Abstract
About 10–20% of patients with Kawasaki disease (KD) are unresponsive to intravenous immunoglobulin (IVIg) and are at increased risk of coronary artery abnormalities (CAAs). Early identification is critical to initiate aggressive therapies, but available scoring systems lack sensitivity in non-Japanese populations. We investigated the accuracy of 3 Japanese scoring systems and studied factors associated with IVIg unresponsiveness in a large multiethnic French population of children with KD to build a new scoring system. Children admitted for KD between 2011–2014 in 65 centers were enrolled. Factors associated with second line-treatment; i.e. unresponsiveness to initial IVIg treatment, were analyzed by multivariate regression analysis. The performance of our score and the Kobayashi, Egami and Sano scores were compared in our population and in ethnic subgroups. Overall, 465 children were reported by 84 physicians; 425 were classified with KD (55% European Caucasian, 12% North African/Middle Eastern, 10% African/Afro-Caribbean, 3% Asian and 11% mixed). Eighty patients (23%) needed second-line treatment. Japanese scores had poor performance in our whole population (sensitivity 14–61%). On multivariate regression analysis, predictors of secondary treatment after initial IVIG were hepatomegaly, ALT level ≥30 IU/L, lymphocyte count <2400/mm3 and time to treatment <5 days. The best sensitivity (77%) and specificity (60%) of this model was with 1 point per variable and cut-off ≥2 points. The sensitivity remained good in our 3 main ethnic subgroups (74–88%). We identified predictors of IVIg resistance and built a new score with good sensitivity and acceptable specificity in a non-Asian population.
Subject terms: Paediatric rheumatic diseases, Vasculitis syndromes
Introduction
Kawasaki disease (KD) is the leading cause of acquired heart disease in childhood in developed countries1. The level of coronary artery involvement mainly determines the prognosis of this systemic vasculitis affecting predominantly young children, although pericarditis, myocarditis and valvular dysfunction are not uncommon1,2. Occasionally, KD can be complicated during the acute phase by shock syndrome3, macrophage activation syndrome4, or myocardial infarction1. Although the mortality rate is relatively low during the acute phase, sudden death due to myocardial ischemia could occur many years later in children or adults with coronary artery sequelae1.
The efficacy of early treatment with intravenous immunoglobulin (IVIg) is well established5,6 and has reduced the prevalence of coronary artery abnormalities (CAAs) from 26–30% to 2.5–5% at 1 month after disease onset6,7. However, 30% to 40% of KD patients develop coronary dilatations within the first days of the disease8. In addition, approximately 10–20% of KD patients is considered resistant to IVIg and has been shown at increased risk of CAAs1,9. Therefore, early identification is critical to initiate more aggressive therapy10,11.
Several scoring systems have been developed in Japanese populations to predict resistance to IVIg therapy and show good sensitivity (77–86%) and specificity (67–86%)12–14. However, they lack sensitivity in North American15,16, European17–19 and other Asian populations20,21. Attempts to develop a scoring system with better performance in American multiethnic and Israeli populations were unsuccessful16,22. Moreover, reliable tools to detect children at high risk of IVIg resistance are essential because this population seems to have increased recently, from 7% to 23% in Japan23 and 10–20% to 38% in San Diego, USA16.
Data on children with KD resistant to IVIg in Europe are scarce. In addition the definition of IVIg resistance based on persistence or recrudescence of fever after completion of IVIG infusion is not homogenous with a period of observation varying from 24 h to 36 h1,24,25. In this study, we analyzed a large multiethnic French population of children with KD. To remain the most in keeping with the current practice, we investigated the necessity of a second-line treatment including possible IVIg re treatment after initial standard of 2 g/kg of IVIg, then we tested the accuracy of the Japanese scoring systems to predict IVIg resistance. Finally we attempted to build a new scoring system predicting secondary treatment in our large group of non-Asian patients with KD.
Methods
Population
Data were extracted from the Kawanet database, a national clinical and biological repository aiming to define the characteristics of KD in France. Patients admitted for KD in 65 French pediatric centers between January 1, 2011 and March 31, 2014 were prospectively or retrospectively enrolled in Kawanet by use of an online electronic case report form (e-CRF) implemented in a Web-secured system (CLEAN Web). The e-CRF contains demographic, clinical, biological, and radiologic information associated with treatments and 3-month follow-up.
The study population was limited to children (≤18 years) and was classified according to the American Heart Association (AHA) criteria in 3 groups: complete KD (fever plus at least 4 of 5 clinical criteria: exanthema, cervical adenitis, conjunctival injection, modification of oral mucosa or modification in extremities), incomplete KD (fever plus <4 clinical criteria and CAAs documented by echocardiography), and others not meeting those criteria. Records for patients in the last group were reviewed during a consensus conference with a nominal group technique by 6 experts from 5 centers (2 pediatricians, 2 pediatric rheumatologists, 1 pediatric infectious disease specialist and 1 pediatric dermatologist) to determine whether patients had probable or a doubtful KD. Participants were given complementary clinical, biological and radiological information on submitted cases. Consensus was obtained if 5/6 experts (83%) provided the same response. Some patients were unclassifiable due to missing data or lack of consensus. Patients with probable KD were taken into account in analyses but those unclassified and doubtful KD were excluded.
Outcome measure and definitions
We analyzed demographic, clinical, biological and radiologic factors associated with unresponsiveness to IVIg. To avoid possible biases and controversies regarding the definition of IVIG resistance, we evaluated current practice decisions and analyzed in our KD cohort, the need for a second course of IVIg or second-line treatment (after the first IVIg infusion) with corticosteroids or anti-tumor necrosis factor agent.
CAAs were defined by detection of a coronary artery dilatation or aneurysms on 2-D echography at diagnosis or early follow-up. Because Z-scores were not available in most cases, coronary lesions were considered internal lumen diameter >3 mm in children <5 years old or >4 mm in children ≥5 years old, internal diameter of a segment measuring ≥1.5 times that of the adjacent segment; or a clearly irregular coronary lumen according to the Japanese Ministry of Health criteria12,14,26. Data on CAAs were reviewed on original echocardiographic reports requested to investigators. Other cardiac complications such as myocarditis, pericarditis, and heart failure were also reported.
