Abstract
We performed two phase I trials of the histone deacetylase inhibitor vorinostat combined with either the vascular endothelial growth factor inhibitor pazopanib (NCT01339871) or the proteasome inhibitor ixazomib (NCT02042989) in patients with metastatic TP53 mutant solid tumors. Both trials followed a 3 + 3 dose-escalation design allowing for a dose expansion cohort of up to 14 additional patients with a specific tumor type. Patients had to have a confirmed TP53 mutation to be enrolled in NCT02042989. Among patients enrolled in NCT01339871, TP53 mutation status was determined for those for whom tumor specimens were available. The results of NCT01339871 were reported previously. Common treatment-related adverse events in NCT02042989 included anemia, thrombocytopenia, fatigue, nausea, vomiting, and diarrhea. Compared with patients with metastatic TP53 hotspot mutant solid tumors who were treated with ixazomib and vorinostat (n = 59), those who were treated with pazopanib and vorinostat (n = 11) had a significantly higher rate of clinical benefit, defined as stable disease lasting ≥6 months or an objective response (3.4% vs. 45%; p < 0.001), a significantly longer median progression-free survival duration (1.7 months [95% confidence interval (CI), 1.1–2.3] vs. 3.5 months [95% CI, 1.7–5.2]; p = 0.002), and a longer median overall survival duration (7.3 months [95% CI, 4.8–9.8] vs. 12.7 months [95% CI, 7.1–18.3]; p = 0.24). Our two phase I trials provide preliminary evidence supporting the use of antiangiogenisis-based therapy in patients with metastatic TP53 mutant solid tumors, especially in those with metastatic sarcoma or metastatic colorectal cancer.
Subject terms: Predictive markers, Phase I trials, Targeted therapies
Introduction
In advanced cancers and those refractory to treatment, many factors may play a contributing role. Tumor cells that survive antiangiogenic therapy and metastasize frequently do so in hypoxic microenvironments1–3. Tumor hypoxia upregulates histone deacetylase (HDAC) activity4, which modulates the overexpression of hypoxia-inducible factor 1α (HIF-1α)5. This hypoxia-mediated increase in HIF-1α promotes tumor progression through the HIF-1α−dependent activation of multiple genes, whose expression enables cancer cells to survive, metastasize, and acquire resistance to antiangiogenic therapy6,7. HDAC5 is a critical player in the p53 acetylation network8,9, and HDAC6 and HDAC8 interact with heat shock protein 90 to facilitate mutant p53 degradation10–12. HDAC inhibition with vorinostat preferentially kills TP53 mutant cancer cells in cell cultures and xenograft models10,11.
The enhanced vascular endothelial growth factor (VEGF) pathway plays an important role in the survival and proliferation of cancer cells with TP53 mutations13,14 and thus represents a potential therapeutic target in TP53 mutant cancers. In cancer cells, TP53 mutations are associated with elevated HIF-1α levels, which augment the HIF-1α−dependent transcriptional activation of the VEGF gene in response to tumor hypoxia15, and mediate resistance to cancer therapy16. In addition, we found that among cancer patients receiving VEGF inhibition−based therapies, the progression-free survival (PFS) durations of patients with mutated TP53 were significantly longer than those of patients with wild-type TP5316–19.
The ability of wild-type p53 protein to induce apoptosis and suppress angiogenesis is of significant scientific merit and urgent clinical interest to develop novel cancer therapeutics. One promising strategy to explore HDAC inhibitor-mediated down-regulation of HIFs for targeting TP53 mutant tumor resistance to antiangiogenic therapy is supported by both preclinical and retrospective clinical findings20–27. To date, the U.S. Food and Drug Administration has approved pazopanib for the treatment of renal cell carcinoma and soft tissue sarcoma; vorinostat for the treatment of primary cutaneous T-cell lymphoma; and ixazomib for the treatment of multiple myeloma. We therefore conducted two phase I trials: one of the HDAC inhibitor vorinostat plus the VEGF inhibitor pazopanib in patients with advanced malignancies (NCT01339871) and another of vorinostat plus the proteasome inhibitor ixazomib in patients with metastatic TP53 mutant solid tumors (NCT02042989).
Results
Patient characteristics
The characteristics of the 78 patients enrolled in the phase I trial of pazopanib and vorinostat were reported previously28. The characteristics of the 59 patients enrolled in the phase I trial of ixazomib and vorinostat are given in Table 1. The phase I trial of ixazomib and vorinostat followed a 3 + 3 dose-escalation design. Patients were enrolled at 4 dose levels. One treatment cycle was 28 days. Oral ixazomib, escalating from 3 to 4 mg, was administered on days 1, 8, and 15, and oral vorinostat, escalating from 100 mg twice daily to 100 mg three times daily, was given on days 1–21. The patients enrolled in the ixazomib and vorinostat trial, whose median age was 59 years (range, 24−76 years), were heavily pretreated; they received a median of 5 systemic therapeutic regimens previously, and 58% had experienced disease progression on VEGF inhibition−based therapy.
Table 1.
