Abstract
High numbers of threatened species might be expected to occur where overall species richness is also high; however, this explains only a proportion of the global variation in threatened species richness. Understanding why many areas have more or fewer threatened species than would be expected given their species richness, and whether that is consistent across taxa, is essential for identifying global conservation priorities. Here, we show that, after controlling for species richness, environmental factors, such as temperature and insularity, are typically more important than human impacts for explaining spatial variation in global threatened species richness. Human impacts, nevertheless, have an important role, with relationships varying between vertebrate groups and zoogeographic regions. Understanding this variation provides a framework for establishing global conservation priorities, identifying those regions where species are inherently more vulnerable to the effects of threatening human processes, and forecasting how threatened species might be distributed in a changing world.
Subject terms: Conservation biology, Ecological modelling, Macroecology
Understanding to what extent geographic patterns in threatened species diversity are driven by environmental features or human activities could aid conservation. Here, Howard et al. investigate broad scale patterns in species richness of threatened vertebrates and test the role of environmental and anthropogenic drivers.
Introduction
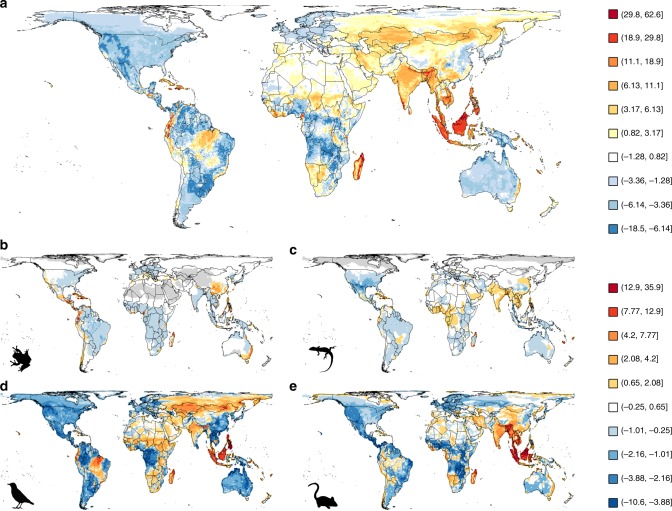
Safeguarding species against threatening processes is a major global priority1–3. Yet, despite the adoption of numerous international agreements aimed at addressing the biodiversity crisis, biodiversity continues to be lost4,5. An ambitious global strategy is now required that clearly defines the actions and policies necessary to restore ecosystems to levels that help both people and nature thrive6–8. This will require the establishment of global conservation priorities, so that limited resources can be focussed on those areas of highest conservation value most at risk from environmental change6,9,10. Currently, conservation plans often target overall species diversity, under the assumption that it can act as an adequate surrogate for other dimensions of biodiversity11–15. Biodiversity indices, however, are seldom spatially congruent11–13,15–17, and total species richness can only partially explain spatial gradients in threatened species richness (Fig. 1, see methods). Areas of South America, sub-Saharan Africa, India and Southeast Asia all have far greater numbers of threatened species than would be expected given the total size of the species pool. Understanding why these areas are hotspots of imperilment and how the mechanisms driving these patterns differ between taxonomic groups, is key for identifying global conservation priorities and the actions and policies required to prevent further loss of biodiversity6–8,18. The processes underlying global patterns of threatened species richness, however, remain largely unexplored.
Fig. 1. Global variation in threatened species richness, having accounted for total species richness.
The maps show the mean residuals from 10 global scale models of threatened species richness when explained by total species richness, for a terrestrial vertebrates, and four separate taxonomic groups: b amphibians, c reptiles, d birds and e mammals. The colour scale indicates model residuals in terms of the number of threatened species and uses Jenks natural breaks81 to determine the break points. Note the scales differ between a, and b–e. Grey indicates areas where there are no species classified as threatened. Model performance was moderate (Table 1 and Supplementary Table 2). Source data are provided as a Source Data file.
Extinction is often driven by threatening human activities19,20. Increasing human populations, long-term land cover change, and pressures from invasive alien species have all been linked to the loss of biodiversity21–23. Environmental conditions can, however, predispose species to the effects of these threatening processes13. Energy availability and habitat heterogeneity have been linked with speciation rates via increases in available niche space and retention of rare species through the provision of refugia, and by providing opportunities for isolation and divergent adaptation24–27. Threatened species richness is likely driven by the interaction between the predisposing environmental conditions that promote the occurrence of extinction-prone, narrow-range endemic species and the threatening human processes that erode biodiversity13. Despite substantial efforts being dedicated to deciphering the determinants of spatial gradients in total species richness24,26, there is only limited understanding of the drivers of spatial gradients in threatened species richness and how they differ from those of total species richness28. If we are to fully understand the distribution of imperilment, we require a global assessment that disentangles the effects of predisposing ecological conditions from those attributable to threatening human processes.
Here, we present a global assessment of the drivers of threatened species richness patterns, revealing the importance of extrinsic threatening processes and predisposing environmental conditions in shaping concentrations of imperilment. Our models of threatened species richness are based on a data set of 26,746 species of terrestrial vertebrates, 20% of which are considered ‘threatened’ with extinction (IUCN Red List status of critically endangered, endangered, or vulnerable). Specifically, we quantify the importance of a suite of environmental and human impact covariates, previously shown to correlate with extinction risk and species richness patterns, in driving spatial variation in global threatened vertebrate species richness. To identify the drivers of threatened species richness independent of the drivers of the size of the species pool, we account for the role of total species richness within our models. We then assess the influence of the covariates on four taxonomic groups individually (amphibians, reptiles, birds and mammals) and at the level of individual zoogeographic regions29 to establish how the drivers of threatened species richness vary between taxa and across space. Finally, we explore how the form of the relationship between these variables and species richness differs when that richness is restricted to threatened species or includes the total species pool.
Our results show that environmental covariates are typically more important than human impacts for explaining global variation in threatened species richness. Nevertheless, we identify substantial variation between zoogeographic regions and taxonomic groups in the importance of individual environmental and human impact variables in driving threatened vertebrate species richness. Specifically, we demonstrate that individual human impact variables can be influential at regional scales. These results suggest that some regions and taxonomic groups may be inherently more vulnerable than others to the effects of threatening human activities, which has important implications for identifying global conservation priorities.
