We report the complete genome sequences of five human coronavirus NL63 (HCoV-NL63) strains obtained using next-generation sequencing. The five HCoV-NL63 strains were obtained from hospitalized children with severe acute respiratory infection detected in Guangdong, China. This study provides several complete genomes of HCoV-NL63 and improves our understanding of HCoV-NL63 evolution in China.
ABSTRACT
We report the complete genome sequences of five human coronavirus NL63 (HCoV-NL63) strains obtained using next-generation sequencing. The five HCoV-NL63 strains were obtained from hospitalized children with severe acute respiratory infection detected in Guangdong, China. This study provides several complete genomes of HCoV-NL63 and improves our understanding of HCoV-NL63 evolution in China.
ANNOUNCEMENT
Human coronavirus NL63 (HCoV-NL63) is a member of the family Coronaviridae, genus Alphacoronavirus, and was first discovered in 2004 (1). HCoV-NL63 is mainly associated with the common cold in children, the elderly, and immunocompromised patients (2, 3). The genome of HCoV-NL63 is about 27 kb with a conserved gene order of 5′-orf1ab-spike (S)-orf3-envelope (E)-membrane (M)-nucleocapsid (N)-poly (A). The species tropism of HCoV-NL63 is determined by spike glycoprotein. HCoV-NL63 and severe acute respiratory syndrome coronavirus (SARS-CoV) share the same cell receptor, angiotensin converting enzyme 2 (ACE-2) (4, 5), for entry into host cells, and HCoV-NL63 is recognized as a common cause of upper respiratory tract infection and has been prevalent worldwide.
Here, nasopharyngeal swab samples were collected from hospitalized children with severe acute respiratory infection in Guangzhou, China, in 2018. This study was performed in strict accordance with human subject protection guidance provided by the Research Ethics Committee of Guangzhou Medical University. The respiratory samples were filtered with 0.22-μm filters, RNA extraction was performed using a Qiagen viral RNA extraction kit, and extracted RNA was used for sequence-independent single-primer amplification (SISPA) (6, 7) as follows: a reverse transcription reaction was performed with SuperScript III reverse transcriptase using a primer containing a fixed sequence, followed by a random hexamer at the 3′ end (FR26RV, GCCGGAGCTCTGCAGATATCNNNNNN). Then, Klenow fragment polymerase (New England Biolabs) was used for DNA synthesis. Finally, PCR amplification was conducted using primers consisting of the fixed portions of the random primers (FR26, GCCGGAGCTCTGCAGATATC). Purified DNA was used for next-generation sequencing (NGS). Libraries were prepared with the Nextera XT kit (Illumina), and paired-end reads (2 × 125 bp) determined using a HiSeq 2500 instrument were used for cleaning and assembling using CLC Genomics Workbench version 11.0. Illumina sequencing yielded about 10 million reads per sample. Reads were assembled into contigs with a de novo assembly model, and the contig sequences were then extracted for subsequent analysis. Partial genome sequences of five HCoV-NL63 strains were obtained by NGS methods. Meanwhile, sets of specific primer pairs were designed and used to amplify the gap region of HCoV-NL63, which was used for genome assemblies using the SeqMan subprogram of the DNAStar software version 7.1.0 with default parameters (Table 1). Finally, five complete genome sequences of HCoV-NL63 were obtained using next-generation sequencing and Sanger sequencing methods together and were designated strains ChinaGD01 (27,531 bp), ChinaGD02 (27,516 bp), ChinaGD03 (27,516 bp), ChinaGD04 (27,532 bp), and ChinaGD05 (27,544 bp). The five HCoV-NL63 strains presented here were aligned using MAFFT version 7.158 (8) and showed 98.5 to ∼99.1% nucleotide homology with the prototype HCoV-NL63 virus (GenBank accession number NC_005831.2) as estimated using MEGA version 5.10 software (9) (Fig. 1).
TABLE 1.
