Abstract
In Taiwan, patients with pulmonary hypertension (PH) related to chronic obstructive pulmonary disease (COPD) are most common PH population (group 3). However, efficacy of medical treatments and optimal prevention methods in this group remain uncertain. Statins such as indirect RhoA/Rho-kinase inhibitors influence one of key signalling pathways that promote PH onset. In this study, we explored protective effects of statins against PH in COPD patients using database from Taiwan National Health Insurance programme from 2002 to 2017. The main outcome was the risk of PH. The Cox proportional-hazards model and the Fine and Gray model were used to adjust covariate and competing risks to estimate the subdistribution hazard ratios (sHRs). 553,617 newly diagnosed COPD patients were stratified by statin users (n = 41,168) and statin nonusers (n = 512,449). After 1:1 propensity score matching of statin users (n = 41,163), and 41,163 statin nonusers were included for outcome analysis. Statin users had a 22% lower risk of PH than nonusers (sHR: 0.78, 95% confidence interval: 0.65–0.94). During subgroup analysis, taking higher daily doses and for a longer duration displayed a more significantly reduced risk of PH (both P for trend <0.001). Statins may have a protective effect against PH that is dose- and time-dependent.
Subject terms: Peripheral vascular disease, Outcomes research
Introduction
Pulmonary hypertension (PH) is a pathophysiologic and hemodynamic condition that increases the pressure level in the pulmonary arteries, veins and/or capillaries. Eventually, under these conditions, the need for the heart ventricles to contract more and more to pump blood through the lungs and heart can result in heart failure, cardiovascular diseases, or respiratory function loss1. In 2018, the sixth World Symposium on PH (WSPH) released an updated statement separating PH patients into five groups according to clinical presentation, hemodynamic characteristics, pathophysiology, and therapeutic strategy2. The incidence of PH is about 2.4 cases per million adults per year in the United States3–6. One Taiwan prevalence survey suggested the patients with PH related to COPD (group 3 in the WSPH classification scheme) is the most common PH population in Taiwan7. Of interest, while the severity of PH among patients in group 3 may be less than that in other PH groups, the three-year survival rate in this group is the lowest across all five PH groups8. This lower survival rate might due to a lack of evidence and efficacy regarding the use of current medical treatments for PH including supportive therapy and certain drugs in PH related to COPD. Besides, there has been no clear prevention strategy revealed that can reduce the risk of PH in COPD patients; currently, only long-term oxygen therapy can improve symptoms. Given these facts, the development of a new treatment in this population is necessary9,10.
There are many studies that have explored the repurposing of PH treatment drugs on the market for other indications. For example, 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors are cholesterol-lowering drugs able to reduce the low-density lipoprotein cholesterol level by around 10% to 50% as well as the triglyceride concentration to a small degree. Because of their good lipid-lowering effects, statins are the first-choice therapeutic modalities in hyperlipidemia patients11. In addition to their lipid-lowering effects, statins also have displayed anti-inflammatory12–14, anti-proliferative15–17 and anti-thrombotic18,19 properties. These effects are associated with the pathophysiology of PH related to COPD. Moreover, statins are indirect RhoA/Rho-kinase inhibitors. Upon blocking the RhoA/Rho-kinase signalling pathway, these medications are capable of stopping vasoconstriction, endothelial nitric oxide synthase, cellular proliferation and apoptosis. However, to date, only in vitro studies have indicated that statins inhibit systemic inflammatory and pulmonary vascular proliferation, and block the RhoA/Rho-kinase signalling pathway; the efficacy of statins in human clinical trials remains unclear.
As such, we conducted a nationwide, population-based retrospective cohort study to explore whether the protective effects of statins could reduce the risk of PH in patients with COPD. Moreover, we compared the protective effects of different types of statins and examined whether such effects were dose- or time-dependent.
Results
Study population
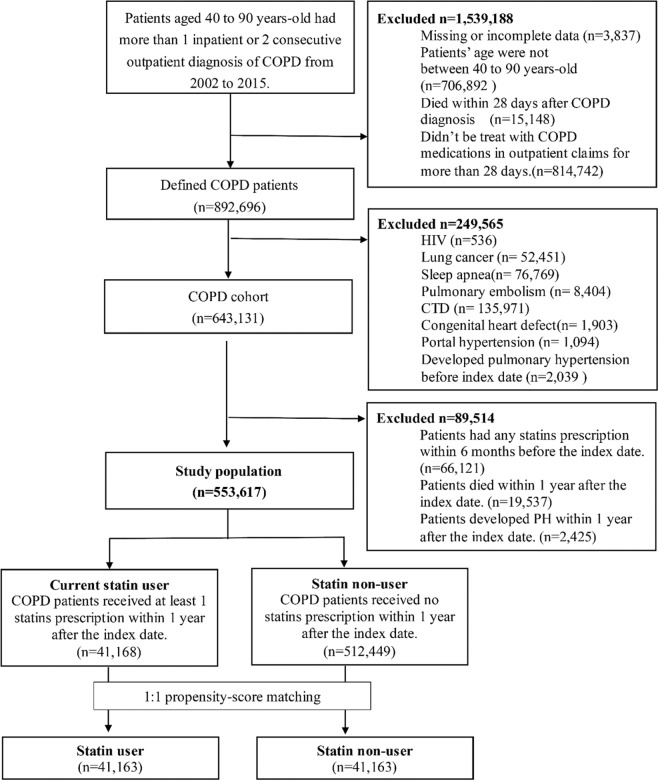
Based on the inclusion and exclusion criteria, a total of 553,617 patients were included in the newly diagnosed COPD cohort (Fig. 1). According to statin exposure, there were s 41,168 statin users and 512,449 nonusers of statins in study population. After 1:1 propensity score (PS) matching, we included 41,163 statin users and 41,163 statin nonusers for PH outcome analysis. Before PH matching, the mean age of patients in the user group (64.60 years) was slightly higher than that in the nonuser group (63.95 years). Because of the indications of statins, patients with statins displayed significantly higher rates of dyslipidemia (P < 0.001), coronary artery disease (P < 0.001), and ischaemic stroke (P < 0.001). Most of the comorbidities were found in significantly higher degrees in the user group than in the nonuser group, with the exception of interstitial pulmonary diseases, asthma and malignant and haemorrhagic stroke. In particular, rates of interstitial pulmonary diseases and malignant stroke were similar between the two groups (P = 0.543 and P = 0.250). Comedication use presented the same trend of comorbidity, while rates of severe and moderate exacerbations of COPD displayed a significant difference (P < 0.001) between the two user groups, although the majority of patients showed no exacerbation in their condition within one year after the index date. After PS matching, there was a significant difference noted in the distribution of comorbidities and concurrent medication use between the two groups. A Cox proportional-hazards (CPH) model was established to adjust all imbalanced characteristics in the following analysis. The details of baseline characteristics of the COPD cohort are presented in Table 1.
Figure 1.
Result of flow chart in study population.
Table 1.