Ethnicity was defined by origin of parents and classified into 5 groups: European Caucasian, North African/Middle Eastern, African/Afro-Caribbean, and Asian (Far East), or mixed (children with parents from different areas). Follow-up was defined by the number of days from diagnosis to the last visit in the center.
Kobayashi, Egami and Sano scores which predict resistance to treatment in Japanese population were studied in our population and in ethnic subgroups. The Egami score (0 to 6 points) includes age at diagnosis ≤6 months (1 point), alanine aminotransferase (ALT) level ≥80 IU/L (2 points), platelet count ≤30.104 IU/L (1 point), C-reactive protein (CRP) level ≥8 mg/dl (1 point), and illness days ≤4 days (1 point), with cut-off ≥312. The Sano score (0 to 3) includes CRP level ≥7 mg/dl (1 point), total bilirubine ≥0.9 mg/dl (1 point) and aspartate aminotransferase (AST) level ≥200 IU/L with cut-off ≥213. The Kobayashi score (0 to 11 points) includes sodium level ≤133 mmol/L (2 points), illness days ≤4 days (2 points), aspartate aminotransferase (AST) level ≥100 IU/L (2 points), neutrophil count ≥80% (2 points), platelet count ≤30.104 IU/L (1 point), CRP level ≥10 mg/dL (1 point), and age ≤12 months (1 point). We investigated the accuracy of the Kobayashi score using a cut-off ≥4 described in the original study published in 2006 in patients treated with IgIV 1 g/kg for 2 consecutive days as well as a cut-off ≥5 as described in the external validation study published in 2011 comparing children treated with IgIV 1 g/kg for 2 consecutive days and 2 g/kg in a single infusion14,27.
Statistical analyses
Descriptive statistics were used for demographic, clinical, biological and radiologic data. Continuous data are described with mean± SD and categorical data with number (%). Data were compared by chi-square test or Fisher’s exact test for categorical variables and Student’s test or Mann-Whitney test for continuous variables. Two quantitative variables (age, and time to treatment) were transformed into clinically relevant classes: age, <1 year, 1–4 years and >5 years; and time to treatment (time between the first day of fever and IVIg treatment), <5 days, 5–10 days and >10 days. Quantitative variables (laboratory variables) were transformed into binary variables by using the receiver operating characteristic (ROC) curve. To obtain the optimized cut-off value, the distance between the point (0,1) and any point on the ROC curve was minimized. The clinical relevance of the threshold was discussed. We estimated odds ratios (ORs) and 95% confidence intervals (CIs). Multivariate analysis involved logistic regression models to identify factors associated with IVIg unresponsiveness. Variables significant at p ≤ 0.20 on univariate analyses were included in the stepwise selection. Potential interactions between variables were tested. The best model was determined by using Akaike Information Criteria. We built a scoring system based on our multivariate analysis for the IVIg resistance model. For each patient, variables retained in the model determined number of criteria. ROC curves were used to obtain the optimized cut-off value for the score maximizing sensitivity and specificity to distinguish unresponsive and responsive patients to the first IVIg infusion. Sensitivity, specificity for our score and the Kobayashi, Egami and Sano scores which predict unresponsiveness to treatment in Japanese populations were studied in our population and in ethnic subgroups.
Statistical analyses involved use of SAS v9.3 (SAS Institute, Cary, NC). All tests were two-tailed, with p < 0.05 considered statistically significant.
Ethics
The study protocol followed ethics guidelines (CPP no.CO-10–002) and was approved by the Comité Consultatif sur le Traitement de l’Information en matière de Recherche dans le domaine de la Santé (Advisory Committee on Information Processing in Research in the Field of Health, no.10.155bis) and the Commission Nationale de l’Informatique et des Libertés (National Commission of Informatics and Freedom, no. DR-2010-032). Informed consent was obtained from parents or legal guardians of children included in the study.
Results
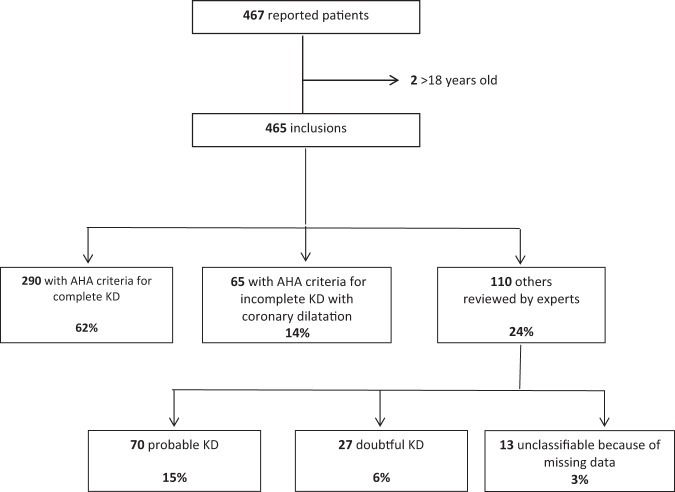
Overall, 84 physicians reported 467 patients from 65 French centers. Two adult patients were not included in the analyses. A total of 355 patients (76%) fulfilled criteria for complete or incomplete KD. Among the 110 other patients, records for 13 were not usable because of missing data. The 97 remaining patients were classified as probable KD, 70 (72%), and doubtful KD, 27 (28%): meaning that in 27/452 (6%) cases, the diagnosis of KD was challenged by experts (Fig. 1).
Figure 1.
Flow of ascertainment of the 467 cases with Kawasaki disease (KD) and classification according to American Heart Association (AHA) criteria and experts.
Characteristics of KD patients
For the 425 patients classified with KD (AHA + experts confirmation), 242 were boys. The mean age at disease onset was 2.8 ± 2.4 years (range 0.1 to 14.4) and mean delay to IVIg treatment 6.6 ± 3.6 days (Table 1). As expected, by definition, the frequency of clinical manifestations and coronary abnormalities differed among complete, incomplete, and probable KD. Patients with incomplete or probable KD were younger than those with complete KD (P = 0.006), and although mean delay to treatment was almost the same for the 3 subgroups, patients with incomplete KD were more frequently treated after 10 days of fever (22% vs. 11% for complete KD and 7% for probable KD) (P = 0.02). Only 3/425 had initial combined therapy with IVIG plus steroids.