Results and discussion
Global drivers of threatened species richness
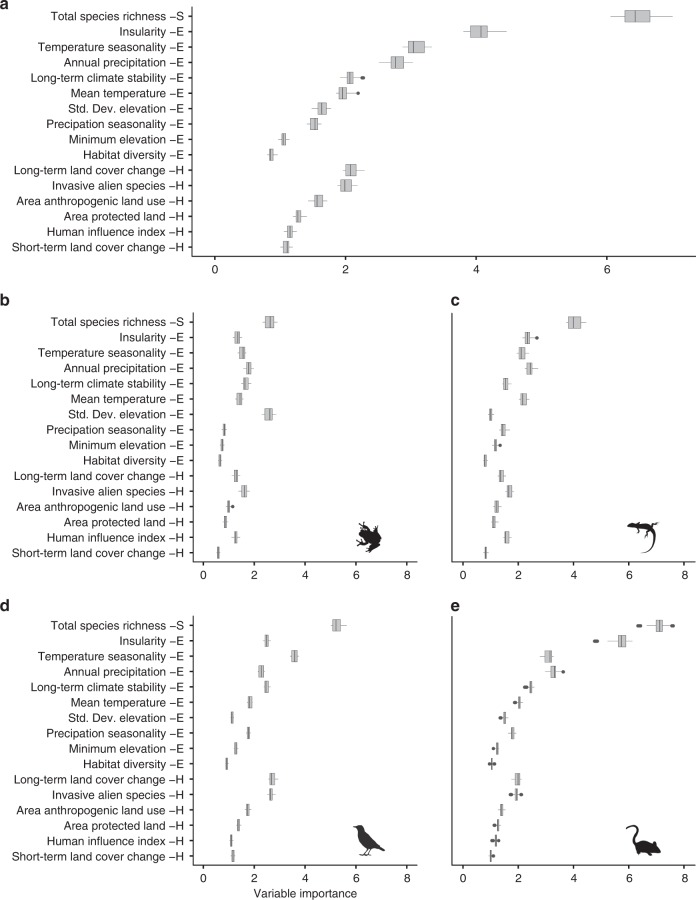
Global models of threatened vertebrate species richness revealed a greater influence of environmental parameters than extrinsic human threats (Fig. 2a, Tables 1 and 2), although human impacts still had an important role. Specifically, insularity, or the area of land mass to which a cell belonged, was of the greatest importance for explaining global threatened vertebrate species richness. Island regions generally support a higher number of endemic, extinction-prone species than continental land masses30 (Supplementary Fig. 10). Two variables associated with the amount of productive energy in a system, namely temperature seasonality and annual precipitation, were also highly influential in our models (ranked 3rd and 4th, respectively, of 16 variables). Given that our models account for total species richness, this result is not a consequence of more productive regions harbouring higher numbers of threatened species simply because they have more species overall. Furthermore, and contrary to previous reports31, the limited influence of human impact variables at a global scale suggests that this result is unrelated to enhanced human influence in areas of greater net primary productivity32–34. Rather, our results suggest that the processes that drive speciation may also be responsible for driving extinction35. Areas of high levels of speciation are associated with small range, specialist species that often persist at low population sizes and are therefore vulnerable to demographic stochasticity or extinction from local catastrophes or human disturbance33,36,37. Of the human impact variables, those of most importance were long-term land cover change and invasive alien species (ranked 6th and 7th, respectively). These results are in line with previous findings, with habitat loss widely cited as the most common cause of species’ extinctions38,39, and the impacts of invasive alien species as the second most common cause40.
Fig. 2. Importance of individual variables for predicting global threatened species richness.
Top panel a indicates the importance of individual variables from the global models of vertebrate threatened species richness, whilst the bottom panels indicate the measures of individual variable importance from the global models of amphibian (b), reptile (c), bird (d) and mammal (e) threatened species richness. Variables are grouped into broader classes, which are indicated by the capital letters on the side of the variable names: Total Species Richness (S), Environmental (E), and Human Impact (H) covariates. Variables are ranked first by groups and then by their median importance in the global model of vertebrate threatened species richness. The vertical line across each box indicates the median and the box boundaries indicate the interquartile range (IQR). Whiskers identify extreme data points that are not more than 1.5 times the IQR on both sides; the dots are more extreme outliers. Source data are provided as a Source Data file.
Table 1.
Performance (R2) of models of threatened species richness.
| Total vertebrates | Amphibians | Reptiles | Birds | Mammals | ||
|---|---|---|---|---|---|---|
| Total species richness only | Global | 0.49 ± 0.03 | 0.04 ± 0.03 | 0.45 ± 0.04 | 0.37 ± 0.08 | 0.36 ± 0.05 |
| Total species richness, environmental and anthropogenic covariates | Global | 0.94 ± 0.01 | 0.72 ± 0.05 | 0.87 ± 0.02 | 0.92 ± 0.01 | 0.94 ± 0.01 |
| Regional | 0.81 ± 0.14 | 0.63 ± 0.20 | 0.70 ± 0.19 | 0.77 ± 0.16 | 0.79 ± 0.15 |
Models are fitted at the global scale with only total species richness as a predictor, and at the global and regional scales using total species richness and a range of environmental and human impact covariates. Each datum represents the mean model performance, measured using R2, and standard deviation, across random forest models (mean ± SD). A full summary of model parameters and of regional level model performance can be found in Supplementary Tables 1 and 2. Source data are provided as a Source Data File.
Table 2.
Comparing the importance of environmental and human impact covariates.
| Estimate | Standard error | z-value | p-value | ||
|---|---|---|---|---|---|
| Total vertebrates (ANOVA: F2,18 = 5817.1, p < 0.01) | Environmental—human impact | 0.58 | 0.05 | 11.64 | <0.01 |
| Total species richness—human impact | 4.93 | 0.05 | 98.69 | <0.01 | |
| Total species richness—environmental | 4.35 | 0.05 | 87.05 | <0.01 | |
| Amphibians (ANOVA: F2,18 = 1673, p < 0.01) | Environmental—human impact | 0.29 | 0.03 | 10.45 | <0.01 |
| Total species richness —human impact | 1.52 | 0.03 | 54.50 | <0.01 | |
| Total species richness—environmental | 1.23 | 0.03 | 44.04 | <0.01 | |
| Reptiles (ANOVA: F2,18 = 2756.4, p < 0.01) | Environmental—human impact | 0.38 | 0.04 | 9.42 | <0.01 |
| Total species richness—human impact | 2.73 | 0.04 | 68.49 | <0.01 | |
| Total species richness—environmental | 2.36 | 0.04 | 59.07 | <0.01 | |
| Birds (ANOVA: F2,18 = 7346.1, p < 0.01) | Environmental—human impact | 0.18 | 0.03 | 5.67 | <0.01 |
| Total species richness—human impact | 3.46 | 0.03 | 107.69 | <0.01 | |
| Total species richness—environmental | 3.28 | 0.03 | 102.03 | <0.01 | |
| Mammals (ANOVA: F2,18 = 4602.9, p < 0.01) | Environmental—human impact | 0.99 | 0.06 | 15.96 | <0.01 |
| Total species richness—human impact | 5.60 | 0.06 | 89.92 | <0.01 | |
| Total species richness—environmental | 4.61 | 0.06 | 73.96 | <0.01 |
Repeated measures ANOVAs, and post-hoc analyses, comparing the mean measures of relative importance of environmental and human impact covariates and total species richness in explaining global threatened vertebrate species richness. Source data are provided as a Source Data File.
