Abstract
Sarcopenia is an age-associated disease characterized by loss of muscle mass and function, but the diagnostic cutoff values remain controversial. To investigate the diagnostic cutoff values and incidence of sarcopenia in a plateau population, the limb skeletal muscle mass, gait speed and grip strength of 2318 Tibetan adults were measured according to the criteria of the Asian Working Group for Sarcopenia. We found that the diagnostic reference values for sarcopenia in the high-altitude population were significantly lower than those in the plain population, and the incidences of sarcopenia in the high-altitude population over 60 years old were 17.2% in men and 36.0% in women, which were significantly higher than those in the plain population. Our study proposes reference values for the diagnosis of sarcopenia in Tibet. We suggest that the cutoff value for sarcopenia in the plateau population should be established based on altitude. Hypoxia may be an important risk factor for sarcopenia.
Subject terms: Anatomy, Musculoskeletal system
Introduction
Sarcopenia is a progressive and generalized skeletal muscle disorder involving the accelerated loss of muscle mass and function that is associated with increased adverse outcomes, including falls, functional decline, frailty, and mortality1. With the ageing of the population, sarcopenia has become one of the major public health problems in the world2,3. Since 2010, the study of sarcopenia in the field of international geriatrics has become increasingly intense, focusing on the diagnostic criteria and pathogenesis of sarcopenia. Both the European and Asian sarcopenia working groups have recommended diagnostic criteria of sarcopenia based on muscle mass, muscle strength and physical function presented by skeletal muscle mass index (SMI), handgrip strength (HS) and gait speed (GS), respectively4,5. Currently, the incidence of sarcopenia and the cutoff values for SMI and HS in different populations are very different. The incidence of sarcopenia is related not only to age but also to racial differences. Ethnic factors may be one of the main reasons for the differences in the incidence of sarcopenia among different populations6–8. The European Working Group on Sarcopenia in Older People (EWGSOP) has suggested that primary sarcopenia is mainly related to ageing and is a phenotype in the process of biological senescence. Ageing is a sequential process of genetic programming, which inevitably results in a decrease in the amount of ageing muscle, but this process is affected by the environment, lifestyle and ageing-related diseases9 and shows significant individual differences. Therefore, ethnic and individual differences should be taken into account in determining diagnostic reference values for sarcopenia, and the best reference values should include a wide range of races and ages. Asia is the most dense and fastest ageing region in the world, and sarcopenia will have a very large impact on the Asian population5. The Asian Working Group for Sarcopenia (AWGS) has recommended that a large number of investigations should be carried out to provide a basis for the establishment of diagnostic criteria for sarcopenia in Asia10. However, there are still no reference values for sarcopenia in the plateau population. At the same time, weight and body composition have been shown to change at high altitudes11,12. Therefore, we investigated the reference values of Tibetans living on the Qinghai-Xizang Plateau in China and provided cutoff value for the diagnosis of sarcopenia in the plateau population.
Materials and Methods
Data sources and study participants
This was a cross-sectional study. In August 2016 and 2017, the survey was carried out with a health examination in Tibet, China. Healthy Tibetans aged over 20 years participated in this study. All participants lived in Tibet, China. The sample consisted of 2318 subjects (900 men and 1418 women), and the average age was 42.33 years. A total of 1355 subjects (555 men and 800 women) were from Lhasa (altitude of 3600 metres), and 963 subjects (345 men and 618 women) were from Shigatse (altitude of 4200 metres). All participants were in good health according to clinical medical evaluations. The following subjects were excluded from the study: 1. individuals with long-term bed rest, sedentary, or extreme weight loss; 2. individuals with heart, lung, liver, kidney, brain diseases, inflammatory reaction diseases, malignant tumours, and endocrine diseases; and 3. individuals with an absorption disorder, gastrointestinal disease, or anorexia drug use. The questionnaires collected information on demographics, lifestyle, history of diseases, physical examinations, activities of daily living and physical performance. These were just to collect demographic information and sample screening. The interviewers were trained before the survey was administered. The study was approved by the Research Ethics Committee of Jinzhou Medical University in accordance with the Declaration of Helsinki. Verbal and written informed consent was obtained from all participants.
Anthropometry
Height was measured using a portable stadiometer (HM200P, American Charder Company, America) and recorded to the nearest 0.1 cm. Body mass index (BMI) was calculated from weight and height.
Measurement of HS
HS was measured using a handheld dynamometer based on strain gauge sensors (CAMRY EH101; Xiangshan, China) to the nearest 0.1 kg. Both hands were tested with the participant seated, elbow flexed at a 110° angle, wrist placed in a neutral position, and the interphalangeal joint of the index finger positioned at a 90° angle. Two readings were obtained for each hand, and the highest value in either hand was used for the analyses. A preliminary study was conducted to assess the reliability of the HS test using the intraclass correlation coefficient. The results showed excellent test-retest reliability of the HS test13.
Measurement of GS
According to the criteria of AWGS, physical performance was assessed using the usual GS. To measure the usual GS, the participants were asked to walk a 5-metre course at their usual speed. Timing commenced when the participants started foot movement and stopped when the foot contacted the ground after completely crossing the 5-metre mark. Canes or walkers were allowed if necessary13.