Table 1.
Characteristics of the 425 patients with Kawasaki disease (KD) according to American Heart Association criteria and experts.
| Variables | Whole KD population n = 425 | Complete KD n = 290 | Incomplete KD n = 65 | Probable KD n = 70 | p |
|---|---|---|---|---|---|
| Male/female, no (sex ratio) | 242/182 (1.3) | 164/125 (1.3) | 38/27 (1.4) | 40/30 (1.3) | 0.97 |
| Age at disease onset, years (mean ± SD) | 2.8 ± 2.4 | 3.0 ± 2.5 | 2.4 ± 2.2 | 2.2 ± 1.6 | 0.006 |
| Delay from fever onset to diagnosis, days (mean ± SD) | 6.0 ± 3.1 | 5.8 ± 3.0 | 6.9 ± 3.5 | 5.9 ± 2.8 | 0.05 |
| Delay from fever onset to IVIg, days (mean ± SD) | 6.6 ± 3.6 | 6.4 ± 3.3 | 7.7 ± 5.2 | 6.5 ± 2.7 | 0.10 |
| Follow-up, days (mean ± SD) | 35.7 ± 55.6 | 33.6 ± 48.5 | 42.7 ± 75.1 | 37.7 ± 61.0 | 0.78 |
| Clinical manifestations, n (%)* | |||||
| Modifications of extremities | 298/424 (70%) | 235 (81%) | 28 (44%) | 35 (50%) | < 0.001 |
| Diffuse exanthema | 320/399 (80%) | 252 (91%) | 30 (50%) | 38 (62%) | < 0.001 |
| Conjunctival injection | 374/421 (89%) | 282 (97%) | 40 (63%) | 52 (78%) | < 0.001 |
| Cervical adenitis >1.5 cm | 219/397 (55%) | 186 (66%) | 20 (34%) | 13 (22%) | < 0.001 |
| Modifications of oral mucosa | 392/418 (94%) | 288 (99%) | 45 (74%) | 59 (88%) | < 0.001 |
| Cheilitis | 350/413 (85%) | 264 (92%) | 35 (57%) | 51 (78%) | < 0.001 |
| Raspberry tongue | 207/371 (56%) | 151 (60%) | 19 (32%) | 37 (63%) | < 0.001 |
| Erythema of the oral mucosa | 275/383 (72%) | 207 (79%) | 30 (52%) | 38 (60%) | < 0.001 |
| Maximum CRP level (mean ± SD) | 156 ± 98 | 157 ± 99 | 157 ± 111 | 152 ± 82 | 0.97 |
| Cardiac complications, n (%)* | |||||
| Coronary dilatation | 163/418 (39%) | 98 (35%) | 65 (100%) | 0 (0%) | < 0.001 |
| Myocarditis | 13/402 (3%) | 7 (3%) | 3 (5%) | 3 (5%) | 0.51 |
| Pericarditis | 75/413 (18%) | 50 (18%) | 14 (22%) | 11 (16%) | 0.67 |
| Acute heart failure | 4/407 (1%) | 3 (1%) | 1 (2%) | 0 (0%) | 0.65 |
| Treatment regimen, n (%)* | |||||
| IVIg | 420/425 (99%) | 287 (99%) | 64 (99%) | 69 (99%) | 1.00 |
| Aspirin | 398/412 (97%) | 270 (97%) | 63 (97%) | 65 (96%) | 0.92 |
| Treated ≥10 days after fever onset | 50/418 (12%) | 31 (11%) | 14 (22%) | 5 (7%) | 0.02 |
| Unresponsiveness to the first IVIg course | 84/348 (23%) | 63 (26%) | 19 (31%) | 12 (20%) | 0.37 |
*Denominators excluded patients with missing or irrelevant information.
IVIg, intravenous immunoglobulin; CRP, C-reactive protein.
Ethnicity
Among the 425 patients classified with KD, 236 (55%) were European Caucasian descendants, 50 (12%) North African/Middle Eastern, 43 (10%) African/Afro-Caribbean, 11 (3%) Asian and 47 (11%) mixed (Table 2). The ethnicity was undetermined in 38 patients (9%). As compared with European Caucasian patients, African/Afro-Caribbean patients less often had cervical adenitis (OR: 0.35, 95% CI 0.17–0.71) and patients with dark skin more often had erythema of the bottom (OR: 2.9, 95% CI 1.4–5.7 for African/Afro-Caribbean and OR: 1.9, 95% CI 1.0–3.8 for North African/Middle Eastern) and perinea desquamation (OR: 9.0, 95% CI 4.3–18.7 for African/Afro-Caribbean and OR: 2.9, 95% CI 1.3–6.3 for North African/Middle Eastern). Time to treatment, necessity of second line treatment after initial IVIG, and cardiac complications were similar in the 3 ethnic subgroups.
Table 2.
Characteristics of patients with KD by ethnic background.
