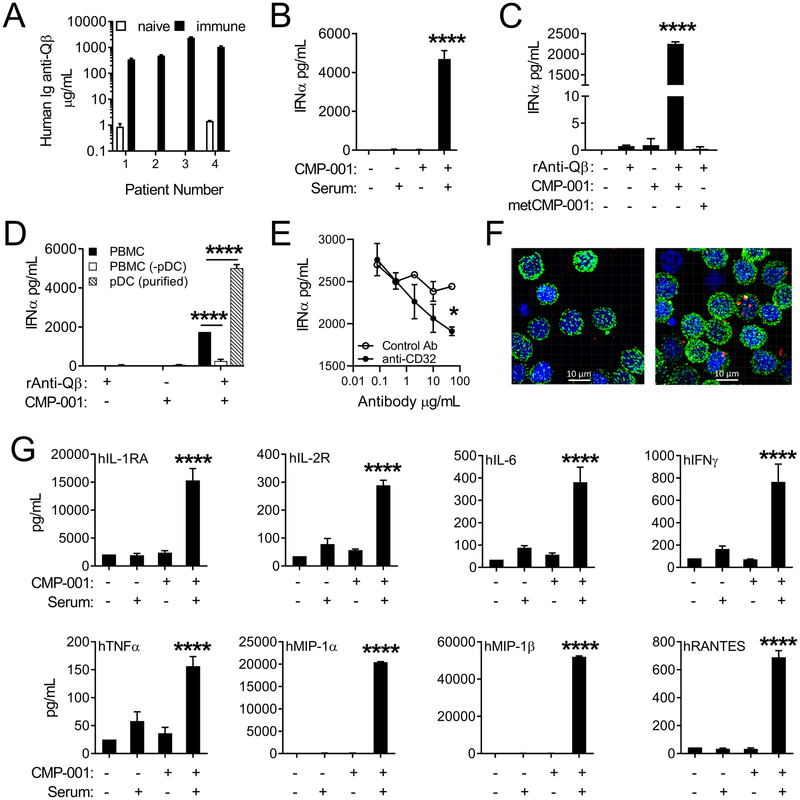
To assess the effect of CMP-001 on the secretion of IFNα and other cytokines, human PBMCs were co-cultured with CMP-001 in the presence or absence or Qβ-immune serum. This serum, which contained high titers of anti-Qβ (Fig. 1A), was obtained from subjects with advanced melanoma being treated with CMP-001 in a phase 1b clinical trial. CMP-001 alone failed to induce production of IFNα, however significant levels of IFNα were produced when the culture included Qβ-immune serum or recombinant anti-Qβ (Figure 1B–C). Incubation of PBMCs with CMP-001 plus pre-immune serum from the same donors (obtained prior to their CMP-001 therapy) had little effect on IFNα production. CpG-methylated control metCMP-001 also had little effect, even in the presence of anti-Qβ (Figure 1C). Depletion of pDCs from the PBMCs significantly reduced the ability of CMP-001 and anti-Qβ to induce IFNα production, while stimulation of enriched pDCs with CMP-001 and anti-Qβ resulted in significant IFNα production (Figure 1D). FcR-blocking antibody (anti-CD32) significantly reduced production of IFNα by PBMCs stimulated with CMP-001 plus recombinant anti-Qβ (Figure 1E). Confocal microscopy revealed that fluorescently tagged CMP-001 is internalized by pDCs only when anti-Qβ is present (Fig. 1F). Additional cytokines/chemokines were also produced by human PBMCs co-cultured with CMP-001 and immune serum, but not with either alone (Figure 1G). These results demonstrate that anti-Qβ opsonization of CMP-001 VLPs is required in order to induce production of pro-inflammatory cytokines/chemokines from immune cells in vitro. This effect is mediated largely by pDCs in human PBMCs.
Figure 1. CMP-001 VLP-induced cytokine production from human PBMCs is dependent on anti-Qβ and pDCs.
A, Ig anti-Qβ levels detected in clinical trial human patient serum before (naïve) and after (immune) treatment with CMP-001 (data is representative of 7 replicate experiments; n=20 patients measured in total). IFNα levels produced by: (B) human PBMCs cultured with or without CMP-001 and immune serum, (C) human PBMCs cultured with or without CMP-001 or methylated CMP-001 (metCMP-001) and recombinant anti-Qβ, (D) human PBMCs, pDC-depleted PBMCs or purified pDCs cultured with or without CMP-001, recombinant anti-Qβ, and immune serum, or (E) human PBMCs cultured with anti-CD32 or control antibody prior to CMP-001 and anti-Qβ (data is representative of 2 replicate experiments; n=2–3 replicates per experimental group). F, Confocal microscopy images from purified pDCs incubated with fluorescently labeled CMP-001 −/+ anti-Qβ (green=BDCA-2, blue=DAPI, red=CMP-001; data is representative of 3 replicate experiments; n=2–3 replicates per experimental group). G, Cytokine levels produced by human PBMCs cultured with or without CMP-001 and immune serum (data is representative of 3 replicate experiments; n=2–3 replicates per experimental group). Data were analyzed using either a one-way ANOVA with Tukey’s multiple comparisons test (C-D and G) or a two-way ANOVA with Sidak’s multiple comparisons test (E-F); *P<0.05; **P<0.01; ****P<0.0001.