Characteristics of patients with confirmed TP53 mutations.
| Characteristic | Clinical Trial | |
|---|---|---|
| Ixazomib + Vorinostat (n = 59) | Pazopanib + Vorinostat (n = 11) | |
| Median age (range), years | 59 (24–76) | 70 (46–78) |
| Gender | ||
| Male | 24 (41) | 5 (45) |
| Female | 35 (59) | 6 (55) |
| Race | ||
| White | 46 (78) | 9 (82) |
| Hispanic | 5 (8) | 0 |
| African American | 4 (7) | 0 |
| Asian | 4 (7) | 2 (2) |
| ECOG performance status score | ||
| 0 | 11 (19) | 0 |
| 1 | 44 (74) | 11 (100) |
| 2 | 4 (7) | 0 |
| Disease type | ||
| Colorectal cancer | 20 (34) | 3 (27) |
| Ovarian cancer | 14 (23) | 3 (27) |
| Breast cancer | 4 (7) | 1 (9) |
| Sarcoma | 4 (7) | 2 (18) |
| Head and neck cancer | 4 (7) | 1 (9) |
| Others* | 13 (22) | 1 (9) |
| Prior chemotherapy | ||
| Median no. of regimens (range) | 5 (0–9) | 4 (0–10) |
| VEGF inhibition−based therapy | 34 (58) | 5 (45) |
| Prior radiation therapy | 33 (56) | 3 (27) |
| Prior surgery | 47 (80) | 10 (91) |
| Median no. of metastasis sites (range) | 3 (1–5) | 3 (2–5) |
| TP53 point mutations | 50 (85) | 9 (82) |
| TP53 hotspot mutations# | 24 (41) | 4 (36) |
| TP53 non-point mutations | 9 (15) | 2 (18) |
Note: All data are no. of patients (%) unless otherwise noted.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; VEGF, vascular endothelial growth factor.
*Includes duodenal, gastric, and pancreatic cancer (n = 2 each) and esophageal cancer, endometrial cancer, non-small cell lung cancer, renal cancer, urachal adenocarcinoma, melanoma, and Mullerian tumor (n = 1 each).
#Mutations at R175, G245, R248, R249, R273, or R282.
Safety evaluation
In the phase I trial of pazopanib and vorinostat, the recommended phase II dosage was 600 mg pazopanib daily in combination with 100 mg vorinostat three times daily28. In the phase I trial of ixazomib and vorinostat, the recommended phase II dosage was 4 mg ixazomib once daily on days 1, 8, and 15 in combination with 100 mg vorinostat three times daily on days 1−21 (dose level 4). The clinically significant grade 2 or higher adverse events experienced by patients treated with ixazomib and vorinostat included anemia, thrombocytopenia, fatigue, anorexia, nausea, vomiting, diarrhea, dehydration, and skin rash (Supplementary Table 1). No treatment-related death or dose-limiting toxicity was observed among these patients. Seven patients, all of whom were enrolled at dose level 4, required dose reductions (18%). The patient withdrawal rates at dose levels 2, 3, and 4 were 13% (1 of 8 patients), 17% (1 of 6 patients), and 31% (12 of 39 patients), respectively.
Efficacy evaluation
Antitumor activity and survival among all patients
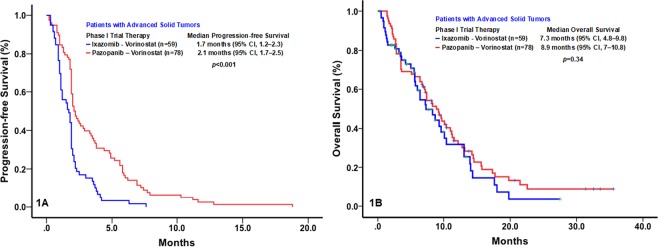
The major clinical outcomes of the 59 patients enrolled in the phase I trial of ixazomib and vorinostat are shown in Table 2. No objective responses were observed in these patients. Compared with patients treated with ixazomib and vorinostat, those treated with pazopanib and vorinostat had a significantly higher rate of clinical benefit, defined as stable disease lasting ≥6 months, a partial response, or a complete response (3.4% vs. 19%; p = 0.007) and a significantly longer median PFS duration (1.7 months [95% confidence interval (CI), 1.1–2.3] vs. 2.1 months [95% CI, 1.7–2.5]; p < 0.001). However, the median OS durations of the patients treated with ixazomib and vorinostat (7.3 months; 95% CI, 4.8–9.8) and those treated with pazopanib and vorinostat (8.9 months; 95% CI, 7.0–10.8) did not differ significantly (p = 0.34) (Fig. 1A,B).
Table 2.