| Variables | European Caucasian | North African/Middle Eastern | African/Afro-Caribbean | P |
|---|---|---|---|---|
| N = 236 | N = 50 | N = 43 | ||
| Demographics | ||||
| Male/female, no. (sex ratio) | 136/100 (1.4) | 29/21 (1.4) | 23/20 (1.2) | 0.87 |
| Age at disease onset, years (mean ± SD) | 3.0 ± 2.6 | 2.9 ± 2.0 | 2.1 ± 1.6 | 0.16 |
| Delay from fever onset to diagnosis, days (mean ± SD) | 6.1 ± 3.1 | 6.1 ± 3.2 | 5.8 ± 2.6 | 0.92 |
| Clinical manifestations, n (%)* | ||||
| Modifications of extremities | 159 (69) | 33 (67) | 35 (81) | 0.22 |
| Diffuse exanthema | 181 (82) | 41 (85) | 31 (72) | 0.24 |
| Conjunctival injection | 211 (90) | 43 (86) | 38 (88) | 0.67 |
| Cervical adenitis >1.5 cm | 126 (57) | 28 (62) | 13 (32) | 0.006 |
| Modifications of oral mucosa | 219 (94) | 46 (96) | 41 (98) | 0.60 |
| Cheilitis | 189 (82) | 43 (90) | 38 (90) | 0.20 |
| Raspberry tongue | 106 (51) | 27 (61) | 23 (62) | 0.27 |
| Erythema | 146 (69) | 33 (77) | 31 (74) | 0.49 |
| Erythema of the bottom | 55 (27) | 17 (41) | 20 (51) | 0.005 |
| Perineal desquamation | 27 (13) | 13 (31) | 25 (58) | < 0.001 |
| Blood tests (mean ± SD) | ||||
| Maximum CRP level (mg/L) | 150 ± 99 | 144 ± 102 | 193 ± 117 | 0.09 |
| Albumin level (g/L) | 32 ± 8 | 33 ± 6 | 27 ± 5 | 0.002 |
| Cardiac complications, n (%)* | ||||
| Coronary artery anomalies | 89 (38) | 16 (33) | 17 (40) | 0.70 |
| Myocarditis | 7 (3) | 2 (4) | 0 (0) | 0.60 |
| Pericarditis | 42 (18) | 8 (17) | 9 (21) | 0.84 |
| Treatment | ||||
| Delay from fever onset to IVIg, days (mean±SD) | 6.6 ± 3.1 | 7.2 ± 4.5 | 6.7 ± 5.5 | 0.56 |
| Time to treatment >10 days, n (%) | 30 (13) | 9 (18) | 3 (7) | 0.27 |
| Unresponsiveness to IVIg, n (%) | 60 (26) | 9 (19) | 10 (23) | 0.55 |
*Denominators excluded patients with missing or irrelevant information.
IVIg, intravenous immunoglobulin; CRP, C-reactive protein.
Unresponsiveness to IVIg
Information on response to the first IVIG treatment was available for 415/425 patients (98%) with KD; 92 (22%) needed second-line treatment. On univariate analysis, IVIg unresponsiveness was strongly associated with clinical or radiological hepatic or cardiac involvement (Table 3). Biologically, IVIg unresponsiveness was associated with inflammation (high CRP and procalcitonin levels, leukocytosis, low albumin level and high lymphocyte count) and hepatic involvement (high AST, ALT and gamma-glutamyl transpeptidase [GGT] levels) (Table 4). Neither disease-onset age nor delay to IVIg infusion >10 days was associated with unresponsiveness to IVIg (Table 3).
Table 3.
Factors associated with unresponsiveness to the first IVIg infusion.
| Variables | Resistance to treatment | |||
|---|---|---|---|---|
| Number of patients (%) | Bivariate analysis | |||
| Yes (n = 92) | No (n = 323) | OR | 95% CI | |
| Demographics | ||||
| Male | 55 (23) | 180 (77) | 1.2 | 0.7–1.9 |
| Female | 37 (21) | 142 (79) | ||
| Age at diagnosis, years | ||||
| < 1 | 28 (28) | 72 (72) | ||
| 1–5 | 51 (19) | 211 (81) | 0.6 | 0.4–1.1 |
| > 5 | 13 (25) | 38 (75) | 0.9 | 0.4–1.9 |
| Clinical manifestations, n (%)* | ||||
| Modifications of extremities | 75 (26) | 210 (74) | 2.8 | 1.5–5.1 |
| Diffuse exanthema | 65 (21) | 249 (79) | 0.6 | 0.4–1.1 |
| Conjunctival injection | 79 (22) | 278 (78) | 1.0 | 0.5–2.1 |
| Cervical adenitis >1.5 cm | 43 (20) | 169 (80) | 0.8 | 0.5–1.3 |
| Modifications of oral mucosa | 81 (21) | 303 (79) | 0.6 | 0.3–1.6 |
| Cheilitis | 76 (22) | 266 (78) | 1.2 | 0.6–2.3 |
| Raspberry tongue | 39 (20) | 161 (80) | 0.8 | 0.5–1.3 |
| Erythema | 55 (20) | 215 (80) | 0.8 | 0.5–1.4 |
| Erythema of the bottom | 30 (28) | 78 (72) | 1.8 | 1.0–3.0 |
| Hepatomegaly | 19 (56) | 15 (44) | 5.5 | 2.7–11.4 |
| Tachycardia | 28 (30) | 65 (70) | 1.7 | 1.0–2.9 |
| Meningeal syndrome | 4 (27) | 11 (73) | 1.3 | 0.4–4.1 |
| Hypotonia | 8 (33) | 16 (67) | 1.8 | 0.7–4.3 |
| Imaging, n (%)* | ||||
| Hydrocholecystis | 11(52) | 10 (48) | 4.3 | 1.7–10.5 |
| Cardiomegaly | 8 (67) | 4 (33) | 7.8 | 2.3–26.5 |
| Coronary artery anomalies | 56 (35) | 105 (65) | 3.3 | 2.0–5.3 |
| Myocarditis | 7 (54) | 6 (46) | 4.6 | 1.5–14.1 |
| Pericarditis | 24 (32) | 51(68) | 2.0 | 1.1–3.4 |
| Treatment, n (%) | ||||
| Time to treatment <5 days | 32 (33) | 66 (67) | 2.1 | 1.2–3.4 |
| Time to treatment >10 days | 13 (26) | 37 (74) | 1.3 | 0.6–2.5 |
| Aspirin with anti-inflammatory dosage | 85 (22) | 297 (78) | 1.0 | 0.4–2.9 |
*Denominators excluded patients with missing or irrelevant information.
Table 4.