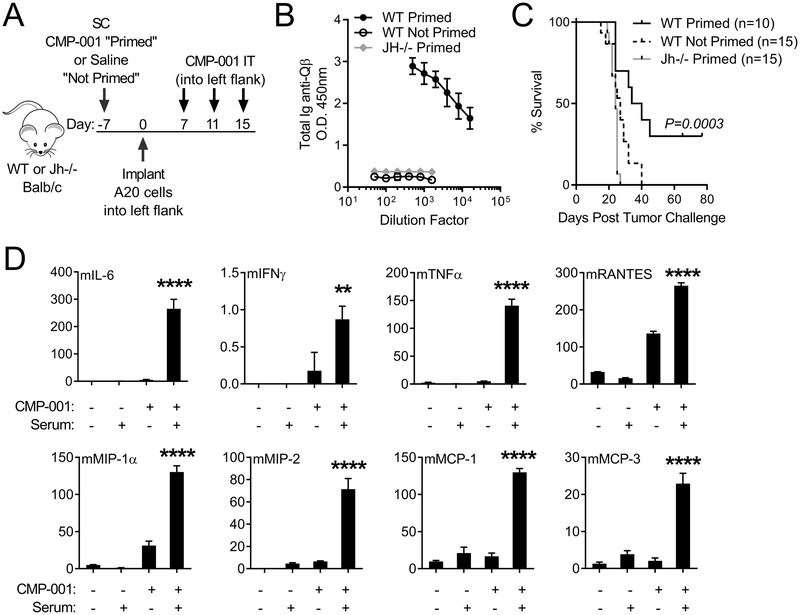
In vivo evaluation in a murine model revealed that wild-type mice “primed” with a single subcutaneous dose of CMP-001 developed an anti-Qβ antibody response, whereas mice that were not primed had no detectable anti-Qβ (Figure 2A–B), a finding which is consistent with the human results. “Primed” mice developed tumor at the same rate as “not primed” mice, however in situ immunization of A20 lymphoma with CMP-001 resulted in a therapeutic response and improved survival in “primed” mice only (Figure 2C). Jh−/− mice, which are B cell deficient, failed to develop an anti-Qβ response after priming with CMP-001 (Figure 2A–B) and in situ immunization of A20 tumors with CMP-001 had no detectable anti-tumor effect in these mice (Figure 2C). Furthermore, both CMP-001 and anti-Qβ immune serum were required to induce cytokine/chemokine production from murine splenocytes (Figure 2D). Thus, in vitro studies in both human and murine systems, and in vivo experiments in a murine tumor model indicate CMP-001 requires anti-Qβ antibody to stimulate immune cells and to effectively induce a therapeutic anti-tumor response. Given these findings, mice were “primed” with CMP-001 to induce an anti-Qβ immune response prior to tumor inoculation for all subsequent experiments.
Figure 2. Anti-Qβ must be present for CMP-001 to induce anti-tumor responses in mice and cytokine production from splenocytes.
A, Treatment schema of WT or Jh−/− Balb/c mice injected SC with CMP-001 (Primed; WT and Jh−/−) or saline (Not Primed; WT) prior to A20 tumor implantation into one flank, collection of serum and subsequent IT CMP-001 treatment. B, Ig anti-Qβ titers in mouse serum detected 10 days after SC administration of CMP-001 (Primed; WT and Jh−/−) or saline (Not Primed; WT); data is representative of 2 replicate experiments; n=5 mice per group. C, Kaplan-Meier survival curves of IT CMP-001 treated WT and Jh−/− Primed and WT Not Primed mice (data is from 2 replicate experiments; n=10–15 mice per group). D, Cytokine levels produced by murine splenocytes cultured with or without CMP-001 and immune serum (data is from one experiment; n=3 replicates per experimental group). Survival data were analyzed using the Logrank test and cytokine data were analyzed using a one-way ANOVA with Tukey’s multiple comparisons test.
CMP-001 is highly stable.
Phosphodiester backbone ODN are rapidly cleaved by nucleases, which has limited their clinical potential compared to nuclease-resistant phosphorothioate backbone ODN (32–34). Packaging of CpG ODN into VLP reduces susceptibility to DNase I digestion outside of cells (31), while maintaining the native DNA backbone required for DNase II cleavage inside the pDC for the type I IFN response (35). Studies were done to assess the functional stability of CMP-001 over time under conditions that would be considered harsh for an immunotherapeutic agent including mechanical stress (centrifugation at >40 x g) for 4 weeks, repeated freeze thaw cycles, and incubation at 40° C for one month. Stressed and unstressed CMP-001, combined with anti-Qβ ab, induced similar levels of IFNα production by normal donor PBMCs (Supplemental Fig. 1), demonstrating CMP-001 is highly stable.
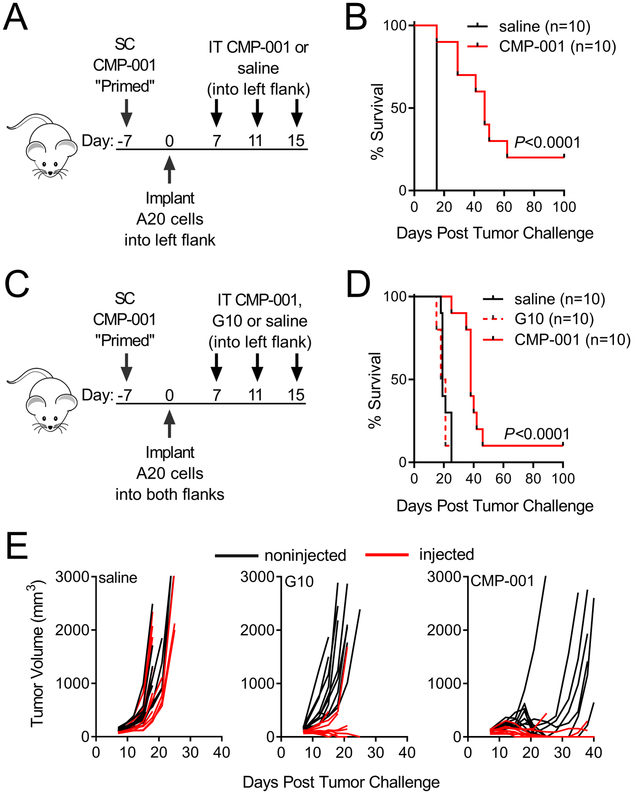
Intratumoral CMP-001 treatment of A20 murine lymphoma results in regression of both injected and noninjected tumors.
