Abstract
The mechanism(s) for sudden death in epilepsy (SUDEP) remain(s) unknown, but seizure spread to brainstem areas serving autonomic and respiratory function is critical. In a rat model, we established a mechanism for SUDEP that involves seizure-induced laryngospasm and obstructive apnea lasting until respiratory arrest. We hypothesized that DBA/2J mice, which display lethal audiogenic seizures, would be protected from death by implanting a tracheal T-tube as a surrogate airway. In a 2 × 2 design, mice were implanted with either open or closed tracheal T-tubes and treated with either low-dose ketamine/xylazine to moderate thoracic spasm during the tonic seizure phase or no drug. Animals receiving both treatments had the highest survival rate, followed by animals receiving the open tube without ketamine/xylazine. The odds ratio for survival was more than twenty times higher with an open T-tube (OR=24.14). The impact of open tracheal tubes indicates that the mechanism of death in DBA/2J mice involves seizure-induced upper airway obstruction until respiratory arrest. These results, our rat work, and our demonstration of inspiratory effort-based electromyographic signals and electrocardiographic abnormalities in rats and humans suggest that seizure-induced laryngospasm and obstructive apnea directly link seizure activity to respiratory arrest in these sudden death examples.
INTRODUCTION
Sudden death in epilepsy (SUDEP) is a major cause of death in persons with epilepsy1. The details of the underlying mechanisms are still being elucidated, but seizure spread to brainstem areas serving autonomic and respiratory function is critical2, 3.
Animal models, such as audiogenic seizure-prone mice, offer insights into potential brainstem activity derangements that may cause a failure to attempt breathing or central apnea. Multiple inbred strains (e.g. the DBA/2 and DBA/1 strains4) and mouse mutants with altered sodium channels (Nav1.1)5, potassium channels (Kv1.1)6, or other proteins (e.g. Fragile X Mental Retardation Protein)7 display severe generalized seizure activity that can frequently be lethal. Several of these strains have been used to identify serotonergic contributions to seizure-induced death4, 8, 9, and to suggest spreading depolarization of brainstem neurons6 as a co-contributor or separate cause of seizure-induced death.
We proposed a mechanism for SUDEP that involves laryngospasm due to seizure spread into brainstem laryngomotor areas, not via reflex, causing obstructive apnea, which lasts sufficiently long to result in respiratory arrest10. Although the MORTEMUS study lacked respiratory recordings, we demonstrated that the same EMG evidence for attempts to breathe during airway occlusion was present11 in the human SUDEP cases illustrated by Ryvlin et al.12 (also shown in13).
To test whether apnea in audiogenic seizure prone mice was central in origin or obstructive, we studied DBA/2J mice implanted with a tracheal T-tube to provide a surrogate airway during seizure activity. Preserving an airway would prevent obstructive apnea from causing death but would not be expected to impact central apnea.
METHODS
All procedures were approved by an Animal Care and Use Committee and conducted in accordance with the United States Public Health Service’s Policy on Humane Care and Use of Laboratory Animals.
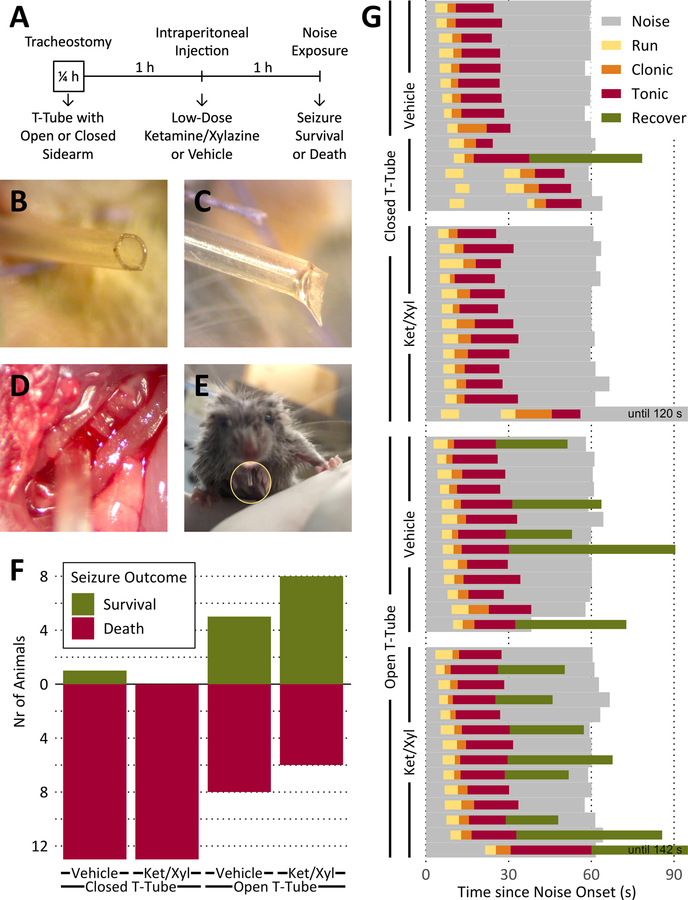
