Abstract
The aim of this study was to measure muscle oxygen saturation (SmO2) dynamics during a climbing specific task until failure in varying conditions. Our prediction was that SmO2 should be a good marker to predict task failure. Eleven elite level climbers performed a finger-hang test on a 23 mm wooden rung under four different weighted conditions, 1. body weight (BW), 2. body weight +20% (BW +20), 3. body weight −20% (BW −20) and 4. body weight −40% (BW −40), maintaining half crimp grip until voluntary exhaustion. During each trial SmO2 and time to task failure (TTF) were measured. TTF was then compared to the minimally attainable value of SmO2 (SmO2min) and time to SmO2min (TTmin). There is a considerable degree of agreement between attainable SmO2min at high intensity conditions (MBW = 21.6% ± 6.4; MBW+20 = 24.0% ± 7.0; MBW−20 = 23.0% ± 7.3). Bland-Altman plot with an a priori set equivalency interval of ±5% indicate that these conditions are statistically not different (MBW-BW + 20 = −2.4%, 95% CI [1.4, −6.2]; MBW−Bw−20 = −1.3, 95% CI [2.5, −5.1]). The fourth and lowest intensity condition (MBW −40 = 32.4% ± 8.8) was statistically different and not equivalent (MBW-BW −40 = −8.8%, 95% CI [−5.0, −12.6]). The same agreement was found between TTF and TTmin for the high intensity conditions plotted via Bland-Altman. While the rate with which oxygen was extracted and utilised changed with the conditions, the attainable SmO2min remained constant at high intensity conditions and was related to TTF.
Subject terms: Metabolism, Predictive markers
Introduction
Failure in elite sport climbing is often associated with a fall as a result of the inability to maintain the required force of isometric muscle contraction following repeated efforts1. For this reason, isometric force production and maintenance in sport climbing has been a growing area of interest for sport scientists and exercise physiologists. This is further underscored by the fact that traditional forms of non-sport specific athletic testing such as VO2max assessments or blood lactate analysis have been unsuccessful in determining sport specific fitness2–5. This is likely due to the highly sport specific muscular demand and muscular development that is required for sport climbing disciplines3,6,7. Near-infrared spectroscopy (NIRS) as a tool to assess local oxidative capacity has proven to be useful8–11 and has rightfully garnered more interest to investigate local muscle physiological dynamics due to its non-invasive and simple application to in vivo and in situ diagnostics. NIRS has been compared to measurements of phosphorus magnetic resonance spectroscopy (P-MRS) to assess PCr dynamics with a good degree of agreement10, as well as in comparison with in situ high-resolution respirometry to assess mitochondrial respiratory capacity11. This capability has allowed a highly focused pursuit in the understanding of local muscular capacity for performance, especially in this case the importance of local oxidative capacity in sport climbing12–16. A greater local oxidative capacity has shown to enhance sport specific strength and endurance when comparing novice and expert climbers13, with a considerable degree of predictive power to determine climbing level; linear regression model predicting Red-point grade (IRCRA Scale) from tissue oxygen resaturation rate: y = −0.351 + 14.121, R2 = 0.2413. Numerous studies look both at isometric holds and repeated or intermittent isometric holding as performance limiting components and their effect on NIRS dynamics12,15,17. Specifically, sustained isometric holds on static gripping tasks have shown to result in NIRS dynamics that indicate loss of force production16. During a finger-hang test, similar to the one conducted in this experiment, Balas et al.18 identified that this test, from a series of tests, explained the greatest portion of the performance variance (70%) in a climbing population. Applying this knowledge to simple in vivo and in situ tests during isometric finger-hangs, it is possible that NIRS dynamics could be used to assess task failure during measurement situations, which in turn could be a useful tool for both training and competition.
For these predictions the minimally attainable muscle oxygen saturation (SmO2min) can be used as a pragmatic surrogate for oxidative capacity8,14,16,19. If oxidative capacity is an individual, but constant volume, then the rate of muscle deoxygenation should increase but SmO2min would remain constant near to the point of task failure. This highlights two research hypothesis; 1. during a sport specific performance task SmO2min remains constant over varying workloads; and 2. time to SmO2min (TTmin) is equal to time to task failure (TTF).