Measurement of appendicular skeletal muscle mass and weight
A bioelectrical impedance analyser (MC-180, Bailida, Japan) was used to measure left upper limb skeletal muscle mass (LUSM), right upper limb skeletal muscle mass (RUSM), left lower limb skeletal muscle mass (LLSM), and right lower limb skeletal muscle mass (RLSM).
Sarcopenia cutoff value determination
According to the recommended diagnostic algorithm from the AWGS5, GS, HS and SMI are the primary indicators for a sarcopenia diagnosis.
SMI cutoff value
SMI was calculated as SMI = ASM/height2 (kg/m2). The appendicular skeletal muscle (ASM) was equal to the total muscle mass of the four limbs. Two standard deviations below the mean SMI in the young reference group (20–40 years old) was used as the SMI cutoff values determination5.
GS cutoff value
Low physical performance was defined as GS less than 0.8 m/s5.
HS cutoff value
The lower 20th percentile of handgrip strength in the study population was used as the cutoff value for low muscle strength5.
Sarcopenia classification
According to the recommended diagnostic algorithm of the AWGS, the subjects with low muscle mass without an impact on muscle strength or physical performance were classified as presarcopenia, and the subjects with low muscle mass along with low muscle strength or low physical performance were considered to have sarcopenia. The subjects without low muscle mass, low HS and low GS were classified as having “no sarcopenia”5.
Statistical analysis
The data, expressed as the mean ± standard deviation, were analyzed by independent t tests in the same age group. The chi-square test was used to determine whether the incidence of sarcopenia differences in the Tibetans occurred among the age groups. All analyses were performed using SPSS (ver. 20.0, IBM Company).
Results
Comparison of height, weight, BMI and limb skeletal muscle mass between Lhasa and Shigatse
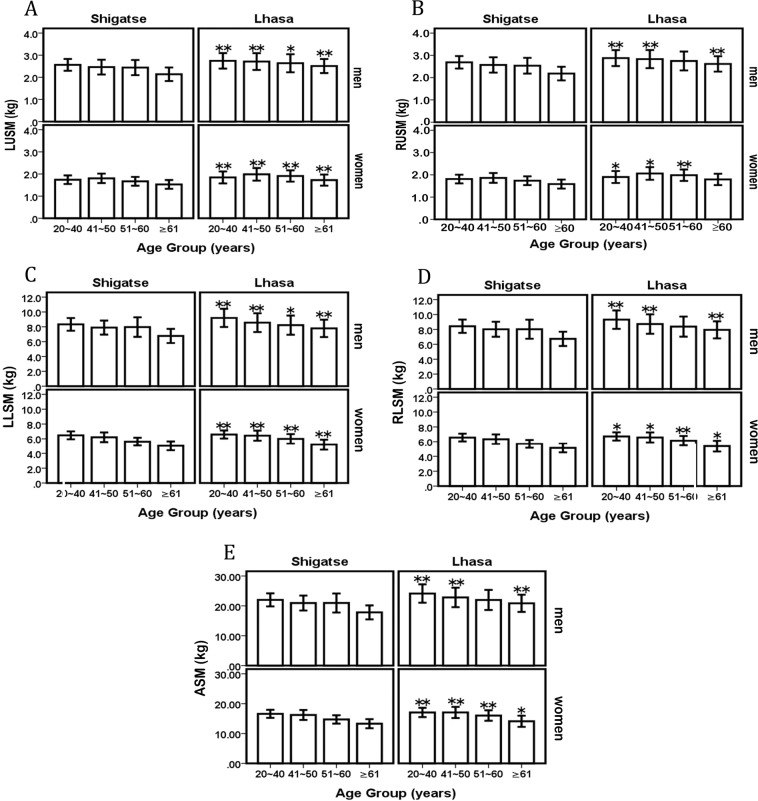
Our results (Table 1) showed that, with the exception of BMI in men aged 51–60 years, the measures of height, weight and BMI in Lhasa were significantly higher than those in Shigatse (P < 0.05), which indicated that altitude had a certain effect on height, weight and BMI. At the higher altitude, height and weight were decreased. Within the same investigation site and for a particular sex, there were significant age differences in height, weight and BMI (P < 0.05), which showed that height decreased with advancing age, and weight initially increased and then decreased. From this study (Table 2; Fig. 1), we found that LUSM, RUSM, LLSM, RLSM and ASM in Lhasa were greater than those in Shigatse at the same age and sex (P < 0.05). This indicated that limb skeletal muscle mass decreased with increasing altitude.
Table 1.
Anthropometry data for the Tibetan participants and comparisons between Lhasa and Shigatse by age and sex (means ± SD).