Major Clinical Outcomes in the Phase I Trial of Ixazomib and Vorinostat (n = 59).
| TP53 mutation | Age | Sex | Race | PS | Pathology | Dose Level | Best response | PFS (mo) | OS (mo) |
|---|---|---|---|---|---|---|---|---|---|
| R273C | 43 | F | A | 1 | CRC | 1 | PD | 1.0 | 3.4+ |
| R273H | 41 | M | W | 0 | Gastric | 1 | PD | 1.9 | 14.6 |
| R175H | 64 | M | W | 1 | CRC | 1 | SD | 3.7 | 7.2 |
| Splice site c.376–1 G > A | 59 | F | W | 1 | SA | 1 | PD | 1.9 | 13.3+ |
| G245S | 64 | M | AA | 1 | Pancreatic | 1 | PD | 0.6 | 1.1 |
| R248W | 60 | F | W | 1 | Breast | 1 | PD | 2.0 | 6.2 |
| R196* | 76 | F | W | 1 | EOC | 2 | PD | 1.1 | 6.3 |
| S127F | 67 | F | W | 1 | Breast | 2 | PD | 0.9 | 2.0 |
| V173M | 55 | F | W | 1 | Breast | 2 | PD | 1.6 | 6.5 |
| H179P | 58 | M | H | 0 | CRC | 2 | SD | 3.0 | 15.0 |
| Q104* | 52 | M | W | 1 | HNC | 2 | Withdrawal | 0.3 | 1.3+ |
| G245S | 56 | F | W | 2 | Urachal | 2 | PD | 2.1 | 18.2 |
| R342* | 40 | M | W | 0 | CRC | 2 | PD | 1.9 | 13.7 |
| V10I | 31 | M | W | 1 | SA | 2 | PD | 1.2 | 10.7+ |
| L111P | 64 | M | W | 2 | Gastric | 3 | PD | 1.0 | 9.7 |
| R280T | 53 | F | H | 1 | Breast | 3 | SD | 3.6 | 7.2 |
| R213L | 75 | M | W | 1 | CRC | 3 | PD | 2.0 | 29.2+ |
| A138V | 60 | M | W | 1 | CRC | 3 | PD | 1.0 | 1.7 |
| V272L | 42 | M | W | 1 | CRC | 3 | SD | 3.5 | 14.6 |
| L201* | 64 | M | W | 1 | CRC | 3 | Withdrawal | 2.3 | 19.9 |
| P278A | 63 | F | W | 1 | EOC | 4 | Withdrawal | 1.2 | 4.7 |
| G245S | 38 | M | AA | 1 | CRC | 4 | PD | 0.9 | 2.1 |
| C135R | 70 | F | W | 1 | Endometrial | 4 | PD | 1.0 | 14.1 |
| S215N | 57 | M | W | 1 | HNC | 4 | PD | 1.8 | 8.4 |
| S241F | 51 | M | AA | 1 | NSCLC | 4 | PD | 2.2 | 5.2 |
| R282W | 60 | M | W | 1 | CRC | 4 | Withdrawal | 1.6 | 3.1 |
| R248W | 60 | F | W | 1 | CRC | 4 | PD | 1.9 | 9.1 |
| R280G | 70 | F | W | 2 | EOC | 4 | SD | 7.6 | 10.2 |
| R175H | 52 | F | W | 0 | CRC | 4 | Withdrawal | 1.1 | 7.4 |
| R248Q | 35 | M | W | 1 | Melanoma | 4 | PD | 0.7 | 4.4 |
| R213* | 48 | F | W | 1 | CRC | 4 | PD | 1.9 | 10.8 |
| C176S, R248Q, R273H | 71 | M | W | 0 | SA | 4 | SD | 3.9 | 18.3 |
| G244S | 66 | F | H | 1 | CRC | 4 | PD | 1.5 | 10.4 |
| R273C | 60 | M | W | 1 | CRC | 4 | PD | 1.1 | 2.5 |
| E336Q/ Y234H | 52 | M | A | 1 | CRC | 4 | Withdrawal | 0.9 | 5.8 |
| C229fs*10 | 64 | F | H | 1 | EOC | 4 | SD | 6.3 | 8.6 |
| E180fs*67 | 58 | F | W | 1 | EOC | 4 | PD | 2.1 | 5.1 |
| R248W | 33 | F | W | 1 | CRC | 4 | Withdrawal | 0.6 | 7.0 |
| Y205H | 76 | M | W | 1 | CRC | 4 | PD | 0.5 | 2.4 |
| Q16*/ R248Q | 63 | F | W | 1 | HNC | 4 | PD | 0.3 | 1.1 |
| R282W | 58 | F | W | 1 | Mullerian | 4 | SD | 4.1 | 11.3 |
| Y220C | 73 | F | AA | 2 | EOC | 4 | Withdrawal | 0.9 | 2.8+ |
| R175H | 50 | F | H | 1 | EOC | 4 | PD | 1.9 | 4.9+ |
| G245R/ K164E | 50 | F | W | 0 | EOC | 4 | SD | 3.8 | 10.8+ |
| R282W | 24 | F | W | 1 | EOC | 4 | Withdrawal | 0.7 | 2.2 |
| H179Y | 67 | F | W | 0 | EOC | 4 | PD | 1.8 | 8.9+ |
| R306* | 42 | M | W | 1 | Renal | 4 | PD | 2.2 | 7.9+ |
| C275F | 64 | F | A | 1 | CRC | 4 | Withdrawal | 0.5 | 6.3+ |
| R273C | 68 | F | A | 0 | Pancreatic | 4 | PD | 1.8 | 2.5+ |
| R175H | 56 | F | W | 1 | Cecum | 4 | Withdrawal | 0.9 | 3.7 |
| R110L | 60 | F | W | 0 | EOC | 4 | PD | 1.9 | 6.6+ |
| L111P | 73 | F | W | 1 | EOC | 4 | Withdrawal | 1.7 | 6.3+ |
| H179R/R273C | 59 | F | W | 1 | EOC | 4 | Withdrawal | 1.2 | 2.8+ |
| R282W | 46 | F | W | 1 | CRC | 4 | SD | 4.2 | 7.2+ |
| R273H | 58 | M | W | 1 | Cecum | 4 | Lost to follow up | 1.1 | 2.8 |
| R273H | 57 | F | W | 0 | HNC | 4 | Withdrawal | 0.3 | 5.7+ |
| Y234N/R158G | 61 | M | W | 0 | Esophageal | 4 | PD | 1.9 | 4.6+ |
| V173L | 57 | F | W | 1 | SA | 4 | PD | 2.5 | 3.3+ |
| P278_G279del | 66 | F | W | 1 | EOC | 4 | PD | 1.1 | 3.5+ |
Abbreviations: PS, ECOG performance status; PFS, progression-free survival; OS, overall survival; mo, month; M, male; F, female; W, white; A, Asian; H, Hispanic; AA, African-American; PD, progression disease; SD, stable disease; + , censored; CRC, colorectal cancer; EOC, epithelial ovarian cancer; SA, sarcoma; HNC, head & neck cancer; NSCLC, non-small cell lung cancer.