IT injection with CMP-001 of mice implanted with one A20 tumor reduced tumor growth and enhanced survival, compared to IT injection of saline (Figure 3A–B). The effect of IT CMP-001 therapy on both injected and noninjected tumors was evaluated by implanting mice with two tumors (one in each flank), but only giving therapeutic IT injection in one tumor (Figure 3C). CMP-001 treatment improved survival significantly in this bilateral model (Figure 3D) although long-term survival was less pronounced than in mice with one tumor. Significantly elevated levels of pro-inflammatory cytokines were detected in the serum from mice treated with IT CMP-001 (Supplemental Fig. 2).
Figure 3. Intratumoral CMP-001 therapy alone extends survival and slows local and distant tumor growth better than soluble G10 CpG ODN.
A-B, Treatment schema and Kaplan-Meier curves of Balb/c mice primed and then implanted on one flank with A20 B lymphoma tumor cells, followed by IT CMP-001 or saline (data is from one experiment; n=10 mice per group). C-D, Treatment schema and Kaplan-Meier curves of Balb/c mice primed and then implanted on both flanks with A20 B lymphoma cells, followed by unilateral IT CMP-001, soluble G10 CpG ODN or saline. E, Tumor volumes (red=injected and black=noninjected) of individual mice after bilateral tumor implantation and treatment with unilateral IT CMP-001, soluble G10 CpG ODN or saline (data is representative of 2 replicate experiments; n=10 mice per group). Survival data were analyzed using the Logrank test.
In pilot studies, comparing IT G10 CpG ODN therapy (the CpG-A component of CMP-001) at doses of 100 μg or 300 μg revealed they were not significantly different in their efficacy (Supplemental Fig. 3A–B). CMP-001 was thus compared to the larger dose of G10 CpG ODN to account for the possibility of its more rapid degradation in vivo. As illustrated in Figure 3D, overall survival of mice treated with 100 μg of CMP-001 was superior to that of mice treated with 300 μg of soluble G10 CpG ODN. The improved response to CMP-001 was particularly notable with respect to its ability to slow the growth of noninjected tumors (Figure 3E).
Anti-PD1 enhances the anti-tumor effect of CMP-001 via a T cell dependent mechanism.
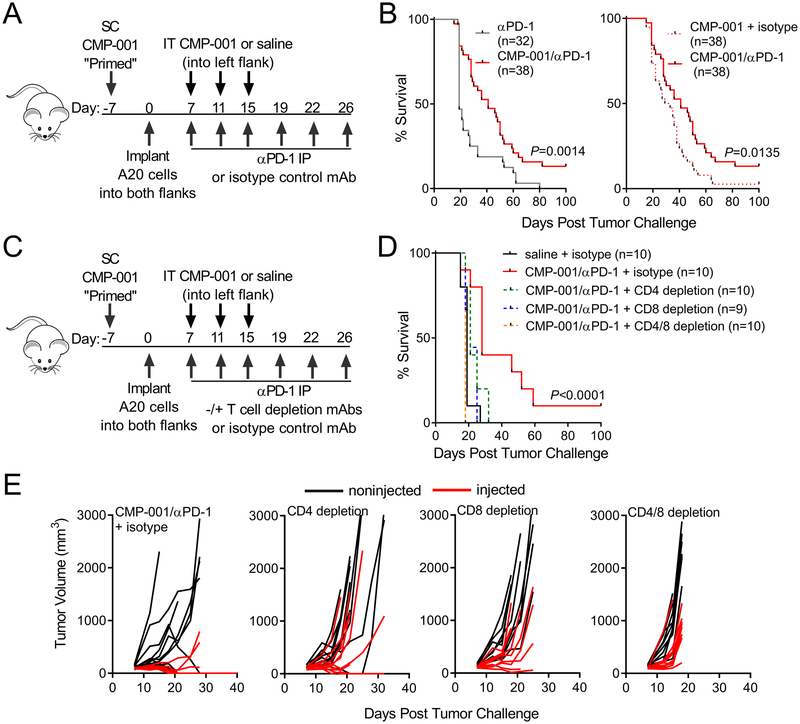
Pilot studies confirmed published data that A20 tumor cells constitutively express PD-L1 (36). In vitro culture of A20 cells with CMP-001 combined with immune serum had no direct impact on A20 viability and only modest impact on PD-L1 surface expression levels (Supplemental Fig. 4A–B). Given the potential inhibitory impact of PD-L1 on T cell anti-tumor responses, systemic anti-PD-1 antibody was added to IT CMP-001 treatment. Results from four replicate mouse experiments demonstrated that the combination of systemic anti-PD-1 and IT CMP-001 significantly enhanced survival compared to either therapy alone (Figure 4A–B).
Figure 4. Checkpoint blockade combined with CMP-001 enhances therapy via T cell dependent anti-tumor responses.
A-B,Treatment schema and Kaplan-Meier curves of Balb/c mice primed and then implanted on both flanks with A20 B lymphoma cells, followed by unilateral IT CMP-001 or saline and IP anti-PD-1 mAb or isotype control (data is from 4 replicate experiments; n=32–38 mice per group). C-D, Treatment schema and Kaplan-Meier curves of Balb/c mice primed and then implanted on both flanks with A20 B lymphoma cells, followed by unilateral IT CMP-001 and IP anti-PD-1 mAb, with and without T cell depletion (+ depleting antibody or isotype control). All depleting or isotype control antibodies were administered starting 2 days prior to the first IT/IP treatment and continued per treatment schedule. E, Tumor volumes (red=injected and black=noninjected) of individual mice after bilateral tumor implantation and combination treatment with CD4, CD8 or CD4 and CD8 T cell depleting antibodies (D-E: data is representative of 2 replicate experiments; n=9–10 mice per group). Survival data were analyzed using the Logrank test.