Variation between taxonomic groups
Models for separate taxa indicated that the dominant processes leading to concentrations of threatened species richness differed substantially between the four vertebrate classes. Specifically, whilst total species richness was the most influential variable for determining the richness of threatened species from all vertebrate classes, the importance of environmental and human impact variables differed substantially between the four groups (Fig. 2). These differences may be driven by varying sensitivities of species to different threatening processes41. For example, for amphibians the diversity of elevation was the most influential variable after total species richness (Fig. 2b), with the highest numbers of threatened amphibians occurring in the most topographically diverse areas, see below. Topographic diversity has been linked with increased speciation rates, and the occurrence of small range, endemic species which are more naturally prone to extinction36,37. This effect, however, was particularly strong in the South American, Amazonian and Australian regions, reflecting the distribution of Chytridiomycosis, an infectious fungal disease partially responsible for the worldwide declines of amphibian populations and prevalent in the montane habitats of those regions42. For birds, temperature seasonality ranked as the most influential variable after total species richness (Fig. 2d), with concentrations of threatened birds occurring in areas of particularly high or low temperature variation, see below. More seasonal environments are often associated with the occurrence of migratory species, which can be more vulnerable than their non-migratory counterparts43. Conversely, benign, less seasonal environments may allow the persistence of naturally rare, extinction prone species44. Differences in the major processes affecting different taxa may also be linked to dispersal ability. More mobile species tend to be less restricted by local heterogeneities in habitat structure, and more likely to be at equilibrium with climatic conditions45. Thus, those habitat variables that vary over a finer resolution, such as topography and human influence, may be more influential in explaining the distribution of threatened amphibians and reptiles, which tend to be less dispersive than birds and mammals.
Spatial variation in the drivers of threat
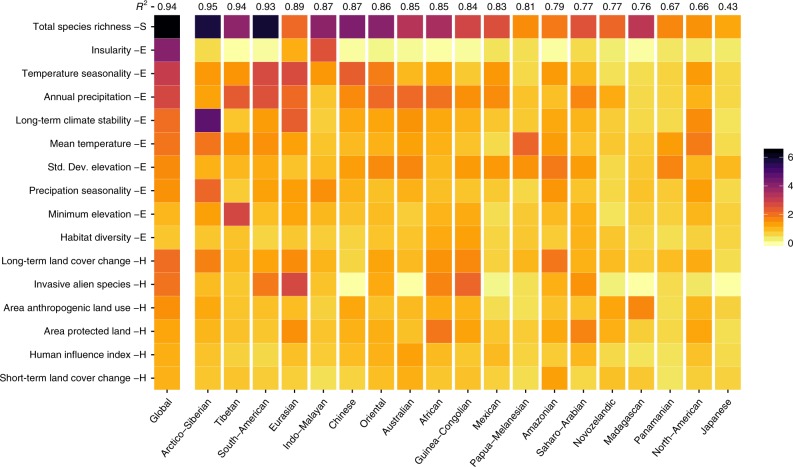
To assess the consistency of drivers of threat across space, we fitted separate models of threatened vertebrate species richness for individual zoogeographic regions29. Apart from the universally high importance of total species richness, the importance of individual environmental and human impact variables in driving threatened vertebrate species richness showed substantial variation between zoogeographic regions, as well as from the global model (Fig. 3). In areas where threatened species richness is greater than expected given the total species richness (Fig. 1a), the most influential variables were often related to threatening human processes. For example, long-term land cover change was of the greatest influence in driving threatened vertebrate species richness in the Amazonian region, whilst the area of human-dominated land uses was the most important variable in Madagascar (Fig. 3). This may be driven by the prevalence of small range, endemic species within these regions, which are vulnerable to the effects of habitat loss33. In contrast, however, across Southeast Asia, variables related to the environment were of greater importance, with insularity in the Indo-Malayan region, temperature seasonality in the Chinese region and annual precipitation in the Oriental region all emerging as important. The finding that human impact variables can be of greater importance than environmental covariates emerges despite the relatively coarse resolution of our analyses. Climatic variables (i.e., temperature and precipitation) can explain a lot of the variation in land cover distribution, including human-related variables46. Hence, climatic variables may be identified as being of greater importance than land cover in coarser resolution analyses. Our large-scale analysis offers important insights into the drivers of broad-scale irreversible changes47. Clearly, our global analysis is less well suited to identifying the drivers of fine-resolution changes in threatened species richness.
Fig. 3. Importance of variables in predicting threatened vertebrate species richness in different zoogeographic regions.
Variables are grouped into broader classes, which are indicated by the capital letters on the side of the variable names: Total Species Richness (S), Environmental (E), and Human Impact (H) covariates. The mean importance of each variable from the models of threatened vertebrate species richness is indicated by the yellow (low importance) to black (high importance) colour gradient. Variables are ordered from top to bottom first by group, and then according to their importance in the global model of threatened vertebrate species richness. Average performance of each regional set of models, measured with R2, is indicated at the top of each column, with regional models ranked by decreasing mean R2. Heat maps of variable importance for the individual taxonomic groups can be found in Supplementary Figs. 5–8. Source data are provided as a Source Data file.
The lack of congruence in the importance of variables across space could be linked to differences between regions in how much ecological factors vary23. For example, in contrast to the global models, insularity was of very low importance in most of the region-level models (Fig. 3). This is surprising, given the importance of insularity in the global models and the role of geographic range size in classifying species’ extinction risk48. This finding is likely because, relative to its variation at a global scale, land mass area shows limited variation within many regions. Similarly, invasive alien species were also of limited importance in some regions. This may be an artefact of the national measurement scale for this covariate, which could limit variation within some regions. Furthermore, the lack of congruence in variable importance may, in part, be attributable to spatial variation in sampling effort. Global data on species occurrence and threat levels are likely to be heterogeneous in both quality and precision. Threatened species richness may be elevated or depressed in proportion to total species richness in regions with incomplete sampling, potentially biasing results. Sampling effort is also likely to vary between taxonomic groups. The completed assessment for the distribution of reptiles is not currently available49. To test for an effect of spatial variation in sampling effort we repeated our analyses including data deficient (DD) species richness under several alternative assumptions of threat status. The distribution of DD species is geographically non-random, with greatest DD species richness in tropical regions50. These additional analyses produced qualitatively similar results that did not substantially differ from the main analysis of absolute threatened species richness (Supplementary Fig. 13), suggesting that our results are robust to spatial variation in sampling effort. Nevertheless, enhanced efforts to address gaps in biodiversity monitoring, such as across tropical regions and for reptiles, will help to address any remaining uncertainties in regional variations in the drivers of imperillment50.