Figure 1.
Kaplan-Meier plots of the rates of progression-free survival (PFS; 1A) and overall survival (OS; 1B) of cancer patients treated with pazopanib and vorinostat (n = 78) or ixazomib and vorinostat (n = 59).
Antitumor activity and survival among patients with tp53 hotspot mutations
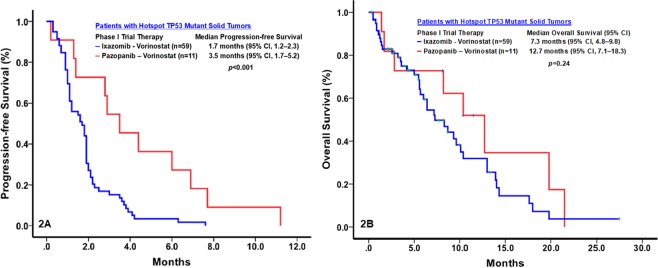
TP53 hotspot mutations were confirmed in all 59 patients enrolled in the phase I trial of ixazomib and vorinostat and in 11 of the 78 patients enrolled in the phase I trial of pazopanib and vorinostat. Compared with patients treated with ixazomib and vorinostat, those treated with pazopanib and vorinostat had a significantly higher rate of clinical benefit (3.4% vs. 45%; p < 0.001) and a significantly longer median PFS duration (1.7 months [95% CI, 1.1–2.3] vs. 3.5 months [95% CI, 1.7–5.2]; p = 0.002). The median OS duration of the patients treated with pazopanib and vorinostat (12.7 months; 95% CI, 7.1–18.3) was longer than that of those treated with ixazomib and vorinostat (7.3 months; 95% CI, 4.8–9.8), but this difference was not significant (p = 0.24) (Fig. 2A,B).
Figure 2.
Kaplan-Meier plots of the rates of progression-free survival (PFS; 2A) and overall survival (OS; 2B) of patients with metastatic TP53 mutant solid tumors treated with pazopanib and vorinostat (n = 11) or ixazomib and vorinostat (n = 59).
Antitumor activity and survival among patients with metastatic sarcoma or colorectal carcinoma with TP53 hotspot mutations
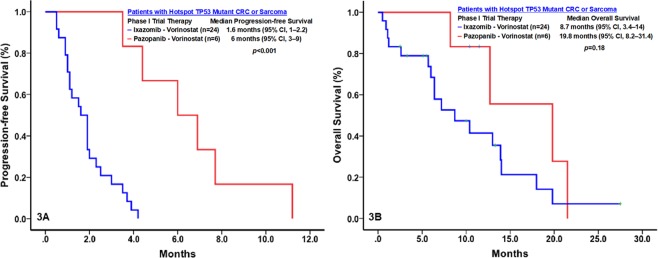
Twenty-four patients enrolled in the phase I trial of ixazomib and vorinostat had colorectal carcinoma (n = 20) or sarcoma (n = 4) with TP53 hotspot mutations, and 6 patients enrolled in the phase I trial of pazopanib and vorinostat had colorectal carcinoma (n = 3) or sarcoma (n = 3) with TP53 hotspot mutations. Compared with patients treated with ixazomib and vorinostat, those treated with pazopanib and vorinostat had a significantly higher rate of clinical benefit (0% vs. 83%; p < 0.001) and a significantly longer median PFS duration (1.6 months [95% CI, 1–2.2] vs. 6.0 months [95% CI, 3.0–9.0]; p < 0.001). The median OS duration of the patients treated with pazopanib and vorinostat (19.8 months, 95% CI, 8.2–31.4) was longer than that of those treated with ixazomib and vorinostat (8.7 months, 95% CI, 3.4–14), but this difference was not significant (p = 0.18) (Fig. 3A,B).
Figure 3.
Kaplan-Meier plots of the rates of progression-free survival (PFS; A) and overall survival (OS; B) of patients with metastatic TP53 mutant sarcoma or colorectal cancer (CRC) treated with pazopanib and vorinostat (n = 6) or ixazomib and vorinostat (n = 24).
