Introduction
Cemiplimab (Libtayo, Regeneron Pharmaceuticals, Tarrytown, NY) is a monoclonal antibody that targets the programmed cell death receptor 1. In 2018, the Food and Drug Administration approved cemiplimab for the treatment of patients with metastatic cutaneous squamous cell carcinoma (SCC) or locally advanced cutaneous SCC that is not a candidate for surgery or radiation.1 Immune checkpoint inhibitors have been implicated in the development of bullous disorders.2,3 We report the first case, to our knowledge, of a patient who developed severe steroid-resistant bullous pemphigoid shortly after initiating therapy with cemiplimab, requiring discontinuation of the programmed cell death receptor 1 inhibitor and treatment with rituximab.
Case report
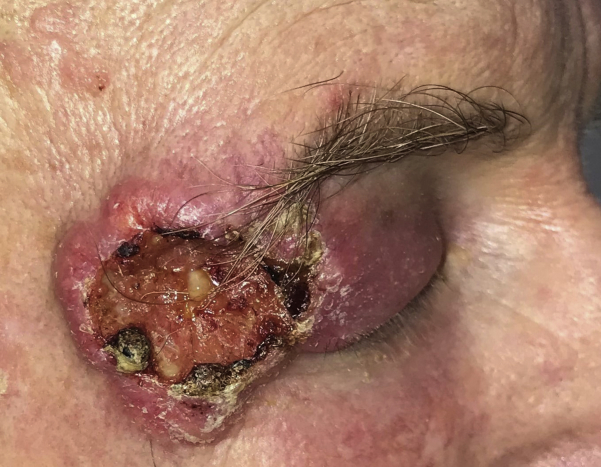
An otherwise healthy 68-year-old man with a history of nonmelanoma skin cancer presented for evaluation of a painless rapidly growing mass on the right temple and lateral periorbital region. Physical examination was notable for an ulcerated skin-colored firm plaque on the right temple, with associated swelling of the right upper eyelid (Fig 1), and biopsy revealed moderately differentiated invasive cutaneous SCC. A computed tomography scan showed a 47 × 15 × 45-mm soft tissue mass invading the extraconal fat superolaterally, with involvement of the lacrimal gland and the eyelid. Positron-emission tomography imaging (with computed tomography) did not show evidence of metastases. The patient received a diagnosis of locally advanced cutaneous SCC. Given the likelihood that surgery would require an orbital exenteration, the patient was offered neoadjuvant therapy with cemiplimab immunotherapy (350-mg intravenous infusion every 3 weeks) before surgery and radiation.
Fig 1.
Cutaneous squamous cell carcinoma. Clinical photograph from the lesion on the right temple and lateral periorbital region.
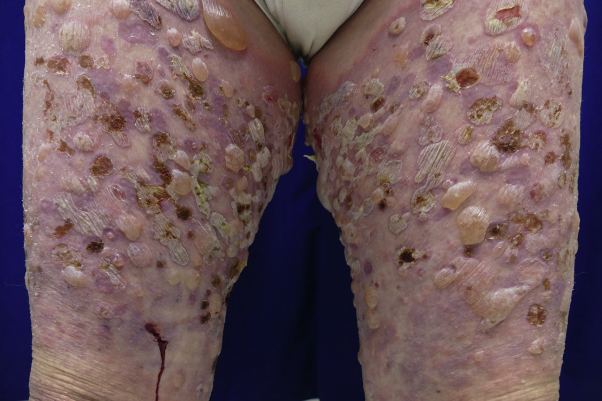
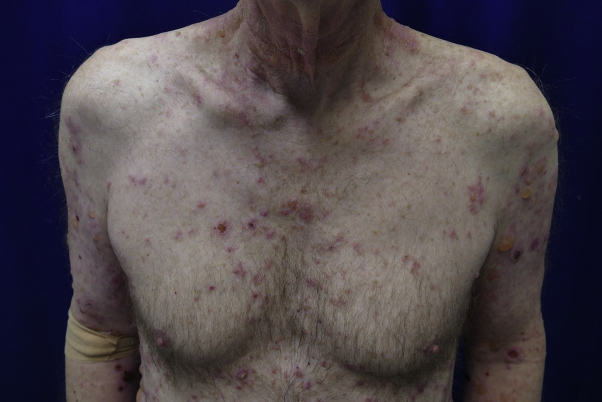
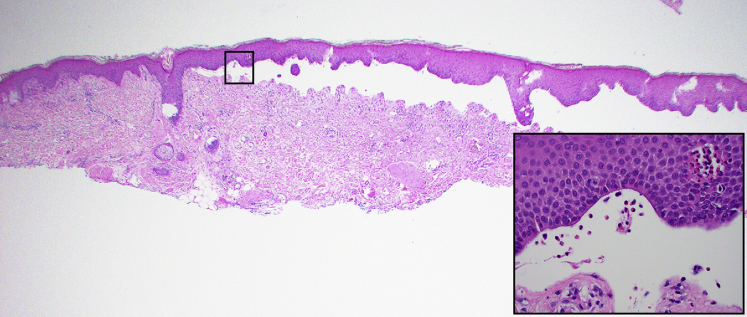
The patient received cemiplimab infusion on days 0, 21, and 42. Before his third infusion on day 42, he developed scattered erythematous macules and edematous papules on the lower extremities and 1 blister on the left ankle, which were treated with topical triamcinolone ointment twice daily. Given the limited body surface area involvement, he continued receiving cemiplimab. However, at approximately day 63 after initiation of cemiplimab, he