Responses of total and threatened species richness differ
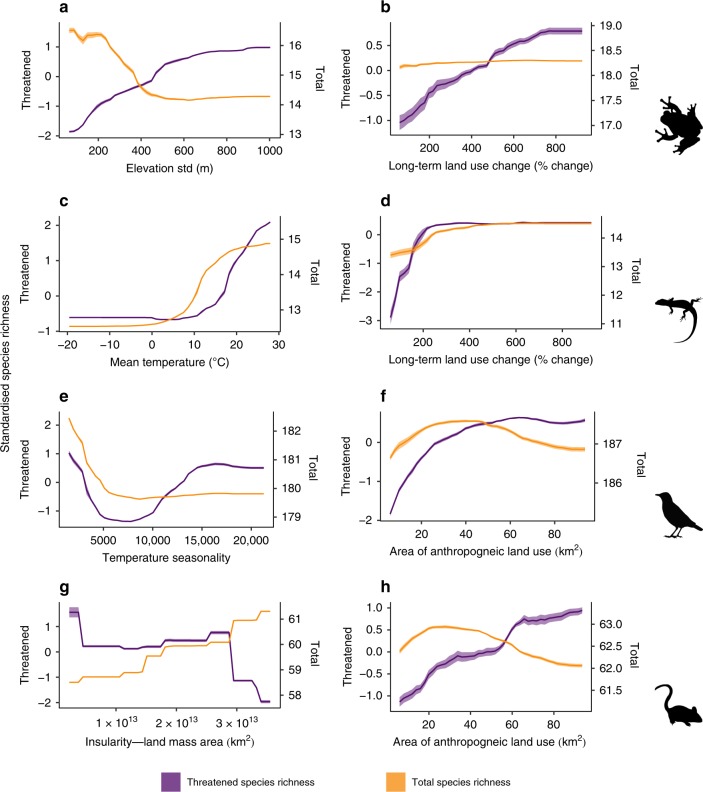
To explore the functional form of relationships between covariates and species richness patterns, we fitted separate models to both threatened and total global vertebrate species richness. In several cases, there were striking differences in the responses of total and threatened species richness to both environmental and human impact processes (Fig. 4). For amphibians, variation in elevation was positively related to threatened species richness, but negatively related to total species richness. Topographically diverse environments promote speciation and diversification through geographic isolation26, yielding narrow-range endemic species, which are more susceptible to extinction owing to a lack of refuges from adverse environmental conditions. The decline in amphibian species richness contrasts with what would be expected from the mid-domain effect (i.e., the peak in species richness towards the centre of a shared geographic domain due to boundary constraints51), but has been reported previously52. It is likely caused by reductions in temperature associated with increasing elevation gradients that occur at too fine a spatial resolution to be reflected by our coarse resolution measure of annual mean temperature53. Threatened amphibian species richness showed a broadly linear positive relationship with long-term land use change, whilst total amphibian species richness remained relatively constant. Similarly, total reptile species richness remained relatively constant with long-term land use change. Threatened reptile species richness, however, showed a positive asymptotic relationship with long-term land use change (Fig. 4). For both birds and mammals, the area of human dominated land uses showed a positive relationship with the number of threatened species in an area, whilst the relationship with total species richness had an intermediate maximum (Fig. 4). These relationships may indicate two processes at work. The initial positive relationship between species richness and human-dominated land uses may reflect how both respond positively to net primary productivity32. The subsequent reduction in total species richness and increase in threatened species richness may be a consequence of the associated increase in habitat loss and fragmentation in areas of extensive human-dominated land uses54,55. The relationship between insularity and mammal species richness also differed between the total and threatened species pools. Whilst the highest total mammal species richnesses were observed in areas with the greatest land mass, the highest threatened species richnesses were observed in the most insular areas. Islands are known to be centres for range-restricted species, more prone to extinction30. They are also, however, acknowledged for having a lower species richness than mainland regions, with species richness increasing with area56,57.
Fig. 4. Relationships between a selection of the most important environmental and human impact covariates and global amphibian, reptile, bird, and mammal species richness.
Lines indicate the mean partial relationships between variables and threatened (purple) and total (orange) species richness from across 10 random forest models for amphibians (a, b), reptiles (c, d), birds (e, f) and mammals (g, h). Shaded areas indicate the standard deviation around the mean partial relationships. The x-axis is limited to the central 90% of a variable’s range. Note the separate y-axes for threatened and total species richness. Plots of all variable relationships can be found in the Supplementary Figs. 10 and 11. Source data are provided as a Source Data file.
Implications for conservation planning
Our global assessment of the drivers of threatened species richness reveals that those environmental characteristics that predispose species assemblages to threat may be more influential than threatening human processes for determining where concentrations of imperilled species occur. This finding does not suggest that threatening human activities are unimportant for driving species’ extinctions; indeed, we shown that human impact variables are influential, and particularly at regional scales. Rather, it suggests that some regions and taxonomic groups may be inherently more vulnerable than others to the effects of those threatening processes. This finding has important implications for conservation planning. Knowing the inherent vulnerability of a region can aid decisions regarding global conservation priorities and could form the basis for a biodiversity conservation roadmap8. For example, areas that are inherently more imperilled might also be at greater risk from the effects of threatening human processes, and could therefore be prioritised for stricter protective actions whilst mitigation—by remediation, for instance—may be more appropriate in less vulnerable areas. Furthermore, we uncover striking variation both between taxonomic groups and across space in the environmental mechanisms that are responsible for driving spatial gradients in threatened species richness. Any future coordinated global conservation strategy must consider the full range of scales at which the drivers of threat operate on biodiversity and the varying sensitivities of species to different threatening processes7,8. Whilst our results cannot supplant local-scale, species-specific assessments, they offer the information necessary to understand the processes acting at a global scale. This is critical, as this is the scale at which large, irreversible changes occur and at which many of the economic and political processes that ultimately drive extinction operate. By identifying the drivers of spatial gradients of threatened species richness, our approach has the potential to inform proactive conservation policy and action to minimise the effects of future environmental change. Combining our models with projections of environmental change would provide a baseline from which to predict how threatened species richness will respond to varying elements of global change and identify where concentrations of imperilment may occur in the future.
Methods
Species richness data
Global distribution data for 6401 species of amphibians, 4450 reptiles and 5312 mammals were obtained from49, whilst distribution data for 10,583 bird species were obtained from58. Data were available as spatial polygons of distribution boundaries, which were projected onto a Behrman equal-area projection and converted to a grid with a cell size of 0.5° at 30°N and 30°S latitude. Where a species’ range polygon intersected with a grid cell, the species was treated as present within the entire cell. For each species, we only considered parts of the range where the species is designated as native, extant and either breeding or resident. Assessments of global extinction risk were taken from the IUCN49. Species richness was calculated as the total number of species present in a grid cell. Threatened species richness was calculated as the total number of threatened species in a grid cell, where threatened species were those classified as either ‘vulnerable’, ‘endangered’ or ‘critically endangered’ by the IUCN. Total species richness and total threatened species richness were also calculated for the four individual taxonomic groups (amphibians, reptiles, birds and mammals, Supplementary Figs. 1 and 2).