T cells and NK cells have both been found to be instrumental in mediating the anti-tumor effects induced by TLR9 agonists used in a variety of routes and strategies (17, 19, 37, 38). The impact of depleting these cells was therefore evaluated. Depletion was carried out and confirmed by flow cytometric analysis of peripheral blood leukocytes (Supplemental Fig. 5A–D). While NK cell depletion had no significant impact on survival (Supplemental Fig. 5E), overall survival was reduced and growth of both injected and noninjected tumors enhanced by depletion of CD4+ or CD8+ T cells (Figure 4D–E), demonstrating T cells play a central role in both the local and the systemic response to combination therapy. Furthermore, mice that remained tumor-free after combination therapy were fully protected upon re-challenge with A20 cells, suggesting that a memory immune response had been induced.
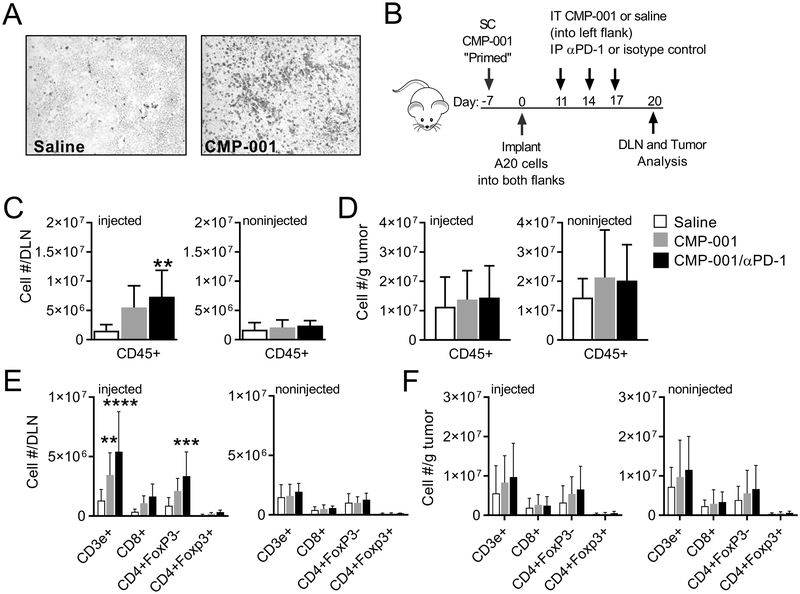
CMP-001 enhances T cell and dendritic cell infiltration into injected A20 tumors and tumor-associated draining lymph nodes.
Initial IHC studies revealed that CD3e+ cells were increased within tumor sections 9 days after IT administration of CMP-001 compared to saline treated tumors (Figure 5A). Given the subjective and non-quantitative nature of IHC field analysis, flow cytometric analysis was used in subsequent studies to provide for a more quantitative assessment of tumor infiltrating lymphocytes (TILs) within injected and noninjected tumors and draining inguinal lymph nodes (DLN). To examine therapy-induced immune cell infiltration, tumors and tumor-associated DLN were harvested 9 days following the first of three administrations of IT saline, CMP-001 or CMP-001 and systemic anti-PD-1 (Figure 5B). Total immune cell infiltrate, as shown by staining for the pan leukocyte marker CD45, was increased in the injected tumor-associated DLN from mice treated with CMP-001 alone or in combination with anti-PD-1, but not in the noninjected tumor-associated DLN or in either tumor (Fig. 5C–D). This observation suggested a local immune response was initiated within the DLN after IT delivery of CMP-001. Immune cell infiltrate in the injected tumor-associated DLN included increased numbers of CD3e+ and CD4+FoxP3- T cells, with CD8+ T cells trending upward (Fig. 5E, injected). Increased numbers of these cells were not observed in the noninjected tumor-associated DLN (Fig. 5E). In contrast, we observed a trend of increasing numbers of CD3e+ and CD4+FoxP3− T cells in both injected and noninjected tumors after combination therapy (Fig. 5F). After treatment the number of mDCs (CD11c+MHCII+) was enhanced in the tumor-associated DLN and trended upwards in both injected and noninjected tumors (Supplemental Fig. 6A–B). Numbers of macrophages (CD11b+F4/80+), monocytic MDSCs (CD11b+Ly6ChiLy6G−) and granulocytic MDSCs (CD11b+Ly6C−Ly6G+) were not significantly altered by treatment in either the DLNs or tumors
Figure 5. IT treatment with CMP-001 enhances T cell and dendritic cell infiltration into injected tumor-associated draining lymph nodes and A20 tumors.
A, Representative immunohistochemistry images from injected tumor sections stained for CD3 (data is from one experiment; n=4 tumors per group). B, Treatment schema of Balb/c mice primed and then implanted on both flanks with A20 B lymphoma cells, followed by unilateral IT saline or CMP-001 and IP anti-PD-1 or isotype control. Both tumors (noninjected and injected) and their corresponding draining inguinal lymph node were harvested 9 days after the first IT treatment and analyzed by flow cytometry. The number of CD45+ cells (C-D) and T cells (E-F) present per draining lymph node or per gram of A20 tumor (noninjected or injected; data is from 2 replicate experiments; n=5–8 tumors or 5–12 draining lymph nodes per group). Data were analyzed by one-way (C-D) or two-way (E-F) ANOVA with Dunnett’s multiple comparisons test; *P<0.05, **P<0.01; ****P<0.0001.
DISCUSSION
The preclinical studies described above were designed to assess the immunologic and therapeutic effects as a cancer immunotherapy of IT delivery of a VLP designated CMP-001 that is composed of the Qβ bacteriophage capsid protein encapsulating a TLR9 agonist. CMP-001 induced cytokine production, including IFNα from plasmacytoid DCs in vitro, but only in the presence of anti-Qβ antibody. The in vivo immunologic and therapeutic response to CMP-001 was also dependent on anti-Qβ antibody. The combination of IT CMP-001 with systemic anti-PD-1 enhanced anti-tumor responses in both injected and noninjected tumors.