Baseline characteristics of COPD patients before and after1-to-1propensity score matching, stratified according to statins using.
| Characteristics n (%) | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| User N = 41,168 | Non-user N = 512,449 | p-value | User N = 41,163 | Non-user N = 41,163 | p-value | |
| Propensity score (SD) | 0.88 (0.08) | 0.93 (0.05) | <0.001 | 0.75 (0.03) | 0.75(0.03) | 1.000 |
| Age group | ||||||
| Mean (SD) | 64.60 (11.06) | 63.95 (12.85) | <0.001 | 64.60 (11.06) | 64.67 (11.27) | 0.370 |
| 40 ≤ age < 50 | 3,937 (9.56) | 83,280 (16.25) | <0.001 | 3,936 (9.56) | 3,936 (9.56) | 1.000 |
| 50 ≤ age < 60 | 10,204 (24.79) | 117,885 (23.00) | 10,204 (24.79) | 10,204 (24.79) | ||
| 60 ≤ age < 70 | 12,356 (30.01) | 119,985 (23.41) | 12,354 (30.01) | 12,354 (30.01) | ||
| 70 ≤ age < 80 | 10,827 (26.30) | 123,860 (24.17) | 10,825 (26.30) | 10,825 (26.30) | ||
| 80 ≤ age | 3,844 (9.34) | 67,439 (13.16) | 3,844 (9.34) | 3,844 (9.34) | ||
| Male | 23,303 (56.60) | 313,271 (61.13) | <0.001 | 23,301 (56.61) | 23,301 (56.61) | 1.000 |
| Insurance premium (TWD) | ||||||
| ≤22,800 TWD | 21,581 (52.42) | 280,896 (54.81) | <0.001 | 21,581 (52.43) | 21,582 (52.43) | 0.994 |
| >22,800 TWD | 19,587 (47.58) | 231,553 (45.19) | 19,582 (47.57) | 19,581 (47.57) | ||
| Urbanization level | ||||||
| Urban | 20,416 (49.59) | 252,309 (49.24) | 0.201 | 20,411 (49.59) | 20,346 (49.43) | 0.646 |
| Suburban | 16,255 (39.48) | 202,886 (39.59) | 16,255 (39.49) | 16,237 (39.45) | ||
| Rural | 4,497 (10.92) | 57,254 (11.17) | 4,497 (10.92) | 4,580 (10.13) | ||
| Comorbidity | ||||||
| Dyslipidemia | 25,510 (61.97) | 34,381 (6.71) | <0.001 | 25,510 (61.97) | 4,603 (61.18) | <0.001 |
| Hypertension | 27,917 (67.81) | 216,905 (42.33) | <0.001 | 27,912 (67.81) | 27,916 (67.82) | 0.976 |
| Diabetes Mellitus | 15,840 (38.48) | 75,574 (14.75) | <0.001 | 15,835 (38.47) | 16,043 (38.97) | 0.137 |
| Obesity | 215 (0.52) | 1,017 (0.20) | <0.001 | 215 (0.52) | 142 (0.34) | <0.001 |
| Chronic kidney disease | 2,010 (4.88) | 12,770 (2.49) | <0.001 | 2,010 (4.88) | 1,668 (4.05) | <0.001 |
| Chronic liver disease | 4,892 (11.88) | 49,970 (9.75) | <0.001 | 4,892 (11.88) | 4,820 (11.71) | 0.437 |
| Arrhythmia | 4,810 (11.68) | 41,636 (8.12) | <0.001 | 4,805 (11.67) | 4,739 (11.51) | 0.472 |
| Interstitial pulmonary diseases | 177 (0.43) | 2,310 (0.45) | 0.543 | 177 (0.43) | 161 (0.39) | 0.383 |
| Asthma | 15,607 (37.91) | 197,980 (38.63) | 0.004 | 15,604 (37.91) | 15,760 (37.29) | 0.263 |
| Malignant | 4,236 (10.29) | 53,653 (10.47) | 0.250 | 4,235 (10.29) | 4,369 (10.61) | 0.127 |
| ASCVD | ||||||
| Coronary artery disease | 13,622 (33.09) | 76,755 (14.98) | <0.001 | 13,618 (33.08) | 8,400 (33.41) | <0.001 |
| Peripheral vascular disease | 1,582 (3.84) | 11,217 (2.19) | <0.001 | 1,582 (3.84) | 1,260 (3.06) | <0.001 |
| Ischemic stroke/TIA | 6,239 (15.15) | 45,500 (8.88) | <0.001 | 6,238 (15.15) | 4,667 (15.34) | <0.001 |
| Hemorrhagic stroke | 583 (1.42) | 8,447 (1.65) | <0.001 | 583 (1.42) | 825 (1.20) | <0.001 |
| Heart failure | 6,155 (14.95) | 42,883 (8.37) | <0.001 | 6,150 (14.94) | 6,152 (14.95) | 0.984 |
| Left ventricular hypertrophy | 435 (1.06) | 3,154 (0.62) | <0.001 | 435 (1.06) | 430 (1.04) | 0.864 |
| Co-medication | ||||||
| Digoxin | 1,902 (4.62) | 17,049 (3.33) | <0.001 | 1,898 (4.61) | 2,045 (4.97) | 0.016 |
| Oral anticoagulant agents | 1,004 (2.44) | 6,669 (1.30) | <0.001 | 1,003 (2.44) | 790 (2.92) | <0.001 |
| Oral antiplatelet agents | 20,260 (49.21) | 115,688 (22.58) | <0.001 | 20,257 (49.21) | 13,406 (49.57) | <0.001 |
| Diuretics | 12,105 (29.40) | 90,184 (17.60) | <0.001 | 12,100 (29.40) | 10,509 (29.53) | <0.001 |
| CCB | 21,022 (51.06) | 162,839 (31.78) | <0.001 | 21,019 (51.06) | 19,511 (51.40) | <0.001 |
| ACEI/ACB | 21,653 (52.60) | 134,311 (26.21) | <0.001 | 21,648 (52.59) | 17,584 (52.72) | <0.001 |
| Beta blocker | 14,761 (35.86) | 97,769 (19.08) | <0.001 | 14,757 (35.85) | 11,917 (35.95) | <0.001 |
| Metformin | 11,085 (26.93) | 44,553 (8.69) | <0.001 | 11,080 (26.92) | 9,567 (26.24) | <0.001 |
| Fibrate | 4,875 (11.84) | 14,045 (2.74) | <0.001 | 4,873 (11.84) | 2,080 (11.05) | <0.001 |
| Other lipid-lowering drugs | 186 (0.45) | 541 (0.11) | <0.001 | 186 (0.45) | 64 (0.16) | <0.001 |
| Medication for COPD | ||||||
| LABA | 933 (2.27) | 11,143 (2.17) | 0.220 | 933 (2.27) | 859 (2.09) | 0.077 |
| LABA/ICS | 4,126 (10.02) | 48,234 (9.41) | <0.001 | 4,125 (10.02) | 3,515 (10.54) | <0.001 |
| LAMA | 1,614 (3.92) | 17,393 (3.39) | <0.001 | 1,614 (3.92) | 1,187 (3.88) | <0.001 |
| LABA/LAMA | 214 (0.52) | 1,814 (0.35) | <0.001 | 214 (0.52) | 132 (0.32) | <0.001 |
| SABA | 3,261 (7.92) | 42,497 (8.29) | 0.008 | 3,260 (7.92) | 3,244 (7.88) | 0.836 |
| SAMA | 1,138 (2.76) | 17,985 (3.51) | <0.001 | 1,137 (2.76) | 1,447 (2.52) | <0.001 |
| SABA/SAMA | 1,588 (3.86) | 19,001 (3.71) | 0.123 | 1,588 (3.86) | 1,407 (3.42) | 0.001 |
| Systemic beta-2 agonists | 10,621 (25.80) | 129,995 (25.37) | 0.052 | 10,618 (25.80) | 10,859 (25.38) | 0.056 |
| ICS | 1,190 (2.89) | 15,262 (2.98) | 0.314 | 1,189 (2.89) | 1,102 (2.68) | 0.065 |
| Methylxanthines | 22,245 (54.03) | 253,261 (49.42) | <0.001 | 22,241 (54.03) | 21,248 (54.62) | <0.001 |
| COPD severity | ||||||
| Moderate exacerbations | ||||||
| 0 | 36,309 (88.20) | 446,596 (87.15) | <0.001 | 30,446 (73.96) | 30,522 (73.15) | 0.799 |
| 1 | 3,385 (8.22) | 41,982 (8.19) | 4,480 (10.88) | 4,469 (10.86) | ||
| ≥2 | 1,474 (3.58) | 23,871 (4.66) | 6,237 (15.15) | 6,172 (15.99) | ||
| Severe exacerbations | ||||||
| 0 | 30,446 (73.96) | 371,050 (72.41) | <0.001 | 36,304 (88.20) | 36,307 (88.20) | 0.995 |
| 1 | 4,485 (10.89) | 55,841 (10.90) | 3,385 (8.22) | 3,387 (8.23) | ||
| ≥2 | 6,237 (15.15) | 85,558 (16.70) | 1,474 (3.58) | 1,469 (3.57) | ||
(COPD = chronic obstructive pulmonary disease; TWD = Taiwan dollars; ASCVD = atherosclerotic cardiovascular disease; TIA = Transient ischemic attack; CCB = calcium channel blocker; ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; LABA = Long-acting β2-aginist; LAMA = Long-acting muscarinic antagonists; SABA = Short-acting β2-aginist; SAMA = Short-acting muscarinic antagonists; ICS = Inhaled corticosteroid).