Environmental covariates
Data on four bioclimatic variables were obtained from WorldClim (Version 1.459); including annual mean temperature, temperature seasonality, annual precipitation and precipitation seasonality. Data were available on a 4.5 km grid for the 30-year period between 1960 and 1990. We derived minimum elevation and standard deviation of elevation using the Global Multi-resolution Terrain Elevation Data 2010 (GMTED201060), available at a resolution of 7.5 arc-seconds. Land cover data were derived from GlobCover version 2.3 2009 (http://due.esrin.esa.int/page_globcover.php), which are available as a raster with 23 land cover classes at a resolution of 300 m. These data were used to estimate the diversity of land cover classes using the Shannon information index61. The total area (km2) of the land mass of which a grid cell is part was calculated to provide a measure of insularity, or how isolated a grid cell is from other land masses. Smaller and more isolated landmasses (i.e., islands) have a smaller value of insularity, whilst grid cells on continental landmass have a larger value. As range size is used as one of the criteria for classifying species’ extinction risk, the inclusion of insularity may introduce circularity into these analyses. However, when compared with mainland areas, islands are known for their higher levels of endemic, range-restricted species that are potentially more vulnerable to imperilment30. By including insularity, we can explore the role of islands in promoting concentrations of threatened species richness alongside the suite of other variables considered here. A measure of temporal climate stability since the last interglacial period (present to 125,000 years ago) was derived from palaeo-climatic data made available by the Bristol Research Initiative for the Global Environment (BRIDGE, http://www.bridge.bris.ac.uk/). Details of the calculation of the model used to derive the paleo-climate data are provided by62,63. Data on precipitation and temperature were sampled at 4,000 year intervals. For each temporal transition, the Euclidean distance was calculated between z-transformed temperature and precipitation in bivariate space. The mean Euclidean distance was used as a measure of long-term climate stability in a grid cell, with smaller values indicating more stable climates64.
Human influence covariates
The total area of land within each grid cell classified as intensively used by humans, as agricultural crop land or as urbanised areas, was obtained from GlobCover 200965. As the occurrence of intensively used lands can manifest in areas with minimal human settlement66, we also included the Human Influence Index (HII, http://sedac.ciesin.columbia.edu/wildareas/) as an additional measure of human impact. As a proxy for protection status, the total area of land falling in the International Union for Conservation of Nature (IUCN) protected area categories I–VI was calculated using the World Database on Protected Areas (WDPA: https://www.protectedplanet.net/). Short-term land cover change was quantified using global land cover data obtained from the European Space Agency Climate Change Initiative (ESA CCI, https://www.esa-landcover-cci.org/?q = node/1). These data were used to calculate the percentage change in the area of human influenced land cover classes between 1992 and 2015. To quantify long-term land cover change, the percentage change in the area of cropland between 1700 and 1992 was estimated using data derived from67. Data on the number of invasive alien species in each country were obtained from68. All human impact and environmental covariate data were aggregated onto the same 0.5° equal area grid used for the species distribution data. Further information on the derivation of explanatory variables can be found in the Supplementary Methods.
Zoogeographic regions
Zoogeographic regions were the 20 regions identified in29. These data were available as range polygons, which were also intersected with the same grid used for the species distribution data. Grid cells were classed as the region with the greatest coverage within the cell. As fewer than 10 cells were classified as belonging to the Polynesian region, this region was excluded from all analyses.
Statistical models
Random Forests were used to assess the potential of the covariates to explain the distribution of threatened species richness. A machine learning technique, random forests are a bootstrapped-based classification and regression tree method that are robust to overfitting, and are recognised as producing good predictive models69. They make fewer assumptions than correlative approaches about the distributions of predictor and response variables. Tests of collinearity between predictor variables revealed that only temperature seasonality and mean annual temperature had an absolute correlation of greater than 0.7 (ρ = 0.85, Supplementary Fig. 4). Models were fitted using the ‘randomForest’ package in R70,71. All analyses were carried out in R 3.3.171.
First, we modelled threatened species richness in relation to total species richness alone. Models were fitted at a global scale for the threatened species richness for the four individual taxonomic groups (amphibians, reptiles, birds and mammals) as well as the total threatened vertebrate species richness (i.e., the four taxonomic groups combined). Second, we modelled threatened species richness in relation to the suite of environmental and human impact covariates detailed above. These models also included total species richness as a covariate, to account for the size of the species pool. This approach enables both the independent effect of the total species richness to be controlled for, whilst also allowing for potential interactions with other covariates72. To explore the potential of taxonomic variation in the drivers of threatened species richness we fitted models both for the total threatened vertebrate species richness as well as for the threatened species richness for the four individual taxonomic groups. To explore spatial variation in the drivers of threatened species richness, we then fitted separate models for the 19 individual zoogeographic regions. Again, we did this for both total threatened vertebrate species richness and for the four individual taxonomic groups. Finally, to verify the results from these models, we also modelled the residual threatened species richness from the first set of models where threated species richness was modelled in relation to total species richness alone (Fig. 1). This third set of models used the same environmental and human impact covariates as before but omitted total species richness. The results from these models were qualitatively very similar to those from the models of absolute threatened species richness and are reported in Supplementary Fig. 12. The model fitting process was the same for all models, regardless of scale or response, and is described below.
To account for potential spatial autocorrelation73, we utilised a blocking method74, to split the data into ten sampling blocks to be used for cross-validation. The creation of each sampling block was based on ecoregions75 (http://www.worldwildlife.org/science/data). Within our study area, each non-contiguous area of an ecoregion was classified as a separate sampling unit. These sampling units were then grouped into ten blocks so that the mean value of each environmental variable was similar across all blocks, but each block covered the full range of environmental variables within the area of study76. As all blocks cover a similar range of environmental data, this method ensures that a similar range of data was used for both calibrating and testing models, whilst also ensuring that the calibration and testing data are spatially segregated77. This method performs well at a large spatial scale, by minimising the influence of spatial autocorrelation whilst allowing models to capture complex spatial processes74.
We used ten-fold cross-validation to find the optimal values of both the number of trees (nt) and the number of predictors (m) used to build each regression tree, which form the random forests. We initially fitted a random forest with nt set to 1000 and m set to 1. The initial model was calibrated on 9 of the 10 sampling blocks and performance was evaluated on the omitted sampling block. We used the coefficient of determination (R2) to assess model performance on the evaluation data. A further 500 trees were then added to the model and the R2 was recalculated on the evaluation data. If the additional trees improved model performance by more than 1% they were accepted. This was repeated iteratively until model performance was not improved by additional trees. We then repeated this process with m set to 2 and 3. This process of fitting models with varying values of nt and m, was repeated ten times, omitting a different sampling block each time. The values of m and nt that maximised R2 across the ten iterations were then used to fit the final set of models. Again, this used a ten-fold cross-validation approach, fitting the model to nine of the ten sampling blocks and evaluating performance on the omitted block using R2, omitting a different sampling block each time. This resulted in ten random forest models of threatened vertebrate species richness. A full summary of final model parameters and performance can be found in Supplementary Tables 1 and 2.