Discussion
Therapeutic targeting of TP53 mutations is a rapidly developing field, and various approaches have undergone clinical evaluation29,30. We have designed and conducted two subsequent phase I clinical trials (NCT01339871, a phase I study of pazopanib and vorinostat in patients with advanced malignancies; and NCT02042989, a phase I study of ixazomib and vorinostat in patients with advanced TP53 mutant malignancies) in order to target patients with metastatic TP53 mutant solid tumors. Our two phase I clinical trials provide preliminary prospective evidence supporting the use of antiangiogenesis-based therapy in patients with TP53 mutations.
Our phase I trial of pazopanib and vorinostat revealed that the combination therapy was more effective in cancer patients with TP53 hotspot mutations than in patients without TP53 hotspot mutations28. Many cancers have TP53 mutations31, many of which produce mutant p53 gain-of-function proteins, leading to tumorigenesis, tumor development, and metastasis32,33. Because they promote tumor growth through VEGF overexpression and increased neovascularization34 and regulate cell cycle arrest, DNA damage repair, cellular senescence, apoptosis, metabolism, stem cell maintenance, tumor invasion, metastasis, and communication with the tumor microenvironment35–37, mutant p53 proteins are potential therapeutic targets16,38. To be effective against TP53 mutant malignancies, a therapy must target many biological pathways simultaneously. The results of our phase I trial of the HDAC inhibitor vorinostat plus the VEGF inhibitor pazopanib support the use of this combination in patients with TP53 mutant malignancies28. These agents likely have antitumor activity through their synergistic antiangiogenic effects, facilitation of mutant p53 degradation10–12, and downregulation of VEGF inhibition−mediated HIF-1α overexpression5,15,27.
Preclinical studies have shown that proteasome inhibition induces p53-dependent and -independent apoptosis and that HDAC inhibition mediates preferential cytotoxicity towards p53 mutant cells. In addition, combined proteasome and HDAC inhibition has synergistic antitumor effects by modulating epigenetic gene expression39,40, posttranslational modifications41, and protein degradation in the proteasome and aggresome pathways42,43, thereby increasing cellular stress and apoptosis44. On the basis of these findings, we conducted a phase I trial of ixazomib and vorinostat in 59 patients with metastatic TP53 mutant solid tumors, expecting to find that the combination had efficacy against these tumors. However, the combination did not elicit an objective response in any of these patients and was associated with poor PFS and OS. These findings led us to initiate the present study to investigate the role of these therapeutic regimens in patients with TP53 mutant malignancies by comparing their antitumor activity and associated survival outcomes between the two phase I trials. Among patients with metastatic solid tumors with TP53 hotspot mutations – particularly patients who had colorectal carcinoma or sarcoma – those treated with pazopanib and vorinostat had a significantly longer median PFS duration and a longer OS duration than did those treated with ixazomib and vorinostat. These findings suggest that vorinostat should be paired with pazopanib, rather than ixazomib, for the treatment of cancers with TP53 mutations.
The present study yielded several additional insights into the use of vorinostat with pazopanib or ixazomib against metastatic solid tumors with TP53 mutations. First, although preclinical evidence suggests that HDAC inhibition induces preferential cytotoxicity towards p53 mutant cells, our findings suggest that the use of vorinostat most likely contributed to the high frequencies of dose reduction and patient withdrawal owing to drug toxicity in the two trials. Thus, additional studies to determine whether lower doses of HDAC inhibitors can have meaningful therapeutic effect against p53 mutant cancer cells may be warranted. Second, many patients can be maintained at lower dose levels. There is no statistical relationship between dose level and major clinical outcomes including antitumor responses and survivals. Third, because p53 is at the hub of numerous signaling pathways triggered by a range of cellular stresses, effective therapeutic strategies against malignancies driven by p53 mutations may require several agents simultaneously targeting multiple p53-regulated downstream pathways.
The present study had several potential limitations that could limit the clinical relevance of its findings. First, as with many early clinical trials, the phase I trials we conducted—despite employing eligibility criteria similar to those used in other phase I trials enrolling patients with advanced solid tumors lacking effective standard therapy—were subject to patient selection bias, which may limit the generalizability of our findings to patients with TP53 mutant malignancies. Second, owing to their small sample sizes, the subgroup analyses could not reliably detect significant differences between groups. Third, because they did not include correlative pharmacokinetics and pharmacodynamics assessments, the phase I trials may not have identified the optimal recommended phase II doses of the drug combinations they tested.