Univariate analysis of laboratory values for unresponsiveness and response to the first IVIG infusion.
| IVIg unresponsive n = 92 | IVIg response n = 323 | P | |||
|---|---|---|---|---|---|
| Number of patients | Number of patients | ||||
| CRP initial (mg/L) | 88 | 152 ± 107 | 291 | 123 ± 83 | 0.04 |
| CRP maximum (mg/L) | 78 | 191 ± 117 | 260 | 146 ± 90 | 0.002 |
| PCT initial (mg/L) | 28 | 3.8 ± 3.0 | 225 | 1.9 ± 2.4 | < 0.001 |
| PCT maximum (mg/L) | 24 | 4.4 ± 3.0 | 248 | 1.9 ± 2.2 | < 0.001 |
| Hemoglobin level (g/dL) | 90 | 10.9 ± 1.5 | 292 | 11.1 ± 1.3 | 0.62 |
| Leukocyte count maximum (/mm3) | 86 | 20377 ± 9310 | 289 | 16281 ± 7378 | < 0.001 |
| Lymphocyte count (/mm3) | 79 | 3206 ± 4696 | 267 | 3992 ± 4118 | < 0.001 |
| Platelet count (x103/mm3) | 89 | 368 ± 156 | 307 | 379 ± 152 | 0.61 |
| AST (UI/L) | 74 | 72 ± 101 | 239 | 54 ± 61 | 0.04 |
| ALT (UI/L) | 72 | 99 ± 120 | 239 | 73 ± 102 | 0.005 |
| GGT (UI/L) | 58 | 94 ± 93 | 160 | 65 ± 72 | 0.02 |
| Albumin (g/L) | 56 | 30 ± 7 | 214 | 32 ± 7 | 0.12 |
| Na + (mmol/L) | 81 | 134 ± 3 | 266 | 135 ± 3 | 0.02 |
| Urea (mmol/L) | 76 | 4.5 ± 4.8 | 249 | 3.8 ± 4.4 | 0.003 |
All data are mean ± SD.
CRP, C-reactive protein level, PCT, procalcitonin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase.
We could estimate the Egami, Sano and Kobayashi scores (with cut-off ≥4 and ≥5) for 328, 211 and 334 patients, respectively (Table 5). All scores had poor performance in detecting unresponsiveness to IVIg in our population with a sensitivity ranging from 14 to 61%. These scores had missed respectively 37/75 (49.3%), 38/44 (86.4%), 32/81 (39.5%) and 46/80 (57.5%) patients unresponsive to IVIg. On stratification by ethnicity, Egami and Kobayashi scores had better sensitivity for African or Afro-Caribbean patients (63 to 88%) than European Caucasians (36 to 53%) or Eastern Caucasian or North African/Middle Eastern patients (33 to 71%). Sano score had poor sensitivity in all our ethnic groups. Asian children were too few to evaluate the performance of the scores.
Table 5.
Sensitivity and specificity of Egami, Sano, Kobayashi and Kawanet scores to predict unresponsiveness to intravenous immunoglobulin in our KD population and by ethnicity.
| Egami n = 320 patients | Sano n = 211 patients | Kobayashi cut-off ≥4 n = 334 patients | Kobayashi cut-off ≥5 n = 334 patients | Kawanet Cut-off ≥2 n = 415 patients | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Se (%) | Sp(%) | Se(%) | Sp(%) | Se(%) | Sp(%) | Se(%) | Sp(%) | Se(%) | Sp(%) | |
| Whole KD population | 51 | 71 | 14 | 86 | 61 | 68 | 43 | 83 | 77 | 60 |
| European Caucasians | 51 | 72 | 18 | 84 | 53 | 71 | 36 | 82 | 74 | 57 |
| Eastern Caucasian or North African/Middle Eastern patients | 33 | 76 | 0 | 89 | 71 | 68 | 57 | 93 | 80 | 65 |
| African or Afro-Caribbean patients | 71 | 82 | 20 | 91 | 88 | 69 | 63 | 85 | 88 | 56 |
Se: sensitivity; Sp: specificity.
Because of the poor sensitivity of these Japanese scores in our population, we developed a new scoring system. On multivariate regression analysis, predictors of secondary treatment after initial IVIG were Hepatomegaly, ALT level ≥30 IU/L, lymphocyte count <2400/mm3 and time to treatment <5 days (Table 6). The best sensitivity (77%) and specificity (60%) of this model was with 1 point per variable and cut-off ≥2 points) with an area under the curve of 0.725. Figure 2 represents the Receiver operator characteristic curve of the Kawanet score. Sensitivity was very good with acceptable specificity in African/Afro-Caribbean population (88% and 56%) and North African/Middle Eastern population (80% and 65%) and remained acceptable in European Caucasian (74% and 57%).
Table 6.
Multivariate logistic regression analysis of predictors of unresponsiveness to IVIg for our patients with KD.
| Variables | OR (95% CI) | P | Points |
|---|---|---|---|
| ALT level > 30 IU/L | 2.4 (1.3–4.5) | 0.008 | 1 |
| Hepatomegaly | 3.0 (1.2–7.4) | 0.020 | 1 |
| Lymphocyte count < 2400/mm3 | 2.2 (1.2–4.0) | 0.010 | 1 |
| Time to treatment < 5 days | 1.9 (1.1–3.5) | 0.032 | 1 |
| Total | 4 | ||
| Cut-off | 2 | ||
| Sensitivity | 77% | ||
| Specificity | 60% |
Figure 2.
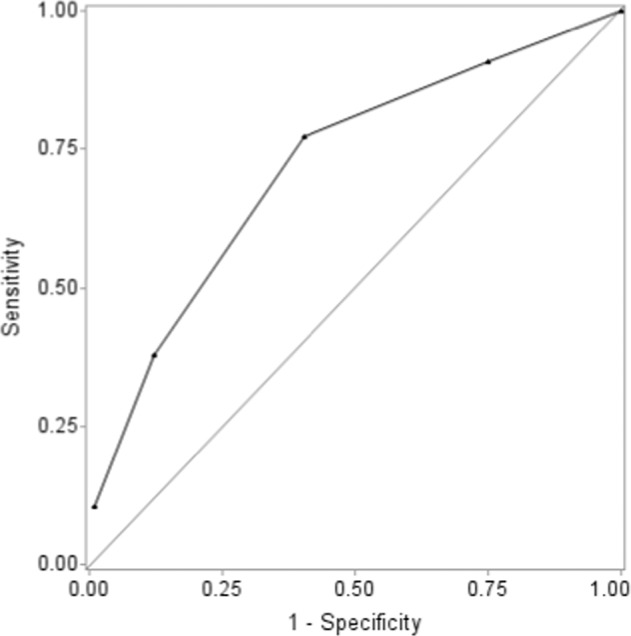
Receiver operator characteristic curve of the Kawanet score.