Methods
Experimental approach to the problem
A repeated measures design was applied to assess muscle oxygen (SmO2) dynamics as measured by NIRS during sport specific finger-hang tests. The purpose of this design was to elicit a force-duration relationship in order to assess physiological and performance related changes over varying intensities. A classical performance-duration relationship is curvilinear flattening to an asymptote with a curve constant. The curve constant represents something in the form of work capacity over this asymptote. SmO2min reflects this work capacity and the change in energetics and metabolite accumulation associated with it20. SmO2min is the primary variable assessed in this study due to the relationship between NIRS signal range and oxidative capacity discussed earlier. Therefore, each participant partook in four trials of the finger-hang test under four separate conditions; body weight (BW), body weight +20% (BW +20%), body weight −20% (BW −20%) and body weight −40% (BW −40%). Each trial was separated by at least 20 minutes of rest, similar to finger-hang test protocols in other studies12,15. All participants started with the BW trial and then the following three trials were in randomized order to eliminate a serial effect. The BW trial was used as the reference trial for comparison against the remaining three trials. Adipose tissue thickness at the sensor location was deemed unproblematic considering the source-detector distance of 25 mm (penetration depth of 12.5 mm)21,22. All participants were asked to refrain from strenuous physical activity 24 hours prior to the experiment, to refrain from alcohol consumption, caffeine consumption and smoking 24 hours prior to the experiment and to maintain individual diet routine.
Participants
The participants were all male elite level climbers (n = 11; ±SD age 24 ± 4.0 years; height 172.9 ± 7.1 cm; weight 65.7 ± 2.5 kg; climbing level: redpoint 27 ± 2.5 IRCRA23). Climbing level was assessed through a questionnaire and was defined by self-reported achievements within the last 3 months. The select population was, therefore well prepared for the physical tasks presented by the data collection minimizing potential risk of injury. All participants were in good health, non-smokers and unmedicated. The participants were informed of the study design and the physical tasks ahead of time and written informed consent was obtained in advance. The study was carried out in accordance with the 1964 Declaration of Helsinki. The University of Bern faculty of Human Sciences Ethics Commission approved the protocol (Nr. 2018-03-00009).
Warm-up
All participants conducted a standard warm-up. First, a 3-minute bike ride with 75 watts resistance at 75–80 rpm. Then two sets of 10 repetitions self-selected (5–10 kg) forearm curls and two sets of 10 repetitions of finger extensions using a TheraBand, followed by self-selected arm-hand-finger mobilisation exercises. Finally, three minutes of self-selected trials on the hang-board to familiarise themselves with the equipment. Participants were informed to avoid over excretion during warm-up. Considering the expertise and fitness level of the participants this was considered unproblematic.
Finger-hang test
A finger-hang test was selected for this study as Balas et al.18 showed that in their model the finger-hang test alone explained approximately 70% of the variance in climbing performance. The finger-hang test was conducted on a commercially available hang-board (AIX, CZ). Participants were informed of the procedure and were instructed to maintain their grip on the hang board with both hands for as long as possible. Minor readjustments during a trial were allowed, as long as both hands remained on the rung and in the same effective position. The hand position applied by all participants was a half crimp; the thumb was not used. A 23 mm rung depth was used for all the trails, following the procedure of a series of previous publication17,24. For every trial the participants were allowed to apply commercially available climbing chalk (magnesium carbonate). Prior to every trial the holding rung was cleaned and cleared of excess chalk. The participants were asked to take a standing position with their arms down and to the side in a natural posture in front of the hang-board where base-line physiological measurements were recorded for 30 seconds. The participants were then asked to prepare themselves on the holding rung. On command the participants lifted their legs and took on the hanging position and the task-time measurement started. The task was considered failed and the timer stopped if either a hand left the hang-board or when a foot returned to the floor. After failure two minutes of data recording followed where the participant again took a natural standing position with arms down and to the side. The same procedure was followed for all the trials and conditions. For the +20%BW condition the participants were equipped with a commercially available climbing harness and the appropriate amount of weight was added using a carabiner and a sling around the belay loop. For the −20%BW and −40%BW conditions a single-wheel pulley system was setup directly behind the hang-board. The participant was attached to one end of the pulley via the climbing harness (belay loop) and the appropriate amount of weight was added to the second end. The pulley system was evaluated and adjusted for mechanical advantage and the appropriate weight was used.