| Age (years) | n | Height (m) | Weight (kg) | BMI (kg/m2) | ||||
|---|---|---|---|---|---|---|---|---|
| Lhasa | Shigatse | Lhasa | Shigatse | Lhasa | Shigatse | Lhasa | Shigatse | |
| Women | ||||||||
| 20~40 | 303 | 282 | 160.40 ± 5.06 | 154.37 ± 4.79** | 57.79 ± 10.19 | 49.89 ± 6.84** | 22.41 ± 3.55 | 20.68 ± 2.39** |
| 41~50 | 221 | 143 | 159.54 ± 5.15 | 154.34 ± 5.07** | 63.57 ± 11.15 | 52.58 ± 7.09** | 24.91 ± 3.89 | 21.15 ± 2.25** |
| 51~60 | 171 | 123 | 157.69 ± 5.88 | 152.08 ± 5.27** | 62.17 ± 9.78 | 50.73 ± 7.49** | 24.99 ± 3.66 | 22.42 ± 4.10** |
| ≥ 61 | 105 | 70 | 152.79 ± 7.56 | 149.08 ± 5.43* | 57.00 ± 9.49 | 47.18 ± 6.31** | 24.44 ± 4.06 | 20.46 ± 2.20** |
| Men | ||||||||
| 20~40 | 221 | 193 | 172.19 ± 5.87 | 165.81 ± 5.80** | 68.03 ± 12.31 | 56.91 ± 7.66** | 22.92 ± 3.88 | 20.93 ± 2.44** |
| 41~50 | 146 | 70 | 169.43 ± 6.56 | 165.96 ± 6.66** | 67.38 ± 11.98 | 58.39 ± 7.72** | 23.44 ± 3.80 | 22.05 ± 2.51** |
| 51~60 | 118 | 53 | 168.20 ± 5.51 | 165.73 ± 6.43* | 66.65 ± 11.86 | 61.36 ± 11.65* | 23.48 ± 3.55 | 21.91 ± 2.94 |
| ≥ 61 | 70 | 29 | 165.31 ± 6.70 | 162.08 ± 5.95* | 65.09 ± 9.72 | 53.83 ± 7.11** | 23.78 ± 3.06 | 21.18 ± 2.39** |
Shigatse compared with Lhasa at the same age: *P < 0.05, **P < 0.01. BMI: body mass index.
Table 2.
Characteristics of limb skeletal muscle mass and comparisons between Lhasa and Shigatse by age and sex (means ± SD, kg).
| Age (years) | n | LUSM | RUSM | LLSM | RLSM | ASM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lhasa | Shigatse | Lhasa | Shigatse | Lhasa | Shigatse | Lhasa | Shigatse | Lhasa | Shigatse | Lhasa | Shigatse | |
| Women | ||||||||||||
| 20~40 | 303 | 282 | 1.84 ± 0.27 | 1.74 ± 0.20** | 1.90 ± 0.27 | 1.81 ± 0.19* | 6.58 ± 0.54 | 6.47 ± 0.53** | 6.72 ± 0.55 | 6.57 ± 0.52* | 17.05 ± 1.55 | 16.59 ± 1.35** |
| 41~50 | 221 | 143 | 1.98 ± 0.28 | 1.80 ± 0.21** | 2.06 ± 0.28 | 1.86 ± 0.22* | 6.43 ± 0.68 | 6.21 ± 0.66** | 6.58 ± 0.68 | 6.34 ± 0.65* | 17.05 ± 1.87 | 16.21 ± 1.67** |
| 51~60 | 171 | 123 | 1.91 ± 0.26 | 1.67 ± 0.20** | 1.98 ± 0.26 | 1.74 ± 0.20** | 5.99 ± 0.65 | 5.61 ± 0.54** | 6.14 ± 0.65 | 5.70 ± 0.54** | 16.02 ± 1.75 | 14.72 ± 1.39** |
| ≥ 61 | 105 | 70 | 1.72 ± 0.25 | 1.53 ± 0.20** | 1.79 ± 0.26 | 1.58 ± 0.20 | 5.20 ± 0.69 | 5.04 ± 0.61** | 5.39 ± 0.74 | 5.14 ± 0.60* | 14.10 ± 1.87 | 13.29 ± 1.52* |
| Men | ||||||||||||
| 20~40 | 221 | 193 | 2.74 ± 0.35 | 2.56 ± 0.27** | 2.88 ± 0.36 | 2.69 ± 0.28** | 9.19 ± 1.23 | 8.32 ± 0.85** | 9.31 ± 1.24 | 8.42 ± 0.89** | 24.13 ± 3.07 | 22.00 ± 2.19** |
| 41~50 | 146 | 70 | 2.71 ± 0.38 | 2.46 ± 0.33** | 2.83 ± 0.41 | 2.57 ± 0.34** | 8.56 ± 1.27 | 7.89 ± 0.94** | 8.72 ± 1.31 | 8.02 ± 1.01** | 22.82 ± 3.27 | 20.94 ± 2.50** |
| 51~60 | 118 | 53 | 2.64 ± 0.41 | 2.44 ± 0.34* | 2.75 ± 0.42 | 2.54 ± 0.35 | 8.22 ± 1.29 | 7.96 ± 1.32* | 8.37 ± 1.35 | 8.02 ± 1.28 | 21.98 ± 3.37 | 20.96 ± 3.19 |
| ≥ 61 | 70 | 29 | 2.51 ± 0.32 | 2.14 ± 0.31** | 2.62 ± 0.35 | 2.18 ± 0.31** | 7.79 ± 1.17 | 6.77 ± 0.95** | 7.94 ± 1.15 | 6.73 ± 0.96** | 20.85 ± 2.87 | 17.82 ± 2.35** |
Shigatse compared with Lhasa in the same age, *P < 0.05, **P < 0.01. LUSM: left upper limb skeletal muscle mass; RUSM: right upper limb skeletal muscle mass; LLSM: left lower limb skeletal muscle mass; RLSM: right lower limb skeletal muscle mass; ASM: appendicular skeletal muscle.