Multiple aspects of the immune response impact on the success or failure of cancer immunotherapy. Optimal anti-tumor T-cell responses require the presentation of tumor-associated antigens by activated DCs expressing costimulatory molecules. This is followed by the activation, proliferation and maintenance of tumor-specific T cells, which is strongly supported by high levels of type I IFN. The location of these immunologic responses (in the tumor, the draining lymph node, distant sites of disease or systemically) can impact on both the success and the toxicity of therapy. Selection of the cancer to be targeted, the agents to combine based on our understanding of cancer immunology and decisions related to the timing, location and dose can all impact on success and toxicity. While the ultimate determination of the success of a given regimen requires clinical evaluation, carefully designed preclinical in vitro and animal model studies can help illustrate the promise of new approaches and combinations, contribute to the design of such studies, and be useful in determining what correlative science should be included to help make clinical trials more informative. The studies described here represent one example of preclinical evaluation of a promising approach that was designed to help translation to the clinic.
CpG ODN were described and evaluated as anti-tumor immunotherapeutic agents in both the laboratory and the clinic in the late 1990s even before their receptor (TLR9) was identified (39, 40). Soon after, various classes of TLR9 agonists were defined (7, 41). While CpG-A TLR9 agonists are potent inducers of IFNα production, DC activation, and indirectly, NK cells (42, 43), they have an unmodified phosphodiester backbone that makes them susceptible to rapid degradation in vivo (32, 33). Thus, the majority of clinical trials of TLR9 agonists to date have been performed with nuclease-resistant, phosphorothioate-modified CpG-B or CpG-C TLR9 agonists (44).
Initial clinical trials that involved systemic administration of CpG-B TLR9 agonists as a monotherapy demonstrated evidence of clinical activity in melanoma, non-Hodgkin lymphoma, cutaneous T-cell lymphoma, renal and basal cell carcinoma (44). However, the number and duration of these responses were limited. Based on positive preclinical data, further development focused on various combination approaches, including chemotherapy, anti-tumor antibody, and cancer vaccines. Unfortunately, larger clinical trials exploring these approaches were negative (45, 46). Studies employing TLR9 agonists as immune adjuvants in cancer vaccines comprised of various tumor-associated antigens showed strong clinical induction of anti-tumor CD4+ and CD8+ T cells but again, few objective responses were seen, and the T cell responses were not sustained, especially within tumors (47).
Human T cells activated by TLR9 agonists express high levels of PD-1. Anti-PD-1 antibody restored T cell function to CD8+ T cells obtained from melanoma patients treated with a TLR9 agonist (30). In addition, tumors that have an IFN gene expression signature and T cells within the tumor, respond better to anti-PD-1 therapy than tumors that lack T cells (29). Combining CMP-001 with anti-PD-1 is therefore a rational approach to induce and maintain anti-tumor T cell responses.
Levy and colleagues explored in situ immunization using TLR9 agonists with encouraging early clinical results (20, 21). The current studies are built on the promise of in situ immunization with TLR9 agonists with two important modifications: 1) CpG-A ODN TLR9 agonist (G10) was used because of its more potent stimulatory effect on pDCs and greater induction of IFNα than other CpG ODN families (7) and 2) A VLP containing the TLR9 agonist was used instead of soluble TLR9 agonists.
One potential limitation to the in situ delivery of soluble TLR9 agonists is their rapid degradation and diffusion out of the tumor (32). Biodegradable nano- and microparticle delivery systems can be used to control the temporal and spatial release of a variety of therapeutic agents, including TLR9 agonists (48). We evaluated such particles in a variety of preclinical murine tumor models and found they can result in anti-tumor responses and safe delivery of chemotherapeutic and immunomodulatory agents (17, 49). These studies demonstrate the potential of combining in situ delivery via particles with systemic therapy to target multiple steps in the immune response, including: 1) inducing tumor antigen uptake and presentation, 2) enhancing T cell activation, and 3) sustaining the T cell response (49).
Use of a VLP (CMP-001) offers a number of theoretical advantages illustrated by the results described here. CMP-001 is a highly stable molecule which increases its practical utility (31). This is particularly important for a VLP containing a CpG-A TLR9 agonist which is vulnerable to nucleases (32). Due to its size compared to a soluble TLR9 agonist, CMP-001 would be expected to have a prolonged residence time within the injected site, and to be taken up by lymphatic vessels leading to increased target cell exposure alongside tumor antigens within tumor draining lymph nodes - an aspect of its mechanism of immune activation that we are studying further. Compared to other forms of TLR9 agonist- containing nano- and microparticles studied by us and others, CMP-001 contains the highest concentration of the TLR9 agonist, with CMP-001 mass being 25% TLR9 agonist. This is several-fold higher than the fraction of TLR9 agonist contained in other particle formulations such as PLGA particles.
Initial in vitro studies with CMP-001 demonstrated little immunostimulatory effect. However, CMP-001 induced very high levels of IFNα when they were opsonized by anti-Qβ antibody. In vitro, anti-Qβ antibody had a significant impact on uptake of CMP-001 by pDCs. Furthermore, blocking FcR and depleting pDCs significantly reduced the ability of CMP-001 and anti-Qβ to induce IFNα production. In vivo confirmation of this finding through use of anti-Fc antibodies or FcRγ−/− mice was not helpful because these manipulations alter the immune response in multiple ways. Nevertheless, together these data provide strong evidence that opsonization of CMP-001 by anti-Qβ results in uptake and production of IFNα by pDCs, which are key steps in the immunostimulatory effects of CMP-001. The CMP-001 itself is highly immunogenic in both mice and humans, thus the first dose of CMP-001 therapy does little to stimulate anti-tumor immunity but does induce a robust anti-Qβ antibody response. The second and subsequent doses of CMP-001 are opsonized by anti-Qβ antibody which allows for uptake by pDCs, induction of IFNα, and successful induction of an anti-tumor response following in situ injection into a tumor. Some studies indicate that the presence or development of anti-viral antibodies is detrimental to therapeutic responses induced by virus-based cancer vaccine systems (50). In contrast, our studies show that the development of antibodies that bind CMP-001 VLPs appears to be essential. This distinction is not surprising given that many virus-based cancer vaccines require viral update by tumor cells which is blocked by antibody, while CMP-001 requires uptake by APCs which is enhanced by antibody. This is consistent with reports that recognition of highly repetitive structures on the surface of VLPs by antibody mediates opsonization and subsequent phagocytosis by APCs (51).
