Abstract
Background
The aim of this study was to clarify the objective therapeutic effects of an acellular technique by ultrapurified alginate (UPAL) gel implantation in canine osteochondral defect models.
Methods
Two osteochondral defects (diameters: 3.0 and 5.0 mm) were created on each patellar groove in both knees of 10 dogs. Defects were divided into four groups (n = 10 each): Group 1, untreated 3.0-mm defect; Group 2, 3.0-mm defect with UPAL gel; Group 3, untreated 5.0-mm defect; and Group 4, 5.0-mm defect with UPAL gel. All surgical procedures were performed by individuals unfamiliar with the technique at an independent institution. Articular surfaces were evaluated grossly and histologically at 27 weeks after operation.
Results
UPAL gel-treated osteochondral defects showed significantly improved gross appearance in Group 4 and histological appearance in Groups 2 and 4. Reparative tissues in the 3.0-mm defect with UPAL gel were replaced by hyaline-like cartilage tissue. The 5.0-mm defects with UPAL gel were mostly covered with fibrocartilaginous tissue, whereas UPAL gel-untreated defects mostly remained uncovered by any tissue.
Conclusions
Although an acellular technique using UPAL gel implantation significantly enhanced osteochondral repair in canines, reparative tissues of the large defect with alginate gel comprised of fibrocartilaginous tissue. This surgical technique is effective, especially for small cartilage injuries. Further improvements are required before clinical application in cases of severe osteochondral defects in humans.
Keywords: Cartilage, Alginate, Acellular technique, Animal model
1. Introduction
Osteochondral and chondral injuries cause pain and the potential for subsequent osteoarthritis (OA). Focal chondral injuries are often identified in arthroscopic procedures in humans [[1], [2], [3]].Although various kinds of surgical procedures have been developed, such as bone marrow stimulation techniques (BMSTs), osteochondral transplantation, and autologous chondrocyte implantation (ACI) to promote cartilage repair, the clinical efficacy remains limited [4,5], suggesting that the improvement of cartilage repair procedures remains an unmet clinical requirement.
The development of an injectable ultrapurified alginate (UPAL) gel as a scaffold material for cartilage tissue engineering has been reported [6]. Although alginates are known to provide favorable biological effects on chondrogenesis for bone marrow stromal cells (BMSCs) [[7], [8], [9]], mitogenic impurities inducing foreign body reactions remain problematic in living bodies [10]. UPAL gel shows greatly reduced levels of impurities. UPAL gel can be used as the carrier for matrix-assisted cell transplantation or acellular osteochondral scaffold for the treatment of osteochondral defects [[11], [12], [13]]. In a canine model, it has been revealed that UPAL gel with autologous BMSCs enhanced osteochondral repair [12,14]. Although UPAL gel without cell transplantation histologically enhanced osteochondral repair compare with osteochondral defect without augmentation, macroscopic and mechanical evaluation showed no significance between the two groups. Size limits for adaptive responses to osteochondral defects also remain unclear. Moreover, ease of applicability by all surgeons has recently gained importance as a step toward clinical application in humans. Hence, animal experiments designed to minimize potential selection bias are required before human clinical trials.
Therefore, we hypothesized that UPAL gel implantation is effective for small or moderate osteochondral defects whereas it has limited indications for large defects. The purpose of this study is to clarify the therapeutic effect of UPAL gel implantation and estimate the adaptive limit of adaptation of this surgical procedure by using large and small osteochondral defect models in canines.
2. Methods
2.1. Materials
We used ultrapurified low-endotoxin (UPLE) alginates (Mochida Pharmaceutical Co.) sterilized by freeze-drying and packaged in sterilized vials. The material based on UPAL gel that was previously reported was used, and its molecular weight was 1700 kDa (Sea Matrix®; Mochida Pharmaceutical Co. Ltd., Tokyo, Japan) [14]. This material was filtered through a 0.22-mm pore size filter and packaged in sterilized vials. Then, 2% w/v alginate solution was prepared by adding normal saline. To prevent the xenobiotic reaction, the endotoxin level of UPAL gel was adjusted to a low enough level (5.76 EU/g).