Figure 1.
Mean changes in limb skeletal muscle mass and comparisons between Lhasa and Shigatse by age and sex. Shigatse compared with Lhasa in the same age: *P < 0.05, **P < 0.01. LUSM: left upper limb skeletal muscle mass; RUSM: right upper limb skeletal muscle mass; LLSM: left lower limb skeletal muscle mass; RLSM: right lower limb skeletal muscle mass; ASM: appendicular skeletal muscle.
Sarcopenia diagnostic cutoff values for Tibetan individuals
The results of HS, GS and SMI measurements are shown in Table 3. We found that HS, GS and SMI decreased with age. GS in Lhasa for those aged from 20 to 50 years was significantly greater than that in Shigatse of the same sex (Table 3; Fig. 2). This suggested that at the higher altitude, physical performance was slower. Especially for Shigatse Tibetan participants aged over 61 years, the GS value (0.87 m/s) was close to 0.8 m/s, which showed that those subjects had sarcopenia.
Table 3.
HS, GS and SMI data and comparisons between Lhasa and Shigatse by age group and sex (means ± SD).
| Age (years) | n | GS (m/s) | HS (kg) | SMI (kg/m2) | ||||
|---|---|---|---|---|---|---|---|---|
| Lhasa | Shigatse | Lhasa | Shigatse | Lhasa | Shigatse | Lhasa | Shigatse | |
| Women | ||||||||
| 20~40 | 303 | 282 | 1.14 ± 0.13 | 1.06 ± 0.16** | 23.30 ± 5.14 | 15.27 ± 5.80** | 6.62 ± 0.50 | 6.96 ± 0.48** |
| 41~50 | 221 | 143 | 1.11 ± 0.12 | 1.01 ± 0.14** | 22.97 ± 5.38 | 13.76 ± 4.66** | 6.69 ± 0.56 | 6.80 ± 0.57 |
| 51~60 | 171 | 123 | 1.04 ± 0.17 | 0.99 ± 0.26 | 19.08 ± 4.60 | 11.68 ± 4.27** | 6.43 ± 0.52 | 6.36 ± 0.49 |
| ≥ 61 | 105 | 70 | 0.91 ± 0.21 | 0.87 ± 0.15 | 14.33 ± 4.38 | 11.14 ± 3.79** | 6.03 ± 0.58 | 5.97 ± 0.50** |
| Men | ||||||||
| 20~40 | 221 | 193 | 1.21 ± 0.15 | 1.17 ± 0.17** | 37.99 ± 7.35 | 24.91 ± 8.69** | 8.13 ± 0.88 | 8.00 ± 0.61 |
| 41~50 | 146 | 70 | 1.15 ± 0.12 | 1.11 ± 0.17** | 35.10 ± 7.95 | 19.74 ± 8.47** | 7.94 ± 0.96 | 7.59 ± 0.66** |
| 51~60 | 118 | 53 | 1.08 ± 0.16 | 1.05 ± 0.18 | 31.63 ± 7.15 | 16.61 ± 7.59** | 7.74 ± 0.90 | 7.61 ± 0.93* |
| ≥ 61 | 70 | 29 | 0.94 ± 0.14 | 0.92 ± 0.15 | 24.46 ± 7.38 | 13.27 ± 6.74** | 7.61 ± 0.81 | 6.78 ± 0.73** |
Shigatse compared with Lhasa at the same age: **P < 0.01. GS: gait speed; HS: handgrip strength; SMI: skeletal muscle mass index.
Figure 2.
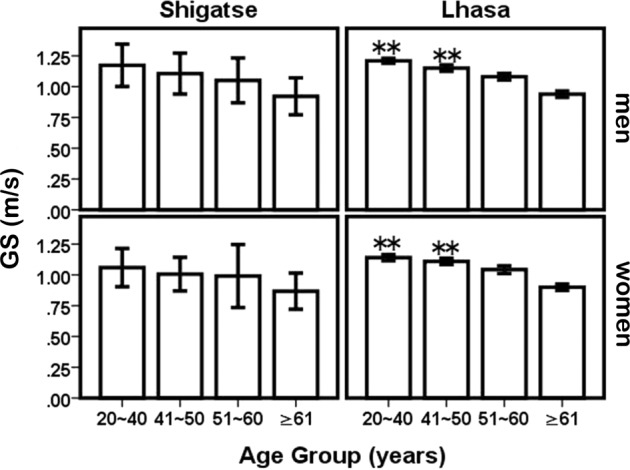
Mean changes in GS and comparisons between Lhasa and Shigatse by age and sex. Shigatse compared with Lhasa in the same age: *P < 0.05, **P < 0.01. GS: gait speed.
HS in Lhasa was greater than that in Shigatse at the same age and sex (P < 0.05) (Table 3; Fig. 3). According to the AWGS strategy, the lower 20th percentile of HS in the study population was used as the cutoff value for low muscle strength. Thus, the cutoff values for HS in Lhasa were 26.7 kg (men) and 15.8 kg (women), and in Shigatse, these values were 13.3 kg (men) and 8.9 kg (women).
Figure 3.

Mean changes in HS and comparisons between Lhasa and Shigatse by age and sex. Shigatse compared with Lhasa in the same age: *P < 0.05, **P < 0.01. HS: handgrip strength.