Near-infrared spectroscopy
NIRS sensors were placed on the forearm flexor digitorum profundus (FDP) muscles, which can be palpated on the anterior side of the forearm one-third between the medial epicondyle of the humerus and the styloid process of the ulna; discussed in point-counterpoint discussion25,26 and considering the importance and relevance of the NIRS probe placement the clear guidelines by Fryer et al.13 was used. The flexor groups, including the FDP have their primary function in finger flexion for the pre-determined task27,28. The sensors were fixed in place using medical adhesive tape (Hypafix; BSN Medical, DE) and were then covered with the compatible commercially available light shield to eliminate possible ambient light intrusion. A commercially available continuous wave NIRS device was used (Moxy Monitor; Fortiori Designs LLC, US) to measure muscle oxygenation. The device uses four wave lengths (680,720,760 and 800 nm) to assess absorbency of oxyhemoglobin (O2Hb) and oxymyoglobin (O2Mb) and deoxyhemoglobin (HHb) and deoxymyoglobin (HMb), via modified Beer-Lambert resulting in a concentration of SmO2 as a percent in the following equation:
Myoglobin and hemoglobin cannot be differentiated using NIRS, and therefore as shown in the equation above, are always evaluated together29. SmO2 is a ratio of relative O2Hb + O2Mb and relative HHb + HMb on an absolute scale of 0–100%21. The device detectors are spaced at 12.5 mm and 25 mm from the emitter. Sampling rate was set at default mode which samples the four wave lengths over 80 cycles for an averaged output every two seconds (0.5 Hz) and gathered using the SWINCO NIRS software (Swinco AG, CH).
Statisical analyses
In order to assess the agreement between trials Bland-Altman plots were constructed to present the data. As the goal of the statistical analyses was to find agreement rather than difference Bland-Altman plots were specifically chosen to address the question, as agreement is not within the scope of traditional inference statistics. Where relevant significance level was set at 0.05. The four conditions were compared for agreement as follows: SmO2min BW against SmO2min of all the remaining conditions and TTF against TTmin for all conditions. Upper and lower limits of agreement were set at 1.96 SD with recommended 95% confidence intervals (CI)30. Additionally, a priori equivalency intervals (EI) were set at the recommended minimally detectable change of ±5%17 in agreement with other device specific recommendations31 for the SmO2min analysis. In order to determine SmO2min a least squares piecewise regression analysis was conducted to find the break point at the minimally attainable SmO2 (Fig. 1). Similar to the results of isometric holds in other data collections24 two clear phases of deoxygenation can be seen and therefore the piecewise regression was set up to establish four knots. TTmin was then set at the corresponding point in time. A Shapiro-Wilks test was selected due to the small sample size to ensure all data were normally distributed. Statistical computations were performed using Microsoft Excel for Windows (Version 16.0.4738.1000) and MathWorks Matlab for Windows (Version 9.3.0.713570 R2017b). For the purpose of this paper the dominant hand was used for statistical analysis, as the dominant hand in comparison to non-dominant hand in climbers shows significant variation in regard to oxygenation kinetics28.
Figure 1.
Piecewise regression analysis identifying SmO2min and TTmin from a select participant for all four trials; (A) body weight; (B) body weight +20%; (C) body weight −20%; (D) body weight −40%. Participants exhibited the expected pattern of two phases of decreasing SmO2 slopes. In order to determine the SmO2min point a four knots solution was applied to identify start and end, as well as shift from phase 1 to phase 2 and then the minimum point in phase 2.
Results
The four test conditions resulted in the expected force-duration relationship predicted by the study design (Table 1). Evaluating the graphical output of the Bland-Altman analysis for SmO2min (Fig. 2) at high intensity a consistent SmO2min is reached regardless of duration of the finger-hang. An acceptable agreement can be discerned in both BW +20 and BW −20 conditions against the BW condition. Both comparisons show no systemic bias against the line of equality indicating no statistical difference, and well bounded upper and lower limits of agreement to the a priori determined EI. In both cases, however statistical equivalency can not be clearly discerned as CI expand beyond the the a priori set EI.
Table 1.