Incidence
A total of 242 (0.59%) users and 338 (0.82%) nonusers of statins, respectively, experienced PH. The mean follow-up times in the user and nonuser groups were 4.13 and 4.28 years. The statin user group displayed a lower incidence rate of PH onset as compared with the nonuser group (1.43 vs. 1.97 per 1,000 person-years). The CPH model analysis findings are summarised in Table 2. In both a univariate analysis [crude hazard ratio (HR): 0.72, 95% confidence interval (CI): 0.61–0.85; P < 0.001) and multivariate analysis (adjusted HR (aHR): 0.76, 95% CI: 0.63–0.93; P = 0.006), a significantly lower HR of PH incidence between statin users and nonusers was observe. There were 6,997 (16.99%) statin users and 5,553 (13.49%) statin nonusers who died within the five-year study period. Death may be a competing risk for a PH event. To adjust the competing risk, we conducted a multivariate with competing risk analysis, where the incidence of PH still presented a significantly lower subdistribution HR (sHR) between statin users and nonusers (sHR: 0.78, 95% CI: 0.65–0.94; P = 0.010) The above results suggest that statins could reduce the risk of PH by approximately 28% to 22% and provide a protective effect against PH (Table 2).
Table 2.
Multivariate cox proportional hazard model analysis for variables related to the risk of PH, stratified according to statins using (aAdjusted for age group, sex, income, comorbidity, co-medication, and COPD severity; bEntry regression model after the stepwise multiple regression analysis; COPD = chronic obstructive pulmonary disease; PH = pulmonary hypertension; HR = hazard ratio; TWD = Taiwan dollars; ASCVD = atherosclerotic cardiovascular disease; TIA = Transient ischemic attack; CCB = calcium channel blocker; ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; * < 0.05; ** < 0.01; *** < 0.001).
| Variables | Crude | p-value | Adjusted | p-value | Subdistribution | p-value |
|---|---|---|---|---|---|---|
| HR (95% CI) | HRa (95% CI) | HRa (95% CI) | ||||
| User vs. non-user | 0.73 (0.61–0.86)*** | <0.001 | 0.76 (0.63–0.93)** | 0.007 | 0.78 (0.65–0.94)* | 0.010 |
| Age groupb | ||||||
| 40 ≤ age < 50 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| 50 ≤ age < 60 | 1.55 (0.95–2.53) | 0.080 | 1.44 (0.88–2.35) | 0.150 | 1.43 (0.87–2.34) | 0.154 |
| 60 ≤ age < 70 | 2.79 (1.76–4.44)*** | <0.001 | 2.05 (1.28–3.29)** | 0.003 | 2.05 (1.27–3.30)** | 0.003 |
| 70 ≤ age < 80 | 4.24 (2.68–6.70)*** | <0.001 | 2.46 (1.53–3.94)*** | <0.001 | 2.35 (1.44–3.82)** | 0.001 |
| 80 ≤ age | 6.53 (4.04–10.55)*** | <0.001 | 2.98 (1.80–4.91)*** | <0.001 | 2.45 (1.46–4.13)** | 0.001 |
| Maleb | 1.19 (1.01–1.41)* | 0.037 | 1.24 (1.05–1.47)* | 0.012 | 1.22 (1.03–1.45)* | 0.020 |
| Insurance premium (NT$)b | ||||||
| ≤22,800 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| >22,800 | 0.48 (0.41–0.58)*** | <0.001 | 0.74 (0.61–0.89)** | 0.002 | 0.80 (0.66–0.96)* | 0.019 |
| Urbanization level | ||||||
| Urban | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Suburban | 1.37 (1.15–1.63)*** | <0.001 | 1.23 (1.03–1.46)* | 0.022 | 1.25 (1.05–1.49)* | 0.011 |
| Rural | 1.18 (0.89–1.55) | 0.250 | 0.96 (0.72–1.26) | 0.753 | 0.97 (0.74–1.29) | 0.851 |
| Comorbidity | ||||||
| Dyslipidemia | 0.59 (0.49–0.71)** | <0.001 | 0.84 (0.67–1.04) | 0.113 | 0.86 (0.69–1.07) | 0.178 |
| Hypertension | 1.32 (1.10–1.58)* | 0.003 | 0.87 (0.70–1.08) | 0.214 | 0.87 (0.69–1.09) | 0.225 |
| Diabetes Mellitus | 1.05 (0.89–1.24) | 0.575 | 1.11 (0.90–1.38) | 0.320 | 1.05 (0.85–1.30) | 0.666 |
| Obesity | 1.54 (0.58–4.13) | 0.387 | 2.23 (0.83–5.99) | 0.112 | 2.30 (0.85–6.21) | 0.100 |
| Chronic kidney disease | 2.06 (1.50–2.84)** | <0.001 | 1.19 (0.86–1.65) | 0.292 | 1.03 (0.74–1.44) | 0.857 |
| Chronic liver disease | 0.85 (0.65–1.12) | 0.244 | 0.91 (0.69–1.19) | 0.496 | 0.89 (0.68–1.17) | 0.409 |
| Arrhythmiab | 2.93 (2.43–3.52)*** | <0.001 | 1.50 (1.22–1.85)*** | <0.001 | 1.49 (1.20–1.84)*** | <0.001 |
| Interstitial pulmonary diseases | 3.39 (1.51–7.57)** | 0.003 | 2.15 (0.96–4.84) | 0.063 | 1.99 (0.88–4.47) | 0.098 |
| Asthmab | 2.00 (1.68–2.39)*** | <0.001 | 1.65 (1.38–1.98)*** | <0.001 | 1.72 (1.43–2.06 *** | <0.001 |
| Malignant | 0.84 (0.62–1.13) | 0.237 | 0.83 (0.61–1.11) | 0.210 | 0.78 (0.57–1.05) | 0.098 |
| ASCVD | ||||||
| Coronary artery disease | 1.62 (1.37–1.92)*** | <0.001 | 0.87 (0.72–1.06) | 0.179 | 0.87 (0.71–1.06) | 0.165 |
| Peripheral vascular disease | 1.02 (0.65–1.61) | 0.933 | 0.74 (0.47–1.17) | 0.193 | 0.70 (0.44–1.12) | 0.137 |
| Ischemic stroke/TIA | 1.10 (0.87–1.40) | 0.432 | 0.75 (0.59–0.97)* | 0.026 | 0.70 (0.55–0.90)** | 0.006 |
| Hemorrhagic stroke | 0.72 (0.32–1.60) | 0.414 | 0.53 (0.24–1.19) | 0.123 | 0.49 (0.22–1.09) | 0.080 |
| Heart failureb | 5.32 (4.45–6.37)*** | <0.001 | 2.17 (1.74–2.71)*** | <0.001 | 2.12 (1.67–2.67)*** | <0.001 |
| Left ventricular hypertrophy | 2.63 (1.58–4.39)*** | <0.001 | 1.21 (0.72–2.04) | 0.464 | 1.23 (0.73–2.09) | 0.443 |
| Co-medication | ||||||
| Digoxinb | 5.06 (4.10–6.25)*** | <0.001 | 1.50 (1.17–1.93)** | 0.001 | 1.43 (1.11–1.85)** | 0.006 |
| Oral anticoagulant agents | 2.96 (2.05–4.27)*** | <0.001 | 1.01 (0.69–1.50) | 0.947 | 1.01 (0.68–1.49) | 0.974 |
| Oral antiplatelet agents | 1.69 (1.44–1.99)*** | <0.001 | 1.12 (0.92–1.36) | 0.275 | 1.12 (0.91–1.36) | 0.285 |
| Diureticsb | 3.83 (3.19–4.61)*** | <0.001 | 1.97 (1.59–2.44)*** | <0.001 | 1.94 (1.57–2.39)*** | <0.001 |