Discussion
Early identification of resistance to IVIg in KD is critical to initiate more effective therapies aimed to limit serious cardiac complications, especially coronary dilatations and aneurysms. Scoring systems have been established and validated in the Japanese population but they have some limitations. First, resistance to IVIG has not a universal definition in terms of persistence or length of reappraisal of fever, second, fever alone may not be the unique indicator of insufficient response as persistence of elevated CRP is associated also with cardiac complications, and finally those scores have not been validated outside the Japanese population15–19. We took the opportunity of the Kawanet, the widest prospective epidemiologic tool ever set up in France, a non-Asian country including multiple ethnic groups and also mixed ethnicities, to evaluate the frequency of secondary treatment after standard treatment i.e.; 2 g/kg of IVIG, and to identify the clinical, biological and radiological variables associated with the use of secondary treatments in our cohort of patients.
Kawanet aimed to analyze the degree of awareness of pediatricians and to have a thorough picture of what is called KD in France, how the disease is managed and the response to standard treatment (IVIg) but it is not a tool dedicated to calculating KD incidence (estimated 9/100,000 children <5 years of age in the Nord Pas de Calais region)28 or prevalence; Although the database contains exclusively e-repository data, a thorough monitoring further ensured the quality of KD cases, and all cases not satisfying the AHA criteria were reviewed and classified by consensus among KD experts, 2 independent of the Kawanet study. We registered 465 patients over a 3-year period, 76% fulfilling the AHA criteria, one third being incomplete or probable cases and significantly younger than complete KD cases (2.4 ± 2.2 and 2.2 ± 1.6 vs. 3.0 ± 2.5 years). For patients without AHA criteria, expert agreement with the referring physicians for KD diagnosis was high, 72%, reflecting a high level of knowledge of KD among French pediatricians, with a possibility of 6% over diagnosis. As a whole, the clinical characteristics of French KD patients agreed with those previously known in terms of sex ratio, age of onset, and distribution of clinical symptoms29–31. Patients of African/Afro-Caribbean ethnicities tended to be younger than European Caucasians and North African/Middle Eastern patients and had significantly less adenitis and more erythema and desquamation of the bottom. Unfortunately, our Asian population (n = 11) was too small to allow any comparison.
Although our KD cohort was early diagnosed and treated with an accurate regimen of IVIg, we observed a high rate of cardiac complications, especially coronary dilatations, 49%, that did not differ among our 3 ethnic populations. This discrepancy with the 26–30% coronary complications without IVIg treatment described in the literature6,7 is due to our definition of cardiac complications — presence of any coronary dilatation on any echocardiography ever, even if the dilatation disappeared — whereas previous rates were estimated at 1 month of disease evolution6,7,32,33. Because of the little published information, we could not confirm a possible reduced or increased risk of coronary dilatation in African/Afro-Caribbean patients34,35. Unfortunately, absence of Z-scores in Kawanet limited a more accurate analysis of cardiac complications1. Therefore, we focused on unresponsiveness to treatment. A relatively high proportion of our patients (22%) required secondary treatment (including IVIg re treatment) after standard treatment with 2 g/kg IVIg. Overall, the Egami, Sano and Kobayashi predicting IVIg resistance did not provide enough performance in our multi ethnic KD population. However, and to our knowledge, for the first time, we observed a better performance of two of these scores in African/Afro-Caribbean patients compared to other ethnic groups, with the exclusion of Asians. Prediction of IVIg resistance is crucial to intensify initial treatment combined with IVIg to prevent cardiac complications12,14,36,37, but none of the currently published scores have good sensitivity in Caucasian populations15–19,36,38. In our multiethnic population, we identified predictive factors of IVIg resistance based on real-life practice, and built a scoring system and obtained for the first time, good sensitivity (77%) and acceptable specificity (60%) in our non-Asian population. The sensitivity remained good in our 3 main ethnic subgroups (74 to 88%). Besides our new score, other predictors of IVIg resistance recently identified include proportion of neutrophils, hemoglobin level, CRP level, procalcitonin level, erythrocyte sedimentation rate, albumin level, creatinine level, sodium level, N-terminal pro-brain natriuretic peptide, interleukin-6 and −10 levels19,39,40 and baseline Z-score ≥2 or echocardiogram alteration, all found to predict high risk KD patients19,41.
The strengths of the Kawanet study are the wide data collection, which was predominantly prospective, the multiethnic distribution of patients, and the involvement of independent experts for case adjudication. In addition, the definition of IVIG unresponsiveness was based on current practice data rather than on any potentially controversial definition. However, the limitations include selection biases: adult patients were not included, potentially most severe patients in intensive care units were not recruited, the recruitment was voluntary and finally, our study could not compare Asian and non-Asian patients. In order to minimize risk of aneurysms associated with unresponsiveness to IVIg19, we maximized sensitivity of the score despite an acceptable slight decrease in specificity.
Conclusion
We identified predictors of IVIg unresponsiveness in our cohort of KD patients and built a new score with good sensitivity and acceptable specificity in the non-Asian population. Our score, which should be tested in other multiethnic KD cohorts, is sensitive enough to be clinically useful in making decisions about initial and alternative treatments in non-Asian populations.