2.2. Experimental canine osteochondral defect model
All surgical procedures were performed at an independent institution (Hamri Co.) using beagle dogs weighing about 15 kg. The age of all dogs is 12–13 months, which is considered as skeletal mature. All animals were anesthetized with intramuscular injection of a mixture of ketamine hydrochloride (Ketalar; Daiichi Sankyo Propharma Co., Tokyo, Japan) and xylazine (Selactal; Bayer Medical Co., Wuppertal, Germany). Anesthesia was maintained with isoflurane (Escain; Mylan Seiyaku, Tokyo, Japan) using an anesthesia inhalation apparatus. Both hind legs were shaved and draped in a sterile fashion. Using standard aseptic techniques, surgery was performed under monitoring of vital signs of respiration and body temperature. After making a 3-cm longitudinal paralateral incision in the lateral aspect of the knee, the patella was everted through a lateral parapatellar approach. Two osteochondral defects (diameters: 3.0 mm and 5.0 mm; depths: 5 mm) in the patellar groove of each knee joint were created in 10 adult beagle dogs (four defects in each dog). Defects were divided into four groups (n = 10 each): Group 1, an untreated 3.0-mm defect; Group 2, a 3.0-mm defect with UPAL gel; Group 3, an untreated 5.0-mm defect; and Group 4, a 5.0-mm defect with UPAL gel. Each knee joint was irrigated with sterile normal saline. Four defects were made for each dog and 3 mm defects were created in the anterior and 5 mm defects were created in the posterior. Four patterns (with or without alginate and two defect sizes) were performed in order so that the total number was same. The measures for animal welfare and pains were taken in accordance with “the guidance for management and use of experimental animals in Tsukuba Research Center, Hamri Co., Ltd. This work was approved by the Animal Experiments Committee of our institutions (#13-H255 and #13-H256).
2.3. Macroscopic, histological, and immunohistochemical evaluations
At sacrifice, all operated knees (10 osteochondral defects in each group) were assessed for gross morphological changes in the cartilage. Distal femora were excised with a power saw and photographed with a digital camera for macroscopic evaluation. For histological evaluation, specimens were fixed with paraformaldehyde, decalcified, and embedded in paraffin. A 5-μm section (1 section from each defect) was obtained from the center portion of each defect. Sections were stained with safranin-O for histomorphometric evaluation. All samples were also immunostained using anti-type II collagen antibody (Kyowa Pharma Chemical, Toyama, Japan) for immunomicroscopic examination. Macroscopic and histological findings were scored using the 8- and 28-point grading scales designed by Niederauer et al. [15] as modifications of the scoring system reported by O'Driscoll [16,17]. Macroscopic scores contain four items and histological scores contain eleven items (Table 1, Table 2). Scores were determined by two independent observers blinded to group allocations. All sample randomization for histological scoring was performed by an independent institution (Hamri Co.). All data are presented as means ± standard error.
Table 1.
Macroscopic evaluation at 27 weeks.
| Findings Group | Group 1. Control φ3 mm × 5 mm | Group 2. UPAL gel φ3 mm × 5 mm | Group 3. Control φ5 mm × 5 mm | Group 4. UPAL gel φ5 mm × 5 mm | |
|---|---|---|---|---|---|
| Number of samples | 10 | 10 | 10 | 10 | |
| Macroscopical findings | |||||
| Edge integration | 0.90 ± 0.18 | 1.20 ± 0.13 | 0.10 ± 0.10 | 0.80 ± 0.13 | ## |
| Smoothness of the cartilage surface | 1.20 ± 0.25 | 1.20 ± 0.13 | 0.20 ± 0.13 | 0.60 ± 0.16 | |
| Cartilage surface, degree of filling | 1.10 ± 0.23 | 1.20 ± 0.20 | 0.2 ± 0.13 | 0.80 ± 0.13 | ## |
| Color of cartilage | 1.20 ± 0.25 | 1.00 ± 0.15 | 0.20 ± 0.13 | 0.70 ± 0.15 | # |
| Total macroscopic score | 4.40 ± 0.85 | 4.60 ± 0.50 | 0.70 ± 0.33 | 2.90 ± 0.48 | ## |
Mean ± Standard error.