| CCB | 1.22 (1.04–1.44)*** | <0.001 | 0.93 (0.77–1.11) | 0.412 | 0.93 (0.77–1.13) | 0.465 |
| ACEI/ACB | 1.75 (1.48–2.07)*** | <0.001 | 1.20 (0.99–1.46) | 0.065 | 1.22 (1.00–1.48) | 0.050 |
| Beta blocker | 1.14 (0.96–1.35) | 0.134 | 0.92 (0.76–1.10) | 0.337 | 0.92 (0.77–1.11) | 0.389 |
| Metformin | 0.74 (0.60–0.91)** | 0.004 | 0.72 (0.55–0.93)* | 0.011 | 0.73 (0.57–0.94)* | 0.015 |
| Fibrate | 0.56 (0.39–0.81)** | 0.002 | 0.81 (0.55–1.18) | 0.275 | 0.82 (0.56–1.20) | 0.313 |
| COPD severity | ||||||
| Moderate exacerbationsb | ||||||
| 0 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| 1 | 2.71 (2.15–3.41)*** | <0.001 | 1.57 (1.23–2.01)*** | <0.001 | 1.55 (1.19–2.02)** | 0.001 |
| ≥2 | 2.77 (2.25–3.42)*** | <0.001 | 1.49 (1.17–1.89)** | 0.001 | 1.42 (1.11–1.83)** | 0.006 |
| Severe exacerbationsb | ||||||
| 0 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| 1 | 3.14 (2.53–3.89)*** | <0.001 | 1.57 (1.23–1.99)*** | <0.001 | 1.45 (1.13–1.87)** | 0.004 |
| ≥2 | 4.84 (3.64–6.43)*** | <0.001 | 1.71 (1.23–2.37)** | 0.001 | 1.33 (0.96–1.85) | 0.089 |
Statin users of an older age; male gender; with lower insurance premiums; who were living in the suburbs; and/or who had a cardiac arrhythmia, asthma or heart failure presented a significantly higher risk of developing PH as compared with nonusers. In contrast, patients with ischaemic stroke or transient ischaemic attack had a lower risk of PH (aHR; 0.75, 95% CI: 0.59–0.97; P = 0.026) Regarding the COPD severity in sHR analysis, only moderate exacerbation and one-time severe exacerbation significantly increased the risk of PH. We next conducted a stepwise multiple regression analysis to elucidate the main factors affecting the incidence rate of PH. Here, age group, gender, insurance premium, arrhythmia, asthma, heart failure, digoxin, diuretic and COPD severity displayed a significantly different risk. These significant factors were subsequently used to construct an adjusted model for use in the following subgroup analysis (Table 2).
Subgroup analysis
The most used statin was atorvastatin, with nearly 40% of statin users taking this medication. As indicated in Table 3, most of the statin used showed a trend of a low risk of PH except lovastatin (aHR: 1.63, 95% CI: 0.95–2.79; P = 0.076) Among all of those being used, pravastatin had the lowest aHR and appeared to significantly reduce the risk of PH by 56% (aHR: 0.44, 95% CI: 0.23–0.86; P = 0.016). After adjusting for all-cause mortality as a competing risk, the trend of the protective effect of each statin did not change.
Table 3.
Subgroup analysis of risk of PH in different kind of statins, stratified according to statins using (aAdjusted for age group, gender, insurance premium, arrhythmia, asthma, heart failure, digoxin, diuretics, and COPD severity; HR = hazard ratio; PH = pulmonary hypertension; PY = person-year; Rate = (event/person-year) *1000; * < 0.05; ** < 0.01; *** < 0.001).
| Kind of statins | User n = 41163 | Non-user n = 41163 | Crude | p-value | Adjusted | p-value | Subdistribution | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Events | Total of PY | Rate | Events | Total of PY | Rate | HR (95% CI) | HRa (95% CI) | HRa (95% CI) | ||||
| simvastatin | 6575 | 42 | 28897.13 | 1.45 | 56 | 27522.95 | 2.03 | 0.72 (0.48–1.07) | 0.102 | 0.66 (0.44–1.00) | 0.050 | 0.71 (0.47–1.06) | 0.094 |
| lovastatin | 4385 | 37 | 19622.88 | 1.89 | 22 | 18513.47 | 1.19 | 1.60 (0.94–2.71) | 0.082 | 1.63 (0.95–2.79) | 0.076 | 1.64 (0.97–2.77) | 0.063 |
| pravastatin | 2588 | 13 | 10740.20 | 1.21 | 28 | 10820.43 | 2.59 | 0.47 (0.24–0.90)* | 0.023 | 0.44 (0.23–0.86)* | 0.016 | 0.45 (0.23–0.89)* | 0.021 |
| fluvastatin or pitavastatin | 4084 | 19 | 15870.42 | 1.20 | 27 | 17050.70 | 1.58 | 0.76 (0.42–1.36) | 0.351 | 0.74 (0.41–1.35) | 0.331 | 0.78 (0.43–1.39) | 0.391 |
| atorvastatin | 16331 | 92 | 66581.49 | 1.38 | 148 | 67789.98 | 2.18 | 0.63 (0.49–0.82)** | 0.001 | 0.63 (0.23–0.82)** | 0.001 | 0.65 (0.50–0.89)** | 0.001 |
| rosuvastatin | 7200 | 39 | 28087.20 | 1.39 | 57 | 30232.80 | 1.89 | 0.74 (0.49–1.11) | 0.142 | 0.75 (0.49–1.13) | 0.167 | 0.77 (0.51–1.17) | 0.227 |
A classification scheme for cumulative defined daily dose (cDDD) including seven levels was included in a multivariate CPH model analysis. The statin nonuser group was employed as a reference group in this analysis. Table 4 revealed that patients with higher cDDDs had lower aHR values of from 1.36 to 0.26 (P for trend ≤0.001). Further, among those patients using more than 180 cDDD, a significantly lower risk of PH was observed (aHR: 0.58, 95% CI: 0.42–0.81; sHR: 0.66, 95% CI: 0.47–0.92). The duration of statin use was calculated by year and divided into six categories. Patients with longer durations of statin use had a lower risk of PH (aHR: 1.15–0.31; P for trend ≤0.001), with a significantly lower risk of PH observed among those using statins for more than one year (aHR: 0.44, 95% CI: 0.31–0.64; sHR: 0.47, 95% CI: 0.32–0.67). Separately, we divided the frequency of use of statins into four levels and conducted a multivariate CPH model analysis. The most common frequency of statin use is 30 defined daily doses (DDDs) per month. Patients using greater numbers of DDDs of statins per month had a lower risk of PH (aHR: 0.81–0.51; P for trend ≤0.001). Further, patients who used more than 20 DDDs of statin per month started to show a significantly lower risk of PH (aHR: 0.51, 95% CI: 0.39–0.67; sHR: 0.54, 95% CI: 0.41–0.71).