Calculating variable importance
The importance of individual variables was calculated using a randomisation approach. We initially assessed model performance using mean squared error (MSE) on predictions made to the full data set. We then randomised a variable, made new predictions and recalculated MSE. The importance of the variable in the model was then calculated using Eq. 1:
| 1 |
where VI is variable importance, MSErand is the mean squared error from the predictions using the randomised variables, and MSEobs is the mean squared error from the predictions with the variable as observed. We repeated the process 1000 times for each variable in the model and reported the mean VI.
To investigate the potential for spatial inconsistencies in the drivers of threatened vertebrate species richness, we repeated the above analyses for individual zoogeographic regions. Both global and zoogeographic regional level analyses were repeated for the four individual taxonomic groups to assess cross-taxon congruence in the drivers of threatened species richness. Owing to the small sample size of some sampling blocks for the individual regions, some models lacked explanatory power (i.e., R2 < 0.2578). These models were excluded from calculations of variable importance and any further analyses. All other models showed medium to excellent performance (Supplementary Table 1). We used repeated measures ANOVAs to identify differences in the explanatory power of environmental and human impact covariates. For this we aggregated the variables into two broad categories (and total species richness), and then took the mean importance across all variables within each category79.
Variable relationships
To explore the functional form of relationships between individual variables and species richness patterns, we fitted separate models to both threatened and total species richness, using the model fitting procedure outlined above. This was performed for the individual taxonomic groups at a global scale, using the same set of predictor variables, barring total species richness. Predictions were then made to a data set where all but the focal variable were held at their mean (or modal) value. This was repeated for each variable for both responses (total and threatened species richness) for the four taxonomic groups. Here, we present a selection of the most important environmental and human impact variables from the taxa specific global models of threatened species richness, for which the response of total species and threatened species richness differs, while also being ranked in the top three of variable importance for each of the environmental and human impact categories. Plots of the relationships between all variables and both threatened and total species richness, along with the measures of variable importance for these additional models, can be found in Supplementary Figs. 9, 10 and 11.
Accounting for data deficient species richness
There is some evidence that data deficient (DD) species are far more likely to be threatened than categorised species80. Furthermore, there is evidence that the distribution of DD species is spatially non-random, with concentrations of DD species occurring throughout low latitude, tropical regions50 (Supplementary Fig. 3). To explore the sensitivity of our results to the inclusion of DD species, we refitted our models, using the process outlined above, but including DD species under several different assumptions of threat status. For this, we randomly classified 0, 50 and 100% of DD species that occur in each grid cell as threatened, and then included these species in our calculations of total and threatened species richness, accordingly. The different assumptions of threat status for the DD species produced qualitatively similar results that did not substantially differ from the main analysis of absolute threatened species richness (Supplementary Fig. 13).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This research was supported by the Inventory, Monitoring & Assessment research staff of the US Forest Service through International Joint Venture Agreement 14-JV-11221636-114 between the Rocky Mountain Research Station and Durham University. We thank BirdLife International for providing bird species distribution data and Stuart H.M. Butchart at Birdlife International for commenting on the manuscript. We also thank Paul J. Valdes from the Bristol Research Initiative for the Global Environment (BRIDGE, http://www.bridge.bris.ac.uk/) for providing paleo-climatic data and commenting on the manuscript.
Source Data
Author contributions
C.H., C.H.F. and P.A.S. designed the study. C.H. performed the analysis. C.H., C.H.F., and P.A.S. wrote the manuscript.
Data availability
The species richness data generated and analysed during this study are included in the Source Data files. The other datasets utilised in this study are derived from published sources, cited in the methods section. The source data underlying Figs. 1–4, Supplementary Figs. 1–13, Tables 1–2, and Supplementary Tables 1–2 are provided as a Source Data file.
Code availability
Code to carry out analyses is publicly available on https://github.com/christinehoward399/Global-Rarity.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Marcel Cardillo, Manuela Gonzalez-Suarez, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-14771-6.
References
- 1.Carvalho SB, et al. Spatial conservation prioritization of biodiversity spanning the evolutionary continuum. Nat. Ecol. Evol. 2017;1:151. doi: 10.1038/s41559-017-0151. [DOI] [PubMed] [Google Scholar]
- 2.CBD (Convention on Biological Diversity). Strategic Plan for Biodiversity 2011-2020, Including Aichi Biodiversity Targets: https://www.cbd.int/sp/. (2011).
- 3.Pimm SL, Russell GJ, Gittleman JL, Brooks TM. The future of biodiversity. Science. 1995;269:347 LP–347350. doi: 10.1126/science.269.5222.347. [DOI] [PubMed] [Google Scholar]
- 4.Tittensor DP, et al. A mid-term analysis of progress toward international biodiversity targets. Science. 2014;346:241–244. doi: 10.1126/science.1257484. [DOI] [PubMed] [Google Scholar]
- 5.Butchart SHM, et al. Global biodiversity: indicators of recent declines. Science. 2010;328:1164–1168. doi: 10.1126/science.1187512. [DOI] [PubMed] [Google Scholar]
- 6.Watson JEM, Venter O. A global plan for nature conservation. Nature. 2017;550:48. doi: 10.1038/nature24144. [DOI] [PubMed] [Google Scholar]
- 7.Mace GM, et al. Aiming higher to bend the curve of biodiversity loss. Nat. Sustain. 2018;1:448–451. doi: 10.1038/s41893-018-0130-0. [DOI] [Google Scholar]
- 8.Arlidge WNS, et al. A global mitigation hierarchy for nature conservation. Bioscience. 2018;68:336–347. doi: 10.1093/biosci/biy029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pimm SL, Jenkins CN, Li BV. How to protect half of Earth to ensure it protects sufficient biodiversity. Sci. Adv. 2018;4:eaat2616. doi: 10.1126/sciadv.aat2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks TM, et al. Global biodiversity conservation priorities. Science. 2006;313:58 LP–58 61. doi: 10.1126/science.1127609. [DOI] [PubMed] [Google Scholar]
