Abstract
Mesial temporal lobe epilepsy (mTLE) is a neurological disorder in which patients suffer from frequent consciousness-impairing seizures, broad neurocognitive deficits, and diminished quality of life. Although seizures in mTLE originate focally in the hippocampus or amygdala, mTLE patients demonstrate cognitive deficits that extend beyond temporal lobe function—such as decline in executive function, cognitive processing speed, and attention—as well as diffuse decreases in neocortical metabolism and functional connectivity. Given prior observations that mTLE patients exhibit impairments in vigilance, and that seizures may disrupt the activity and long-range connectivity of subcortical brain structures involved in vigilance regulation, we propose that subcortical activating networks underlying vigilance play a critical role in mediating the widespread neural and cognitive effects of focal mTLE. Here, we review evidence for impaired vigilance in mTLE, examine clinical implications and potential network underpinnings, and suggest neuroimaging strategies for determining the relationship between vigilance, brain connectivity, and neurocognition in patients and healthy controls.
Keywords: activating, arousal, consciousness, seizure, subcortical
1 ∣. INTRODUCTION
Epilepsy is a common and debilitating neurological disorder that affects a similar number of patients as breast cancer in women or lung cancer in men.1 Mesial temporal lobe epilepsy (mTLE) is the most common epilepsy syndrome in adults, and approximately 40% of mTLE patients continue to suffer from seizures despite optimal medical therapy.1,2 mTLE is classified as a “focal” epilepsy, as seizures originate from a discreet brain region termed the epileptogenic zone, which is the hippocampus and/or amygdala in mTLE. Increasingly, however, mTLE is being recognized as a brain network disorder, and the detrimental effects of seizures extend far beyond the mesial temporal lobe.3 mTLE patients often experience a wide range of cognitive, psychiatric, and behavioral problems that interfere with activities of daily living, limit the ability to work, affect interpersonal relationships, and dramatically impair quality of life.4,5 Therefore, it is clinically important to study not only seizure origination and propagation pathways in mTLE, but also network perturbations underlying its cognitive and behavioral sequelae.
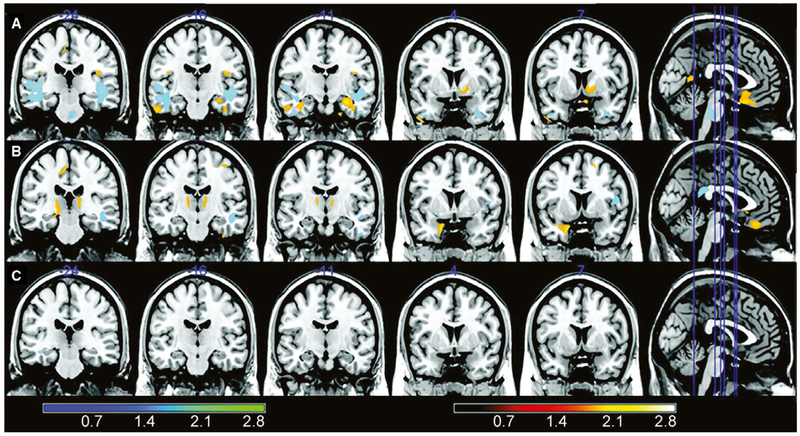
Some neurocognitive impairments in mTLE can be explained by temporal lobe dysfunction, and may help diagnostically to localize seizure onset for surgery. For instance, verbal memory or language decline are common in mTLE of the language dominant side, whereas visuospatial memory problems are found in nondominant mTLE.6,7 However, many neurocognitive deficits in mTLE cannot be explained by temporal lobe disruption,8,9 and may more often be associated with frontal or parietal lobe dysfunction. These include problems with executive function, cognitive processing, concentration, and social recognition.10-12 Furthermore, mTLE patients demonstrate diffuse neocortical hypometabolism, numerous brain connectivity perturbations, and multifocal gray matter atrophy.3,13,14 In one study using voxel-based morphometry analysis of serial magnetic resonance imaging (MRI) scans in mTLE patients, gray matter reduction was noted not only in limbic structures, but also in frontal, parietal, and occipital cortices, as well as bilateral thalamus (Figure 1).15 These observations may suggest a common subcortical source (or sources) of widespread network dysfunction in mTLE.
FIGURE 1.
Voxel-based morphometry (VBM) analyses comparing first and second magnetic resonance imaging (MRI) of left and right mesial temporal lobe epilepsy (mTLE) patients and controls. The VBM analysis (paired t test of MRI1 vs MRI2) of patients with left mTLE (row A) demonstrated significant gray matter reduction in the ipsilateral hippocampus, parahippocampal gyrus, and temporal lobes; bilateral frontal regions and cerebellum; and contralateral occipital region, fusiform gyrus, and cingulate. Patients with right mTLE (row B) had a significant reduction in gray matter volume in the ipsilateral uncus, fusiform gyrus, cerebellum, and occipital and frontal regions; bilateral thalamus and frontal region; and contralateral parietal region. A paired t test comparing the first and the second MRI of the control group (row C) showed only a small significant area of volume reduction in the right temporal lobe white matter. The minimum interval between the baseline and follow-up MRI was 7 months (range = 7-85 months, median = 39 months, SD = 27.5 months). Colored bars show z scores. Hot colors indicate gray matter; cold colors indicate white matter. Modified with permission from Coan et al15
In mTLE patients16,17 and rodent models of limbic seizures,18-20 our group and other collaborators previously demonstrated network alterations involving subcortical brain structures important for vigilance regulation, and have found relationships between these network changes and neuropsychological deficits.16,17 In general, these “subcortical activating structures” may refer to nuclei in the brainstem ascending reticular activating system (ARAS), the basal forebrain region including nucleus basalis, the intralaminar thalamic nuclei, the pulvinar, and the posterior hypothalamus.21-23 Taking recent findings from animal and human studies together with observations of psychomotor slowing, sustained attention problems, and excessive daytime sleepiness in mTLE,24-26 we propose that networks underlying vigilance play a critical role in mediating global network effects of focal mTLE. Specifically, recurrent consciousness-impairing focal seizures may lead to abnormal connectivity between subcortical vigilance structures and neocortex, causing vigilance impairments that contribute to poor neurocognitive function in mTLE. Because the majority of mTLE studies focus on temporal lobe and limbic structures, the role of subcortical vigilance centers in mTLE has been underexamined despite indications of its potential importance. In the remainder of this article, we will review evidence for impaired vigilance in mTLE, examine clinical implications and potential network underpinnings, and suggest neuroimaging strategies to further evaluate vigilance in patients and healthy controls. We will primarily focus this review on mTLE and not include other focal epilepsy syndromes (eg, lateral temporal, focal neocortical). The networks discussed herein have been best characterized in mTLE, although the differential effects of other epilepsy syndromes on vigilance will be an important focus in other works.
2 ∣. WHAT IS VIGILANCE?