* p < 0.05, **: p < 0.01 vs. the Group 1.
# p < 0.05, ##: p < 0.01 vs. the Group 3.
Table 2.
Histological and total evaluations at 27 weeks.
| Findings Group | Group 1. Control φ3 mm × 5 mm | Group 2. UPAL gel φ3 mm × 5 mm | Group 3. Controlφ5 mm × 5 mm | Group 4. UPAL gel φ5 mm × 5 mm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of samples | 10 | 10 | 10 | 10 | ||||||||||
| Histological findings | ||||||||||||||
| Nature of Predominant Tissue | 1.10 ± 0.23 | 2.20 ± 0.20 | ** | 0.50 ± 0.17 | 1.50 ± 0.17 | ## | ||||||||
| Surface regularity | 2.00 ± 0.39 | 2.80 ± 0.13 | 0.60 ± 0.22 | 2.00 ± 0.21 | ## | |||||||||
| Structural integrity, homogeneity | 1.20 ± 0.25 | 1.70 ± 0.15 | 0.30 ± 0.15 | 0.90 ± 0.23 | # | |||||||||
| Thickness | 0.50 ± 0.17 | 1.20 ± 0.20 | * | 0.00 ± 0.00 | 0.50 ± 0.17 | # | ||||||||
| Bonding to adjacent cartilage | 1.40 ± 0.22 | 1.30 ± 0.15 | 0.90 ± 0.23 | 1.30 ± 0.15 | ||||||||||
| Hypocellularity | 0.40 ± 0.16 | 1.20 ± 0.20 | ** | 0.00 ± 0.00 | 0.60 ± 0.16 | ## | ||||||||
| Chondrocyte clustering | 0.50 ± 0.17 | 1.40 ± 0.22 | ** | 0.00 ± 0.00 | 0.60 ± 0.16 | ## | ||||||||
| Adjacent cartilage degeneration | 1.40 ± 0.16 | 1.60 ± 0.22 | 1.30 ± 0.21 | 1.70 ± 0.15 | ||||||||||
| Reconstruction of subchondral bone | 1.10 ± 0.28 | 2.20 ± 0.20 | ** | 0.20 ± 0.13 | 0.90 ± 0.31 | |||||||||
| Inflammatory response in subchondral bone region | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | ||||||||||
| Safranin-O staining | 0.90 ± 0.28 | 2.00 ± 0.21 | ** | 0.20 ± 0.13 | 1.00 ± 0.21 | ## | ||||||||
| Total histological score | 12.50 ± 1.77 | 19.60 ± 1.33 | ** | 6.00 ± 0.76 | 13.00 ± 1.37 | ## | ||||||||
| Overall score | 16.90 ± 2.42 | 24.20 ± 1.49 | * | 6.70 ± 0.79 | 15.90 ± 1.57 | ## | ||||||||
Mean ± Standard error.
* p < 0.05, **: p < 0.01 vs. the Group 1.
# p < 0.05, ##: p < 0.01 vs. the Group 3.
The stored histological images were analyzed by ImageJ of the National Institute of Health (http://imagej.nih.gov/ij/). The hyaline-like repaired area, which had been stained brown by anti-type II collagen antibody immunostaining, was then highlighted for measurement. Col-2 positive ratio was determined as the ratio of collagen type 2 staining area in healing area to the total healing area of cartilage defects. All analyses were performed in a blinded fashion by one investigator (R.B.).
2.4. Statistical analysis
Statistical comparisons were performed between Groups I and II and between Groups III and IV. Equality of variance was tested using an F-test. When a set of variances was homogeneous, the parameters were analyzed using Student's t-test. When a set of variances was inhomogeneous, the parameters were analyzed using Welch's t-test. Differences were considered significant for values of P < 0.05.
3. Results
All surgeries were successfully performed by an independent institution without obvious perioperative complications. At 27 weeks postoperatively, no obvious complications such as infection, excessive joint fluid or joint contracture were observed.