The reference value of SMI refers to the mean SMI value in the study population aged 20 to 40 years old. From our results (Table 3; Fig. 4), there was a significant difference in SMI between Lhasa and Shigatse in women aged 20–40 years (P < 0.05). However, there was no difference in men. Therefore, the common SMI reference value of 8.07 ± 0.77 kg/m2 was used to diagnose sarcopenia in men. In women, the SMI reference values in Lhasa and Shigatse were 6.62 ± 0.50 kg/m2 and 6.96 ± 0.48 kg/m2, respectively. Thus, in men, the SMI cutoff value was 6.53 kg/m2; in women, the cutoff values in Lhasa and Shigatse were 5.62 kg/m2 and 6.0 kg/m2, respectively.
Figure 4.

Mean changes in SMI and comparisons between Lhasa and Shigatse by age and sex. Shigatse compared with Lhasa in the same age: *P < 0.05, **P < 0.01. SMI: skeletal muscle mass index.
Incidence of sarcopenia among the Tibetan participants
According to the methods of AWGS and the cutoff values obtained in this study, the incidence of sarcopenia in the Tibetan participants was determined as shown in Table 4. Among Tibetans over the age of 40 years, the incidence of sarcopenia in women was 13.3% and that in men was 8.6%. The incidence of sarcopenia increased significantly with age, especially in women, where these values increased from 3.8% to 36% across ages. The incidence of sarcopenia was significantly higher in Shigatse than in Lhasa (P < 0.05).
Table 4.
Incidence of sarcopenia in Tibetans and comparisons among the different ages by region.
| Age (years) | Lhasa | Shigatse | Tibet | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sarcopenia | Sarcopenia | Sarcopenia | |||||||||||||
| NO (%) | YES (%) | n1 | χ2 | P | NO (%) | YES (%) | n2 | χ2 | P | Total | NO (%) | YES (%) | χ2 | P | |
| Women | |||||||||||||||
| 41~50 | 216 (97.7) | 5 (2.3) | 221 | 61.76 | 0.000 | 134 (93.7) | 9 (6.3) | 143 | 56.419 | 0.000 | 364 | 350 (96.2) | 14 (3.8) | 107.01 | 0.000 |
| 51~60 | 165 (96.5) | 6 (3.5) | 171 | 95 (77.2) | 28 (32.9) | 123 | 294 | 260 (88.4) | 34 (11.6) | ||||||
| ≥61 | 78 (74.3) | 27 (25.7) | 105 | 34 (48.6) | 36 (51.4) | 70 | 175 | 112 (64) | 63 (36.0) | ||||||
| total | 459 (92.4) | 38 (7.6) | 497 | 263 (78.3) | 73 (21.7) | 336 | 833 | 722 (86.7) | 111 (13.3) | ||||||
| Men | |||||||||||||||
| 41~50 | 138 (94.5) | 8 (5.5) | 146 | 1.654 | 0.437 | 66 (94.3) | 4 (5.7) | 70 | 19.584 | 0.000 | 216 | 204 (94.4) | 12 (5.6) | 11.963 | 0.003 |
| 51~60 | 108 (91.5) | 10 (8.5) | 118 | 50 (94.3) | 3 (5.7) | 53 | 171 | 158 (92.4) | 13 (7.6) | ||||||
| ≥61 | 63 (90.0) | 7 (10.0) | 70 | 19 (65.5) | 10 (34.5) | 29 | 99 | 82 (82.8) | 17 (17.2) | ||||||
| total | 309 (92.5) | 25 (7.5) | 334 | 135 (88.8) | 17 (11.2) | 152 | 486 | 444 (91.4) | 42 (8.6) | ||||||
n1, n2 and n3 were the number of subjects of different age groups by sex and region, respectively. χ2 and P were the comparison of Incidence on sarcopenia.
Discussion
Hypoxic environments have been shown to influence a person’s body composition (e.g., reductions in body weight, fat-free mass, fat mass, muscle mass and/or body water)14. Tibetans live on the Qinghai-Xizang Plateau, which is a typical plateau environment. Our results (Tables 1 and 2) showed that the height, weight, BMI and limb skeletal muscle mass of Tibetans were reduced with increasing altitude. This was in accordance with a previous study15–17.
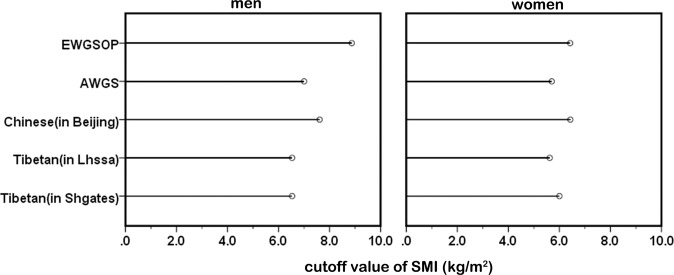
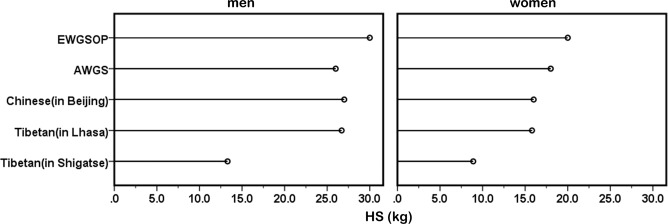
The AWGS has suggested that the cutoff values for sarcopenia may be different because of ethnicities, body size, lifestyles, and cultural backgrounds5. To date, there are no specific cutoff values for the plateau population. In this study, we established cutoff values for sarcopenia in Tibetans living at altitudes over 3000 metres. By comparison, it was found that the cutoff values for SMI and HS in Tibetans were lower than those suggested by the EWGSOP and AWGS and for the Chinese population in Beijing (Table 5; Figs. 5 and 6). The cutoff values from the EWGSOP and AWGS and for the Chinese population in Beijing were not applicable to Tibetans.