Although definitions of “vigilance” vary, it is often defined as “the ability to sustain attention to a task.”27 Whereas both “vigilance” and “arousal” involve a state of neocortical activation, the latter refers more simply to the physiological sleep-wake cycle without requiring behavioral responsiveness.27,28 “Alertness” also overlaps with “arousal” but requires a cognitive component, which may be phasic (relating to a specific response) or tonic.29,30 In addition, “attention” refers to cortical activation that enhances information processing that may be selective (to a particular factor or time) or sustained.24,25 Vigilance will herein be considered synonymous with “sustained attention” or “tonic alertness,” as is common in the literature.31 Nonetheless, sustained attention is closely intertwined with arousal and sleep-wake states, as the ability to maintain arousal is a key component of the ability to maintain attention to a task31; therefore, studies of arousal will also be emphasized below. Vigilance (and arousal) are often measured statically using a psychomotor vigilance task (PVT) or other tasks requiring sustained attention, or dynamically using electroencephalography (EEG) or eye behavior.32,33
3 ∣. CLINICAL STUDIES DEMONSTRATING IMPAIRED VIGILANCE IN EPILEPSY
Several studies have noted impairment of vigilance in mTLE and other focal epilepsies, particularly in individuals suffering from frequent consciousness-impairing focal seizures. One longitudinal study demonstrated marked declines in psychomotor speed and sustained attention in 50 patients over 5 years that was related to disease duration and severity.24 Another investigation found sustained attention deficits in epilepsy patients suffering from predominantly consciousness-impairing focal seizures.25 Many other studies have further demonstrated impaired sustained attention in focal epilepsy.34-36 Furthermore, although antiepileptic medications have known cognitive side effects, several studies have shown that psychomotor speed and attentional deficits in epilepsy cannot be fully explained by medications alone, and are also present in patients not on medications.37-39 For instance, one study of 50 patients and 69 controls showed progressive psychomotor slowing in patients that was not related to medication levels,26 and an aforementioned longitudinal investigation of epilepsy patients demonstrated that decline of sustained attention was not related to medication type, number, or changes.24 To our knowledge, the potential differential effects of right versus left mTLE on vigilance have not yet been explored in detail.
Next, focal epilepsy patients often suffer from excessive daytime sleepiness (EDS) and sleep-wake disturbances that further suggest problems with arousal networks. In one study of 39 mTLE patients, the most frequent complaint (85%) was EDS, and parasomnias and decreased rapid eye movement sleep were also common.40 Many other studies have found high rates of EDS, sleep fragmentation, and disturbed sleep architecture in focal epilepsy, as well as increased risk of obstructive sleep apnea.41-43 Furthermore, several investigations have found that EDS and sleep disturbances are not solely related to antiepileptic medications,44-46 but instead are closely associated with severity and/or duration of disease,45-47 and may be accompanied by decline in quality of life.44,48 Overall, these clinical studies suggest potential impairment of vigilance networks in focal epilepsy patients.
4 ∣. THE EXTENDED NETWORK INHIBITION HYPOTHESIS
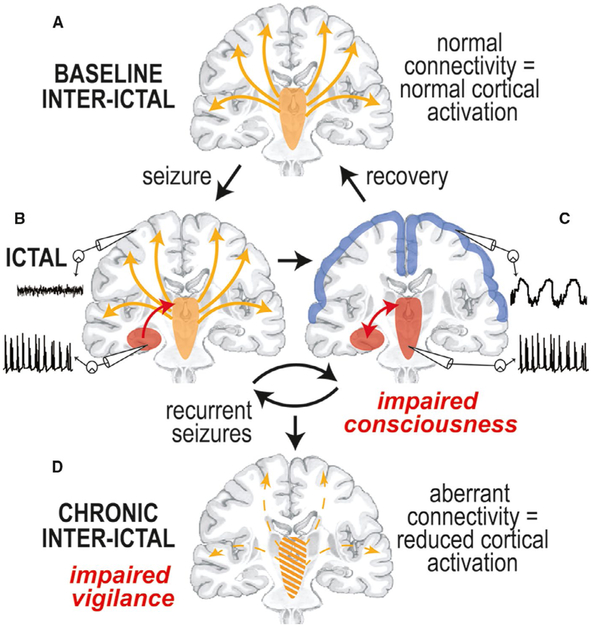
Mechanistically, how might mTLE lead to subcortical network connectivity problems and impaired vigilance? We postulate that limbic seizures directly disrupt subcortical structures involved in vigilance, and that over time, recurrent seizures (“ictal” events) may lead to chronic long-term (“interictal”) disruption of subcortical-cortical network connectivity. The Network Inhibition Hypothesis was first proposed by Blumenfeld to explain ictal neocortical inhibition during limbic seizures,49,50 and we have since extended this hypothesis to help explain interictal subcortical-cortical connectivity reductions in mTLE (Figure 2).
FIGURE 2.
The “Extended Network Inhibition Hypothesis” addressing impaired vigilance in mesial temporal lobe epilepsy. Description is provided in the text. Modified with permission from Englot and Blumenfeld50
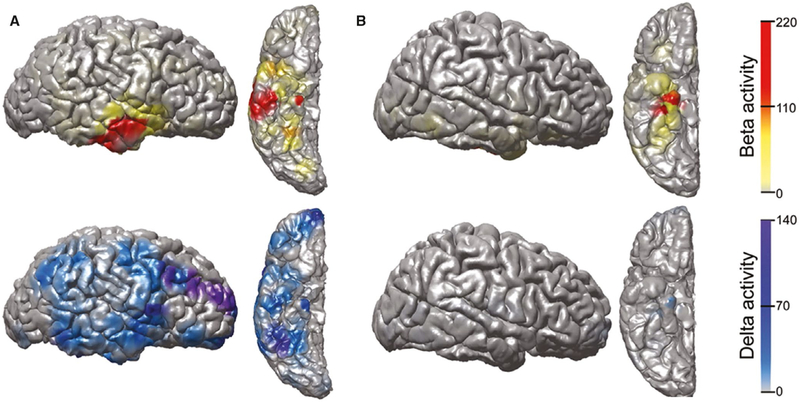
At baseline, vigilance requires normal neocortical activation via direct and indirect ascending excitatory projects from subcortical regions (Figure 2A). During transition to the ictal period, seizure activity begins in the hippocampus, and may initially remain confined to the hippocampus and not disturb normal cortical activity, generating a small focal seizure with spared consciousness (Figure 2B). However, when seizure activity spreads to involve subcortical activating structures, the normal excitatory input from the subcortical regions to the neocortex is perturbed, and the neocortex defaults to a sleeplike inhibited state, resulting in a focal seizure with impaired consciousness (Figure 2C). This state of ictal neocortical inhibition was demonstrated in previous human intracranial EEG studies, which showed that consciousness-impairing focal seizures are accompanied by slow delta-range sleeplike rhythms in frontoparietal neocortex (Figure 3A), whereas smaller consciousness-sparing focal seizures show preserved normal neocortical EEG rhythms (Figure 3B).51
FIGURE 3.
Ictal neocortical slow activity from intracranial electroencephalographic (EEG) recordings during a consciousness-impairing, but not consciousness-sparing, focal seizure in mesial temporal lobe epilepsy. A, During a consciousness-impairing focal seizure, large elevations in fast beta activity are seen in the mesial and lateral temporal lobe during the event, representing regions of seizure onset and propagation, respectively. Increased delta activity is most dramatic in the frontal and parietal association cortices, where there is no fast seizure activity, as well as in the mesial and lateral temporal lobe, where fast seizure activity is also present. The occipital and perirolandic areas are relatively spared. B, During a consciousness-sparing focal seizure, elevations in fast beta activity are seen in the mesial temporal lobe, the site of seizure onset, with minimal lateral temporal involvement. Increased delta activity is seen in the mesial temporal lobe, in the same region as the ictal beta activity, but the neocortex is relatively spared. Data shown are fractional change in beta- or delta-range EEG signal power during the entire seizures versus a 60-second uninterrupted baseline, overlaid on a three-dimensional reconstruction of the patient's preimplant magnetic resonance imaging. Only the ipsilateral (right) hemisphere is shown. Modified with permission from Englot et al51
The withdrawal of afferent excitation during consciousness-impairing focal seizures is also associated with reduced neocortical cerebral blood flow, which is distinct from increased cerebral blood flow seen in brain areas directly impacted by seizure spread.52 Furthermore, previous rodent studies showed that decreased neuronal firing, cerebral metabolism, and excitatory neurotransmission are observed in the neocortex during this state of withdrawn excitation.18,20 It was also demonstrated that reduced neocortical activity only occurs when seizure activity spreads to subcortical activating structures—such as basal forebrain, thalamus, and brainstem ARAS nuclei—and both neocortical inhibition and behavioral impairment can be averted by preventing seizure spread from the hippocampus to subcortical activating structures with a fornix lesion.19,20 These findings strongly support the network inhibition hypothesis ictally, but what about the interictal period?