Total scores including macroscopic and histological findings showed that reparative tissues of the 3.0- and 5.0-mm defects in UPAL-treated Groups 2 and 4 were significantly enhanced compared with control groups. Although no significant difference in total macroscopic score was seen between Group 1 (4.40 ± 0.85) and Group 2 (4.60 ± 0.50, P > 0.05; Table 1), total histological score was significantly higher in Group 2 (19.60 ± 1.33) than in Group 1 (12.50 ± 1.77, P < 0.01; Table 2).
For the 5.0-mm defect groups, total macroscopic and histological scores were significantly higher in Group 4 (total macroscopic score, 2.90 ± 0.48; total histological score, 13.00 ± 1.37) than in Group 3 (total macroscopic score, 0.70 ± 0.33; total histological score, 6.00 ± 0.76; P < 0.01 each).
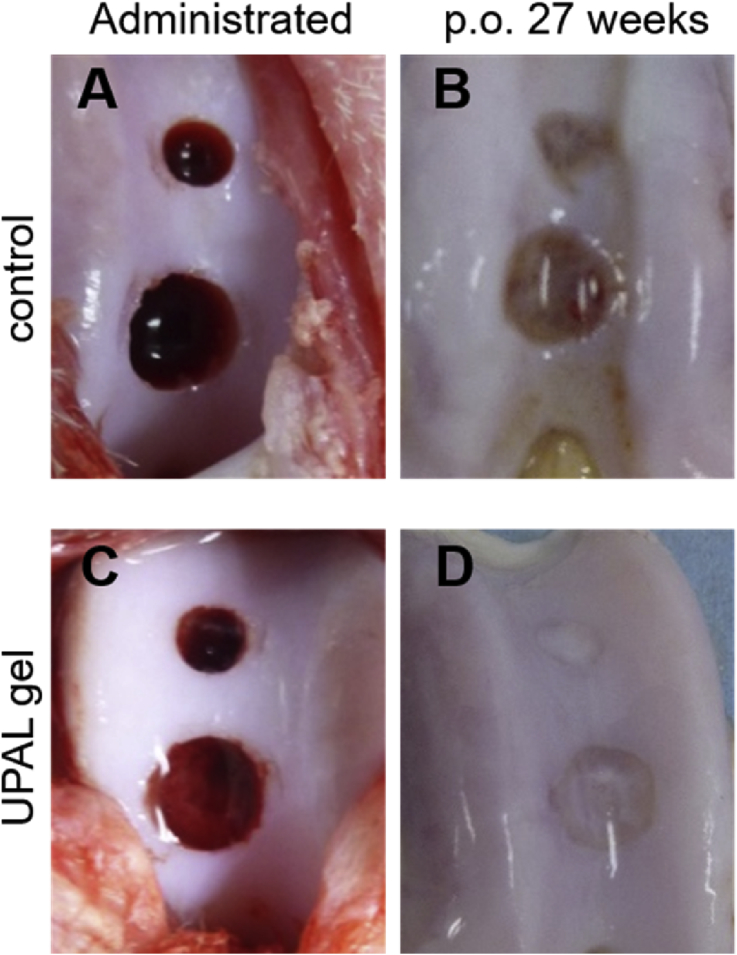
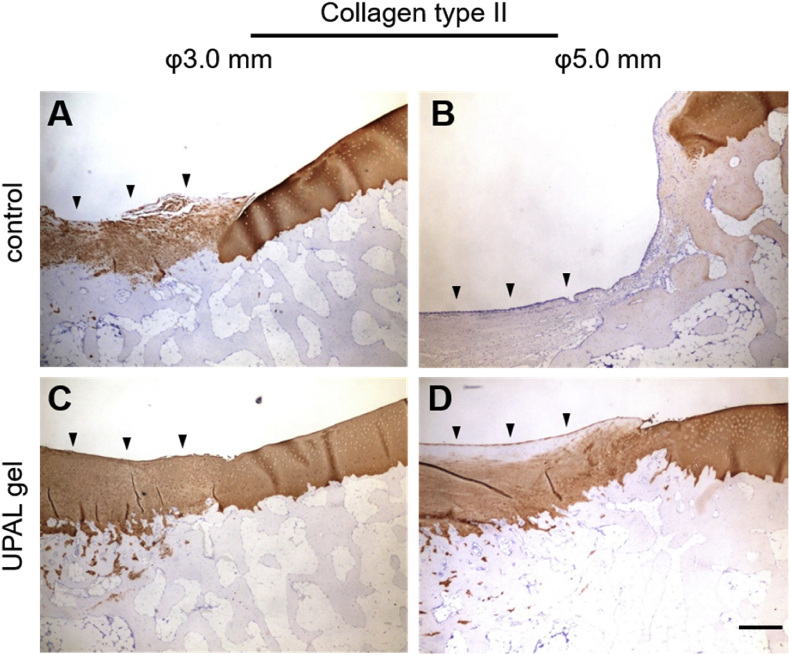
Reparative tissues with the 3.0-mm diameter defects treated with UPAL gel in Group 2 showed substitution of firm, smooth hyaline-like cartilage tissue that had integrated into host tissues (Fig. 1). Although the 5.0-mm defects treated with UPAL gel in Group 4 showed successful coverage by reparative tissue, whereas untreated defects in Group 3 mostly remained uncovered by any tissue at all, histological examination of the 5.0-mm defects in Group 4 mostly showed fibrocartilaginous repair (Fig. 2). The immunohistochemistry also