Acknowledgements
The sponsor was Assistance Publique-Hôpitaux de Paris (Département de la Recherche Clinique et du Développement). We acknowledge also for their collaboration Agbo-Kpati Placide (Lagny), Agostini Helene (Kremlin Bicêtre), Armangaud Didier (Poissy), Armangaud Jean-Baptiste (Paris), Grimprel Emmanuel (Paris), Ballot Claire (Besançon), Barrey Catherine (Bry-sur-Marne), Benoist Grégoire (Boulogne-Billancourt), Bonnet Mathilde (Lille), Borm Bettina (Bézier), Bosdure Emmanuelle (Marseille), Bro Bru Cécile (Grenoble), Brochard Karine (Toulouse), Brosset Philippe (Limoges), Daltroff Gérard (Belfort), Dallochio Aymeric (Tulle), Decobert Marion (Orsay), Dejode Cecile (Annemasse), Desdoits Alexandra (Caen), Eyssette-Guerreau Stéphanie (Pontoise), Faye Albert (Paris), Gajdos Vincent (Clamart), Gilles Isabelle (Evreux), Gilton-Bott Lucie (Nîmes), Hentgen Véronique (Le Chesnay), Higel Laetitia (Strasbourg), Launay Elise (Nantes), Leblanc Antoine (Evry), Lechevalier Pauline (Paris), Mahe Emmanuel (Argenteuil), Merlin Etienne (Clermont-Ferrand), Mosca Alexis (Evry), Orzechowski Christine (Bry-sur-Marne), Poignant Sylvaine (Cholet), Rivière Marie-Françoise (Bourges), Talmud Deborah (Orléans), Uettwiller Florence (Tours). We also thank for their contribution Armelle Arnoux, Caroline Galeotti, Anna Auguste, Isabelle Pruvost, Alexandre Belot, Kandara Khaled, Violaine Bresson, Ashnaf Al Junaidi, Caroline Laffort, Amandine Fichet, Marie Baret, Pierre Emmanuel Seguela, David Rosselini, Raphaël Teissier, Eve Goulais, Marie Line Jacquemont, Julie Chaix, Marie-Amélie Dubois, Cristelle Parache, Eric Moulene, Maxime Veques, Claire Dauphin, Agnès Mourcia, Aurelia Carbasse, Anne Laure Jurquet, Emilie Sauvaget, Elisabeth Pinlou, Marie Baills, Marion Lagree, Murielle Lalande, Benedicte Romefort, Candice Meyzer, Mélanie Cochez, Sarah Cohen, Isabelle Michelet, Céline Castaings-Carlioz, Isabelle Melki, Bérangère Koehl, Sophie Dugue, Renaud Blonde, Charlène Goma, Tu-Anh Tran, Virginie Lambert. The study was funded by a grant from Programme Hospitalier de Recherche Clinique-PHRC 2009 (French Ministry of Health) and the French Society of Rheumatology (SFR).
Author contributions
Dr. Piram and Pr Koné-Paut conceived of and designed the study, designed the data collection instrument, participated in the consensus conference, drafted the initial manuscript, and reviewed and revised the manuscript, approved the final manuscript as submitted and agree to be accountable for all aspects of the work. Ms. Darce Bello coordinated and supervised data collection and reviewed and revised the manuscript, approved the final manuscript as submitted and agree to be accountable for all aspects of the work. Pr. Cimaz participated in the consensus conference, critically reviewed the manuscript for important intellectual content, approved the final manuscript as submitted and agree to be accountable for all aspects of the work. Ms Piedvache analyzed the data and reviewed and revised the manuscript. approved the final manuscript as submitted and agree to be accountable for all aspects of the work. Dr. Tellier, Dr Di Filippo, Pr Boralevi, Dr. Madhi, Dr Meinzer participated to acquisition of data, reviewed and revised the manuscript, approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McCrindle BW, et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 2.Dionne A, Dahdah N. Myocarditis and Kawasaki disease. Int. J. rheumatic Dis. 2017;21:45–49. doi: 10.1111/1756-185X.13219. [DOI] [PubMed] [Google Scholar]
- 3.Tissandier C, et al. Kawasaki shock syndrome complicating a recurrence of Kawasaki disease. Pediatrics. 2014;134:e1695–1699. doi: 10.1542/peds.2014-0004. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Gong F, Zhu W, Fu S, Zhang Q. Macrophage activation syndrome in Kawasaki disease: more common than we thought? Semin. arthritis rheumatism. 2015;44:405–410. doi: 10.1016/j.semarthrit.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Furusho K, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984;2:1055–1058. doi: 10.1016/S0140-6736(84)91504-6. [DOI] [PubMed] [Google Scholar]
- 6.Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J. pediatrics. 1997;131:888–893. doi: 10.1016/S0022-3476(97)70038-6. [DOI] [PubMed] [Google Scholar]
- 7.Mori M, et al. Meta-analysis of the results of intravenous gamma globulin treatment of coronary artery lesions in Kawasaki disease. Mod. Rheumatol. 2004;14:361–366. doi: 10.3109/s10165-004-0324-3. [DOI] [PubMed] [Google Scholar]
- 8.Tulloh RMR, et al. Kawasaki disease: a prospective population survey in the UK and Ireland from 2013 to 2015. Arch. Dis. Child. 2018;104:640–646. doi: 10.1136/archdischild-2018-315087. [DOI] [PubMed] [Google Scholar]
- 9.Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics. 2000;105:E78. doi: 10.1542/peds.105.6.e78. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi T, et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet. 2012;379:1613–1620. doi: 10.1016/S0140-6736(11)61930-2. [DOI] [PubMed] [Google Scholar]
- 11.Eleftheriou D, et al. Management of Kawasaki disease. Arch. Dis. Child. 2014;99:74–83. doi: 10.1136/archdischild-2012-302841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egami K, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J. pediatrics. 2006;149:237–240. doi: 10.1016/j.jpeds.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 13.Sano T, et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur. J. pediatrics. 2007;166:131–137. doi: 10.1007/s00431-006-0223-z. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–2612. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 15.Sleeper LA, et al. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J. pediatrics. 2011;158:831–835. doi: 10.1016/j.jpeds.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tremoulet AH, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J. pediatrics. 2008;153:117–121. doi: 10.1016/j.jpeds.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Manubens J, et al. Role of the Egami score to predict immunoglobulin resistance in Kawasaki disease among a Western Mediterranean population. Rheumatol. Int. 2016;36:905–910. doi: 10.1007/s00296-016-3499-y. [DOI] [PubMed] [Google Scholar]