Mean (±SD) for minimally attainable muscle oxygenation (SmO2min), time to SmO2min (TTmin) and time to failure (TTF) during the four experimental conditions. For statistical analysis see text.
| Trial 1: BW | Trial 1: BW +20 | Trial 1: BW −20 | Trial 1: BW −40 | |
|---|---|---|---|---|
| SmO2min (%) | 21.6 ± 6.4 | 24.0 ± 7.0 | 23.0 ± 7.3 | 30.4 ± 9.1 |
| TTmin (s) | 66.9 ± 20.8 | 39.7 ± 12.8 | 67.5 ± 33.1 | 112.6 ± 107.8 |
| TTF (s) | 77.5 ± 27.8 | 47.7 ± 12.4 | 106.4 ± 24.5 | 311.5 ± 209.0 |
Figure 2.
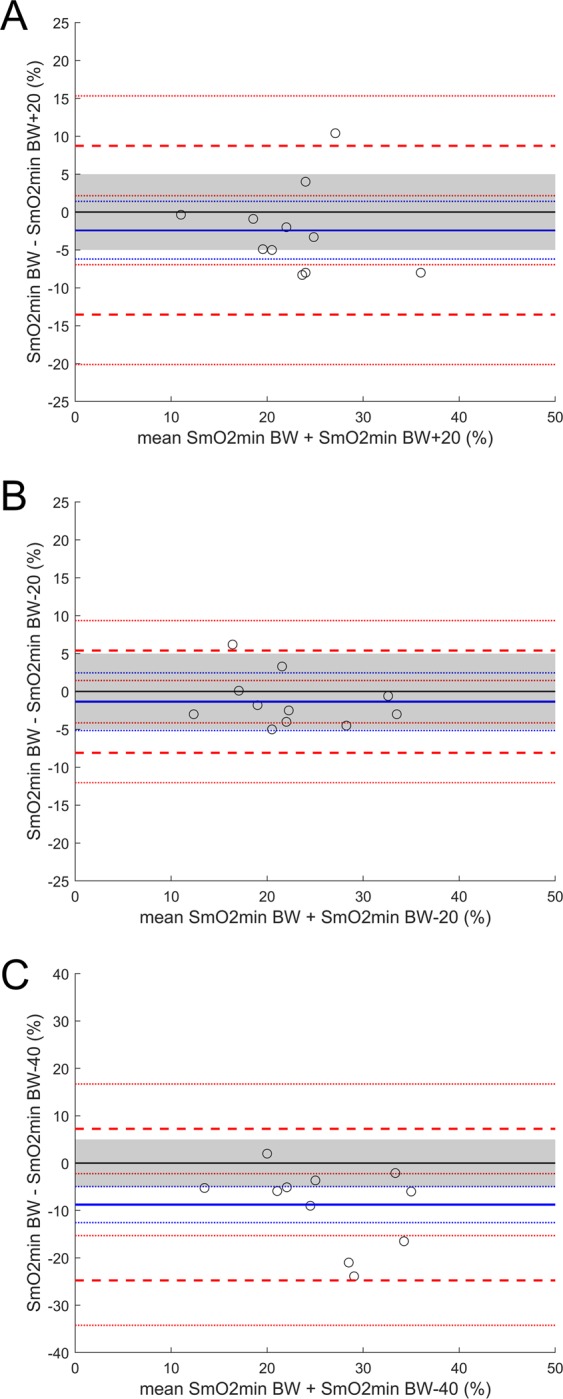
Bland-Altman plot looking at agreement between SmO2min during the four trial conditions with a priori equivalency interval (EI) set at ±5% (shaded area). The solid blue line identifies the mean bias (MB) and the dotted blue lines the 95% CI. The dashed red lines identify the upper and lower limits of agreement at ±1.96 SD with 95% CI. (A) MBW-BW + 20 = −2.4%, 95% CI [1.4, −6.2]; (B) MBW-Bw−20 = −1.3, 95% CI [2.5, −5.1]; (C) MBW-BW −40 = −8.8%, 95% CI [−5.0, −12.6].
For the BW −40 condition against the BW condition there was no statistical equivalency and a clear difference, with a systemic bias towards higher SmO2min values for the BW −40 condition (Fig. 2). As with SmO2min, for the high intensity trials, TTF and TTmin are comparable indicating SmO2min capacity and rate of depletion as a potential predictor of task failure. Comparison between BW and BW +20 (Fig. 3) show a good degree of agreement. These two conditions show no clear systemic bias from the line of equality and should not be considered statistically different, while a claim of equality remains absent. In both cases upper and lower limits of agreement remain well bounded with a tendency to greater values for TTF. While BW −40% condition to a much greater extent than the BW −20% condition, both are statistically different and not equivalent (Fig. 3). This illustrates, when comparing all conditions, a clear bias towards increasing TTF vs. TTmin with increasing intensity.