revealed that the 3.0-mm defects treated with UPAL gel in Group 2 showed the deeply-stained reparative tissue by type-2 collagen immunostaining, whereas the 5.0-mm defects treated with UPAL gel in Group 4 showed partially-stained tissue in the defects (Fig. 3).
Fig. 1.
Macroscopic appearance. Macroscopic appearance at 0 weeks (A, C) and 27 weeks (B, D).
Fig. 2.
Histological examinations at 27 weeks. (A-D) Control group and (E-H) UPAL gel group. Low magnification of 3-mm diameter defects (A, E) and 5-mm diameter defects (C, G). Scale bar, 1 mm. High magnification of 3-mm diameter defects (B, F) and 5-mm diameter defects (D, H). Scale bar, 200 μm.
Fig. 3.
Immunostaining analysis. Immunostaining of type II collagen in 3-mm diameter defects (A, C) and 5-mm diameter defects (B, D) at 27 weeks after surgery. Scale bar, 100 μm.
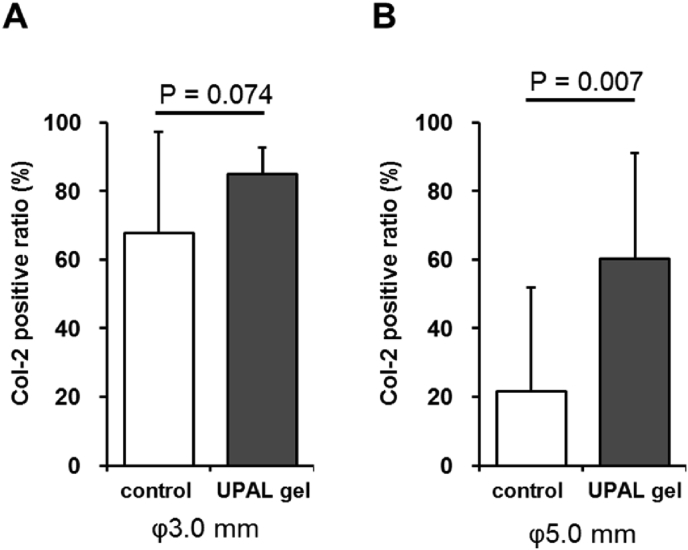
The quantitative evaluation of hyaline-like cartilage healing, which was surmised by the anti-type 2 collagen immunostaining, revealed that the area of hyaline-like cartilage healing of the 5.0-mm defects in UPAL-treated Groups 4 were significantly wide compared with control groups (Fig. 4).
Fig. 4.
Quantitative evaluation of hyaline-like cartilage healing. The ratio of collagen type 2 staining area in healing area to the total healing area of osteochondral defects in 3-mm diameter defects (A) and 5-mm diameter defects (B).