- 18.Davies S, et al. Predicting IVIG resistance in UK Kawasaki disease. Arch. Dis. Child. 2015;100:366–368. doi: 10.1136/archdischild-2014-307397. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Cooke E, et al. Epidemiological and clinical features of Kawasaki disease in Spain over 5 years and risk factors for aneurysm development. (2011–2016): KAWA-RACE study group. PLoS One. 2019;14:e0215665. doi: 10.1371/journal.pone.0215665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song R, Yao W, Li X. Efficacy of Four Scoring Systems in Predicting Intravenous Immunoglobulin Resistance in Children with Kawasaki Disease in a Children’s Hospital in Beijing, North China. J. pediatrics. 2017;184:120–124. doi: 10.1016/j.jpeds.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Shin J, Lee H, Eun L. Verification of Current Risk Scores for Kawasaki Disease in Korean Children. J. Korean Med. Sci. 2017;32:1991–1996. doi: 10.3346/jkms.2017.32.12.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bar-Meir, M. et al. Prediction of Resistance to Intravenous Immunoglobulin in Children With Kawasaki Disease. Journal of the Pediatric Infectious Diseases Society (2017). [DOI] [PubMed]
- 23.Kibata T, et al. Coronary artery lesions and the increasing incidence of Kawasaki disease resistant to initial immunoglobulin. Int. J. cardiology. 2016;214:209–215. doi: 10.1016/j.ijcard.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Research Committee of the Japanese Society of Pediatric, C. & Cardiac Surgery Committee for Development of Guidelines for Medical Treatment of Acute Kawasaki, D. Guidelines for medical treatment of acute Kawasaki disease: report of the Research Committee of the Japanese Society of Pediatric Cardiology and Cardiac Surgery (2012 revised version) Pediatr. Int. 2014;56:135–158. doi: 10.1111/ped.12317. [DOI] [PubMed] [Google Scholar]
- 25.Baek JS, et al. Coronary artery status of patients with transient fever 24–36 h after first IVIG infusion did not differ from that seen in responsive patients. Pediatr. Rheumatol. Online J. 2018;16:83. doi: 10.1186/s12969-018-0301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Research Committee on Kawasaki disease. Report of subcommittee on standardization of diagnostic criteria and reporting of coronary artery lesions in Kawasaki disease. Tokyo, Japan, Ministry of Health and Welfare. (1994).
- 27.Seki M, et al. External validation of a risk score to predict intravenous immunoglobulin resistance in patients with kawasaki disease. Pediatric Infect. Dis. J. 2011;30:145–147. doi: 10.1097/INF.0b013e3181f386db. [DOI] [PubMed] [Google Scholar]
- 28.Heuclin T, et al. Increased detection rate of Kawasaki disease using new diagnostic algorithm, including early use of echocardiography. J. pediatrics. 2009;155(695–699):e691. doi: 10.1016/j.jpeds.2009.04.058. [DOI] [PubMed] [Google Scholar]
- 29.Bayers S, Shulman ST, Paller AS. Kawasaki disease: part I. Diagnosis, clinical features, and pathogenesis. J. Am. Acad. Dermatol. 2013;69(501):e501–511. doi: 10.1016/j.jaad.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Maggio MC, Corsello G, Prinzi E, Cimaz R. Kawasaki disease in Sicily: clinical description and markers of disease severity. Ital. J. Pediatr. 2016;42:92. doi: 10.1186/s13052-016-0306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saundankar J, et al. The epidemiology and clinical features of Kawasaki disease in Australia. Pediatrics. 2014;133:e1009–1014. doi: 10.1542/peds.2013-2936. [DOI] [PubMed] [Google Scholar]
- 32.Newburger JW, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N. Engl. J. Med. 1986;315:341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 33.Chbeir D, et al. Kawasaki disease: abnormal initial echocardiogram is associated with resistance to IV Ig and development of coronary artery lesions. Pediatr. Rheumatol. Online J. 2018;16:48. doi: 10.1186/s12969-018-0264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porcalla AR, Sable CA, Patel KM, Martin GR, Singh N. The epidemiology of Kawasaki disease in an urban hospital: does African American race protect against coronary artery aneurysms? Pediatr. Cardiol. 2005;26:775–781. doi: 10.1007/s00246-005-0916-5. [DOI] [PubMed] [Google Scholar]
- 35.Clark DE, et al. Predictors of Intravenous Immunoglobulin Nonresponse and Racial Disparities in Kawasaki Disease. Pediatric Infect. Dis. J. 2018;37:1227–1234. doi: 10.1097/INF.0000000000002019. [DOI] [PubMed] [Google Scholar]
- 36.Rigante D, et al. Critical Overview of the Risk Scoring Systems to Predict Non-Responsiveness to Intravenous Immunoglobulin in Kawasaki Syndrome. Int. J. Mol. Sci. 2016;17:278. doi: 10.3390/ijms17030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogata S, et al. Corticosteroid pulse combination therapy for refractory Kawasaki disease: a randomized trial. Pediatrics. 2012;129:e17–23. doi: 10.1542/peds.2011-0148. [DOI] [PubMed] [Google Scholar]
- 38.Berdej-Szczot E, et al. Risk factors of immunoglobulin resistance and coronary complications in children with Kawasaki disease. Kardiol. Pol. 2017;75:261–266. doi: 10.5603/KP.a2016.0179. [DOI] [PubMed] [Google Scholar]
- 39.Xie T, et al. Predictors for intravenous immunoglobulin resistance and coronary artery lesions in Kawasaki disease. Pediatr. Rheumatol. Online J. 2017;15:17. doi: 10.1186/s12969-017-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y, et al. Interleukin-6 is prone to be a candidate biomarker for predicting incomplete and IVIG nonresponsive Kawasaki disease rather than coronary artery aneurysm. Clin. Exp. Med. 2019;19:173–181. doi: 10.1007/s10238-018-00544-5. [DOI] [PubMed] [Google Scholar]
- 41.Son MBF, et al. Predicting Coronary Artery Aneurysms in Kawasaki Disease at a North American Center: An Assessment of Baseline z Scores. J. Am. Heart Assoc. 2017;6:6. doi: 10.1161/JAHA.116.005378. [DOI] [PMC free article] [PubMed] [Google Scholar]