Figure 3.
Bland-Altman plot looking at agreement between TTF and TTmin during the four trial conditions. The solid blue line identifies the mean bias (MB) and the dashed blue lines the 95% CI. The dashed red lines identify the upper and lower limits of agreement at ±1.96 SD with 95% CI. (A) MBW TTF-BW TTmin = 10.6 s, 95% CI [19.4, 1.9]; (B) MBW + 20 TTF-BW + 20 TTmin = 8.0 s, 95% CI [16.5, −0.5]; (C) MBW−20 TTF-BW−20 TTmin = 38.9 s, 95% CI [62.8, 15.0]; (D) MBW −40 TTF-BW −40 TTmin = 158.1 s, 95% CI [265.0, 51.2].
Discussion
The results of this study show that for the three high intensity conditions, isometric muscle contraction results in the same SmO2min value at the point of failure. These results can be further extrapolated to confirm the relationship between TTF and TTmin, which also show good agreement. Considering these findings, a value as simple as SmO2min and rate of SmO2 change can be used to potentially predict failure during isometric muscle contraction. Similar results were reported during sustained static gripping tasks16, where maximum and minimum values for deoxyhemoglobin and oxyhemoglobin respectively were correlated to loss of force production.
In order to maintain isometric contraction a continuous utilization of energy stores must take place. This ATP breakdown is buffered by high energy phosphate transfer from PCr to ADP. Evidence supports that PCr is not only reconstituted via oxidative means but dependent on oxygen availability32,33. Considering the importance of oxidative capacity on energy flux in contracting muscles it stands to reason that muscle oxygenation as measured by NIRS is a suitable tool to assess and predict muscle contraction failure for a given task. This argument is further enhanced by the relationship between muscle oxidative capacity as measured by P-MRS and NIRS. For this reason, a plethora of studies have demonstrated the diagnostic qualities of NIRS, from threshold testing34,35, muscle damage analysis36 and high intensity interval response37,38. Similar physiological reasoning has been applied to NIRS measurements during sport climbing, highlighting the importance of specific NIRS derived parameters17. SmO2min as an indicator of muscle oxidative capacity and a predictor of performance for climbing specifically is discussed by both Balas et al.12 and Fryer et al.13. However, in a recent publication Balas et al.17 moves forward with caution in applying SmO2min as a reliable marker as in their findings it had a low reliability and large variation. It is important to note that Balas et al.17 identify the parameter as Tissue Saturation Index (TSI) minimum rather than SmO2min, which already may go a long way in clarifying the contradictory results. The results of this study show a consistency in SmO2min at high intensity efforts. The NIRS device used in this experiment presumes to isolate the muscle tissue through an a priori Monte Carlo modelling for different tissue properties and photon path-length variations using the four wavelengths identified21. In this way, variations such as cutaneous and subcutaneous blood flow, as well as dilution from low metabolically active signals can be minimized and the resulting parameter is called SmO2. The device used by Balas et al.17 assumes homogeneity and infinite tissue and therefore does not distinguish between muscle tissue, adipose and skin resulting in the parameter TSI22. This discrepancy may be the reason for the discussion at hand. Here it is important to note that there is no consensus gold standard for SmO2 or TSI measures.
While for the high intensity conditions the hypothesis holds true, it is evidently clear that at lower intensities the paradigm fails to be applicable. This is potentially caused by a multitude of factors. The first which needs to be further evaluated is a study design limitation, in that performance was controlled externally, but not effectively measured. Considering the experimental population, if the task is set at merely maintaining hand position and not falling, the ability to release tension between the left and right hand could explain the volatility in the NIRS data at low intensity. Both sustained static gripping16 and in repeated hand grip activity14 showed changes in NIRS signal with decreases in performance output; therefore, it is safe to assume that if performance output does not remain constant SmO2 dynamics will vary. It is almost certain that at low intensities force exerted on the wooden rung was not constant between left and right hand. Tightly linked to the first comment, lower contraction force or changing contraction force offers the increased opportunity for reperfusion. Numerous studies discuss the effect of blood flow and blood volume changes on the NIRS signal and its confounding effects39,40. It is highly likely that in comparison to the higher intensity bouts, the low intensity bouts allow for a greater degree or chance of muscle reperfusion. More investigation is needed into the ability to predict performance at lower and varying intensity work rates using NIRS.