At the end of a consciousness-impairing focal seizure, vigilance is slowly restored as subcortical activating structures regain normal cortical excitation (from Figure 2C to 2A). Over time, however, recurrent consciousness-impairing focal seizures may lead to progressive dysfunction of subcortical activating structures and chronically reduced connectivity between these regions and the neocortex (Figure 2D). Decreased connectivity between subcortical activating structures and the neocortex may produce long-term reductions in neocortical activation, neocortical activity, and impairment of vigilance (Figure 2D). Progressive long-term neocortical dysfunction is supported by previous magneto-encephalographic studies demonstrating decreased interictal frontoparietal-insular functional connectivity in mTLE patients that is quantitatively related to higher frequency of consciousness-impairing seizures and longer duration of epilepsy.53
5 ∣. INTERICTAL CONNECTIVITY PERTURBATION OF SUBCORTICAL VIGILANCE NETWORKS IN MTLE
Our Extended Network Inhibition Hypothesis posits that mTLE may result in long-term interictal connectivity problems between subcortical vigilance structures and the neocortex, which may contribute to neurocognitive deficits and diffuse neural perturbations. It is worthwhile to note that given the novelty of this field, and the modest number of studies exploring vigilance networks in mTLE to date, our hypothesis remains preliminary at this time. Important subcortical structures that contribute to vigilance include ARAS, basal forebrain, intralaminar thalamic nuclei, pulvinar, and posterior hypothalamus.21-23 Neuroanatomically, mammalian cognitive functions are typically localized to neocortical networks. However, there is evidence that subcortical vigilance networks may also influence cognition and behavior. For example, atrophy of the basal forebrain and its projections has been demonstrated in individuals with mild cognitive impairment.54 Patients with Parkinson disease treated with pedunculopontine nucleus (PPN) deep brain stimulation for motor symptoms have been shown to also have improvements in executive function and working memory,55 as well as attentional processing.56 Human functional MRI (fMRI) studies have demonstrated reduced activation of the ventral tegmental area (VTA) during decision-making in schizophrenia.57 In rats, high-frequency stimulation of the central intralaminar thalamic nucleus may result in improved performance on attention and memory tasks.28 In summary, relating subcortical vigilance center connectivity to neurocognitive function may be relatively novel, but potential relationships should not be surprising, given that ascending excitatory projections are required for normal neocortical activation.
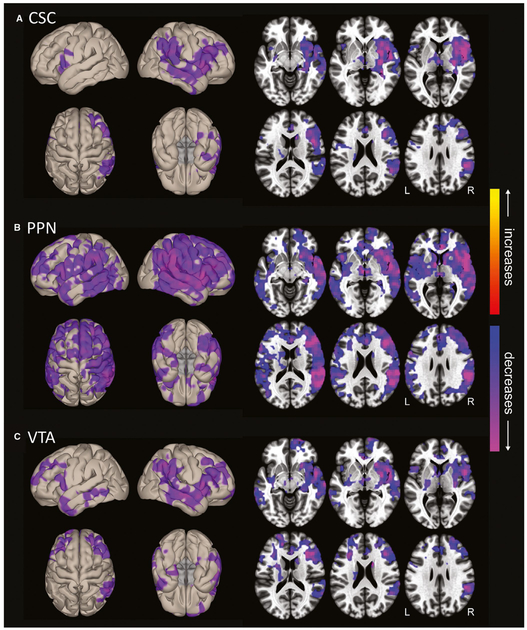
Recently, our group examined fMRI functional connectivity patterns of eight pontomesencephalic brainstem ARAS nuclei in preoperative mTLE patients versus controls. Overall, mean ARAS connectivity was significantly lower in mTLE patients than controls, and the greatest connectivity perturbations were observed in the cuneiform/subcuneiform nuclear complex, PPN, and VTA.16 Across the brain, the largest decreases in functional connectivity seeded from ARAS in mTLE patients were found in frontoparietal association neocortex, posterior temporal cortex, and insula (Figure 4).17 Marked decreases in diffusion tensor imaging structural connectivity between these three ARAS nuclei and the neocortex were also noted in patients versus controls.17 Importantly, decreases in fMRI functional connectivity between ARAS nuclei and frontoparietal neocortex were associated with worse performance in attention, cognitive processing speed, and executive function, along with other domains such as verbal and visuospatial memory.16 We also noted relationships between higher frequency of consciousness-impairing seizures and greater magnitude of both ARAS functional16 and structural connectivity17 impairments. Furthermore, another group recently reported reductions in gray matter connectivity in both the rostral brainstem (where ARAS nuclei are located) and caudal brainstem in focal epilepsy patients (Figure 5).58 These connectivity reductions were correlated with impaired heart rate variability, suggesting brainstem perturbations in these patients may also contribute to the autonomic instability reported in these patients.59
FIGURE 4.
Ascending reticular activating system (ARAS) functional connectivity decreases in mesial temporal lobe epilepsy patients. Surface (left) and axial (right) views are shown of voxelwise functional connectivity differences in patients compared to controls, seeded from cuneiform/subcuneiform nucleus (CSC; A), pedunculopontine nucleus (PPN; B), and ventral tegmental area (VTA; C). Seed-to-voxel functional connectivity (bivariate correlation) maps comparing patients and controls (t test) were generated for each ARAS region using the CONN toolbox 17 (https://www.nitrc.org/projects/conn/). In all three regions, connectivity decreases in patients are observed in insular, frontal, temporal, and parietal neocortical areas, with larger changes on the right side. Decreases appear most prominent in PPN-seeded connectivity maps, also involving subcortical structures such as thalamus and basal ganglia. No increases are seen in patients. Data represent t tests in 26 patients versus 26 matched controls (parametric cluster threshold level P < .01, with false discovery rate correction of multiple comparisons to reduce the false-positive rate). Modified with permission from Englot et al17
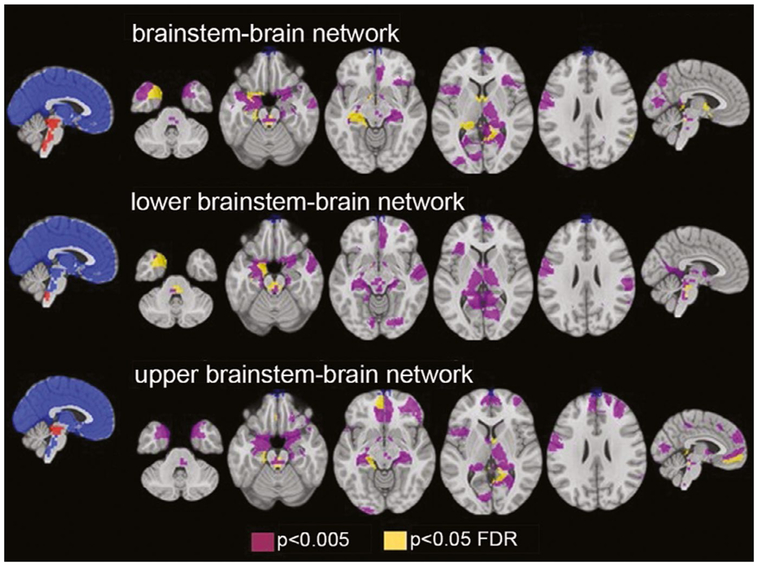
FIGURE 5.