Table 4.
Subgroup analysis of risk of PH, stratified according to classification of cDDD, duration of statins use and intensity (aAdjusted for age group, gender, insurance premium, arrhythmia, asthma, heart failure, digoxin, diuretics, and COPD severity.; HR = hazard ratio; COPD = chronic obstructive pulmonary disease; PH = pulmonary hypertension; cDDD = cumulative defined daily doses; PY = person-year; Rate = (event/person-year) *1000; * < 0.05; ** < 0.01; *** < 0.001).
| Group | N | Events | Total of PY | Rate | Crude | p-value | Adjusted | p-value | Subdistribution | p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HRa (95% CI) | HRa (95% CI) | ||||||||
| cDDD | ||||||||||
| Non-user | 41163 | 338 | 171937.85 | 1.97 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| cDDD < 28 | 4992 | 61 | 19423.87 | 3.14 | 1.60 (1.21–2.09)** | 0.001 | 1.36 (1.03–1.79)* | 0.031 | 1.36 (1.02–1.82)* | 0.038 |
| 28 ≤ cDDD < 90 | 7147 | 59 | 26901.31 | 2.19 | 1.11(0.84–1.47) | 0.450 | 1.06 (0.80–1.40) | 0.672 | 1.11 (0.83–1.48) | 0.489 |
| 90 ≤ cDDD < 180 | 6221 | 43 | 23988.18 | 1.79 | 0.91 (0.66–1.25) | 0.560 | 0.89 (0.65–1.23) | 0.479 | 0.94 (0.67–1.30) | 0.695 |
| 180 ≤ cDDD < 365 | 8472 | 40 | 34531.87 | 1.16 | 0.59 (0.42–0.82)** | 0.002 | 0.58 (0.42–0.81)** | 0.001 | 0.66 (0.47–0.92)* | 0.015 |
| 365 ≤ cDDD < 730 | 8495 | 25 | 37496.93 | 0.67 | 0.34 (0.23–0.51)*** | <0.001 | 0.37 (0.24–0.55)*** | <0.001 | 0.43 (0.28–0.65)*** | <0.001 |
| 730 ≤ cDDD | 5836 | 14 | 27464.22 | 0.51 | 0.26 (0.15–0.44)*** | <0.001 | 0.26 (0.15–0.45)*** | <0.001 | 0.32 (0.19–0.55)*** | <0.001 |
| p for trend test | <0.001 | |||||||||
| Duration of statins use (year) | ||||||||||
| Non-user | 41163 | 338 | 171929.9123 | 1.97 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| year < 0.5 | 13448 | 125 | 51095.84931 | 2.45 | 1.24 (1.01–1.52)* | 0.039 | 1.15 (0.93–1.41) | 0.203 | 1.12 (0.91–1.38) | 0.301 |
| 0.5 ≤ year < 1 | 7249 | 45 | 27638.78082 | 1.63 | 0.83 (0.61–1.13) | 0.229 | 0.81 (0.59–1.10) | 0.178 | 0.80 (0.58–1.09) | 0.157 |
| 1 ≤ year < 2 | 8931 | 32 | 37213.73973 | 0.86 | 0.44 (0.30–0.63)*** | <0.001 | 0.44 (0.31–0.64)*** | <0.001 | 0.47 (0.32–0.67)*** | <0.001 |
| 2 ≤ year < 3 | 5125 | 22 | 23182.96986 | 0.95 | 0.48 (0.31–0.74)** | 0.001 | 0.51 (0.33–0.79)** | 0.002 | 0.57 (0.37–0.88)* | 0.011 |
| 3 ≤ year | 6410 | 18 | 30677.67397 | 0.59 | 0.30 (0.19–0.48)*** | <0.001 | 0.31 (0.19–0.49)*** | <0.001 | 0.35 (0.22–0.56)*** | <0.001 |
| p for trend test | <0.001 | |||||||||
| Intensity (cDDD/month) | ||||||||||
| Non-user | 41163 | 338 | 171929.9123 | 1.97 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| <10 | 4021 | 32 | 15812.85206 | 2.02 | 1.03 (0.72–1.48) | 0.884 | 0.81 (0.56–1.16) | 0.252 | 0.79 (0.55–1.15) | 0.216 |
| 10 ≤ intensity < 20 | 21619 | 149 | 91493.42192 | 1.63 | 0.83 (0.68–1.01) | 0.056 | 0.83 (0.69–1.01) | 0.064 | 0.87 (0.71–1.05) | 0.142 |
| 20≤ | 15523 | 61 | 62502.73973 | 0.98 | 0.50 (0.38–0.65)*** | <0.001 | 0.51 (0.39–0.67)*** | <0.001 | 0.54 (0.41–0.71)*** | <0.001 |
| p for trend test | <0.001 | |||||||||
Sensitivity analysis
As compared with the original definition of a PH event, a more precise definition did not change the trend of observing a protective effect against PH. Moreover, the sensitivity analysis had a lower risk of PH when compared with the original definition of a PH event in the statin user group (aHR: 0.70, 95% CI: 0.56–0.87 vs. aHR: 0.76, 95% CI: 0.63–0.93) Besides, extending the one-year confirmation period to three years and conducting a longer or shorter duration of observation did not have much of an influence on the outcome of PH risk. Findings of the sensitivity analysis are shown in Supplementary Tables 1 and 2.
Discussion
During the five-year study observation period, the statin user group displayed a lower incidence rate of PH as compared with in the nonuser group (1.43 vs. 1.97 per 1,000 person-years; P < 0.001). After adjusting for age, sex, income, comorbidity, comedication use, COPD severity and competing risks, the finding was that statin use reduced the incidence rate of PH by 22% among COPD patients (sHR: 0.78, 95% CI: 0.65–0.94; P = 0.010). These results support the suggestion that statins offer a protective effect against PH in COPD patients.