Patients and Methods
Trial design and patient enrollment
The trial of pazopanib and vorinostat enrolled patients with metastatic solid tumors from April 2011 to December 2013, whereas the trial of ixazomib and vorinostat enrolled patients with metastatic solid tumors carrying TP53 hotspot mutations, defined as positive cytoplasmic staining by immunohistochemistry and/or next gene mutation sequencing from July 2014 to February 2017. For both trials, patients were age ≥18 years, with a histologically confirmed advanced malignancy and without a standard therapy that improved survival ≥3 months. All eligible patients also had measurable or evaluable disease that had progressed prior to enrollment and an Eastern Cooperative Oncology Group performance status score of ≥245. Additional eligibility criteria included adequate marrow function (absolute neutrophil count ≥1,000/μl and platelet count ≥75,000/μl), a calculated creatinine clearance rate of ≥30 ml/min, a total bilirubin level ≤1.5 × the upper limit of the normal (ULN), and alanine aminotransferase and aspartate aminotransferase levels ≤3 × the ULN. Patients were excluded if they had clinically significant cardiovascular disease; had active uncontrolled central nervous system involvement; had active serious infection requiring systemic antibiotics; had known gastrointestinal disease or other condition that could interfere with swallowing or oral absorption; were pregnant or lactating; had not recovered from previous cancer therapeutics; or were unwilling or unable to give written informed consent. Both trials were conducted at MD Anderson and were approved by MD Anderson’s Institutional Review Board. Informed consent was obtained from all study participants, and all methods were performed in accordance within the relevant guidelines and regulations.
Evaluation of toxicity and efficacy
Patients who had received at least one dose of any of the study agents were considered evaluable for drug safety. Toxicity severity was graded according to the Common Terminology Criteria for Adverse Events, version 4.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40). Dose-limiting toxicity was defined as treatment-related grade 4 hematologic toxicity lasting >1 week; grade 4 nausea or vomiting lasting >3 days despite appropriate medical intervention; grade 4 fatigue or hypertension; or grade 3 or higher non-hematologic toxicity occurring within the initial 28-day treatment cycle. The maximum tolerated dose was defined as the dose level below that at which >33% of patients experienced dose-limiting toxicity.
Patients who received at least one dose of any of the study agents were considered evaluable for drug efficacy. Patients receiving therapy underwent radiographic imaging studies every 8 weeks. We used Response Evaluation Criteria in Solid Tumors 1.1 to assess tumor responses46.
Molecular assays for TP53 mutations
Archival formalin-fixed, paraffin-embedded tissue blocks, core biopsy specimens, or surgical resection specimens were used for TP53 mutation assessment. TP53 mutation assessment was performed in a Clinical Laboratory Improvement Amendment−certified molecular diagnostic laboratory at MD Anderson Cancer Center (for hotspots 2 [1–20], 4 [68–113], 5 [126–138], 5 [149–187], 6 [187–223], 7 [225–258], 8 [263–307], and 10 [332–367]), as well as at Foundation Medicine (for the entire coding sequence), as described previously47–49.
Statistical considerations
Both phase I trials followed a modified zone-based 3 + 3 dose-escalation design50. An additional cohort of up to 3 patients was allowed per dose level as needed for safety assessment. If clinical benefit was observed in patients with a specific type of cancer, a dose expansion cohort of up to 14 patients was permitted at the highest dose level considered to be safe at the time of patient entry, as described previously51. Continuous data were summarized using medians, ranges, and 95% CIs. Categorical data were summarized using frequencies and percentages. Differences in categorical variables were assessed using the Fisher exact test. PFS was defined as the time from the date of initial trial therapy (cycle 1 day 1) to the date of death or tumor progression. OS was defined as the time from the date of initial trial therapy to the date of death or last radiographic assessment. Patients who had no evidence of disease progression or were alive at the end of the study period were censored at the date of last radiographic assessment. The Kaplan-Meier method was used to estimate PFS and OS, and log-rank tests were used to compare PFS and OS distributions between the treatment groups. Differences between the treatment groups were assessed using two-sided t-tests; p values < 0.05 were considered significant. Statistical analyses were performed using SPSS Statistics, version 24 (IBM, Armonk, NY).
Supplementary information
Acknowledgements
The authors thank the patients who participated in the clinical trials and their families; the clinical research personnel and clinical staff in MD Anderson’s Clinical Center for Targeted Therapy for their comprehensive patient care and service; and Joe Munch in Scientific Publications, Research Medical Library, at MD Anderson for editing the manuscript. The authors also thank Takeda Pharmaceutical for supporting the phase I trial of ixazomib and vorinostat through a grant (to SF) and by providing ixazomib.
Author contributions
Yudong Wang: Data collection, analysis, and interpretation; manuscript writing; final manuscript approval; and provision of study materials and patients. Filip Janku: Data collection, manuscript writing, final manuscript approval, and provision of study materials and patients. Sarina Piha-Paul: Data collection, manuscript writing, final manuscript approval, and provision of study materials and patients. Kenneth Hess: Data collection, assembly, analysis, and interpretation. Russell Broaddus: Data collection, manuscript writing, final manuscript approval, and provision of study materials and patients. Lidong Liu: Data collection and assembly. Naiyi Shi: Data collection and assembly. Michael Overman: Data collection, manuscript writing, final manuscript approval, and provision of study materials and patients. Scott Kopetz: Data collection, manuscript writing, final manuscript approval, and provision of study materials and patients. Vivek Subbiah: Data collection, analysis, and interpretation; final manuscript approval; and provision of study materials and patients. Aung Naing: Study conception and design; data collection, analysis, and interpretation; manuscript writing; final manuscript approval; and provision of study materials and patients. David Hong: Manuscript editing and provision of study materials and patients. Apostolia-Maria Tsimberidou: Data collection, analysis, and interpretation; manuscript writing; final manuscript approval; and provision of study materials and patients. Daniel Karp: Data collection, manuscript writing, final manuscript approval, and provision of study materials and patients. James Yao: Data collection, manuscript writing, final manuscript approval, and provision of study materials and patients. Siqing Fu: Study conception and design; data collection, analysis, and interpretation; manuscript writing; final manuscript approval; and provision of study materials and patients.