Brainstem atrophy in focal epilepsy patients destabilizes brainstem-brain interactions. Data demonstrate reductions in gray matter connectivity between brainstem and other brain regions that were present in epilepsy patients and correlated with impaired heart rate variability. Functional connectivity measurements are taken from 12 focal epilepsy patients undergoing task-free functional magnetic resonance imaging. FDR, false discovery rate. Modified with permission from Mueller et al58
In another recent study, we found that fMRI functional connectivity between the central lateral thalamic nucleus (an intralaminar nucleus known to receive input from ARAS nuclei and project broadly to cortex60) and the occipital lobe was abnormal and more positive in mTLE patients compared to controls.61 Whereas fMRI correlations between ARAS and frontoparietal neocortex are typically positive in healthy controls, fMRI correlations between thalamus and occipital lobe in healthy controls are known to be strongly negative—a pathway that may be related to visuoperceptual attention and the posterior dominant EEG rhythm.62-64 Patients also had abnormal connectivity between ARAS and central lateral thalamic nucleus, and abnormal brainstem-thalamic connectivity was associated with impaired performance on visuoperceptual attention tasks.61 Overall, these studies suggest long-term abnormalities in subcortical-cortical vigilance networks in mTLE. Taken together, (1) progressive neurocognitive deficits, (2) vigilance and wakefulness problems, (3) diffuse neocortical hypometabolism, and (4) widespread cortical gray matter atrophy in mTLE are observations that helped prompt the Extended Network Inhibition Hypothesis to explain long-term interictal neocortical dysfunction in this disorder.
6 ∣. NEUROIMAGING STUDIES OF VIGILANCE
Although studies relating brain connectivity to vigilance state have not been performed in epilepsy patients, to our knowledge, recent work by our group and others has begun to probe dynamic network fluctuations related to vigilance in healthy controls and nonhuman primates. Such studies have focused largely on the dimension of vigilance relating to arousal, or drowsiness and sleep-wake states.32,33,65Although scalp EEG has long been the primary technique for studying arousal in the human brain, fMRI studies have begun to demonstrate how activity and network connectivity across the whole brain—and at millimeter-scale spatial resolution—are modulated with levels of wakefulness in the healthy brain. State-dependent fMRI signals have been investigated by experimentally inducing different arousal states (including pre- versus postcaffeine66 and sleep deprivation), or by recording established indicators of natural fluctuations in arousal throughout the fMRI scan (including EEG and pupil dilation).67-69
Drawing upon such neuroimaging techniques, we and others have demonstrated that changes in arousal are accompanied by changes in coherence within and between major cortical resting-state networks.33,70,71 For instance, by assessing fMRI functional connectivity in temporal sliding windows, we observed that the strength of connectivity between default mode and dorsal attention networks varied together with EEG spectral markers of arousal (Figure 6).32 In addition, ongoing fluctuations in arousal have been consistently found to covary with widespread modulation of cortical fMRI signal levels, together with opposing signal changes in subcortical structures such as thalamus and brainstem.33,71,72 By combining pharmacological inactivation and fMRI in macaque monkeys, we demonstrated causal evidence that focal disruptions of one major arousal region—the nucleus basalis—elicited marked, large-scale alterations in cortical fMRI signals, particularly during states of reduced arousal (Figure 7).73 Together, these findings offer more direct evidence that subcortical arousal nuclei may drive widespread fluctuations in brain activity, and emphasize the need to more fully characterize subcortical, vigilance-dependent connections in the human brain.
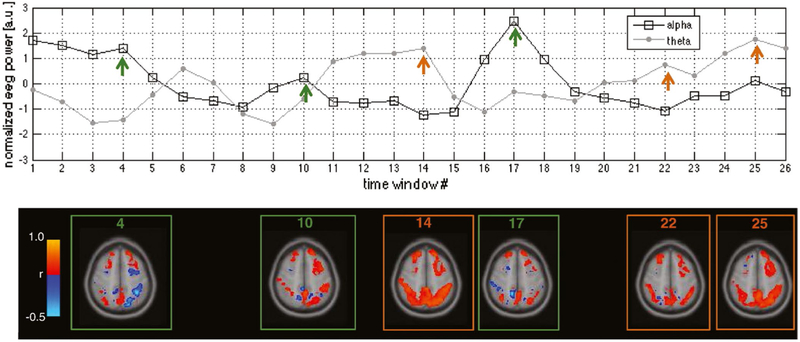
FIGURE 6.
Vigilance-dependent cortical connectivity. Top: Temporally normalized fluctuations in electroencephalographic (EEG) alpha and theta power, measured in window sizes of 40 seconds throughout a functional magnetic resonance imaging (fMRI) scan. Arrows indicate windows selected for visualization of seed-based correlations in the bottom panel. Bottom: Temporal variations in fMRI functional connectivity, calculated within the indicated time windows. Functional connectivity was calculated with respect to a seed region in the default mode network (posterior cingulate cortex), and correlations were computed for each voxel in the default mode network and dorsal attention network. This figure shows data from a single subject, and is intended as an illustration. Adapted with permission from Chang et al32
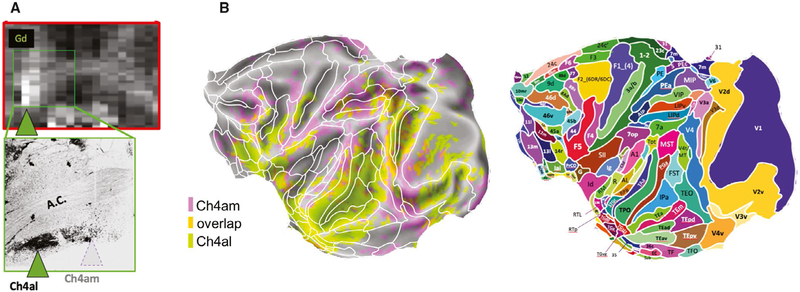
FIGURE 7.
Disruption of subcortical neural activity alters cortical functional magnetic resonance imaging (fMRI) patterns. Transient pharmacological inactivation of two subregions in the nucleus basalis, Ch4al and Ch4am (A), induced spatially distinct changes in the amplitude of cortical fMRI signals (B) in the macaque. For anatomic reference, superimposed on the map in B are areal boundaries of the Saleem and Logothetis atlas. Adapted with permission from Turchi et al73
Whereas brain regions implicated in arousal state have been identified in animal studies, much less is known about the network-level interactions between vigilance/arousal systems and areas supporting high-level cognition in humans.74,75 Most human fMRI studies do not measure or account for these states, due in part to the practical challenges of setting up and monitoring arousal indicators (such as EEG) in the MRI scanner. Furthermore, to our knowledge, those studies that do examine state-dependent connectivity have focused heavily on neocortical networks and often do not segment small subcortical structures (eg, Chang et al,32,68,76 Tagliazucchi et al,32,68,76 Wang et al32,68,76), owing in part to technical challenges of acquiring high-quality fMRI data in small brainstem and forebrain nuclei.77,78 Ongoing developments in image acquisition, noise reduction, and advanced segmentation techniques may allow for more readily imaging subcortical arousal regions that are prone to signal dropout and physiological artifacts.79-81 Finally, because components of vigilance relating to cognition, motivation, and task performance are not directly probed during the task-free resting-state condition, future studies can also incorporate externally focused tasks during EEG-fMRI.