The ability to evaluate task success and failure using NIRS, during sport specific applications, could provide training and competition insight. It could provide trainers and athletes pacing strategies or rest planning strategies. Furthermore, it could provide alternative metrics to coordinate trainings which include interval type sessions. While more applied research is needed to find the true cost-benefit analysis of these tools, it is a conceivable path forward.
Conclusion
A growing body of research supports the reliable application of NIRS to assess performance in sport climbing13,17,41,42. NIRS specific parameters can reasonably be applied to training processes and performance diagnostics to further develop athletes and educate coaches on physiological processes. While it is not clear which dynamics or specific parameters will be most beneficial in terms of diagnostics or training coordination it is clear that a relationship exists. In the specific case of this investigation it would appear that simply understanding the limit of SmO2 available to an athlete an a priori in situ prediction could be made with a certain degree of confidence at high intensity efforts. This can then be applied as mentioned to training coordination and diagnostics, or perhaps even in-field or in-event failure predictions.
Acknowledgements
The research was supported by Idiag AG (CH). Further, we would like to thank colleagues from our institute for know-how and insight which helped in the developed of procedures and data collection and analysis.
Author contributions
Study design, ethical considerations, and participant requirement followed by data collection was conducted by all authors. Data management was conducted by A.F. and S.P. Data analysis and statistical methodology was conducted by A.F. and D.E. A.F. wrote the manuscript with input from S.P. and D.E. All authors partook in editing and manuscript discussion. R.L. was instrumental in additional data collection and analysis during the manuscript review process and was therefore added as an additional author during this process.
Competing interests
The first author is a co-developer of the NIRS device (Moxy Monitor; Fortiori Designs LLC, USA) used in this paper. The first author in addition to his position as an assistant at the University of Bern is an employee of the European retailer (Idiag AG, CH) of the NIRS device used in this paper.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giles LV, Rhodes EC, Taunton JE. The physiology of rock climbing. Sports Med. 2006;36:529–545. doi: 10.2165/00007256-200636060-00006. [DOI] [PubMed] [Google Scholar]
- 2.Baláš J, et al. The Relationship between Climbing Ability and Physiological Responses to Rock Climbing. Sci. World J. 2014;2014:1–6. doi: 10.1155/2014/678387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Geus B, Villanueva O’Driscoll S, Meeusen R. Influence of climbing style on physiological responses during indoor rock climbing on routes with the same difficulty. Eur. J. Appl. Physiol. 2006;98:489–496. doi: 10.1007/s00421-006-0287-5. [DOI] [PubMed] [Google Scholar]
- 4.Sheel AW. Physiology of sport rock climbing. Br J Sports Med. 2004;38:355–359. doi: 10.1136/bjsm.2003.008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watts PB. Physiology of difficult rock climbing. Eur J Appl Physiol. 2004;91:361–372. doi: 10.1007/s00421-003-1036-7. [DOI] [PubMed] [Google Scholar]
- 6.Fanchini M, Violette F, Impellizzeri FM, Maffiuletti NA. Differences in Climbing-Specific Strength Between Boulder and Lead Rock Climbers. J Strength Cond Res. 2013;27:310–314. doi: 10.1519/JSC.0b013e3182577026. [DOI] [PubMed] [Google Scholar]
- 7.Green JG, Stannard SR. Active Recovery Strategies and Handgrip Performance in Trained Vs. Untrained Climbers. J Strength Cond Res. 2010;24:494–501. doi: 10.1519/JSC.0b013e3181c06af3. [DOI] [PubMed] [Google Scholar]