Competing interests
The competing and non-financial interests of the authors are listed below. This study used MD Anderson’s Clinical Research Facility, which is supported by the NIH through MD Anderson’s Cancer Center Support Grant (P30CA016672). MD Anderson receives funds from Takeda Pharmaceutical Company and the National Institutes of Health/NCI (grant #P30CA016672), which in turn funded Dr. Fu’s research for this manuscript. Drs. Vivek Subbiah and David Hong have both received clinical trial research funding from Takeda Pharmaceutical Company and the NIH/NCI through MD Anderson. Drs. Filip Janku, Sarina Piha-Paul, Michael Overman, Russell Broaddus, Scott Kopetz, Aung Naing, Apostolia-Maria Tsimberidou, Daniel Karp, and James Yao receive partial funding for their clinic research from the NIH/NCI (grant #P30CA016672). All other authors have no competing interests to declare.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-58366-z.
References
- 1.Paez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebos JM, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonsen TG, Gaustad JV, Rofstad EK. Development of hypoxia in a preclinical model of tumor micrometastases. Int. J. Radiat. Oncol. Biol. Phys. 2010;76:879–888. doi: 10.1016/j.ijrobp.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 4.Fath DM, et al. Histone deacetylase inhibitors repress the transactivation potential of hypoxia-inducible factors independently of direct acetylation of HIF-alpha. J. Biol. Chem. 2006;281:13612–13619. doi: 10.1074/jbc.M600456200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SH, et al. Regulation of the HIF-1alpha stability by histone deacetylases. Oncol. Rep. 2007;17:647–651. [PubMed] [Google Scholar]
- 6.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Sen N, Kumari R, Singh MI, Das S. HDAC5, a key component in temporal regulation of p53-mediated transactivation in response to genotoxic stress. Mol. Cell. 2013;52:406–420. doi: 10.1016/j.molcel.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Marks PA, Richon VM, Miller T, Kelly WK. Histone deacetylase inhibitors. Adv. Cancer Res. 2004;91:137–168. doi: 10.1016/S0065-230X(04)91004-4. [DOI] [PubMed] [Google Scholar]
- 10.Li D, Marchenko ND, Moll UM. SAHA shows preferential cytotoxicity in mutant p53 cancer cells by destabilizing mutant p53 through inhibition of the HDAC6-Hsp90 chaperone axis. Cell Death Differ. 2011;18:1904–1913. doi: 10.1038/cdd.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan W, Liu S, Xu E, Zhang J, Zhang Y, Chen X, Chen X. Histone deacetylase inhibitors suppress mutant p53 transcription via histone deacetylase 8. Oncogene. 2012;32(5):599–609. doi: 10.1038/onc.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blagosklonny MV, et al. Depletion of mutant p53 and cytotoxicity of histone deacetylase inhibitors. Cancer Res. 2005;65:7386–7392. doi: 10.1158/0008-5472.CAN-04-3433. [DOI] [PubMed] [Google Scholar]
- 13.Joshi H, Bhanot G, Borresen-Dale AL, Kristensen V. Potential tumorigenic programs associated with TP53 mutation status reveal role of VEGF pathway. Br. J. Cancer. 2012;107:1722–1728. doi: 10.1038/bjc.2012.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montero E, Abreu C, Tonino P. Relationship between VEGF and p53 expression and tumor cell proliferation in human gastrointestinal carcinomas. J. cancer Res. Clin. Oncol. 2008;134:193–201. doi: 10.1007/s00432-007-0270-5. [DOI] [PubMed] [Google Scholar]
- 15.Ravi R, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes. Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 16.Said R, et al. Characteristics and survival of patients with advanced cancer and p53 mutations. Oncotarget. 2014;5:3871–3879. doi: 10.18632/oncotarget.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, et al. Survival of patients with metastatic leiomyosarcoma: the MD Anderson Clinical Center for targeted therapy experience. Cancer Med. 2016;5:3437–3444. doi: 10.1002/cam4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, et al. Antiangiogenesis and gene aberration-related therapy may improve overall survival in patients with concurrent KRAS and TP53 hotspot mutant cancer. Oncotarget. 2017;8:33796–33806. doi: 10.18632/oncotarget.16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, et al. Outcome analysis of Phase I trial patients with metastatic KRAS and/or TP53 mutant non-small cell lung cancer. Oncotarget. 2018;9:33258–33270. doi: 10.18632/oncotarget.25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraisl P, Mazzone M, Schmidt T, Carmeliet P. Regulation of angiogenesis by oxygen and metabolism. Dev. Cell. 2009;16:167–179. doi: 10.1016/j.devcel.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Mazumdar J, Dondeti V, Simon MC. Hypoxia-inducible factors in stem cells and cancer. J. Cell Mol. Med. 2009;13:4319–4328. doi: 10.1111/j.1582-4934.2009.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dokmanovic M, et al. Histone deacetylase inhibitors selectively suppress expression of HDAC7. Mol. cancer therapeutics. 2007;6:2525–2534. doi: 10.1158/1535-7163.MCT-07-0251. [DOI] [PubMed] [Google Scholar]
- 23.Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 2010;20:51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson PG, Anderson KC. Bortezomib: a novel therapy approved for multiple myeloma. Clin. Adv. Hematol. Oncol. 2003;1:596–600. [PubMed] [Google Scholar]
- 25.Pili, R. et al. Combination of the histone deacetylase inhibitor vorinostat with bevacizumab in pretreated patients with renal cell carcinoma: Safety, efficacy, and pharmacodynamic results. Genitourinary Cancers Symposium, Abstract 350 (2010).