Overall, a growing body of research indicates that human brain signals and large-scale networks can be extensively modulated with vigilance. This highlights the importance of monitoring vigilance in neuroimaging studies, and of investigating subcortical arousal networks—especially in neurological disorders that may involve altered vigilance regulation.
7 ∣. RELATING VIGILANCE, CONNECTIVITY, AND NEUROCOGNITION IN MTLE
As discussed above, we hypothesize that widespread cognitive deficits observed in mTLE may arise from seizure-induced disruptions to subcortical brain structures involved in vigilance regulation. However, the degree to which subcortical activating networks mediate the neural and cognitive effects of focal mTLE has not yet been directly examined. Multimodal neuroimaging, combined with in-depth neurocognitive assessments, may provide fruitful avenues for bridging this gap and identifying connectivity perturbations that influence vigilance state and contribute to neurocognitive decline.
To provide a baseline for assessing mTLE patients, it is important to first obtain a more complete characterization of vigilance-dependent functional connectivity in healthy controls. However, to date, detailed knowledge of the connections between subcortical vigilance centers and neocortical networks implicated in attentional and cognitive processing is currently lacking. Further studies that incorporate concurrent recordings of EEG and fMRI may provide a means for characterizing fMRI connectivity across a spectrum of vigilance states (Figure 8), and for determining interregional fMRI connections that are most strongly modulated with vigilance. Such information would enable vigilance-dependent network dynamics to be directly compared with those of mTLE patients, and acquisition of diffusion tensor imaging data would additionally allow for relating structural connectivity disturbances with aberrant vigilance-dependent functional connectivity.
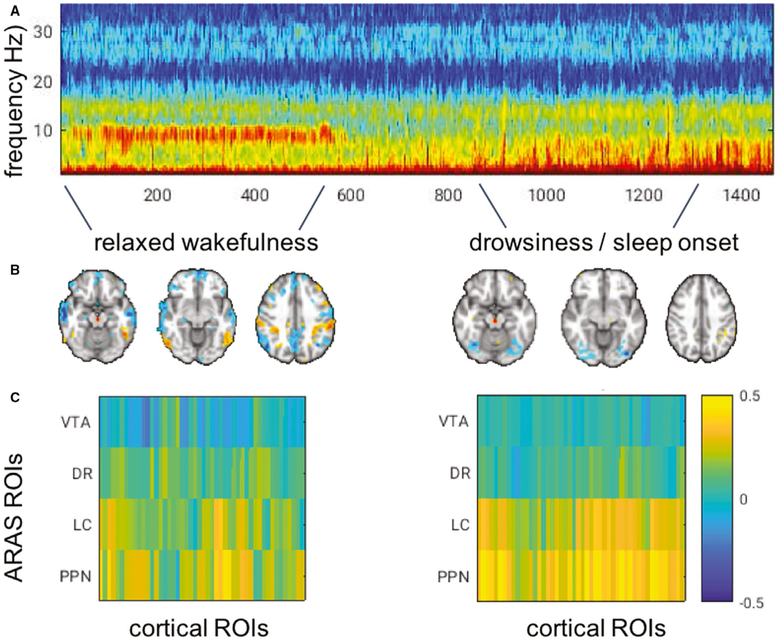
FIGURE 8.
Subcortical to cortical functional connectivity across vigilance states. Example scan from one control subject illustrates how (A) electroencephalogram (EEG) acquired during functional magnetic resonance imaging indicates a clear shift from higher vigilance to sleep onset, indicated by the loss of alpha power; and (B, C) functional connectivity (correlation) can be calculated between individual ascending reticular activating system (ARAS) nuclei and cortex during epochs corresponding to different EEG-defined arousal states. B, Correlations from a seed region in the ARAS ventral tegmental area (VTA). C, Correlation between ARAS nuclei and 48 cortical regions of interest (ROIs). DR, dorsal raphe; LC, locus coeruleus; PPN, pedunculopontine nucleus
Furthermore, whereas neuropsychological decline in mTLE patients has been well documented, the relationship between neurocognition and vigilance has not been investigated in detail. In addition to neuroimaging studies, we suggest that neuropsychological evaluations may be related to standard behavioral tests of vigilance and arousal, including a PVT82,83 and the Epworth Sleepiness Scale (ESS)—a questionnaire to measure excessive daytime sleepiness.84,85 Modeling factors such as ESS and PVT scores could enable the statistical decomposition of neurocognitive deficits into components that are more closely linked to altered vigilance, and those that are more closely related to seizure focus (eg, executive function, cognitive processing). In such a framework, one may also attempt to partial out the effects of vigilance to isolate purely cortical effects, to the extent that such effects are linearly separable.
Finally, neuropsychological test results may be integrated with functional neuroimaging data to examine how vigilance-related cognitive deficits in mTLE are linked with disturbances in subcortical-to-cortical brain connectivity. We hypothesize that the neurocognitive deficits that are most closely related to vigilance will show the strongest statistical associations with disrupted subcortical-cortical connectivity.
8 ∣. REAL-WORLD CLINICAL SIGNIFICANCE OF STUDYING VIGILANCE NETWORKS IN MTLE
Many studies in mTLE focus on limbic networks and seizure origination and propagation pathways, given clear clinical implications for diagnosis, localization, and treatment in this disorder. However, we believe that investigating vigilance networks in mTLE has several clinically relevant implications.
8.1 ∣. Uncovering neuromodulation targets
Although seizures are refractory to medications in 40% of mTLE patients, many of these patients are candidates for resection or ablation of the mesial temporal lobe, leading to a 60%-80% chance of seizure freedom.1,86 For those who are not candidates for resection (eg, epileptogenic hippocampus is dominant for memory), palliative neurostimulation treatment may significantly reduce seizure frequency and severity, and improve attention and cognition.87-89 Studies of vigilance networks may help uncover novel neuromodulation targets in subcortical activating structures to reduce morbidity. For example, previous rats studies have shown that deep brain stimulation (DBS) of intralaminar thalamus and PPN can prevent behavioral impairment and neocortical inhibition in limbic seizures.90 Also, in humans with Parkinson disease, DBS with a PPN target has been shown to improve vigilance, working memory, executive function, and sleep-wake disturbances.55,56 To our knowledge, at least one study evaluating the role of DBS in preserving consciousness during seizures in human epilepsy patients is currently in its early stages.
8.2 ∣. Understanding and preventing sudden unexpected death in epilepsy
mTLE patients have a 5-10 times increased mortality rate compared to the general population, in large part due to sudden unexpected death in epilepsy (SUDEP).1 It has been proposed that SUDEP mechanisms may involve dysfunction of excitatory serotonergic projections from brainstem ARAS nuclei, resulting in depressed arousal and breathing.91 This hypothesis is supported by recent experiments showing diminished single unit brainstem recordings during and after seizures in a rodent model of mTLE.92 Furthermore, studies of mouse models have demonstrated higher risk of SUDEP during impaired vigilance state,93 and brainstem atrophy has been observed in MRI of mTLE patients who ultimately succumbed to SUDEP.94 Thus, studying arousal system dysfunction has important implications for understanding and preventing this devastating consequence of mTLE.