- 11.Grenyer R, et al. Global distribution and conservation of rare and threatened vertebrates. Nature. 2006;444:93–96. doi: 10.1038/nature05237. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins CN, Pimm SL, Joppa LN. Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl. Acad. Sci. USA. 2013;110:E2602 LP–E2602610. doi: 10.1073/pnas.1302251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orme CDL, et al. Global hotspots of species richness are not congruent with endemism or threat. Nature. 2005;436:1016–1019. doi: 10.1038/nature03850. [DOI] [PubMed] [Google Scholar]
- 14.Pimm S. L., Jenkins C. N., Abell R., Brooks T. M., Gittleman J. L., Joppa L. N., Raven P. H., Roberts C. M., Sexton J. O. The biodiversity of species and their rates of extinction, distribution, and protection. Science. 2014;344(6187):1246752–1246752. doi: 10.1126/science.1246752. [DOI] [PubMed] [Google Scholar]
- 15.Veach V, Di Minin E, Pouzols FM, Moilanen A. Species richness as criterion for global conservation area placement leads to large losses in coverage of biodiversity. Divers. Distrib. 2017;23:715–726. doi: 10.1111/ddi.12571. [DOI] [Google Scholar]
- 16.Roll U, et al. The global distribution of tetrapods reveals a need for targeted reptile conservation. Nat. Ecol. Evol. 2017;1:1677–1682. doi: 10.1038/s41559-017-0332-2. [DOI] [PubMed] [Google Scholar]
- 17.Westgate MJ, Barton PS, Lane PW, Lindenmayer DB. Global meta-analysis reveals low consistency of biodiversity congruence relationships. Nat. Commun. 2014;5:3899. doi: 10.1038/ncomms4899. [DOI] [PubMed] [Google Scholar]
- 18.Le Saout S, et al. Protected areas and effective biodiversity conservation. Science. 2013;342:803 LP–803805. doi: 10.1126/science.1239268. [DOI] [PubMed] [Google Scholar]
- 19.Tilman D, et al. Future threats to biodiversity and pathways to their prevention. Nature. 2017;546:73. doi: 10.1038/nature22900. [DOI] [PubMed] [Google Scholar]
- 20.Di Marco M, Venter O, Possingham HP, Watson JEM. Changes in human footprint drive changes in species extinction risk. Nat. Commun. 2018;9:4621. doi: 10.1038/s41467-018-07049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardillo M, et al. Human population density and extinction risk in the world’s carnivores. PLoS Biol. 2004;2:e197. doi: 10.1371/journal.pbio.0020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pacifici M, et al. Species/’ traits influenced their response to recent climate change. Nat. Clim. Change. 2017;7:205–208. doi: 10.1038/nclimate3223. [DOI] [Google Scholar]
- 23.Davies RG, et al. Human impacts and the global distribution of extinction risk. Proc. R. Soc. B Biol. Sci. 2006;273:2127 LP–2122133. doi: 10.1098/rspb.2006.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawkins BA, et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology. 2003;84:3105–3117. doi: 10.1890/03-8006. [DOI] [Google Scholar]
- 25.Rohde K. Latitudinal gradients in species diversity: the search for the primary cause. Oikos. 1992;65:514–527. doi: 10.2307/3545569. [DOI] [Google Scholar]
- 26.Stein A, Gerstner K, Kreft H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014;17:866–880. doi: 10.1111/ele.12277. [DOI] [PubMed] [Google Scholar]
- 27.Mittelbach G, et al. Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol. Lett. 2007;10:315–331. doi: 10.1111/j.1461-0248.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- 28.Howard C, Flather CH, A. Stephens P. What drives at-risk species richness? Environmental factors are more influential than anthropogenic factors or biological traits. Conserv. Lett. 2018;0:e12624. [Google Scholar]
- 29.Holt BG, et al. An update of Wallace’s zoogeographic regions of the world. Science. 2013;339:74 LP–74 78. doi: 10.1126/science.1228282. [DOI] [PubMed] [Google Scholar]
- 30.Kier G, et al. A global assessment of endemism and species richness across island and mainland regions. Proc. Natl. Acad. Sci. USA. 2009;106:9322–9327. doi: 10.1073/pnas.0810306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerr JT, Currie DJ. Effects of human activity on global extinction risk. Conserv. Biol. 2018;9:1528–1538. doi: 10.1046/j.1523-1739.1995.09061528.x. [DOI] [Google Scholar]
- 32.Luck GW. The relationships between net primary productivity, human population density and species conservation. J. Biogeogr. 2007;34:201–212. doi: 10.1111/j.1365-2699.2006.01575.x. [DOI] [Google Scholar]
- 33.Vamosi JC, Vamosi SM. Extinction risk escalates in the tropics. PLoS One. 2008;3:e3886. doi: 10.1371/journal.pone.0003886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chown SL, van Rensburg BJ, Gaston KJ, Ana SLR, van Jaarsveld AS. Energy, species richness, and human population size: conservation implications at a national scale. Ecol. Appl. 2003;13:1233–1241. doi: 10.1890/02-5105. [DOI] [Google Scholar]
- 35.Weir JT, Schluter D. The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science. 2007;315:1574 LP–1571576. doi: 10.1126/science.1135590. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg DA, Mooers AØ. Linking speciation to extinction: diversification raises contemporary extinction risk in amphibians. Evol. Lett. 2017;1:40–48. doi: 10.1002/evl3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies TJ, et al. Extinction risk and diversification are linked in a plant biodiversity hotspot. PLoS Biol. 2011;9:e1000620. doi: 10.1371/journal.pbio.1000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilcove DS, Rothstein D, Dubow J, Phillips A, Losos E. Quantifying threats to imperiled species in the United States: Assessing the relative importance of habitat destruction, alien species, pollution, overexploitation, and disease. Bioscience. 1998;48:607–615. doi: 10.2307/1313420. [DOI] [Google Scholar]
- 39.Flather CH, Knowles MS, Kendall IA. Threatened and endangered species geography. Bioscience. 1998;48:365–376. doi: 10.2307/1313375. [DOI] [Google Scholar]
- 40.Bellard C, Cassey P, Blackburn TM. Alien species as a driver of recent extinctions. Biol. Lett. 2016;12:20150623. doi: 10.1098/rsbl.2015.0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ducatez Simon, Shine Richard. Drivers of Extinction Risk in Terrestrial Vertebrates. Conservation Letters. 2017;10(2):186–194. doi: 10.1111/conl.12258. [DOI] [Google Scholar]
- 42.Scheele BC, et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science. 2019;363:1459 LP–1451463. doi: 10.1126/science.aav0379. [DOI] [PubMed] [Google Scholar]
- 43.Bairlein F. Migratory birds under threat. Science. 2016;354:547 LP–547548. doi: 10.1126/science.aah6647. [DOI] [PubMed] [Google Scholar]
- 44.Harrison S, Viers JH, Thorne JH, Grace JB. Favorable environments and the persistence of naturally rare species. Conserv. Lett. 2008;1:65–74. doi: 10.1111/j.1755-263X.2008.00010.x. [DOI] [Google Scholar]
- 45.Arita HT, Rodríguez P. Local-regional relationships and the geographical distribution of species. Glob. Ecol. Biogeogr. 2004;13:15–21. doi: 10.1111/j.1466-882X.2004.00067.x. [DOI] [Google Scholar]