The pathophysiology in PH related to COPD is complex and caused by multiple mechanisms. Pulmonary vascular remodelling, parenchymal lung destruction and hypoxia are the three known main independent mechanisms in PH related to COPD. Among these, pulmonary vascular remodelling and parenchymal lung destruction are observed early on during the course of PH. These conditions may also be associated with findings of systemic inflammation and endothelial cell dysfunction that are involved in the pathophysiology of COPD as well. If COPD continues to deteriorate toward hypoxemia, the onset of hypoxia causes pulmonary arterial vasoconstriction9. The protective effect of statins may be attributed to their anti-inflammatory effect that functions by limiting immune cell activation and reducing inflammatory cytokines12–14. A controlled pilot study showed that atorvastatin treatment in COPD patients significantly reduced the neutrophil count in sputum by 34% and the CD45+ cell count by 57% in lung biopsies (P = 0.008)13. Moreover, statins are the same as endothelin-1 receptor antagonists, which are specific drugs used for PH treatment in that endothelin-1 receptor antagonists are indirect RhoA/Rho-kinase inhibitors20. RhoA is one of the Rho G proteins, which are intracellular messengers. In the RhoA/Rho-kinase signalling pathway, RhoA activates the downstream effectors Rho-kinase I (ROCK-I) and Rho-kinase II (ROCK-II) and causes vasoconstriction, endothelial nitric oxide synthase, cellular proliferation and apoptosis. The expression of the signalling pathway is common in hypoxic lungs. Statins can decrease the progression of an early process in the cholesterol biosynthetic pathway as well as inhibit the synthesis of isoprenoids, which are prerequisite posttranslational lipid attachments necessary for Rho activation20. Statins also have anti-thrombotic effects: they inhibit the platelet-derived growth factor signal and reduce platelet thrombus formation in patients with idiopathic pulmonary arterial hypertension. Fluvastatin has shown beneficial effects on chronic hypoxia-induced PH by limiting endothelial nitric oxide synthase activity18,19. In summary, because of their role as indirect RhoA/Rho-kinase inhibitors and other pharmacological activities, we hypothesised that statins may have potential therapeutic benefits in PH.
To our knowledge, this study was the first to investigate whether statins can reduce the incidence of PH in the COPD population. The only other similar study involving patients with severe COPD supported that statin use is associated with a significantly lower PAWP (12 ± 5 vs. 15 ± 6 mmHg; P = 0.002) and PAPm (26 ± 7 vs. 29 ± 7 mmHg; P = 0.002) outcomes21. Findings of PAWP values over 15 mmHg and PAPm values over 20 mmHg lead to placement in the WSPH’s group 3. So, the reduction of PAWP and PAPm via statin use may support that statins have an association with the reduction of PH incidence. In this manner, these results complement our study findings. However, the incidence of group 3 PH is still unknown. One study reported an incidence of 14% among elderly patients older than 65 years, while the ASPIRE registry data showed that 56.7% of COPD patients had group 3 PH8,22. It is difficult to compare the incidence and prevalence rates among these studies and our research because the study populations vary. In our multiple regression analysis, older age, male gender, lower insurance premiums, living in the suburbs, having an arrhythmia, having asthma, having heart failure and COPD severity were4 risk factors for PH development. According to the pathophysiology and disease progression of PH, these risk factors appear reasonable9. However, patients with ischaemic stroke or transient ischaemic attack had a lower risk of PH (aHR: 0.75, 95% CI: 0.59–0.97; P = 0.026) The reasoning for this perhaps is that patients who experience ischaemic stroke have a ninefold higher mortality rate as compared with patients without stroke: in other words, they die before PH occurs, leading to a lower reported incidence of PH23. After adjusting the all-cause mortality as a competing risk, statin users still showed a significantly lower risk of PH in comparison with nonusers (sHR: 0.78, 95% CI: 0.65–0.94; P = 0.010).
During subgroup analysis, the results indicated dose- and time-dependent effects existed for statin use in that the statins achieved the protective effect against PH until patients achieved greater than 180 cDDD, reached one year of use, or used more than 20 DDDs per month. Among patients with greater cDDDs and longer durations of use, a lower risk of PH incidence could be observed. To understand the association between cDDD and the duration of statin use, this study completed a subgroup analysis of intensity, referring to the cDDD per month. The normal cDDD per month is 30 DDDs. However, the DDD is an average maintenance dose per day, so it may represent a moderate statin dose according to the drug’s lipid-lowering effects11,24. Patients who used more than 20 DDDs of statin per month displayed a significantly lower risk of PH (sHR: 0.54, 95% CI: 0.41–0.71). The exploration of the dose- and time-dependent effects of statins has been common in studies involving different populations, making the results more robust25–27.
The study is the first to explore the protective effects of statins against PH in COPD patients. In the study design, many important strengths can be observed. The Nation Health Insurance (NHI) database is one of the largest and most comprehensive medical population databases in the world, so the study had access to a larger sample size than other studies. Specifically, 41,163 COPD patients and 1,325 patients with PH related to COPD were included in the final analysis of this study. This is in comparison with other investigations, where the sample size of patients with PH related to COPD was only 40 to 60 patients28–33. This extensive sample size may provide enough power for statistical analysis. Another strength is the long observation time. The study relied on the database from 2002 to 2017 and included a five-year observation period, ensuring the availability of enough PH events for analysis. Further, the results were robust across several different definitions of statin drug exposure and observation duration.
In contrast, however, this study also had several limitations. The study population and outcomes were all defined based on the International Classification of Diseases, ninth revision, clinical modification (ICD-9-CM) or the International Classification of Diseases, 10th revision, clinical modification in the admissions record rather than on clinical diagnosis because the NHI database lacked examination results and laboratory data. Therefore, the definition of PH in this study was established according to a Chang et al. study that used the same NIH database. This previous study verified the accuracy of the diagnosis code (i.e., ICD-9-CM) by chart review in a medical centre hospital. The positive predictive value of using diagnosis codes in this regard was reported as up to 94.9%7. Then, the current study also used the second definition that combined the diagnosis codes and examinations to enhance the correct rate of PH diagnosis. Such examinations like right-heart catheterisation, echocardiographic, and chest X-ray were recommended by the 2015 European Society of Cardiology/European Respiratory Society guidelines for the diagnosis and treatment of pulmonary hypertension10. The limitations of the NHI database also include no access to smoking status, lifestyle, lung function or PH severity. The study also could not confirm that patients were taking treatments for PH or not. The reason for this is that patients in this population often do not fit the payment rules of PH-specific therapy in the NHI programme. So, many used those drugs at their own expense, which would not be recorded in the NIH database.
In conclusion, statins may have a protective effect against PH through reducing the incidence of PH in patients with COPD. Moreover, the protective effect was dose- and time-dependent. An age of more than 60 years, male gender, low income, heart failure, arrhythmia, asthma and COPD severity were risk factors for PH. However, we could not identify a consistent benefit in protective effect between different kinds of statins. Further randomised controlled trials involving different statins and accurate statin exposure control are required.
Methods
Data sources
Taiwan established the NHI programme in 1995. The Taiwan NIH programme, which covers 99.6% of 23 million Taiwanese and 93% of hospitals, clinics and pharmacies, represents one of the largest and most comprehensive medical population databases in the world. We used the full population database with all its medical records and multiple cause of death datasets from the NHI programme from 2002 to 201734. To maintain the privacy of patients, the identity numbers of patients, medical institutions and medical providers were encrypted through the application of anonymous codes. All researchers in Taiwan are required to follow the Computer-processed Personal Data Protection Law and should not attempt to decrypt and impair the privacy of patients. We independently conducted this study at a subcenter of the Health and Welfare Data Science Centers at Kaohsiung Medical University. This study was approved by the institutional review board of Kaohsiung Medical University Chung-Ho Memorial Hospital (KMUHIRB-EXEMPT(I)-20190032).
Study population
We identified newly diagnosed COPD patients from both outpatient and inpatient visit records from January 1, 2002 to December 31, 2015 (ICD-9-CM codes 490, 491, 492 and 496). Patients with more than one inpatient diagnosis or more than two consecutive outpatient diagnoses of COPD and who were treated using COPD medications according to outpatient claims for more than 28 days within one year after the primary COPD diagnosis date were defined as COPD patients. Patients with other etiologies of PH10 (Supplementary Table 3) and lung cancer (ICD-9-CM code 162) were excluded (n = 249,565). Patients who developed PH before their COPD diagnosis (n = 2,425) or died within 28 days after their COPD diagnosis (n = 15,148) were also excluded. The date of the primary diagnosis of COPD was defined as the index date.