8.3 ∣. Behavioral or pharmacological therapies
Whereas cognitive behavioral therapies aimed at improving attention and executive function are relatively common in patients with attention-deficit/hyperactivity disorder, they are less often considered in epilepsy treatment.95,96 Nonetheless, recent studies suggest that behavioral and psychological treatments may enhance quality of life in epilepsy.97 Medications to improve alertness might also be explored in this patient population, although the potential for lowering seizure threshold would need to be cautiously considered. Defining vigilance impairments in mTLE and associated network disturbances may open the door for further behavioral or pharmacological therapies in this disorder.
8.4 ∣. Earlier surgery
The average duration of epilepsy prior to surgery is 20 years, and only approximately 1% of potential candidates are referred for surgery.1,98 Uncovering strong evidence of progressive decline in network connectivity and cognition in epilepsy patients may provide further impetus to speed the pathway to surgery in refractory patients. Revealing progressive disturbances in network connectivity, cognition, and/or vigilance with recurrent seizures may prompt earlier surgical evaluation and intervention to prevent further decline.
9 ∣. POTENTIAL CONFOUNDING EFFECTS OF MEDICATIONS
mTLE patients take antiepileptic medications that may influence vigilance level, neurocognitive performance, and potentially even brain connectivity. This is likely a critical confounder in vigilance studies in mTLE, but we do not know to what extent, because nearly all behavioral and neuroimaging studies of refractory epilepsy have been performed with patients on medications. Nevertheless, it is unlikely that diseased-related effects of mTLE are solely related to medications. In one recent neuroimaging study of mTLE patients after epilepsy surgery, we noted that certain functional connectivity alternations recovered to levels resembling those of controls in patients who became free of seizures, regardless of whether they were still taking medications.99 Furthermore, several studies have noted impaired vigilance,26,39 neurocognitive deficits,24,37,38 and EDS44-46 in epilepsy patients that are not fully explained by medications and often present in the absence of medications. Therefore, distinguishing the contributions of medications and subcortical network impairments to vigilance and cognition is critical to understand their clinical implications in mTLE treatment. One possible strategy to address the confounding effects of medications might be to repeat neuroimaging network studies in patients who are admitted to the epilepsy monitoring unit and temporarily weaned off of medications, to evaluate for differences in functional connections. Finally, it is important to note that even if certain vigilance and connectivity problems result from medication effects, these will be equally important to understand given their clinical implications in mTLE treatment.
10 ∣. CONCLUSION
Although it is not yet clear why mTLE patients demonstrate broad neurocognitive problems that extend beyond the temporal lobe, studies in both animals and humans have begun to point to a critical role for subcortical activating networks underlying vigilance. At the same time, fMRI studies have revealed that dynamic changes in vigilance level are accompanied by widespread changes in brain activity and functional connectivity that are driven in part by subcortical arousal regions. Taking these findings together, we propose that seizure-induced damage to subcortical activating networks contributes to diffuse neural and neurocognitive dysfunction in mTLE. We suggest that multimodal neuroimaging studies of vigilance-dependent brain connectivity, combined with in-depth neurocognitive assessments, may allow for directly linking subcortical-to-neocortical functional connectivity, vigilance states, and neurocognition. Such studies would help to fill a critical gap in our understanding of the network mechanisms underlying neurocognitive dysfunction in mTLE, as well as help to inform clinical treatment decisions.
Key Points.
mTLE patients demonstrate cognitive deficits, neocortical atrophy, and hypometabolism that extend beyond the temporal lobe
The role of subcortical vigilance networks in contributing to pathophysiology and cognitive problems in mTLE is underappreciated
Progressive problems with vigilance and arousal in mTLE cannot be explained by antiepileptic medications alone
Developing novel treatments for epilepsy requires an understanding of both seizure networks and disturbances in distal connections
Acknowledgments
Funding information
Support was received from the National Institutes of Health: R00 NS097618 (D.J.E.), R01 NS108445 (V.L.M.), R01 NS110130 (V.L.M.), K22 ES028048 (C.C.), and R01 NS112252 (D.J.E. and C.C.).
Footnotes
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Engel J Jr. What can we do for people with drug-resistant epilepsy? The 2016 Wartenberg Lecture. Neurology. 2016;87:2483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Englot DJ, Chang EF. Rates and predictors of seizure freedom in resective epilepsy surgery: an update. Neurosurg Rev. 2014;37(3):389–404; discussion 404–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Englot DJ, Konrad PE, Morgan VL. Regional and global connectivity disturbances in focal epilepsy, related neurocognitive sequelae, and potential mechanistic underpinnings. Epilepsia. 2016;57:1546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gleissner U, Helmstaedter C, Schramm J, Elger CE. Memory outcome after selective amygdalohippocampectomy: a study in 140 patients with temporal lobe epilepsy. Epilepsia. 2002;43:87–95. [DOI] [PubMed] [Google Scholar]
- 5.Caplan R. Epilepsy, language, and social skills. Brain Lang. 2019;193:18–30. [DOI] [PubMed] [Google Scholar]
- 6.Helmstaedter C, Kockelmann E. Cognitive outcomes in patients with chronic temporal lobe epilepsy. Epilepsia. 2006;47(Suppl 2): 96–8. [DOI] [PubMed] [Google Scholar]
- 7.Hoppe C, Elger CE, Helmstaedter C. Long-term memory impairment in patients with focal epilepsy. Epilepsia. 2007;48(Suppl 9):26–9. [DOI] [PubMed] [Google Scholar]
- 8.Bell B, Lin JJ, Seidenberg M, Hermann B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol. 2011;7:154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tailby C, Kowalczyk MA, Jackson GD. Cognitive impairment in epilepsy: the role of reduced network flexibility. Ann Clin Transl Neurol. 2018;5:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gargaro AC, Sakamoto AC, Bianchin MM, et al. Atypical neuropsychological profiles and cognitive outcome in mesial temporal lobe epilepsy. Epilepsy Behav. 2013;27:461–9. [DOI] [PubMed] [Google Scholar]
- 11.Zhao F, Kang H, You L, Rastogi P, Venkatesh D, Chandra M. Neuropsychological deficits in temporal lobe epilepsy: a comprehensive review. Ann Indian Acad Neurol. 2014;17:374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stretton J, Thompson PJ. Frontal lobe function in temporal lobe epilepsy. Epilepsy Res. 2012;98:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvim MKM, Coan AC, Campos BM, et al. Progression of gray matter atrophy in seizure-free patients with temporal lobe epilepsy. Epilepsia. 2016;57:621–9. [DOI] [PubMed] [Google Scholar]
- 14.Aparicio J, Carreño M, Bargalló N, et al. Combined 18F-FDG-PET and diffusion tensor imaging in mesial temporal lobe epilepsy with hippocampal sclerosis. Neuroimage Clin. 2016;12:976–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coan AC, Appenzeller S, Bonilha L, Li LM, Cendes F. Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology. 2009;73:834–42. [DOI] [PubMed] [Google Scholar]