Primers used for the genome sequencing of HCoV-NL63
| Primer | Sequence (5′–3′) | Target (nucleotide position) or amplification method | Size (bp) |
|---|---|---|---|
| 1F | CCTGGCCTCTTGCTTTTTCACATGT | 20504 | 1,686 |
| 1R | ACTTCGACGGTTGAGAAACAAATAG | 22189 | 1,686 |
| 2F | CGCGTTAAGAGTGGTTCACCAGGTG | 22059 | 1,623 |
| 2R | CAAAGCTGCAAGCCGTCCAGTAATT | 23681 | 1,623 |
| 3F | TTCAATTCAAGCCGATCAACAAGTT | 23624 | 1,600 |
| 3R | GTCATCAATTAATCGAAGGAACATC | 25223 | 1,600 |
| 4F | CGAAGAGCCTGTTGTTGGTATAGTC | 25153 | 1,690 |
| 4R | AACACGCTTCCAACGAGGTTTCTTC | 26849 | 1,690 |
| 5F | CCAGGGCTGATAAGCCTTCTCAGTT | 26800 | 754 |
| 5R | GTGTATCCATATCAAAAACAATATC | 27553 | 754 |
| 6F | TGAGGATGTTTGTGTTTGTTTTGAC | 18864 | 1,710 |
| 6R | GTCAGGAACACCTAATTGTAACATA | 20573 | 1,710 |
| 7F | TGCGTGGTTGGTTGGGTATGGATGT | 17234 | 1,682 |
| 7R | ACGCTCATACGAACCCTGAATACTA | 18915 | 1,682 |
| 8F | ATTCAGCAACTGGTTCCTTAGATGT | 15479 | 1,808 |
| 8R | GTTATCGCCACAAACATGAGCACTT | 17286 | 1,808 |
| 9F | CTCCCTACTATGACACAGCTGAATC | 14005 | 1,596 |
| 9R | AGCCGCAAAGAGTCTAAGTGTATCT | 15600 | 1,596 |
| 10F | GACCGTACAACTATTCAAAGTGTTG | 12398 | 1,659 |
| 10R | GTTCTTTACCACTAATAGCATACTT | 14056 | 1,659 |
| 11F | GGGCTATGGCTAATGGTTATACAAG | 9801 | 1,435 |
| 11R | TTTGCGATATTCATGGCACGCTTCA | 11235 | 1,435 |
| 12F | ACCCTTCAGAGTGTTGCTTCATCAT | 11090 | 1,378 |
| 12R | AGTCGAGCTGCACTAGAACCCCTTG | 12467 | 1,378 |
| 13F | CAACCACTGTAACTAGCTTTCATGG | 7758 | 2,103 |
| 13R | CTGCCAAAATAGAATAGCACTCAAC | 9860 | 2,103 |
| 14F | GTCAAAAGGGTGATGCTGAAGAGGC | 5424 | 2,394 |
| 14R | TCAACTGACCATTCTCAATGTACTT | 7817 | 2,394 |
| 15F | TAGAGATGAATTGGGTGTTCGTGTT | 3424 | 2,043 |
| 15R | GGTCCAACATCACCTGTAACAAATT | 5466 | 2,043 |
| 16F | GCAGATGTTCCAGATGCTTTTCAAT | 1637 | 1,864 |
| 16R | GCAACTGTACAAGTGTGGTACTAAT | 3500 | 1,864 |
| 17F | CAGCAATTATGTTCTTCAGGACTTT | 565 | 1,118 |
| 17R | GTGTAAATGTGCGATAAACTGATTG | 1682 | 1,118 |
| 18F | CTTAAAGAATTTTTCTATCTATAGA | 1 | 1,056 |
| 18R | CATGCACCAACACTCCAACTCTCAG | 1056 | 1,056 |
| GSP 1 | CGAAGAGCCTGTTGTTGGTATAGTC | 3′ RACEa | Unknown |
| AP | GGCCACGCGTCGACTAGTACTTTTTTTTTTTTTTTTT | 3′ RACE | Unknown |
| GSP 2 | CCAGGGCTGATAAGCCTTCTCAGTT | Nested PCR | Unknown |
| AUAP | GGCCACGCGTCGACTAGTAC | Nested PCR | Unknown |
| AAP | GGCCACGCGTCGACTAGTACGGGGGGGGGG | 5′ RACE | Unknown |
| GSP 1 | GTGTAAATGTGCGATAAACTGATTG | 5′ RACE | Unknown |
| AUAP | GGCCACGCGTCGACTAGTAC | Nested PCR | Unknown |
| GSP 2 | CATGCACCAACACTCCAACTCTCAG | Nested PCR | Unknown |
| GSP3 | CCATGGCCAAAAACAACATCAAAGT | Nested PCR | Unknown |
RACE, rapid amplification of cDNA ends.