Table 5.
Diagnostic cutoff value for sarcopenia in different populations.
| SMI (kg/m2) | HS (kg) | |||
|---|---|---|---|---|
| men | women | men | women | |
| EWGSOP4 | <8.87 | <6.42 | <30 | <20 |
| AWGS5 | <7.0 | <5.7 | <26 | <18 |
| Chinese (in Beijing)33 | <7.61 | <6.43 | <27 | <16 |
| Tibetan (in Lhasa) | <6.53 | <5.62 | <26.7 | <15.8 |
| Tibetan (in Shigatse) | <6.53 | <6.0 | <13.3 | <8.9 |
SMI: skeletal muscle mass index; HS: handgrip strength.
Figure 5.
Comparison of SMI reference values among the different populations. SMI: skeletal muscle mass index.
Figure 6.
Comparison of HS reference values among the different populations. HS: handgrip strength.
There was no significant difference in SMI reference values between Lhasa and Shigatse in Tibetan men; thus, the common reference value 8.07 ± 0.77 kg/m2 was used. However, in Tibetan women, the SMI reference values in Shigatse were greater than those in Lhasa, and the respective reference values should be used. The reason that the SMI reference values in Shigatse were higher than those in Lhasa may be that (1) the participants in Shigatse were from agricultural and pastoral areas, and the participants in Lhasa were from towns, which led to the ASM proportion of the weight being higher in Shigatse (33.93%) than in Lhasa (29.50%); (2) the height of the Tibetans in Shigatse (154.37 cm) was lower than that of those in Lhasa (160.40 cm). We suggest that the cutoff values for sarcopenia in the plateau population should be established according to altitude, such as moderate altitude: 1500–3500 m; high altitude: 3500–5300 m; extreme altitude: >5300 m.
Studies have shown that in plateau environments, weight loss is mainly caused by loss of fat mass18, while in pathological hypoxia (such as chronic obstructive pulmonary disease), weight loss is mainly due to losses in fat-free mass, such as muscle19. In the process of ageing, the structural and functional changes of skeletal muscles are similar to those of hypoxia11, which has been considered to be the cause of muscle atrophy and decreased contractility6. Therefore, some researchers believe that hypoxia may be the main cause of sarcopenia during ageing. Kayser’s study showed that body weight loss was initially due to water loss, followed by loss of fat mass and muscle mass and that more body weight was lost than fat20. Other studies have shown that the incidence of sarcopenia in patients with chronic obstructive pulmonary disease was higher than that in normal people21. The results showed that the skeletal muscle mass of the extremities in individuals in Shigatse (4200 m) was lower than that in those in Lhasa city (3600 m), and the incidence of sarcopenia in Shigatse was greater than that in Lhasa. The results indicated that hypoxia on the plateau has an important effect on the mass and function of skeletal muscle. Based on the comparison of the incidence of sarcopenia (Table 6), the incidence of sarcopenia in the plateau population was higher than that in the plain population. It has been reported that the incidences of sarcopenia in Beijing adults aged 60 years or older were 11.3% for men and 18.7% for women22, and in Taiwan, they were 8.2% for men and 6.5% for women23. However, in this study, we found that the incidences of sarcopenia in Tibetans were 17.2% for men and 36.0% for women. The incidences of sarcopenia in Tibet were higher than those in other parts of China and Asia. Compared to Beijing and Taiwan, the odd ratios were 1.42 (for men) and 3.65 (for women) and 7.53 (for men) and 26.00 (for women), respectively, which indicated that hypoxia might be a risk factor for sarcopenia. Hypoxia may be the main cause of sarcopenia in the ageing process. In the ageing process, changes in skeletal muscle structure and function are similar to those observed in hypoxia24. Studies have shown that for every 1000 feet above sea level, the maximum oxygen consumption decreases by 3.2%, which is similar to the change in maximum oxygen consumption during ageing25. Chronic hypoxia exposure caused by a high-altitude environment accelerates the decomposition of skeletal muscle. At the same time, protein synthesis was inhibited, resulting in a decline in skeletal muscle mass26. Hypoxia can increase the concentration of hormones in the blood and inhibit the endocrine response6, while changes in endocrine function (such as testosterone, oestrogen, adrenocortical hormone, insulin, etc.) and skeletal muscle content and function are closely related to the occurrence of sarcopenia8. Wandrag L27 showed that FFM loss was associated with increased levels of biomarkers related to the NO pathway (e.g., nitrite), oxidative stress (e.g., 4-HNE), inflammation (e.g., IL-6) and metabolic efficiency (e.g., GLP-1 and insulin) in a hypobaric hypoxic environment. These factors may be the reason for the high incidence of sarcopenia in the plateau environment.