Given the rapid growth of A20 murine tumors and a desire to study the biology of CMP-001 therapy in this model, we provided the “priming” dose of CMP-001 to induce an anti-Qβ immune response prior to tumor inoculation. Most human tumors grow at a slower rate than the murine A20 tumor model providing a window for induction of an anti-Qβ response in patients with the first dose of CMP-001 serving to induce anti-Qβ antibody production. A phase I trial of in situ immunization with CMP-001 combined with systemic pembrolizumab (anti-PD-1) for treatment of stage IV skin melanoma has recently been initiated (Clinical trial identifier ) influenced in part on these results.
Treatment regimens for patients with B cell lymphoma generally include anti-CD20 mAb. These patients have suppressed B cell compartments and a limited ability to generate a primary antibody response to new antigens due to both the underlying disease and the anti-CD20 mAb therapy. We have demonstrated that generation of anti-Qβ antibody is necessary for CMP-001 to have a therapeutic effect. This will impact on patient selection for clinical evaluation of CMP-001 in lymphoma, as patients currently receiving anti-CD20 mAb are unlikely to be able to generate an anti-Qβ antibody response. B cell recovery typically begins 6 months after anti-CD20 mAb is discontinued (52), with humoral responses to influenza vaccine being limited within this timeframe (53). Since low levels of anti-Qβ are sufficient for CMP-001 to be opsonized and activate pDCs, we predict most B cell lymphoma patients who are 6 months out from anti-CD20 mAb will produce enough anti-Qβ antibody following the first dose of CMP-001 to allow for a therapeutic response. Based on the preclinical data presented here, a clinical study in lymphoma subjects who have relapsed or refractory disease and are at least 6 months out from their last anti-CD20 mAb therapy has recently been opened.
Supplementary Material
Key Points.
Immunostimulation by CMP-001 requires anti-Qβ antibody
In mice, IT CMP-001 induces lymphoma regression that is enhanced by anti-PD-1
Acknowledgements
Data presented herein were obtained:
With the assistance of the Comparative Pathology Core, which is a research core in the Pathology Department at the University of Iowa.
At the Flow Cytometry Facility, which is a Carver College of Medicine / Holden Comprehensive Cancer Center core research facility at the University of Iowa.1
In the University of Iowa Central Microscopy Research Facilities.2
Disclosures
Research was supported in part by funding from Checkmate Pharmaceuticals.
Aaron Morris and Arthur M. Krieg are employed by Checkmate Pharmaceuticals.
Grant Support:
Research reported in this publication was supported by P50 CA97274, P30 CA86862, and the Leukemia and Lymphoma Society TRP 6522–17
Footnotes
The facility is funded through user fees and the generous financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veteran’s Administration Medical Center.
This instrumentation was purchased with funding from the NIH SIG grant 1 S10 RR025439–01.
REFERENCES
- 1.Dyck L, and Mills KH. 2017. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol. [DOI] [PubMed] [Google Scholar]
- 2.Spranger S, Luke JJ, Bao R, Zha Y, Hernandez KM, Li Y, Gajewski AP, Andrade J, and Gajewski TF. 2016. Density of immunogenic antigens does not explain the presence or absence of the T-cell-inflamed tumor microenvironment in melanoma. Proc Natl Acad Sci U S A 113: E7759–E7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gosu V, Basith S, Kwon OP, and Choi S. 2012. Therapeutic applications of nucleic acids and their analogues in Toll-like receptor signaling. Molecules 17: 13503–13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartmann G, Battiany J, Poeck H, Wagner M, Kerkmann M, Lubenow N, Rothenfusser S, and Endres S. 2003. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-alpha induction in plasmacytoid dendritic cells. Eur J Immunol 33: 1633–1641. [DOI] [PubMed] [Google Scholar]
- 5.Marshall JD, Fearon K, Abbate C, Subramanian S, Yee P, Gregorio J, Coffman RL, and Van Nest G. 2003. Identification of a novel CpG DNA class and motif that optimally stimulate B cell and plasmacytoid dendritic cell functions. J Leukoc Biol 73: 781–792. [DOI] [PubMed] [Google Scholar]
- 6.Vollmer J, Weeratna R, Payette P, Jurk M, Schetter C, Laucht M, Wader T, Tluk S, Liu M, Davis HL, and Krieg AM. 2004. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur J Immunol 34: 251–262. [DOI] [PubMed] [Google Scholar]
- 7.Krieg AM 2002. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 20: 709–760. [DOI] [PubMed] [Google Scholar]
- 8.Marshall JD, Fearon KL, Higgins D, Hessel EM, Kanzler H, Abbate C, Yee P, Gregorio J, Cruz TD, Lizcano JO, Zolotorev A, McClure HM, Brasky KM, Murthy KK, Coffman RL, and Nest GV. 2005. Superior activity of the type C class of ISS in vitro and in vivo across multiple species. DNA Cell Biol 24: 63–72. [DOI] [PubMed] [Google Scholar]
- 9.Sun S, Zhang X, Tough D, and Sprent J. 2000. Multiple effects of immunostimulatory DNA on T cells and the role of type I interferons. Springer Semin Immunopathol 22: 77–84. [DOI] [PubMed] [Google Scholar]
- 10.McCluskie MJ, and Krieg AM. 2006. Enhancement of infectious disease vaccines through TLR9-dependent recognition of CpG DNA. Curr Top Microbiol Immunol 311: 155–178. [DOI] [PubMed] [Google Scholar]