Baseline characteristics and COPD severity
Baseline characteristics and COPD severity were confirmed by medical records in the one year after the index date according to ICD-9-CM codes or ICD-10-CM codes. The demographic data contained age, age group, gender, urbanisation level35,36 and insurance premium information. To adjust for other confounders that might influence the outcome, we listed comorbidities including a high risk of atherosclerotic cardiovascular disease (ASCVD)24 and other medications used in the treatment of PAH [e.g., digoxin, calcium channel blockers, warfarin]. Detailed baseline characteristics and definitions are shown in Supplementary Table 4. The assessment of exacerbation risk in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines was used to define the severity of COPD. The category of severe exacerbations refers to exacerbations leading to emergency room or hospital admission, while moderate exacerbations were those not leading to hospital admission but where patients were treated with SABA plus antibiotics or oral corticosteroids37. Because the index date was the first COPD diagnosis date, the severity of COPD was confirmed at one year after the index date.
The baseline characteristics of the original cohort were used to perform PS matching by multivariate logistic regression (via OneToManyMTCH, a SAS procedure; SAS Institute, Cary, NC, USA). Data on age group, gender, insurance premium, urbanisation, hypertension, diabetes, heart failure, left ventricular hypertrophy and COPD severity were used to calculate the propensity score. We adopted a 1:1 matching scheme to generate the statin user and nonuser groups for analysis after follow-up38.
Exposure and outcomes assessment
For the statin exposure definition, to solve the issue of reverse causality, we established a six-month drug washout period according to the pharmacokinetic and pharmacodynamic properties of statins26,27. Patients with any level of statin exposure during the washout period were excluded. Patients needed to use at least one statin in the one year after COPD diagnosis to be defined as statin users. Conversely, patients who never received prescriptions for statins in the one year after COPD diagnosis were defined as statin nonusers.
The incidence of PH was the primary outcome in the study. To identify the event of PH, we used the diagnosis code (i.e., 416.0, 416.8, or 416.9 in ICD-9-CM; I270, I272, I278, or I279 in ICD-10-CM) in the medical records to define the event of PH. Then, we applied two criteria to confirm patients truly had PH. For the first one, patients were required to have more than one inpatient diagnosis or emergency room admission for PH or more than two consecutive outpatient diagnoses of PH in one year. For the second, in the sensitivity analysis, patients had to undergo diagnostic examinations for PH (e.g., right-heart catheterisation, echocardiography, or chest X-ray) and receive a diagnosis of PH at the same time. We used the first criterion in the main analysis4,7.
To assess the influence of incidence among different kinds of statins, doses and time frames, this study included subgroup analyses. There were seven statins that were prescribed in Taiwan during the study period. The patients’ statin group was determined based on their most-used statin during the five-year observation period. We analysed the dose-dependent effect by considering the DDD, which was established by the World Health Organization to standardise the doses of different kinds of statins. The cDDD was the total amount of statin exposure during the five-year observation period and was divided into six levels (i.e., ≤28, 28–90, 90–180, 180–365, 365–730, and ≥730 cDDD). Additionally, the duration of statin use was calculated by year and divided into five levels (i.e., ≤0.5, 0.5–1, 1–2, 2–3, and ≥3 years). The intensity was calculated by dividing the cDDD by the duration of statin use during the whole five-year observation period. Then, we divided such into three levels to compare weather the intensity could affect the protective effect of statins39 (i.e., ≤10, 10–20, and ≥20 cDDD/month).
Follow-up time
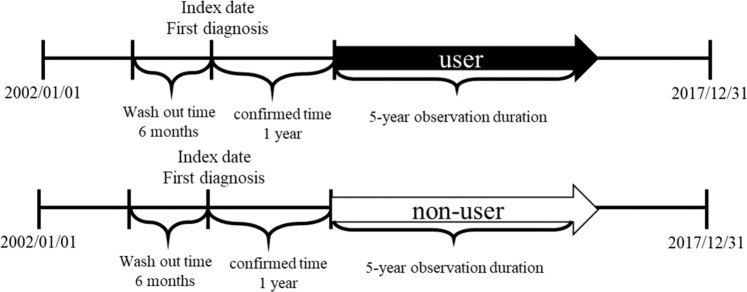
The five-year follow-up period began at one year after the index date. Users and nonusers alike stopped participating at the occurrence of PH or censoring. If patients died before the occurrence of PH or were not diagnosed with PH by the end of the observation period, they were defined as being censors. The definition of censors was the same in both the user and nonuser groups. During the observation period, any statin exposure would not change the user group (Fig. 2).
Figure 2.
We set a five-year observation duration to reduce the influence of switching to different statins or discounting taking statins. The follow-up start form one year after index date and to the five years after index date.
Statistical analysis
For baseline characteristics, continuous variables were presented as means (standard deviations) and categorical variables were presented as percentages. Continuous variables were analysed by Student’s t-test or analysis of variance and categorical variables were analysed by Fisher’s exact test or chi-squared test if the value was less than 30. The crude incidence of PH was estimated as the total number of events during the five-year observation period divided by total person-years. This study conducted a CPH model to estimate the HR between the two user groups. The multivariate model was adjusted according to demographic characteristics, gender, comorbidities, comedication use and COPD severity to calculate the aHR. To reduce the influence of interaction and collinear effects between characteristics, stepwise selection was performed to select important factors to construct the multivariate regression model for subgroup analysis. Because of the competing risk of death, the Fine and Gray competing risk model was used to estimate the sHR in the primary outcome40.
All of the above analyses were performed using the SAS version 9.4. software program (SAS Institute, Cary, NC, USA). Statistical significance was determined as two-tailed and α = 0.05.
Supplementary information
Acknowledgements
This study was based in part on data from the NHIRD provided by the Bureau of National Health Insurance (BNHI) of the Ministry of Health and Welfare. The conclusions presented in this study are those of the authors and do not necessarily reflect the views of the BNHI, the the Ministry of Health and Welfare. This work was supported by the Kaohsiung Medical University (KMU-Q106011). We are grateful to Kaohsiung Medical University for providing administrative and funding support.
Author contributions
All authors conceived the study design; Wen-Ting conducted the experiment and wrote the manuscripts. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-59828-0.