- 16.Englot DJ, D’Haese P-F, Konrad PE, et al. Functional connectivity disturbances of the ascending reticular activating system in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2017;88:925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Englot DJ, Gonzalez HFJ, Reynolds BB, et al. Relating structural and functional brainstem connectivity to disease measures in epilepsy. Neurology. 2018;91:e67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008;28:9066–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Englot DJ, Modi B, Mishra AM, DeSalvo M, Hyder F, Blumenfeld H. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci. 2009;29:13006–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motelow J, Li W, Zhan Q, et al. Decreased subcortical cholinergic arousal in focal seizures. Neuron. 2015;85:561–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage. 2008;42:1127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–73. [PubMed] [Google Scholar]
- 23.Jones BE. Arousal systems. Front Biosci. 2003;8:s438–51. [DOI] [PubMed] [Google Scholar]
- 24.Piazzini A, Turner K, Chifari R, Morabito A, Canger R, Canevini MP. Attention and psychomotor speed decline in patients with temporal lobe epilepsy: a longitudinal study. Epilepsy Res. 2006;72:89–96. [DOI] [PubMed] [Google Scholar]
- 25.Stella F, Maciel JA. Attentional disorders in patients with complex partial epilepsy. Arq Neuropsiquiatr. 2003;61:335–8. [DOI] [PubMed] [Google Scholar]
- 26.Sung C, Jones JE, Jackson DC, et al. Age-accelerated psychomotor slowing in temporal lobe epilepsy. Epilepsy Res. 2013;103:231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parasuraman R. Consistency of individual differences in human vigilance performance: an abilities classification analysis. J Appl Psychol. 1976;61:486–92. [PubMed] [Google Scholar]
- 28.Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000;101:243–76. [DOI] [PubMed] [Google Scholar]
- 29.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. [DOI] [PubMed] [Google Scholar]
- 30.Nebes RD, Brady CB. Phasic and tonic alertness in Alzheimer's disease. Cortex. 1993;29:77–90. [DOI] [PubMed] [Google Scholar]
- 31.Oken BS, Salinsky MC, Elsas SM. Vigilance, alertness, or sustained attention: physiological basis and measurement. Clin Neurophysiol. 2006;117:1885–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang C, Liu Z, Chen MC, Liu X, Duyn JH. EEG correlates of time-varying BOLD functional connectivity. Neuroimage. 2013;72:227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang C, Leopold DA, Schölvinck ML, et al. Tracking brain arousal fluctuations with fMRI. Proc Natl Acad Sci U S A. 2016;113:4518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gascoigne MB, Smith ML, Barton B, Webster R, Gill D, Lah S. Attention deficits in children with epilepsy: preliminary findings. Epilepsy Behav. 2017;67:7–12. [DOI] [PubMed] [Google Scholar]
- 35.Mirsky AF, Duncan CC, Myslobodsky MS. Petit mal epilepsy: a review and integration of recent information. J Clin Neurophysiol. 1986;3:179–208. [PubMed] [Google Scholar]
- 36.Loiseau P, Signoret JL, Strube E. Attention problems in adult epileptic patients. Acta Neurol Scand Suppl. 1984;99:31–4. [DOI] [PubMed] [Google Scholar]
- 37.Elger CE, Helmstaedter C, Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3:663–72. [DOI] [PubMed] [Google Scholar]
- 38.Hirsch E, Schmitz B, Carreno M. Epilepsy, antiepileptic drugs (AEDs) and cognition. Acta Neurol Scand Suppl. 2003;180:23–32. [DOI] [PubMed] [Google Scholar]
- 39.Brodie MJ, McPhail E, Macphee GJA, Larkin JG, Gray JMB. Psychomotor impairment and anticonvulsant therapy in adult epileptic patients. Eur J Clin Pharmacol. 1987;31:655–60. [DOI] [PubMed] [Google Scholar]
- 40.de Almeida CA, Lins OG, Lins SG, et al. Sleep disorders in temporal lobe epilepsy. Arq Neuropsiquiatr. 2003;61:979–87. [DOI] [PubMed] [Google Scholar]
- 41.Zanzmera P, Shukla G, Gupta A, et al. Markedly disturbed sleep in medically refractory compared to controlled epilepsy—a clinical and polysomnography study. Seizure. 2012;21:487–90. [DOI] [PubMed] [Google Scholar]
- 42.Carrion MJM, Nunes ML, Martinez JVL, Portuguez MW, da Costa JC. Evaluation of sleep quality in patients with refractory seizures who undergo epilepsy surgery. Epilepsy Behav. 2010;17:120–3. [DOI] [PubMed] [Google Scholar]
- 43.Klobucnikova K, Siarnik P, Sivakova M, et al. Sleep-disordered breathing and excessive daytime sleepiness in patients with epilepsy—a polysomnographic study. Neuro Endocrinol Lett. 2016;37:313–7. [PubMed] [Google Scholar]
- 44.Kwan P, Yu E, Leung H, Leon T, Mychaskiw MA. Association of subjective anxiety, depression, and sleep disturbance with quality-of-life ratings in adults with epilepsy. Epilepsia. 2009;50:1059–66. [DOI] [PubMed] [Google Scholar]
- 45.Gammino M, Zummo L, Bue AL, et al. Excessive daytime sleepiness and sleep disorders in a population of patients with epilepsy: a case-control study. J Epilepsy Res. 2016;6:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Im H-J, Park S-H, Baek S-H, et al. Associations of impaired sleep quality, insomnia, and sleepiness with epilepsy: a questionnaire-based case-control study. Epilepsy Behav. 2016;57:55–9. [DOI] [PubMed] [Google Scholar]
- 47.Chen NC, Tsai MH, Chang CC, et al. Sleep quality and daytime sleepiness in patients with epilepsy. Acta Neurol Taiwan. 2011;20:249–56. [PubMed] [Google Scholar]
- 48.de Weerd AL, de Haas S, Otte A, et al. Subjective sleep disturbance in patients with partial epilepsy: a questionnaire-based study on prevalence and impact on quality of life. Epilepsia. 2004;45:1397–404. [DOI] [PubMed] [Google Scholar]
- 49.Norden AD, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002;3:219–31. [DOI] [PubMed] [Google Scholar]
- 50.Englot DJ, Blumenfeld H. Consciousness and epilepsy: why are complex-partial seizures complex? Prog Brain Res. 2009;177:147–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Englot DJ, Yang LI, Hamid H, et al. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 2010;133:3764–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blumenfeld H, McNally KA, Vanderhill SD, et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 2004;14:892–902. [DOI] [PubMed] [Google Scholar]
- 53.Englot DJ, Hinkley LB, Kort NS, et al. Global and regional functional connectivity maps of neural oscillations in focal epilepsy. Brain. 2015;138:2249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cantero JL, Zaborszky L, Atienza M. Volume loss of the nucleus basalis of Meynert is associated with atrophy of innervated regions in mild cognitive impairment. Cereb Cortex. 2017;27:3881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alessandro S, Ceravolo R, Brusa L, et al. Non-motor functions in parkinsonian patients implanted in the pedunculopontine nucleus: focus on sleep and cognitive domains. J Neurol Sci. 2010;289:44–8. [DOI] [PubMed] [Google Scholar]
- 56.Fischer J, Schwiecker K, Bittner V, et al. Modulation of attentional processing by deep brain stimulation of the pedunculopontine nucleus region in patients with parkinsonian disorders. Neuropsychology. 2015;29:632–7. [DOI] [PubMed] [Google Scholar]
- 57.Rausch F, Mier D, Eifler S, et al. Reduced activation in ventral striatum and ventral tegmental area during probabilistic decision-making in schizophrenia. Schizophr Res. 2014;156:143–9. [DOI] [PubMed] [Google Scholar]
- 58.Mueller SG, Bateman LM, Nei M, Goldman AM, Laxer KD. Brainstem atrophy in focal epilepsy destabilizes brainstem-brain interactions: preliminary findings. Neuroimage Clin. 2019;23:101888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nayak CS, Sinha S, Nagappa M, Thennarasu K, Taly AB. Lack of heart rate variability during sleep-related apnea in patients with temporal lobe epilepsy (TLE)—an indirect marker of SUDEP? Sleep Breath. 2017;21:163–72. [DOI] [PubMed] [Google Scholar]