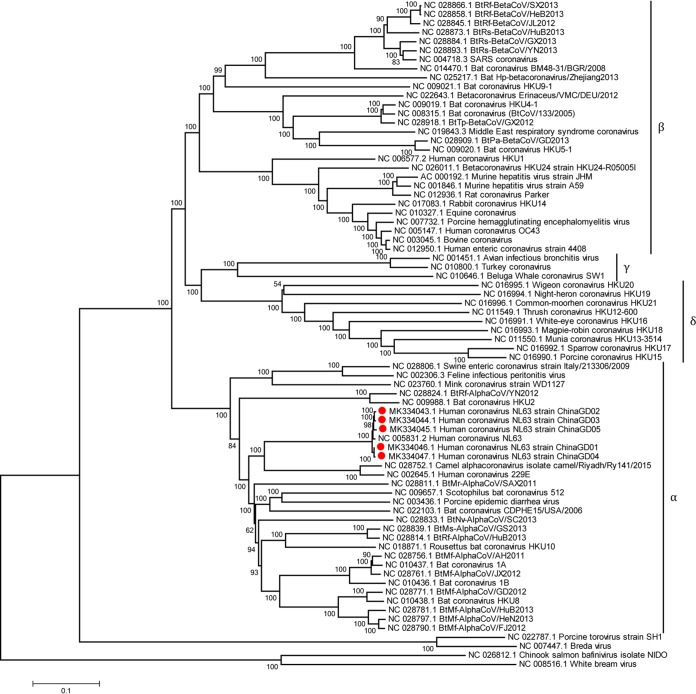
FIG 1.
Phylogenetic analysis of HCoV-NL63 based on complete genomes. The complete genomes of 69 coronavirus references were obtained from the GenBank database, and multiple alignments were performed using MAFFT version 7.158 with default parameters. The phylogenetic tree was constructed using the neighbor-joining method with 1,000 bootstraps in MEGA version 5.10 with default parameters. The numbers at the nodes represent bootstrap support. Bootstrap values greater than 70% were considered statistically significant for grouping. The HCoV-NL63 strains presented in this study are marked with red circles.
Only two complete genome sequences of HCoV-NL63 associated with acute respiratory illness have been obtained and reported in China. The complete genome sequence data from our study will provide insight into the evolution and genetic diversity of HCoV-NL63 in China.
Data availability.
The complete genome sequences of the five newly identified HCoV-NL63 strains have been deposited in GenBank under the accession numbers MK334043, MK334044, MK334045, MK334046, and MK334047. The sequencing reads are available in the SRA database under BioProject accession number PRJNA601331.
ACKNOWLEDGMENTS
This research was supported by grants from the National Key Research and Development Program of China (2018YFC1200100), National Natural Science Foundation of China (NSFC 81702047, 81772191, 91842106, and 8181101118), State Key Laboratory of Respiratory Disease (SKLRD-QN-201715 and SKLRD-QN-201912), National Key Technology R&D Program (2018YFC1311900), Guangdong Science and Technology Foundation (2019B030316028), 2015 Thousand Talents Plan Award of China, China Postdoctoral Science Foundation, and the Ph.D. Start-up Fund of the Natural Science Foundation of Guangdong Province, China.
REFERENCES
- 1.van der Hoek L, Pyrc K, Jebbink MF, Vermeulen-Oost W, Berkhout RJ, Wolthers KC, Wertheim-van Dillen PM, Kaandorp J, Spaargaren J, Berkhout B. 2004. Identification of a new human coronavirus. Nat Med 10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Hoek L, Pyrc K, Berkhout B. 2006. Human coronavirus NL63, a new respiratory virus. FEMS Microbiol Rev 30:760–773. doi: 10.1111/j.1574-6976.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaiser L, Regamey N, Roiha H, Deffernez C, Frey U. 2005. Human coronavirus NL63 associated with lower respiratory tract symptoms in early life. Pediatr Infect Dis J 24:1015–1017. doi: 10.1097/01.inf.0000183773.80217.12. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann H, Pyrc K, van der Hoek L, Geier M, Berkhout B, Pöhlmann S. 2005. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci U S A 102:7988–7993. doi: 10.1073/pnas.0409465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. 2005. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reyes GR, Kim JP. 1991. Sequence-independent, single-primer amplification (SISPA) of complex DNA populations. Mol Cell Probes 5:473–481. doi: 10.1016/s0890-8508(05)80020-9. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Zhu N, Li Y, Lu R, Wang H, Liu G, Zou X, Xie Z, Tan W. 2016. Metagenomic analysis of viral genetic diversity in respiratory samples from children with severe acute respiratory infection in China. Clin Microbiol Infect 22:458.e451–458.e459. doi: 10.1016/j.cmi.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The complete genome sequences of the five newly identified HCoV-NL63 strains have been deposited in GenBank under the accession numbers MK334043, MK334044, MK334045, MK334046, and MK334047. The sequencing reads are available in the SRA database under BioProject accession number PRJNA601331.