- 8.Hamaoka T, McCully KK, Quaresima V, Yamamoto k, Chance B. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt. 2007;12:062105. doi: 10.1117/1.2805437. [DOI] [PubMed] [Google Scholar]
- 9.Nagasawa T, et al. A practical indicator of muscle oxidative capacity determined by recovery of muscle O 2 consumption using NIR spectroscopy. Eur J Sport Sci. 2003;3:1–10. doi: 10.1080/17461390300073207. [DOI] [Google Scholar]
- 10.Ryan TE, Southern WM, Reynolds MA, McCully KK. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J Appl Physiol. 2013;115:1757–1766. doi: 10.1152/japplphysiol.00835.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan TE, Brophy P, Lin C-T, Hickner RC, Neufer PD. Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: a comparison with in situ measurements. J Physiol. 2014;592:3231–3241. doi: 10.1113/jphysiol.2014.274456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baláš J, et al. Active recovery of the finger flexors enhances intermittent handgrip performance in rock climbers. Eur J Sport Sci. 2016;16:764–772. doi: 10.1080/17461391.2015.1119198. [DOI] [PubMed] [Google Scholar]
- 13.Fryer S, et al. Forearm muscle oxidative capacity index predicts sport rock-climbing performance. Eur J Appl Physiol. 2016;116:1479–1484. doi: 10.1007/s00421-016-3403-1. [DOI] [PubMed] [Google Scholar]
- 14.Nakada M, et al. Relationships between Force Curves and Muscle Oxygenation Kinetics during Repeated Handgrip. J Physiol Anthropol Appl Human Sci. 2004;23:191–196. doi: 10.2114/jpa.23.191. [DOI] [PubMed] [Google Scholar]
- 15.Philippe M, Wegst D, Müller T, Raschner C, Burtscher M. Climbing-specific finger flexor performance and forearm muscle oxygenation in elite male and female sport climbers. Eur J Appl Physiol. 2012;112:2839–2847. doi: 10.1007/s00421-011-2260-1. [DOI] [PubMed] [Google Scholar]
- 16.Yamaji S, Demura S, Nagasawa Y, Nakada M. Relationships between decreasing force and muscle oxygenation kinetics during sustained static gripping. J Physiol Anthropol Appl Human Sci. 2004;23:41–47. doi: 10.2114/jpa.23.41. [DOI] [PubMed] [Google Scholar]
- 17.Baláš J, Kodejška J, Krupková D, Hannsmann J, Fryer S. Reliability of Near-Infrared Spectroscopy For Measuring Intermittent Handgrip Contractions in Sport Climbers. J Strength Cond Res. 2018;32:494–501. doi: 10.1519/JSC.0000000000002341. [DOI] [PubMed] [Google Scholar]
- 18.Baláš J, Pecha O, Martin AJ, Cochrane D. Hand–arm strength and endurance as predictors of climbing performance. Eur J Sport Sci. 2012;12:16–25. doi: 10.1080/17461391.2010.546431. [DOI] [Google Scholar]
- 19.Ferrari M, Muthalib M, Quaresima V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos Trans R Soc A Math Phys Eng Sci. 2011;369:4577–4590. doi: 10.1098/rsta.2011.0230. [DOI] [PubMed] [Google Scholar]
- 20.Jones AM, Wilkerson DP, DiMenna F, Fulford J, Poole DC. Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. Am J Physiol Regul Integr Comp Physiol. 2008;294:585–593. doi: 10.1152/ajpregu.00731.2007. [DOI] [PubMed] [Google Scholar]
- 21.Feldmann A, Schmitz R, Erlacher D. Near-infrared spectroscopy-derived muscle oxygen saturation on a 0% to 100% scale: reliability and validity of the Moxy Monitor. J Biomed Opt. 2019;24:1–11. doi: 10.1117/1.JBO.24.11.115001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Beekvelt MC, Borghuis MS, van Engelen BG, Wevers RA, Colier WN. Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscle. Clin Sci. 2001;101:21–28. doi: 10.1042/cs20000247. [DOI] [PubMed] [Google Scholar]
- 23.Draper, N. et al. Comparative grading scales, statistical analyses, climber descriptors and ability grouping: International Rock Climbing Research Association Position Statement. Sports Technology, 10.1080/19346182.2015.1107081 (2016).