- 11.Scheiermann J, and Klinman DM. 2014. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine 32: 6377–6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melisi D, Frizziero M, Tamburrino A, Zanotto M, Carbone C, Piro G, and Tortora G. 2014. Toll-Like Receptor 9 Agonists for Cancer Therapy. Biomedicines 2: 211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumgaertner P, Costa Nunes C, Cachot A, Maby-El Hajjami H, Cagnon L, Braun M, Derre L, Rivals JP, Rimoldi D, Gnjatic S, Abed Maillard S, Marcos Mondejar P, Protti MP, Romano E, Michielin O, Romero P, Speiser DE, and Jandus C. 2016. Vaccination of stage III/IV melanoma patients with long NY-ESO-1 peptide and CpG-B elicits robust CD8+ and CD4+ T-cell responses with multiple specificities including a novel DR7-restricted epitope. Oncoimmunology 5: e1216290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Legat A, Maby-El Hajjami H, Baumgaertner P, Cagnon L, Abed Maillard S, Geldhof C, Iancu EM, Lebon L, Guillaume P, Dojcinovic D, Michielin O, Romano E, Berthod G, Rimoldi D, Triebel F, Luescher I, Rufer N, and Speiser DE. 2016. Vaccination with LAG-3Ig (IMP321) and Peptides Induces Specific CD4 and CD8 T-Cell Responses in Metastatic Melanoma Patients--Report of a Phase I/IIa Clinical Trial. Clin Cancer Res 22: 1330–1340. [DOI] [PubMed] [Google Scholar]
- 15.Kim YH, Girardi M, Duvic M, Kuzel T, Link BK, Pinter-Brown L, and Rook AH. 2010. Phase I trial of a Toll-like receptor 9 agonist, PF-3512676 (CPG 7909), in patients with treatment-refractory, cutaneous T-cell lymphoma. J Am Acad Dermatol 63: 975–983. [DOI] [PubMed] [Google Scholar]
- 16.Witzig TE, Wiseman GA, Maurer MJ, Habermann TM, Micallef IN, Nowakowski GS, Ansell SM, Colgan JP, Inwards DJ, Porrata LF, Link BK, Zent CS, Johnston PB, Shanafelt TD, Allmer C, Asmann YW, Gupta M, Ballas ZK, Smith BJ, and Weiner GJ. 2013. A phase I trial of immunostimulatory CpG 7909 oligodeoxynucleotide and 90 yttrium ibritumomab tiuxetan radioimmunotherapy for relapsed B-cell non-Hodgkin lymphoma. Am J Hematol 88: 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makkouk A, Joshi VB, Wongrakpanich A, Lemke CD, Gross BP, Salem AK, and Weiner GJ. 2015. Biodegradable microparticles loaded with doxorubicin and CpG ODN for in situ immunization against cancer. AAPS J 17: 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sagiv-Barfi I, Kohrt HE, Burckhardt L, Czerwinski DK, and Levy R. 2015. Ibrutinib enhances the antitumor immune response induced by intratumoral injection of a TLR9 ligand in mouse lymphoma. Blood 125: 2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varghese B, Widman A, Do J, Taidi B, Czerwinski DK, Timmerman J, Levy S, and Levy R. 2009. Generation of CD8+ T cell-mediated immunity against idiotype-negative lymphoma escapees. Blood 114: 4477–4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe RT, Knox SJ, Shin LK, Wapnir I, Tibshirani RJ, and Levy R. 2010. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol 28: 4324–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YH, Gratzinger D, Harrison C, Brody JD, Czerwinski DK, Ai WZ, Morales A, Abdulla F, Xing L, Navi D, Tibshirani RJ, Advani RH, Lingala B, Shah S, Hoppe RT, and Levy R. 2012. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood 119: 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Campos J, Gallotta M, Gong M, Crain C, Naik E, Coffman RL, and Guiducci C. 2016. Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells. Proc Natl Acad Sci U S A 113: E7240–E7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Song W, Czerwinski DK, Varghese B, Uematsu S, Akira S, Krieg AM, and Levy R. 2007. Lymphoma immunotherapy with CpG oligodeoxynucleotides requires TLR9 either in the host or in the tumor itself. J Immunol 179: 2493–2500. [DOI] [PubMed] [Google Scholar]
- 24.Houot R, and Levy R. 2009. T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood 113: 3546–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozlovska TM, Cielens I, Vasiljeva I, Strelnikova A, Kazaks A, Dislers A, Dreilina D, Ose V, Gusars I, and Pumpens P. 1996. RNA phage Q beta coat protein as a carrier for foreign epitopes. Intervirology 39: 9–15. [DOI] [PubMed] [Google Scholar]
- 26.Braun M, Jandus C, Maurer P, Hammann-Haenni A, Schwarz K, Bachmann MF, Speiser DE, and Romero P. 2012. Virus-like particles induce robust human T-helper cell responses. Eur J Immunol 42: 330–340. [DOI] [PubMed] [Google Scholar]
- 27.Casale TB, Cole J, Beck E, Vogelmeier CF, Willers J, Lassen C, Hammann-Haenni A, Trokan L, Saudan P, and Wechsler ME. 2015. CYT003, a TLR9 agonist, in persistent allergic asthma - a randomized placebo-controlled Phase 2b study. Allergy 70: 1160–1168. [DOI] [PubMed] [Google Scholar]
- 28.Lonberg N, and Korman AJ. 2017. Masterful Antibodies: Checkpoint Blockade. Cancer Immunol Res 5: 275–281. [DOI] [PubMed] [Google Scholar]
- 29.Granier C, De Guillebon E, Blanc C, Roussel H, Badoual C, Colin E, Saldmann A, Gey A, Oudard S, and Tartour E. 2017. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open 2: e000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fourcade J, Sun Z, Pagliano O, Chauvin JM, Sander C, Janjic B, Tarhini AA, Tawbi HA, Kirkwood JM, Moschos S, Wang H, Guillaume P, Luescher IF, Krieg A, Anderson AC, Kuchroo VK, and Zarour HM. 2014. PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8(+) T cells induced by melanoma vaccines. Cancer Res 74: 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storni T, Ruedl C, Schwarz K, Schwendener RA, Renner WA, and Bachmann MF. 2004. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J Immunol 172: 1777–1785. [DOI] [PubMed] [Google Scholar]