- 60.Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–40. [DOI] [PubMed] [Google Scholar]
- 61.González HFJ, Chakravorti S, Goodale SE, et al. Thalamic arousal network disturbances in temporal lobe epilepsy and improvement after surgery. J Neurol Neurosurg Psychiatry. 2019;90:1109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feige B, Scheffler K, Esposito F, Di Salle F, Hennig J, Seifritz E. Cortical and subcortical correlates of electroencephalographic alpha rhythm modulation. J Neurophysiol. 2005;93:2864–72. [DOI] [PubMed] [Google Scholar]
- 63.Goldman RI, Stern JM, Engel J, Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13:2487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zou Q, Long X, Zuo X, et al. Functional connectivity between the thalamus and visual cortex under eyes closed and eyes open conditions: a resting-state fMRI study. Hum Brain Mapp. 2009;30:3066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verweij IM, Romeijn N, Smit DJA, Piantoni G, Van Someren EJW, van der Werf YD. Sleep deprivation leads to a loss of functional connectivity in frontal brain regions. BMC Neurosci. 2014;15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong CW, Olafsson V, Tal O, Liu TT. Anti-correlated networks, global signal regression, and the effects of caffeine in resting-state functional MRI. Neuroimage. 2012;63:356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Larson-Prior LJ, Power JD, Vincent JL, et al. Modulation of the brain's functional network architecture in the transition from wake to sleep. Prog Brain Res. 2011;193:277–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tagliazucchi E, von Wegner F, Morzelewski A, Borisov S, Jahnke K, Laufs H. Automatic sleep staging using fMRI functional connectivity data. Neuroimage. 2012;63:63–72. [DOI] [PubMed] [Google Scholar]
- 69.Yellin D, Berkovich-Ohana A, Malach R. Coupling between pupil fluctuations and resting-state fMRI uncovers a slow build-up of antagonistic responses in the human cortex. Neuroimage. 2015;106:414–27. [DOI] [PubMed] [Google Scholar]
- 70.Allen EA, Damaraju E, Eichele T, Wu L, Calhoun VD. EEG signatures of dynamic functional network connectivity states. Brain Topogr. 2018;31:101–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ong JL, Kong D, Chia TTY, Tandi J, Thomas Yeo BT, Chee MWL. Co-activated yet disconnected—neural correlates of eye closures when trying to stay awake. Neuroimage. 2015;118:553–62. [DOI] [PubMed] [Google Scholar]
- 72.Olbrich S, Mulert C, Karch S, et al. EEG-vigilance and BOLD effect during simultaneous EEG/fMRI measurement. Neuroimage. 2009;45:319–32. [DOI] [PubMed] [Google Scholar]
- 73.Turchi J, Chang C, Ye FQ, et al. The basal forebrain regulates global resting-state fMRI fluctuations. Neuron. 2018;97:940–52.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bianciardi M, Toschi N, Edlow BL, et al. Toward an in vivo neuroimaging template of human brainstem nuclei of the ascending arousal, autonomic, and motor systems. Brain Connect. 2015;5:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Satpute AB, Kragel PA, Barrett LF, Wager TD, Bianciardi M. Deconstructing arousal into wakeful, autonomic and affective varieties. Neurosci Lett. 2019;693:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang C, Ong JL, Patanaik A, Zhou J, Chee MWL. Spontaneous eyelid closures link vigilance fluctuation with fMRI dynamic connectivity states. Proc Natl Acad Sci U S A. 2016;113:9653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang C, Raven EP, Duyn JH. Brain-heart interactions: challenges and opportunities with functional magnetic resonance imaging at ultra-high field. Philos Trans A Math Phys Eng Sci. 2016;374(2067):20150188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brooks JCW, Faull OK, Pattinson KTS, Jenkinson M. Physiological noise in brainstem FMRI. Front Hum Neurosci. 2013;7:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Y, D'Haese P-F, Newton A, Dawant B. Thalamic nuclei segmentation in clinical 3T T1-weighted images using high-resolution 7T shape models. Prog Biomed Opt Imaging Proc SPIE. 2015;9415:9. [Google Scholar]
- 80.Markello RD, Spreng RN, Luh W-M, Anderson AK, De Rosa E. Segregation of the human basal forebrain using resting state functional MRI. Neuroimage. 2018;173:287–97. [DOI] [PubMed] [Google Scholar]
- 81.Gonzalez-Castillo J, Panwar P, Buchanan LC, et al. Evaluation of multi-echo ICA denoising for task based fMRI studies: block designs, rapid event-related designs, and cardiac-gated fMRI. Neuroimage. 2016;141:452–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 83.Basner M, Mollicone D, Dinges DF. Validity and sensitivity of a brief psychomotor vigilance test (PVT-B) to total and partial sleep deprivation. Acta Astronaut. 2011;69:949–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johns M, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep. 1997;20:844–9. [DOI] [PubMed] [Google Scholar]
- 85.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. [DOI] [PubMed] [Google Scholar]
- 86.Englot DJ, Lee AT, Tsai C, et al. Seizure types and frequency in patients who “fail” temporal lobectomy for intractable epilepsy. Neurosurgery. 2013;73:838–44. [DOI] [PubMed] [Google Scholar]
- 87.Loring DW, Kapur R, Meador KJ, Morrell MJ. Differential neuropsychological outcomes following targeted responsive neurostimulation for partial-onset epilepsy. Epilepsia. 2015;56:1836–44. [DOI] [PubMed] [Google Scholar]
- 88.Salanova V, Witt T, Worth R, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84:1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Englot DJ, Birk H, Chang EF. Seizure outcomes in nonresective epilepsy surgery: an update. Neurosurg Rev. 2017;40:181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kundishora AJ, Gummadavelli A, Ma C, et al. Restoring conscious arousal during focal limbic seizures with deep brain stimulation. Cereb Cortex. 2017;27:1964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Richerson GB, Buchanan GF. The serotonin axis: shared mechanisms in seizures, depression, and SUDEP. Epilepsia. 2011;52(Suppl 1):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhan Q, Buchanan GF, Motelow JE, et al. Impaired serotonergic brainstem function during and after seizures. J Neurosci. 2016;36:2711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hajek MA, Buchanan GF. Influence of vigilance state on physiological consequences of seizures and seizure-induced death in mice. J Neurophysiol. 2016;115:2286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mueller SG, Bateman LM, Laxer KD. Evidence for brainstem network disruption in temporal lobe epilepsy and sudden unexplained death in epilepsy. Neuroimage Clin. 2014;5:208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Knouse LE, Teller J, Brooks MA. Meta-analysis of cognitive-behavioral treatments for adult ADHD. J Consult Clin Psychol. 2017;85:737–50. [DOI] [PubMed] [Google Scholar]
- 96.Kemper AR, Maslow GR, Hill S, et al. Attention Deficit Hyperactivity Disorder: Diagnosis and Treatment in Children and Adolescents. Rockville, MD: Agency for Healthcare Research and Quality; 2018. [PubMed] [Google Scholar]
- 97.Michaelis R, Tang V, Wagner JL, et al. Cochrane systematic review and meta-analysis of the impact of psychological treatments for people with epilepsy on health-related quality of life. Epilepsia. 2018;59:315–32. [DOI] [PubMed] [Google Scholar]
- 98.Englot DJ, Ouyang D, Garcia PA, Barbaro NM, Chang EF. Epilepsy surgery trends in the United States, 1990–2008. Neurology. 2012;78:1200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonzalez HFJ, Goodale SE, Jacobs ML, et al. Brainstem functional connectivity disturbances in epilepsy may recover after successful surgery. Neurosurgery. 2019. [Epub ahead of print]. 10.1093/neuros/nyz128 [DOI] [PMC free article] [PubMed] [Google Scholar]