- 26.Dasari, A. et al. A phase I safety and tolerability study of vorinostat (V) in combination with sorafenib (S) in patients with advanced solid tumors, with exploration of two tumor-type specific expanded cohorts at the recommended phase II dose (renal and non-small cell lung carcinoma). Journal of Clinical Oncology, 28:15s, Suppl; abstract 2562 (2010).
- 27.Ellis L, Hammers H, Pili R. Targeting tumor angiogenesis with histone deacetylase inhibitors. Cancer Lett. 2009;280:145–153. doi: 10.1016/j.canlet.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu S, et al. Phase I study of pazopanib and vorinostat: a therapeutic approach for inhibiting mutant p53-mediated angiogenesis and facilitating mutant p53 degradation. Ann. Oncol. 2015;26:1012–1018. doi: 10.1093/annonc/mdv066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen L, et al. The fundamental role of the p53 pathway in tumor metabolism and its implication in tumor therapy. Clin. Cancer Res. 2012;18:1561–1567. doi: 10.1158/1078-0432.CCR-11-3040. [DOI] [PubMed] [Google Scholar]
- 30.Vousden KH, Ryan KM. p53 and metabolism. Nat. Rev. Cancer. 2009;9:691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- 31.Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nat. 2009;458:1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell. 2014;25:304–317. doi: 10.1016/j.ccr.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leroy B, et al. The TP53 website: an integrative resource centre for the TP53 mutation database and TP53 mutant analysis. Nucleic acids Res. 2013;41:D962–969. doi: 10.1093/nar/gks1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambs S, et al. p53 and vascular endothelial growth factor regulate tumor growth of NOS2-expressing human carcinoma cells. Nat. Med. 1998;4:1371–1376. doi: 10.1038/3957. [DOI] [PubMed] [Google Scholar]
- 35.Yamakuchi M, et al. P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc. Natl Acad. Sci. USA. 2010;107:6334–6339. doi: 10.1073/pnas.0911082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat. Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 37.Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer. 2014;14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Said R, et al. P53 mutations in advanced cancers: clinical characteristics, outcomes, and correlation between progression-free survival and bevacizumab-containing therapy. Oncotarget. 2013;4:705–714. doi: 10.18632/oncotarget.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitsiades CS, et al. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc. Natl Acad. Sci. USA. 2004;101:540–545. doi: 10.1073/pnas.2536759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitsiades N, et al. Molecular sequelae of histone deacetylase inhibition in human malignant B cells. Blood. 2003;101:4055–4062. doi: 10.1182/blood-2002-11-3514. [DOI] [PubMed] [Google Scholar]
- 41.Mitsiades N, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc. Natl Acad. Sci. USA. 2002;99:14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hideshima T, Richardson PG, Anderson KC. Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma. Mol. Cancer Ther. 2011;10:2034–2042. doi: 10.1158/1535-7163.MCT-11-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Catley L, et al. Aggresome induction by proteasome inhibitor bortezomib and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood. 2006;108:3441–3449. doi: 10.1182/blood-2006-04-016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pei XY, Dai Y, Grant S. Synergistic induction of oxidative injury and apoptosis in human multiple myeloma cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitors. Clin. cancer research: an. Off. J. Am. Assoc. Cancer Res. 2004;10:3839–3852. doi: 10.1158/1078-0432.CCR-03-0561. [DOI] [PubMed] [Google Scholar]
- 45.Oken MM, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 46.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 47.Singh RR, et al. Clinical validation of a next-generation sequencing screen for mutational hotspots in 46 cancer-related genes. J. Mol. diagnostics: JMD. 2013;15:607–622. doi: 10.1016/j.jmoldx.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Frampton GM, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol. 2013;31:1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacConaill LE. Existing and emerging technologies for tumor genomic profiling. J. Clin. oncology: Off. J. Am. Soc. Clin. Oncol. 2013;31:1815–1824. doi: 10.1200/JCO.2012.46.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang X, Biswas S, Oki Y, Issa JP, Berry DA. A parallel phase I/II clinical trial design for combination therapies. Biometrics. 2007;63:429–436. doi: 10.1111/j.1541-0420.2006.00685.x. [DOI] [PubMed] [Google Scholar]
- 51.Moroney J, et al. Phase I study of the antiangiogenic antibody bevacizumab and the mTOR/hypoxia-inducible factor inhibitor temsirolimus combined with liposomal doxorubicin: tolerance and biological activity. Clin. cancer research: an. Off. J. Am. Assoc. Cancer Res. 2012;18:5796–5805. doi: 10.1158/1078-0432.CCR-12-1158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.