- 32.Sands H, Gorey-Feret LJ, Cocuzza AJ, Hobbs FW, Chidester D, and Trainor GL. 1994. Biodistribution and metabolism of internally 3H-labeled oligonucleotides. I. Comparison of a phosphodiester and a phosphorothioate. Mol Pharmacol 45: 932–943. [PubMed] [Google Scholar]
- 33.Mutwiri GK, Nichani AK, Babiuk S, and Babiuk LA. 2004. Strategies for enhancing the immunostimulatory effects of CpG oligodeoxynucleotides. J Control Release 97: 1–17. [DOI] [PubMed] [Google Scholar]
- 34.Senti G, Johansen P, Haug S, Bull C, Gottschaller C, Muller P, Pfister T, Maurer P, Bachmann MF, Graf N, and Kundig TM. 2009. Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: a phase I/IIa clinical trial. Clin Exp Allergy 39: 562–570. [DOI] [PubMed] [Google Scholar]
- 35.Chan MP, Onji M, Fukui R, Kawane K, Shibata T, Saitoh S, Ohto U, Shimizu T, Barber GN, and Miyake K. 2015. DNase II-dependent DNA digestion is required for DNA sensing by TLR9. Nat Commun 6: 5853. [DOI] [PubMed] [Google Scholar]
- 36.Tang H, Liang Y, Anders RA, Taube JM, Qiu X, Mulgaonkar A, Liu X, Harrington SM, Guo J, Xin Y, Xiong Y, Nham K, Silvers W, Hao G, Sun X, Chen M, Hannan R, Qiao J, Dong H, Peng H, and Fu YX. 2018. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J Clin Invest 128: 580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moga E, Alvarez E, Canto E, Vidal S, Rodriguez-Sanchez JL, Sierra J, and Briones J. 2008. NK cells stimulated with IL-15 or CpG ODN enhance rituximab-dependent cellular cytotoxicity against B-cell lymphoma. Exp Hematol 36: 69–77. [DOI] [PubMed] [Google Scholar]
- 38.Schettini J, Kidiyoor A, Besmer DM, Tinder TL, Roy LD, Lustgarten J, Gendler SJ, and Mukherjee P. 2012. Intratumoral delivery of CpG-conjugated anti-MUC1 antibody enhances NK cell anti-tumor activity. Cancer Immunol Immunother 61: 2055–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krieg AM 1996. Lymphocyte activation by CpG dinucleotide motifs in prokaryotic DNA. Trends Microbiol 4: 73–76. [DOI] [PubMed] [Google Scholar]
- 40.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, and Klinman DM. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374: 546–549. [DOI] [PubMed] [Google Scholar]
- 41.Krieg AM 2001. Now I know my CpGs. Trends Microbiol 9: 249–252. [DOI] [PubMed] [Google Scholar]
- 42.Krug A, Rothenfusser S, Hornung V, Jahrsdorfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, and Hartmann G. 2001. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol 31: 2154–2163. [DOI] [PubMed] [Google Scholar]
- 43.Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg AM, and Hartmann G. 2001. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol 31: 3026–3037. [DOI] [PubMed] [Google Scholar]
- 44.Krieg AM 2012. CpG still rocks! Update on an accidental drug. Nucleic Acid Ther 22: 77–89. [DOI] [PubMed] [Google Scholar]
- 45.Hirsh V, Paz-Ares L, Boyer M, Rosell R, Middleton G, Eberhardt WE, Szczesna A, Reiterer P, Saleh M, Arrieta O, Bajetta E, Webb RT, Raats J, Benner RJ, Fowst C, Meech SJ, Readett D, and Schiller JH. 2011. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 29: 2667–2674. [DOI] [PubMed] [Google Scholar]
- 46.Manegold C, van Zandwijk N, Szczesna A, Zatloukal P, Au JS, Blasinska-Morawiec M, Serwatowski P, Krzakowski M, Jassem J, Tan EH, Benner RJ, Ingrosso A, Meech SJ, Readett D, and Thatcher N. 2012. A phase III randomized study of gemcitabine and cisplatin with or without PF-3512676 (TLR9 agonist) as first-line treatment of advanced non-small-cell lung cancer. Ann Oncol 23: 72–77. [DOI] [PubMed] [Google Scholar]
- 47.Appay V, Jandus C, Voelter V, Reynard S, Coupland SE, Rimoldi D, Lienard D, Guillaume P, Krieg AM, Cerottini JC, Romero P, Leyvraz S, Rufer N, and Speiser DE. 2006. New generation vaccine induces effective melanoma-specific CD8+ T cells in the circulation but not in the tumor site. J Immunol 177: 1670–1678. [DOI] [PubMed] [Google Scholar]
- 48.Joshi VB, Geary SM, and Salem AK. 2013. Biodegradable particles as vaccine antigen delivery systems for stimulating cellular immune responses. Hum Vaccin Immunother 9: 2584–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Makkouk A, Joshi VB, Lemke CD, Wongrakpanich A, Olivier AK, Blackwell SE, Salem AK, and Weiner GJ. 2015. Three steps to breaking immune tolerance to lymphoma: a microparticle approach. Cancer Immunol Res 3: 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiocca EA, and Rabkin SD. 2014. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res 2: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohsen MO, Gomes AC, Vogel M, and Bachmann MF. 2018. Interaction of Viral Capsid-Derived Virus-Like Particles (VLPs) with the Innate Immune System. Vaccines (Basel) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leandro MJ, Cambridge G, Ehrenstein MR, and Edwards JC. 2006. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum 54: 613–620. [DOI] [PubMed] [Google Scholar]
- 53.Yri OE, Torfoss D, Hungnes O, Tierens A, Waalen K, Nordoy T, Dudman S, Kilander A, Wader KF, Ostenstad B, Ekanger R, Meyer P, and Kolstad A. 2011. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood 118: 6769–6771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.