4. Discussion
Our resent study revealed that implantation of UPAL gel without cell transplantation performed by individuals unfamiliar with the required surgical technique macroscopically and histologically enhanced cartilage repair of osteochondral defects in this canine model. For small defects, cartilage healing with UPAL in Group 2 was significantly superior compared with the untreated Group 1 in terms of histological appearance, but no significant differences were apparent macroscopically. When comparing the histological evaluation of both groups finely, it can be seen that osteochondral defects are buried in some regenerative tissues in both Group 1 and Group 2. However, regarding the quality of the regenerated tissue, a hyaline-like cartilage tissue that is deeply stained by Safranin O was obtained by filling the osteochondral defect in the UPAL gel, whereas regenerative tissue in group 1 was mainly composed of fibrous tissue. Thus, we speculated that the macroscopic scoring system could not distinguish between groups because reparative fibrous tissue grew over the small defect in the untreated group.
On the other hand, use of UPAL gel led to macroscopically and histologically enhanced osteochondral repair in large defects, possibly because large defects in the untreated Group 3 showed poor cartilage repair at the macroscopic level, whereas most large defects in the UPAL-treated Group 4 were filled with reparative tissue. In addition, the area of hyaline-like cartilage healing of the 5.0-mm defects in UPAL-treated group were significantly wider than that in control groups, suggesting that UPAL gel implantation could enhance both quantitative and qualitative healing of an articular cartilage defects. We previously reported that UPAL gel enhances cell proliferation and chondrogenic differentiation of BM-MSCs [14]. Moreover, we have already demonstrated the BM-MSCs recruited from bone marrow could penetrate into the defect covered with UPAL [13]. From these previous findings, we speculate that the UPAL gel implantation sustained migrated cells, possibly including BM-MSCs, to enhance cartilage regeneration. UPAL gel also makes migrated cells differentiate into chondrocytes, resulting in the enhancement of cartilage repair.
Although UPAL gel significantly enhanced osteochondral repair in large defects, reparative tissues of the large defects with UPAL gel showed fibrocartilaginous tissue and were sometimes insufficient. Recently, acellular chondrogenesis with a hydrogel is one of the promising techniques for the treatment of chondral defects in the joints [[18], [19], [20]]. This option enhances the regenerative power by the capacity of biologic scaffolds to fill the defect. A recent systematic review and meta-analysis demonstrated promising data in animal studies: they found that the implantation of acellular biomaterials significantly improved cartilage regeneration by 15.6% compared to untreated empty defect controls. Furthermore, the addition of biologics to biomaterials significantly improved cartilage regeneration compared to control biomaterials [21]. However, those authors also revealed that implantation of cellular biomaterials significantly improved cartilage regeneration by 18.6% compared to acellular biomaterials in their systematic review [22]. Based on our current results, there is a limit to cartilage regeneration without cell transplantation, and some additional treatment will be required for large defects. Indications for the surgical use of UPAL gel in humans would require establishment of the severity of osteochondral defects most appropriate for optimal use in clinical applications.
This study was regarded as a pilot study ahead of potential clinical application. Hence, this large-animal study was designed to minimize bias. In translational studies using animals, performance bias should be considered when developers or their immediate collaborators act as operators [23]. To minimize such sources of bias, all surgical procedures and sample randomization for histological scoring were performed by an independent institution in this study. UPAL gels presented high cartilage regeneration ability in this animal study conducted by these rigorous study designs. These results were possibly due to high structural compatibility of alginate, ease of handling, and simplicity of the surgical procedure.
Some limitations to this study need to be considered. First, we created two osteochondral defects in each knee. Anatomical features, particularly healing potential, may have differed between defects. As we completely randomized the positioning of osteochondral defects and grouping, bias resulting from differences in healing potential between defect positions should have been minimal. Second, although our previous and current studies showed no obvious adverse effects from the use of UPAL gel, elucidation of the metabolic pathway underlying the activity of UPAL gel is essential for future clinical trials in human. Finally, assessments from this current study are premised on the assessment of canine models. Load bearing in canines may differ markedly from that in humans because of the relatively lower weight or quadrupedalism. Bipedal animals such as primate models may be better suited to testing the clinical applicability of UPAL gel.