Our study was a 2×2 fully-crossed design, with 54 animals divided into 4 groups of 13 or 14 each: Equal numbers of animals were implanted with a T-tube with either an open sidearm (to provide a surrogate airway during seizure) or a closed sidearm (surgery control). Equal numbers again were injected 1 h later with either low-dose ketamine/xylazine (to moderate thoracic spasm) or saline (vehicle control). Another 1 h later, audiogenic seizures were induced by noise exposure, and survival or death was scored as the outcome (Fig. 1A). Death was determined from observation of complete ear relaxation without spontaneous breathing for ≥ 1 min, followed by necropsy. All animals were tested in room air.
Tracheal implants in DBA/2J mice protect against death from audiogenic seizures.
A. Time course of individual experiments.
B. Open sidearm of a tracheal implant.
C. Closed sidearm of a tracheal implant.
D. T-tube implanted into the exposed trachea (running from bottom left to top right) during surgery.
E. DBA/2 mouse after recovery from surgery with tracheal implant protruding from the neck (inside yellow circle).
F. Increased survival rates from audiogenic seizures after implanting DBA/2 mice with closed or open tracheal T-tubes (as a surrogate airway during seizure-induced laryngospasm) and injecting with vehicle or low dose ketamine/xylazine (Ket/Xyl; to moderate thoracic spasm).
G. No change in course of seizure phases with either T-tube or drug treatment. There were no statistical differences in the durations of the clonic or tonic phases between groups. One animal received a 2 min tone, but ear relaxation indicating imminent death occurred in < 1 min. Noise = noise source on; Run = uncontrolled, wild running; Clonic = mouse on its side with clonic limb movements; Tonic = full tonic hindlimb extension until ear relaxation (endpoint for mice that die); Recover = period until righting (survivors only).
T-shaped tracheal tubes were made from 22 gauge polyurethane angiocaths, with one end of a 7–8 mm percutaneous sidearm glued with cyanoacrylate at a right angle into an opening centered in a 2–3 mm intratracheal piece while the other end was also left open (Fig. 1B) or heat-sealed (Fig. 1C). The T-tubes were implanted under isoflurane anesthesia (2–4% in oxygen) into a ventral tracheotomy window between the 2nd and 5th tracheal rings (Fig. 1D) of DBA/2 seizure-prone mice (6–11 g, 8.2 ± 0.8 g; 19–22 days old, 20.1 ± 0.7 days old; 19 female, 35 male; Jackson Laboratories, Bar Harbor, ME) and secured with a figure-of-eight stitch of 7–0 polyglactin suture through the strap muscles above the T-tube. After the skin was similarly closed, the open or closed end of the sidearm protruded by ~ 5 mm (Fig. 1E). Surgeries lasted 10–30 min/animal (15 ± 3.9 min/animal). The immediate post-operative survival rate was 100%, and no laryngospasms or other respiratory abnormalities were observed.
A single dose of either ketamine/xylazine (1/10th of anesthetic dose: 8.5 mg/kg and 1.5 mg/kg) or saline (vehicle) was injected intraperitoneally to moderate ictal spastic contractions of the thoracic skeletal musculature (thoracic spasm), a condition similar to electrical asphyxiation14, 15 and a possible confound of the open-vs.-closed-T-tube comparison.
The rationale for the ketamine/xylazine treatment came from our experience in pilot experiments where animals were implanted with T-tubes under a combination of ketamine (85 mg/kg) and xylazine (15 mg/kg) anesthesia. Animals tested too early after surgery did not display seizure activity in response to the seizure-inducing tone, however, seizures induced postoperatively in these animals suggested that a low dose of this combination moderated ictal thoracic spasm. T-tube implantation under isoflurane anesthesia (2–4% in oxygen) permitted faster recovery and earlier responsiveness to tone presentation and was used for all study animals.