- 24.Kodejška J, Michailov ML, Baláš J. Forearm muscle oxygenation during sustained isometric contractions in rock climbers. AUC Kinanthro. 2016;51:48–55. doi: 10.14712/23366052.2015.31. [DOI] [Google Scholar]
- 25.Halsey T, Callender N. Comment on: Forearm oxygenation and blood flow kinetics during a sustained contraction in multiple ability groups of rock climbers. J Sports Sci. 2016;34:2153–2153. doi: 10.1080/02640414.2016.1227467. [DOI] [PubMed] [Google Scholar]
- 26.Fryer S, Stoner L. Rebuttal: near-infrared spectroscopy derived forearm oxygenation does predict rock climbing performance. J Sports Sci. 2016;34:2154–2154. doi: 10.1080/02640414.2016.1238132. [DOI] [PubMed] [Google Scholar]
- 27.Deyhle MR, et al. Relative Importance of Four Muscle Groups for Indoor Rock Climbing Performance. J Strength Cond Res. 2015;29:2006–2014. doi: 10.1519/JSC.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 28.Giles D, et al. Differences in Oxygenation Kinetics Between the Dominant and Nondominant Flexor Digitorum Profundus in Rock Climbers. Int J Sports Physiol Perform. 2017;12:137–139. doi: 10.1123/ijspp.2015-0651. [DOI] [PubMed] [Google Scholar]
- 29.Jöbsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 30.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet (London, England) 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 31.McManus CJ, Collison J, Cooper CE. Performance comparison of the MOXY and PortaMon near-infrared spectroscopy muscle oximeters at rest and during exercise. J Biomed Opt. 2018;23:015007. doi: 10.1117/1.JBO.23.1.015007. [DOI] [PubMed] [Google Scholar]
- 32.Haseler LJ, Hogan MC, Richardson RS. Skeletal muscle phosphocreatine recovery in exercise-trained humans is dependent on O 2 availability. J Appl Physiol. 1999;86:2013–2018. doi: 10.1152/jappl.1999.86.6.2013. [DOI] [PubMed] [Google Scholar]
- 33.Kemp GJ, Taylor DJ, Radda GK. Control of phosphocreatine resynthesis during recovery from exercise in human skeletal muscle. NMR Biomed. 1993;6:66–72. doi: 10.1002/nbm.1940060111. [DOI] [PubMed] [Google Scholar]
- 34.Grassi B, Quaresima V, Marconi C, Ferrari M, Cerretelli P. Blood lactate accumulation and muscle deoxygenation during incremental exercise. J Appl Physiol. 1999;87:348–355. doi: 10.1152/jappl.1999.87.1.348. [DOI] [PubMed] [Google Scholar]
- 35.Wang B, et al. Differences between the Vastus Lateralis and Gastrocnemius Lateralis in the Assessment Ability of Breakpoints of Muscle Oxygenation for Aerobic Capacity Indices During an Incremental Cycling Exercise. J Sports Sci Med. 2012;11:606–13. [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmadi S, Sinclair PJ, Foroughi N, Davis GM. Monitoring muscle oxygenation after eccentric exercise-induced muscle damage using near-infrared spectroscopy. Appl Physiol Nutr Metab. 2008;33:743–752. doi: 10.1139/h08-048. [DOI] [PubMed] [Google Scholar]
- 37.Jones B, Hamilton DK, Cooper CE. Muscle Oxygen Changes following Sprint Interval Cycling Training in Elite Field Hockey Players. PLoS One. 2015;10:e0120338. doi: 10.1371/journal.pone.0120338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zorgati H, et al. Effect of pedaling cadence on muscle oxygenation during high-intensity cycling until exhaustion: a comparison between untrained subjects and triathletes. Eur J Appl Physiol. 2015;115:2681–2689. doi: 10.1007/s00421-015-3235-4. [DOI] [PubMed] [Google Scholar]
- 39.Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. J Appl Physiol. 2012;113:175–183. doi: 10.1152/japplphysiol.00319.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wunsch SA, Muller-Delp J, Delp MD. Time course of vasodilatory responses in skeletal muscle arterioles: role in hyperemia at onset of exercise. Am J Physiol Heart Circ Physiol. 2000;279:H1715–1723. doi: 10.1152/ajpheart.2000.279.4.H1715. [DOI] [PubMed] [Google Scholar]
- 41.Crenshaw AG, Elcadi GH, Hellstrom F, Mathiassen SE. Reliability of near-infrared spectroscopy for measuring forearm and shoulder oxygenation in healthy males and females. Eur J Appl Physiol. 2012;112:2703–2715. doi: 10.1007/s00421-011-2244-1. [DOI] [PubMed] [Google Scholar]
- 42.Fryer S, et al. Differences in forearm strength, endurance, and hemodynamic kinetics between male boulderers and lead rock climbers. Eur J Sport Sci. 2017;17:1177–1183. doi: 10.1080/17461391.2017.1353135. [DOI] [PubMed] [Google Scholar]