In conclusion, we clarified that an acellular technique using UPAL gel implantation significantly enhanced small and large osteochondral repair in canines. Although alginate gel histologically enhanced osteochondral repair in large defects, reparative tissues in large defects with alginate gel were fibrocartilaginous. Further technical improvements or material modifications may be required before clinical application can be countenanced for cases of severe osteochondral defects in humans.
Funding
This work was funded by Mochida Pharmaceutical Co., Ltd., and was supported in part by the Newly Extended Technology Transfer Program of the Japan Science and Technology Agency.
Conflict of interest
This work was supported by Mochida Pharmaceutical Co., Ltd.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Curl W.W., Krome J., Gordon E.S., Rushing J., Smith B.P., Poehling G.G. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997 Aug;13(4):456–460. doi: 10.1016/s0749-8063(97)90124-9. 1997. [DOI] [PubMed] [Google Scholar]
- 2.Widuchowski W., Widuchowski J., Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007 Jun;14(3):177–182. doi: 10.1016/j.knee.2007.02.001. Epub 2007 Apr 10 2007. [DOI] [PubMed] [Google Scholar]
- 3.Hjelle K., Solheim E., Strand T., Muri R., Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002 Sep;18(7):730–734. doi: 10.1053/jars.2002.32839. 2002. [DOI] [PubMed] [Google Scholar]
- 4.Lim H.C., Bae J.H., Song S.H., Park Y.E., Kim S.J. Current treatments of isolated articular cartilage lesions of the knee achieve similar outcomes. Clin Orthop Relat Res. 2012 Aug;470(8):2261–2267. doi: 10.1007/s11999-012-2304-9. Epub 2012 Mar 16 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magnussen R.A., Dunn W.R., Carey J.L., Spindler K.P. Treatment of focal articular cartilage defects in the knee: a systematic review. Clin Orthop Relat Res. 2008 Apr;466(4):952–962. doi: 10.1007/s11999-007-0097-z. Epub 2008 Jan 12 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Igarashi T., Iwasaki N., Kawamura D., Kasahara Y., Tsukuda Y., Ohzawa N. Repair of articular cartilage defects with a novel injectable in situ forming material in a canine model. J Biomed Mater Res. 2012 Jan;100(1):180–187. doi: 10.1002/jbm.a.33248. Epub 2011 Oct 23 2012. [DOI] [PubMed] [Google Scholar]
- 7.Chang S.C., Rowley J.A., Tobias G., Genes N.G., Roy A.K., Mooney D.J. Injection molding of chondrocyte/alginate constructs in the shape of facial implants. J Biomed Mater Res. 2001 Jun 15;55(4):503–511. doi: 10.1002/1097-4636(20010615)55:4<503::aid-jbm1043>3.0.co;2-s. 2001. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S.B., Meirisch C.M., Wilson H.A., Diduch D.R. The use of absorbable co-polymer pads with alginate and cells for articular cartilage repair in rabbits. Biomater. 2003 Jul;24(15):2653–2660. doi: 10.1016/s0142-9612(03)00058-9. 2003. [DOI] [PubMed] [Google Scholar]
- 9.Paige K.T., Cima L.G., Yaremchuk M.J., Schloo B.L., Vacanti J.P., Vacanti C.A. De novo cartilage generation using calcium alginate-chondrocyte constructs. Plast Reconstr Surg. 1996 Jan;97(1):168–178. doi: 10.1097/00006534-199601000-00027. Discuss 179-80 1996. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann U., Thurmer F., Jork A., Weber M., Mimietz S., Hillgartner M. A novel class of amitogenic alginate microcapsules for long-term immunoisolated transplantation. Ann N Y Acad Sci. 2001 Nov:944199–944215. doi: 10.1111/j.1749-6632.2001.tb03833.x. 2001. [DOI] [PubMed] [Google Scholar]