Audiogenic seizures were induced by exposure to 1 min of 115–120 dB noise from a personal alarm device (PAL-1, Tbotech Safety & Security, Bonita Springs, FL) in a ~1 cu ft acrylic chamber (Ikea SYNAS LED lighting box) lit from below and two sides, and the outcome (survival or death) was scored. Video (Canon Powershot S100) of the animals’ behavior, starting with 2 min of silence before noise exposure and continuing for at least 1 min afterwards, was used to measure the duration of each seizure phase (running, clonic, tonic, and recovery or death4). Animals that survived the audiogenic seizures were euthanized with a barbiturate overdose (Euthasol, 150 mg/kg). Every animal was examined postmortem to confirm the T-tube’s patency and secure placement in the trachea.
Exact procedures in StatXact software (Cytel, Cambridge, MA) were used to estimate the common odds ratio for the two-way contingency table of seizure outcome vs. T-tube treatment stratified by drug treatment and to perform a Cochran-Armitage trend test of drug effects. Fisher’s exact tests16 were performed with R software (https://www.r-project.org).
RESULTS
Open tracheal tubes increased survival rates in mice experiencing audiogenic seizures that included tonic hindlimb extension. Open tube implants in animals given a single postoperative 1/10th dose of ketamine/xylazine (“low-dose” k/x) protected 8/14 animals from dying and protected 5/13 animals given saline (Fig. 1F). The impact of the tracheal implant by itself on survival was highly significant (p=0.0004 for the common odds ratio for both drug strata; odds ratio = 24.14). While low-dose ketamine/xylazine by itself did not protect any animal against dying (odds ratio = 1.47, NS), the k/x treatment did have a secondary protective effect in combination with the open T-tube treatment (Cochran-Armitage trend test, two-sided p=0.0004, p=0.0301 with the single treatment order reversed; Fisher’s Exact tests with Bonferoni-Holm corrections for multiple comparisons: p=0.0072 k/x open vs k/x closed, p=0.0006 for all open vs all closed). Neither the T-tube nor the drug treatment changed the time course of the audiogenic seizure phases (Fig. 1G).
As an alternative method for minimizing confounding factors such as thoracic spasm or tube malfunction, we retested a set of 16 animals (5 from the original set) that survived an initial seizure with an open tube implant and no drug. When the tracheal tube was sealed before the second test, every animal died as a result of the seizure (n=7). By comparison, only 1 of 9 animals died when retested with the tube still open (Fisher exact p= 0.0014; odds ratio using 0.5 to correct for 0 = 112.0).
CONCLUSIONS
Survival tracheostomy could prevent death in DBA/2J mice experiencing audiogenic seizure activity that would otherwise be lethal indicating that obstructive apnea occurs during audiogenic seizures in mice. Some animals implanted with open tracheal tubes, however, still died. The basis for these deaths was severe seizure-associated spastic contraction of the skeletal musculature of the thorax and, normally, these two mechanisms co-exist. Whereas a low dose of ketamine/xylazine appeared to reduce the contribution of thoracic spasm to death, it was not necessary or sufficient for survival. Whereas NMDA receptor antagonists have been shown to prevent spreading depolarization and apnea17, this cannot be the explanation for the survival in k/x treated animals. Only eliminating obstructive apnea with the surrogate airway saved lives. The secondary test, which focused on animals that survived one seizure with an open tube (and thus naturally had an incidental level of thoracic spasm or any other confound) all died when retested with a closed tube demonstrates the protective effect of the surrogate airway very clearly.
Our results show that seizure activity in DBA/2J mice prevents breathing by (1) inducing laryngospasm to cause obstructive apnea10 and (2) thoracic spasm to restrict respiratory movements until respiratory arrest occurs. If seizure activity induced central apnea, which progressed to respiratory arrest, there would be no benefit of the open tube in any animals. Our results suggest that alterations in serotonergic neuronal activity3, 8, 9, 18, 19 and/or spreading depolarization6 may be the mechanism of respiratory arrest after a period of seizure-induced obstructive apnea. Based on our data for DBA/2 mice, our work on urethane anesthetized rats, and our demonstration of inspiratory effort EMG signals and EKG abnormalities in rats and humans, we conclude that seizure-induced laryngospasm and associated obstructive apnea are the direct links between seizure activity and respiratory arrest in these examples of sudden death.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH/NINDS (NS104796) and philanthropic contributions.