References
- 1.Hsu CH, et al. 2014 Guidelines of Taiwan Society of Cardiology (TSOC) for the Management of Pulmonary Arterial Hypertension. Acta Cardiol. Sin. 2014;30:401–444. [PMC free article] [PubMed] [Google Scholar]
- 2.Simonneau Gérald, Montani David, Celermajer David S., Denton Christopher P., Gatzoulis Michael A., Krowka Michael, Williams Paul G., Souza Rogerio. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. European Respiratory Journal. 2019;53(1):1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badesch DB, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137:376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 4.Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur. Respir. J. 2007;30:104–109. doi: 10.1183/09031936.00092306. [DOI] [PubMed] [Google Scholar]
- 5.McGoon MD, et al. Pulmonary arterial hypertension: epidemiology and registries. J. Am. Coll. Cardiol. 2013;62:D51–59. doi: 10.1016/j.jacc.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Ling Y, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am. J. Respir. Crit. Care Med. 2012;186:790–796. doi: 10.1164/rccm.201203-0383OC. [DOI] [PubMed] [Google Scholar]
- 7.Chang, W. T. et al. Prognostic Factors in Patients With Pulmonary Hypertension-A Nationwide Cohort Study. J Am Heart Assoc5, 10.1161/JAHA.116.003579 (2016). [DOI] [PMC free article] [PubMed]
- 8.Hurdman J, et al. ASPIRE registry: assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur. Respir. J. 2012;39:945–955. doi: 10.1183/09031936.00078411. [DOI] [PubMed] [Google Scholar]
- 9.Cuttica MJ. Pulmonary hypertension associated with lung diseases and hypoxemia. Heart Fail. Rev. 2016;21:299–308. doi: 10.1007/s10741-016-9551-x. [DOI] [PubMed] [Google Scholar]
- 10.Galie N, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur. Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 11.Wierzbicki AS, Poston R, Ferro A. The lipid and non-lipid effects of statins. Pharmacology Therapeutics. 2003;99:95–112. doi: 10.1016/s0163-7258(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 12.So JY, Dhungana S, Beros JJ, Criner GJ. Statins in the treatment of COPD and asthma-where do we stand? Curr. Opin. Pharmacol. 2018;40:26–33. doi: 10.1016/j.coph.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Mroz RM, et al. Anti-inflammatory effects of atorvastatin treatment in chronic obstructive pulmonary disease. A controlled pilot study. J. Physiol. Pharmacol. 2015;66:111–128. [PubMed] [Google Scholar]
- 14.Maneechotesuwan K, Wongkajornsilp A, Adcock IM, Barnes PJ. Simvastatin Suppresses Airway IL-17 and Upregulates IL-10 in Patients With Stable COPD. Chest. 2015;148:1164–1176. doi: 10.1378/chest.14-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun X, Ku DD. Rosuvastatin provides pleiotropic protection against pulmonary hypertension, right ventricular hypertrophy, and coronary endothelial dysfunction in rats. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H801–809. doi: 10.1152/ajpheart.01112.2007. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura T, et al. Simvastatin attenuates smooth muscle neointimal proliferation and pulmonary hypertension in rats. Am. J. Respir. Crit. Care Med. 2002;166:1403–1408. doi: 10.1164/rccm.200203-268OC. [DOI] [PubMed] [Google Scholar]
- 17.Seeger H, Mueck AO, Lippert TH. Fluvastatin increases prostacyclin and decreases endothelin production by human umbilical vein endothelial cells. Int. J. Clin. Pharmacol. Ther. 2000;38:270–272. doi: 10.5414/cpp38270. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda T, et al. Inhibitory effects of simvastatin on platelet-derived growth factor signaling in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. J. Cardiovasc. Pharmacol. 2010;55:39–48. doi: 10.1097/FJC.0b013e3181c0419c. [DOI] [PubMed] [Google Scholar]
- 19.Murata T, et al. Statin protects endothelial nitric oxide synthase activity in hypoxia-induced pulmonary hypertension. Arterioscler. Thromb. Vasc. Biol. 2005;25:2335–2342. doi: 10.1161/01.ATV.0000186184.33537.48. [DOI] [PubMed] [Google Scholar]
- 20.Xing XQ, et al. Statins may ameliorate pulmonary hypertension via RhoA/Rho-kinase signaling pathway. Med. Hypotheses. 2007;68:1108–1113. doi: 10.1016/j.mehy.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 21.Reed RM, et al. Statin therapy is associated with decreased pulmonary vascular pressures in severe COPD. COPD. 2011;8:96–102. doi: 10.3109/15412555.2011.558545. [DOI] [PubMed] [Google Scholar]
- 22.Pugh ME, et al. Causes of pulmonary hypertension in the elderly. Chest. 2014;146:159–166. doi: 10.1378/chest.13-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdel-Qadir H, et al. Importance of Considering Competing Risks in Time-to-Event Analyses: Application to Stroke Risk in a Retrospective Cohort Study of Elderly Patients With Atrial Fibrillation. Circ. Cardiovasc. Qual. Outcomes. 2018;11:e004580. doi: 10.1161/CIRCOUTCOMES.118.004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grundy, S. M. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation, CIR0000000000000625, 10.1161/CIR.0000000000000625 (2018).
- 25.Langballe R, et al. Statin use and risk of contralateral breast cancer: a nationwide cohort study. Br. J. Cancer. 2018;119:1297–1305. doi: 10.1038/s41416-018-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu JC, et al. Statins dose-dependently exert a chemopreventive effect against lung cancer in COPD patients: a population-based cohort study. Oncotarget. 2016;7:59618–59629. doi: 10.18632/oncotarget.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu JC, et al. Statins dose-dependently exert a significant chemopreventive effect on colon cancer in patients with chronic obstructive pulmonary disease: A population-based cohort study. Oncotarget. 2016;7:65270–65283. doi: 10.18632/oncotarget.11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arian A, Moghadam SG, Kazemi T, Hajihosseini M. The Effects of Statins on Pulmonary Artery Pressure in Patients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Trial. J. Res. Pharm. Pract. 2017;6:27–30. doi: 10.4103/2279-042X.200985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chogtu B, et al. A prospective, randomized study: Evaluation of the effect of rosuvastatin in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Indian. J. Pharmacol. 2016;48:503–508. doi: 10.4103/0253-7613.190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moosavi SAJ, Raji H, Faghankhani M, Yazdani R, Esmaeili M. Evaluation of the Effects of Atorvastatin on the Treatment of Secondary Pulmonary Hypertension due to Chronic Obstructive Pulmonary Diseases: A Randomized Controlled. Trial. Iran. Red. Crescent Me. 2013;15:649–654. doi: 10.5812/ircmj.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng WJ, et al. Atorvastatin in pulmonary arterial hypertension (APATH) study. Eur. Respir. J. 2012;40:67–74. doi: 10.1183/09031936.00149011. [DOI] [PubMed] [Google Scholar]
- 32.Wilkins MR, et al. Simvastatin as a treatment for pulmonary hypertension trial. Am. J. Respir. Crit. Care Med. 2010;181:1106–1113. doi: 10.1164/rccm.2009111-699OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee TM, Chen CC, Shen HN, Chang NC. Effects of pravastatin on functional capacity in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Clin. Sci. 2009;116:497–505. doi: 10.1042/CS20080241. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh C-Y, et al. Taiwan’s National Health Insurance Research Database: past and future. Clin. Epidemiol. 2019;11:349–358. doi: 10.2147/clep.s196293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng BR, et al. Rural-urban disparities in family physician practice patterns: A nationwide survey in Taiwan. Int. J. Health Plann Manage. 2018 doi: 10.1002/hpm.2662. [DOI] [PubMed] [Google Scholar]
- 36.Liu CY, et al. development stratification of Taiwan townships into sampling design of large scale health interview survey. J. Health Manag. 2006;4:1–22. [Google Scholar]
- 37.Wang MT, et al. Association of Cardiovascular Risk With Inhaled Long-Acting Bronchodilators in Patients With Chronic Obstructive Pulmonary Disease: A Nested Case-Control Study. JAMA Intern. Med. 2018;178:229–238. doi: 10.1001/jamainternmed.2017.7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.S., L. in Proceedings of the 29th SAS Users Group International Conference (Canada, 2004).
- 39.Chang YT, et al. Dose-Dependent Relationship Between Metformin and Colorectal Cancer Occurrence Among Patients with Type 2 Diabetes-A Nationwide Cohort Study. Transl. Oncol. 2018;11:535–541. doi: 10.1016/j.tranon.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.