- 11.Baba R., Onodera T., Momma D., Matsuoka M., Hontani K., Elmorsy S. A novel bone marrow stimulation technique augmented by administration of ultrapurified alginate gel enhances osteochondral repair in a rabbit model. Tissue Eng C Methods. 2015;21 doi: 10.1089/ten.tec.2015.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baba R., Onodera T., Matsuoka M., Hontani K., Joutoku Z., Matsubara S. Bone marrow stimulation technique augmented by an ultrapurified alginate gel enhances cartilage repair in a canine model. Am J Sports Med. 2018;46 doi: 10.1177/0363546518770436. [DOI] [PubMed] [Google Scholar]
- 13.Sukegawa A., Iwasaki N., Kasahara Y., Onodera T., Igarashi T., Minami A. Repair of rabbit osteochondral defects by an acellular technique with an ultrapurified alginate gel containing stromal cell-derived factor-1. Tissue Eng. 2012;18 doi: 10.1089/ten.TEA.2011.0380. [DOI] [PubMed] [Google Scholar]
- 14.Igarashi T., Iwasaki N., Kasahara Y., Minami A. A cellular implantation system using an injectable ultra-purified alginate gel for repair of osteochondral defects in a rabbit model. J Biomed Mater Res. 2010;94:844–855. doi: 10.1002/jbm.a.32762. [doi] [DOI] [PubMed] [Google Scholar]
- 15.Niederauer G.G., Slivka M.A., Leatherbury N.C., Korvick D.L., Harroff H.H., Ehler W.C. Evaluation of multiphase implants for repair of focal osteochondral defects in goats. Biomater. 2000 Dec;21(24):2561–2574. doi: 10.1016/s0142-9612(00)00124-1. 2000. [DOI] [PubMed] [Google Scholar]
- 16.Hoemann C., Kandel R., Roberts S., Saris D.B., Creemers L., Mainil-Varlet P. International cartilage repair society (ICRS) recommended guidelines for histological endpoints for cartilage repair studies in animal models and clinical trials. Cartilage. 2011;2:153–172. doi: 10.1177/1947603510397535. [doi]10.1177_1947603510397535 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Driscoll S.W., Keeley F.W., Salter R.B. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Jt Surg Am. 1986 Sep;68(7):1017–1035. 1986. [PubMed] [Google Scholar]
- 18.de Queiroz A.A.B., Debieux P., Amaro J., Ferretti M., Cohen M. Hydrogel implant is as effective as osteochondral autologous transplantation for treating focal cartilage knee injury in 24 months. Knee Surg Sports Traumatol Arthrosc. 2018;26:2934–2941. doi: 10.1007/s00167-018-4834-5. [DOI] [PubMed] [Google Scholar]
- 19.Pipino G., Risitano S., Alviano F., Wu E.J., Bonsi L., Vaccarisi D.C. Microfractures and hydrogel scaffolds in the treatment of osteochondral knee defects: a clinical and histological evaluation. J Clin Orthop Trauma. 2019;10:67–75. doi: 10.1016/j.jcot.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shive M.S., Stanish W.D., McCormack R., Forriol F., Mohtadi N., Pelet S. BST-CarGel(R) treatment maintains cartilage repair superiority over microfracture at 5 Years in a multicenter randomized controlled trial. Cartilage. 2015;6:62–72. doi: 10.1177/1947603514562064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pot M.W., Gonzales V.K., Buma P., IntHout J., van Kuppevelt T.H., de Vries R.B.M. Improved cartilage regeneration by implantation of acellular biomaterials after bone marrow stimulation: a systematic review and meta-analysis of animal studies. PeerJ. 2016;4 doi: 10.7717/peerj.2243. e2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pot M.W., van Kuppevelt T.H., Gonzales V.K., Buma P., IntHout J., de Vries R.B.M. Augmented cartilage regeneration by implantation of cellular versus acellular implants after bone marrow stimulation: a systematic review and meta-analysis of animal studies. PeerJ. 2017;5 doi: 10.7717/peerj.3927. e3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paradis C. Bias in surgical research. Ann Surg. 2008;248:180–188. doi: 10.1097/SLA.0b013e318176bf4b. [pii] [DOI] [PubMed] [Google Scholar]