Footnotes
CONFLICTS OF INTEREST
None of the authors has conflicts to disclose.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention Lancet Neurol. 2016. September;15(10):1075–1088. [DOI] [PubMed] [Google Scholar]
- 2.Barot N, Nei M. Autonomic aspects of sudden unexpected death in epilepsy (SUDEP) Clin Auton Res. 2018. November 19. [DOI] [PubMed] [Google Scholar]
- 3.Richerson GB, Buchanan GF. The serotonin axis: Shared mechanisms in seizures, depression, and SUDEP Epilepsia. 2011. January;52 Suppl 1:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faingold C, Tupal S, N’Gouemo P. Genetic Models of Reflex Epilepsy and SUDEP in Rats and Mice In: Pitkänen A, Buckmaster PS, Galanopoulou AS, Moshé SL, Models of seizures and epilepsy. Second edition ed. London: Elsevier/Academic Press; 2017. p. 441–453. [Google Scholar]
- 5.Escayg A, Goldin AL. Sodium channel SCN1A and epilepsy: mutations and mechanisms Epilepsia. 2010. September;51(9):1650–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aiba I, Noebels JL. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models Sci Transl Med. 2015. April 8;7(282):282ra246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong J, Chuang SC, Bianchi R, Zhao W, Paul G, Thakkar P, et al. Regulatory BC1 RNA and the fragile X mental retardation protein: convergent functionality in brain PloS one. 2010. November 23;5(11):e15509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tupal S, Faingold CL. Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice Epilepsia. 2006. January;47(1):21–26. [DOI] [PubMed] [Google Scholar]
- 9.Uteshev VV, Tupal S, Mhaskar Y, Faingold CL. Abnormal serotonin receptor expression in DBA/2 mice associated with susceptibility to sudden death due to respiratory arrest Epilepsy Res. 2010. February;88(2–3):183–188. [DOI] [PubMed] [Google Scholar]
- 10.Nakase K, Kollmar R, Lazar J, Arjomandi H, Sundaram K, Silverman J, et al. Laryngospasm, central and obstructive apnea during seizures: Defining pathophysiology for sudden death in a rat model Epilepsy Res. 2016. October 27;128:126–139. [DOI] [PubMed] [Google Scholar]
- 11.Stewart M, Kollmar R, Nakase K, Silverman J, Sundaram K, Orman R, et al. Obstructive apnea due to laryngospasm links ictal to postictal events in SUDEP cases and offers practical biomarkers for review of past cases and prevention of new ones Epilepsia. 2017;58(6):e87–e90. [DOI] [PubMed] [Google Scholar]
- 12.Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study Lancet Neurol. 2013. October;12(10):966–977. [DOI] [PubMed] [Google Scholar]
- 13.Lacuey N, Vilella L, Hampson JP, Sahadevan J, Lhatoo SD. Ictal laryngospasm monitored by video-EEG and polygraphy: a potential SUDEP mechanism Epileptic Disord. 2018. April 1;20(2):146–150. [DOI] [PubMed] [Google Scholar]
- 14.Lee WR, Zoledziowski S. Effects of Electric Shock on Respiration in the Rabbit Br J Ind Med. 1964. April;21:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee WR. The Mechanisms of Death from Electric Shock Med Sci Law. 1965. January;5:23–28. [DOI] [PubMed] [Google Scholar]
- 16.Agresti A. Categorical data analysis. 3rd ed Hoboken, NJ: Wiley; 2013. [Google Scholar]
- 17.Jansen NA, Schenke M, Voskuyl RA, Thijs RD, van den Maagdenberg A, Tolner EA. Apnea Associated with Brainstem Seizures in Cacna1a (S218L) Mice Is Caused by Medullary Spreading Depolarization J Neurosci. 2019. November 27;39(48):9633–9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchanan GF, Murray NM, Hajek MA, Richerson GB. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality J Physiol. 2014. October 1;592(19):4395–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mooney S, Kollmar R, Gurevich R, Tromblee J, Banerjee A, Sundaram K, et al. An oxygen-rich atmosphere or systemic fluoxetine extend the time to respiratory arrest in a rat model of obstructive apnea Neurobiol Dis. 2019. November 20;134:104682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.