- 46.Thuiller W, Araujo MB, Lavorel S. Do we need land-cover data to model species distributions in Europe? J. Biogeogr. 2004;31:353–361. doi: 10.1046/j.0305-0270.2003.00991.x. [DOI] [Google Scholar]
- 47.Keil P, et al. Spatial scaling of extinction rates: Theory and data reveal nonlinearity and a major upscaling and downscaling challenge. Glob. Ecol. Biogeogr. 2018;27:2–13. doi: 10.1111/geb.12669. [DOI] [Google Scholar]
- 48.Mace GM, et al. Quantification of extinction risk: IUCN’s System for classifying threatened species. Conserv. Biol. 2008;22:1424–1442. doi: 10.1111/j.1523-1739.2008.01044.x. [DOI] [PubMed] [Google Scholar]
- 49.IUCN. The IUCN Red List of Threatened Species Version 2016-1. http://www.iucnredlist.org (2016). [DOI] [PMC free article] [PubMed]
- 50.Collen B, Ram M, Zamin T, McRae L. The tropical biodiversity data gap: addressing disparity in global monitoring. Trop. Conserv. Sci. 2008;1:75–88. doi: 10.1177/194008290800100202. [DOI] [Google Scholar]
- 51.Colwell RK, Lees DC. The mid-domain effect: geometric constraints on the geography of species richness. Trends Ecol. Evol. 2000;15:70–76. doi: 10.1016/S0169-5347(99)01767-X. [DOI] [PubMed] [Google Scholar]
- 52.Khatiwada JR, et al. Amphibian community structure along elevation gradients in eastern Nepal Himalaya. BMC Ecol. 2019;19:19. doi: 10.1186/s12898-019-0234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kluge J, Kessler M, Dunn RR. What drives elevational patterns of diversity? A test of geometric constraints, climate and species pool effects for pteridophytes on an elevational gradient in Costa Rica. Glob. Ecol. Biogeogr. 2006;15:358–371. doi: 10.1111/j.1466-822X.2006.00223.x. [DOI] [Google Scholar]
- 54.Pautasso M. Scale dependence of the correlation between human population presence and vertebrate and plant species richness. Ecol. Lett. 2007;10:16–24. doi: 10.1111/j.1461-0248.2006.00993.x. [DOI] [PubMed] [Google Scholar]
- 55.Polaina E, González-Suárez M, Kuemmerle T, Kehoe L, Revilla E. From tropical shelters to temperate defaunation: The relationship between agricultural transition stage and the distribution of threatened mammals. Glob. Ecol. Biogeogr. 2018;27:647–657. doi: 10.1111/geb.12725. [DOI] [Google Scholar]
- 56.Whittaker, R. J. & Fernández-Palacios, J. M. Island biogeography: ecology, evolution, and conservation (Oxford University Press, 2007).
- 57.Rosenzweig, M. L., L, R. M. & Press, C. U. Species Diversity in Space and Time (Cambridge University Press, 1995).
- 58.BirdLife International and NatureServe. Bird species distribution maps of the world. Version 6.0. Cambridge, UK: BirdLife International; 2016. [Google Scholar]
- 59.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- 60.Danielson, J. J. & Gesch, D. B. Global multi-resolution terrain elevation data 2010 (GMTED2010). (2011).
- 61.Magurran, A. E. Measuring biological diversity. (Wiley, 2013). [DOI] [PubMed]
- 62.Singarayer JS, Valdes PJ. High-latitude climate sensitivity to ice-sheet forcing over the last 120 kyr. Quat. Sci. Rev. 2010;29:43–55. doi: 10.1016/j.quascirev.2009.10.011. [DOI] [Google Scholar]
- 63.Davies-Barnard T, Ridgwell A, Singarayer J, Valdes P. Quantifying the influence of the terrestrial biosphere on glacial–interglacial climate dynamics. Clim. 2017;13:1381–1401. [Google Scholar]
- 64.Voskamp A, Baker DJ, Stephens PA, Valdes PJ, Willis SG. Global patterns in the divergence between phylogenetic diversity and species richness in terrestrial birds. J. Biogeogr. 2017;44:709–721. doi: 10.1111/jbi.12916. [DOI] [Google Scholar]
- 65.Arino, O. et al. GlobCover: ESA service for global land cover from MERIS. in 2007 IEEE international geoscience and remote sensing symposium 2412–2415 (IEEE, 2007).
- 66.Radeloff VC, et al. Housing growth in and near United States protected areas limits their conservation value. Proc. Natl. Acad. Sci. USA. 2010;107:940–945. doi: 10.1073/pnas.0911131107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramankutty N, Foley JA. Estimating historical changes in global land cover: croplands from 1700 to 1992. Glob. Biogeochem. Cycles. 1999;13:997–1027. doi: 10.1029/1999GB900046. [DOI] [Google Scholar]
- 68.Turbelin AJ, Malamud BD, Francis RA. Mapping the global state of invasive alien species: patterns of invasion and policy responses. Glob. Ecol. Biogeogr. 2017;26:78–92. doi: 10.1111/geb.12517. [DOI] [Google Scholar]
- 69.Breiman L. Random forests. Mach. Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 70.Liaw, A. & Wiener, M. Classification and regression by randomForest. R News2, (2002).
- 71.R Core Team. R: A language and environment for statistical computing. (2019).
- 72.Freckleton RP. On the misuse of residuals in ecology: regression of residuals vs. multiple regression. J. Anim. Ecol. 2002;71:542–545. doi: 10.1046/j.1365-2656.2002.00618.x. [DOI] [Google Scholar]
- 73.Araújo MB, Guisan A. Five (or so) challenges for species distribution modelling. J. Biogeogr. 2006;33:1677–1688. doi: 10.1111/j.1365-2699.2006.01584.x. [DOI] [Google Scholar]
- 74.Bagchi R, et al. Evaluating the effectiveness of conservation site networks under climate change: accounting for uncertainty. Glob. Chang. Biol. 2013;19:1236–1248. doi: 10.1111/gcb.12123. [DOI] [PubMed] [Google Scholar]
- 75.Olson DM, et al. Terrestrial ecoregions of the world: A new map of life on earth. Bioscience. 2001;51:933–938. doi: 10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2. [DOI] [Google Scholar]
- 76.Moore, R. T. blockTools: Blocking, assignment, and diagnosing interference in randomized experiments. R package version 0.6-1. (2014).
- 77.Hijmans RJ. Cross‐validation of species distribution models: removing spatial sorting bias and calibration with a null model. Ecology. 2012;93:679–688. doi: 10.1890/11-0826.1. [DOI] [PubMed] [Google Scholar]
- 78.Cohen, J. Statistical power analysis for the behavioral sciences (L. Erlbaum Associates, 1988).
- 79.Ishwaran H. Variable importance in binary regression trees and forests. Electron. J. Stat. 2007;1:519–537. doi: 10.1214/07-EJS039. [DOI] [Google Scholar]
- 80.Jetz W, Freckleton RP. Towards a general framework for predicting threat status of data-deficient species from phylogenetic, spatial and environmental information. Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20140016. doi: 10.1098/rstb.2014.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jenks, G. F. Visualizing statistical distributions and generalizing process. in Annals of the Association of American Geographers vol. 57 179 (Blackwell, 1967).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The species richness data generated and analysed during this study are included in the Source Data files. The other datasets utilised in this study are derived from published sources, cited in the methods section. The source data underlying Figs. 1–4, Supplementary Figs. 1–13, Tables 1–2, and Supplementary Tables 1–2 are provided as a Source Data file.
Code to carry out analyses is publicly available on https://github.com/christinehoward399/Global-Rarity.