Table 6.
Comparison of the incidence of sarcopenia in the population over 60 years of age in different areas.
| Sex | Sarcopenia | Tibet (%) | Beijing (%)22 | Taiwan (%)23 |
|---|---|---|---|---|
| Men | Yes | 17.2 | 11.3 | 8.2 |
| No | 82.8 | 88.7 | 91.8 | |
| Women | Yes | 36.0 | 18.7 | 6.5 |
| No | 64 | 81.3 | 93.5 |
OR (Beijing, men) = 1.42. OR (Beijing, women) = 3.65.
OR (Taiwan, men) = 7.53. OR (Taiwan, women) =26.00.
One of the limitations of this study was that in the plateau environment, the reliability of bioimpedance analysis (BIA) needs further research to be verified. Dual-energy X-ray (DXA) is the most accurate equipment to measure skeletal muscle mass. The medical and economic conditions are poor in Tibet, and therefore, there is little DXA equipment available in Tibet, and it would not have been convenient to carry. However, because the Tibetans lived in a scattered dispersal pattern, the BIA was suitable for measuring skeletal muscle mass in this study. Some studies have shown that there is a good correlation between BIA results under standard conditions and MRI prediction results28. However, the use of BIA for the assessment of body composition in plateaus has been challenged, as changes in hydration may influence the accuracy of the measurements29,30. The second limitation of this study was that the sample size for those over the age of 61 was small. According to China’s sixth census data, Tibet’s population that is over 65 years old accounts for only 5.09%, and the average life expectancy is 68.2 years31. This was consistent with studies that have shown that when the altitude was higher, life expectancy was shorter32. This led to difficulty in investigating individuals over 61 years old, especially in Shigatse, which is located 4200 metres above sea level. The insufficiency of the sample size of those over 61 years old may have led to a certain deviation in the incidence of sarcopenia in Tibet. At the same time, among those who were 51–60 years old, our results showed that the incidence of sarcopenia (32.9%) in Shigatse (above 4200 metres) was significantly higher than that (3.5%) in Lhasa (above 3600 metres). Therefore, we suggest that the investigation of the incidence of sarcopenia in plateaus above 4000 metres should be carried out from the age of 50.
Our findings provide reference values and the incidence of sarcopenia in Tibetans. We suggest that the cutoff values for sarcopenia in plateau populations should be established based on the altitude. Hypoxia may be an important risk factor for sarcopenia, but the mechanism is to be further studied.
Acknowledgements
We are grateful to the Lhasa Tama Community Health Service Center and the Saga County People’s Hospital for their help. We also would like to thank Professor Wei Liu for his assistance in preparing the final version of the manuscript. This study was supported by the National Natural Science Foundation of China for funding the project (31571233) and the Biological Anthropology Innovation Team Project of JZMU (JYLJ201702).
Author contributions
Youfeng Wen conceived and designed the study. Liping Ye, Jie Yao, Xin Li, Yingying Liu, Jia Song and Zhengqi Sun performed the experiments. Ying Chen and Youfeng Wen analysed and validated the data. Liping Ye and Youfeng Wen were the major contributors in writing the manuscript. All the authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 2.Alfonso J, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014;43(6):748–759. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J.Appl. Physiol. 2000;89(1):81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft AJ, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen LK, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J. Am Med. Dir. Assoc. 2014;15(2):95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Tanimoto Y, et al. Sarcopenia and falls in community-dwelling elderly subjects in japan: defining sarcopenia according to criteria of the european working group on sarcopenia in older people. Arch. Gerontol. Geriatr. 2014;59(2):295–299. doi: 10.1016/j.archger.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Alemán-Mateo H, Ruiz Valenzuela RE. Skeletal muscle mass indices in healthy young Mexican adults aged 20–40 years: implications for diagnoses of sarcopenia in the elderly population. Scientific.World. Journal. 2014;2014:1–5. doi: 10.1155/2014/672158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnholt KE, et al. Endocrine responses to acute and chronic high-altitude exposure (4,300 meters): modulating effects of caloric restriction. Am. J. Physiol. Endocrinol. Metab. 2006;290(6):1078–1088. doi: 10.1152/ajpendo.00449.2005. [DOI] [PubMed] [Google Scholar]
- 9.Clynes MA, Edwards MH, Buehring B, Dennison EM, Cooper C. Definitions of sarcopenia: associations with previous falls and fracture in a population sample. Calcif. Tissue International. 2015;97(5):445–452. doi: 10.1007/s00223-015-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckinx F, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J. Cachexia Sarcopenia Muscle. 2018;9(2):269–278. doi: 10.1002/jcsm.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaccagni L, Barbieri D, Cogo A, Gualdirusso E. Anthropometric and body composition changes during expeditions at high altitude. High. Alt. Med. Biol. 2014;15(2):176–182. doi: 10.1089/ham.2013.1133. [DOI] [PubMed] [Google Scholar]
- 12.Boyer SJ, Blume FD. Weight loss and changes in body composition at high altitude. J. Appl. Physiol. 1984;57(5):1580–1585. doi: 10.1152/jappl.1984.57.5.1580. [DOI] [PubMed] [Google Scholar]
- 13.Gao L, et al. Prevalence of sarcopenia and associated factors in chinese community-dwelling elderly: comparison between rural and urban areas. JAMDA. 2015;16(11):1003. doi: 10.1016/j.jamda.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Hamad N, Travis SPL. Weight loss at high altitude: pathophysiology and practical implications. Eur. J. Gastroenterol. Hepatol. 2006;18(1):5–10. doi: 10.1097/00042737-200601000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Bianba. et al. Anthropometric measures of 9- to 10-year-old native tibetan children living at 3700 and 4300 m above sea level and han chinese living at 3700 m. Medicine. 94(42) (2015). [DOI] [PMC free article] [PubMed]
- 16.Tripathy V, Gupta R. Growth among tibetans at high and low altitudes in india. Am. J. Hum. Biol. 2010;19(6):789–800. doi: 10.1002/ajhb.20638. [DOI] [PubMed] [Google Scholar]
- 17.Sitko S, Cirer-Sastre R. López Laval,I. Effects of high altitude mountaineering on body composition: a systematic review. Nutr. Hosp. 2019;36(5):1189–1195. doi: 10.20960/nh.02582. [DOI] [PubMed] [Google Scholar]
- 18.Sherf-Dagan S, et al. Prospective Longitudinal Trends in Body Composition and Clinical Outcomes 3 Years Following Sleeve Gastrectomy. Obes. Surg. 2019;11(11):1–9. doi: 10.1007/s11695-019-04057-2. [DOI] [PubMed] [Google Scholar]
- 19.Jones SE, et al. Sarcopenia in copd: prevalence, clinical correlates and esponse to pulmonary rehabilitation. Thorax. 2015;70(3):213–218. doi: 10.1136/thoraxjnl-2014-206440. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, et al. Sarcopenia: an independent predictor of mortality in community-dwelling older korean men. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69(10):1244–1252. doi: 10.1093/gerona/glu050. [DOI] [PubMed] [Google Scholar]
- 21.Costa TMDRL, Costa FM, Thaísa HJ, Moreira CA, Victória ZCB. Body composition and sarcopenia in patients with chronic obstructive pulmonary disease. Endocrine. 2018;60(5):95–102. doi: 10.1007/s12020-018-1533-4. [DOI] [PubMed] [Google Scholar]
- 22.Xia Z, et al. Analysis of the dietary factors on sarcopenia in elderly in Beijing. Wei Sheng Yan Jiu. 2016;45(3):388–93. [PubMed] [Google Scholar]
- 23.Wu IC, et al. Epidemiology of sarcopenia among community-dwelling older adults in Taiwan: a pooled analysis for a broader adoption of sarcopenia assessments. Geriatr. Gerontol. Int. 2014;14(Suppl):52–60. doi: 10.1111/ggi.12193. [DOI] [PubMed] [Google Scholar]
- 24.Di GC, Petruccelli G, Bianchi G, Cacchio M, Verratti V. Does hypoxia cause sarcopenia? prevention of hypoxia could reduce sarcopenia. J. Biol. Regul. Homeost. Agents. 2009;23(1):55–58. [PubMed] [Google Scholar]
- 25.Hepple RT, Hagen JL, Krause DJ. Oxidative capacity interacts with oxygen delivery to determine maximal O(2) uptake in rat skeletal muscles in situ. J. Physiol. 2002;541(3):1003–1012. doi: 10.1113/jphysiol.2001.013649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan Tb, Shi YF, Li JL. Effects of hypoxia on body composition and protein catabolism of skeletal muscle. J. Cap. Inst. Phys. Education. 2005;17(3):113–115. [Google Scholar]
- 27.Wandrag L, et al. Does hypoxia play a role in the development of sarcopenia in humans? mechanistic insights from the caudwell xtreme everest expedition. Redox Biology. 2017;13:60–68. doi: 10.1016/j.redox.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000;89(2):465–71. doi: 10.1152/jappl.2000.89.2.465. [DOI] [PubMed] [Google Scholar]
- 29.Boos CJ, et al. Comparison of two methods of assessing total body water at sea level and increasing high altitude. Clin. Physiol. Funct. Imaging. 2014;34(6):478–484. doi: 10.1111/cpf.12121. [DOI] [PubMed] [Google Scholar]
- 30.Fulco CS, Hoyt RW, Baker-Fulco CJ, Gonzalez J, Cymerman A. Use of bioelectrical impedance to assess body composition changes at high altitude. J. Appl. Physiol. 1992;72(6):2181–2187. doi: 10.1152/jappl.1992.72.6.2181. [DOI] [PubMed] [Google Scholar]
- 31.Population Census Office under the State Council & Department of Population and Employment Statistics National Bureau of Statistica. Tabulation on the 2010 Population Census of the People’s Republic of China Book I. China Statistics Press, 265-291(Beijing, 2012).
- 32.Zha X, Tian Y, Gao X, Wang W, Yu C. Quantitatively evaluate the environmental impact factors of the life expectancy in tibet, china. Env. Geochem. Health. 2019;41(3):1507–1520. doi: 10.1007/s10653-018-0211-z. [DOI] [PubMed] [Google Scholar]
- 33.Zeng P, et al. Differences in body composition and physical functions associated with sarcopenia in Chinese elderly: reference values and prevalence. Arch. Gerontol. Geriatr. 2015;60(1):118–123. doi: 10.1016/j.archger.2014.08.010. [DOI] [PubMed] [Google Scholar]