was noted to have innumerable intact, tense, fluid-filled vesicles; bullae; and erosions involving an estimated 60% of his body surface area (Figs 2 and 3). The conjunctival, oral, genital, and perianal mucosal surfaces were not affected. A skin biopsy from lesional and perilesional skin revealed a subepidermal blister with eosinophilic spongiosis and dermal eosinophilia (Fig 4). Direct immunofluorescence demonstrated linear deposition of IgG and C3 in the basement membrane zone, whereas the IgA result was negative. Salt-split skin analysis revealed staining on the epidermal side of the basement membrane. Serum anti-BP180 IgG antibody levels were elevated, at 169 units/mL. Serum anti-BP230 IgG antibodies were not detected. Peripheral blood absolute eosinophil levels were elevated, at 1600 cells/μL. The patient received a diagnosis of cemiplimab-induced bullous pemphigoid, scored as a grade 3 immune-related adverse event in accordance with the Common Terminology Criteria for Adverse Events version 4.03.
Fig 2.
Bullous pemphigoid. Clinical photograph from the lower extremities.
Fig 3.
Bullous pemphigoid. Clinical photograph from the chest and upper extremities.
Fig 4.
Bullous pemphigoid. Lesional and perilesional skin on the anterior surface of the right thigh. (Hematoxylin-eosin stain; original magnification ×20.) Inset shows the subepidermal blister containing eosinophils and the overlying epidermis with eosinophilic spongiosis. (Hematoxylin-eosin stain; original magnification ×400.)
Cemiplimab infusions were discontinued and the patient began receiving prednisone 60 mg daily for 2 weeks, without resolution of blistering. His prednisone level was then increased to 40 mg twice daily for 2 weeks, with stabilization of his blisters. On tapering his prednisone, the blistering recurred, and therefore a lymphoma-based dosing regimen of rituximab (375 mg/m2 weekly) for 4 weeks was administered.4,5 The onset of new blistering subsided after the rituximab and prednisone dose was successfully tapered during 3 months. At the 6-month follow-up, the patient was free of new blisters, with no need for additional systemic therapy.
Discussion
Immune checkpoint inhibitors include agents that inhibit programmed cell death receptor 1 (nivolumab, pembrolizumab, and cemiplimab), programmed death ligand 1 (atezolizumab, avelumab, and durvalumab), and cytotoxic T-lymphocyte–associated protein 4 (ipilimumab). These anticancer therapies interfere with inhibitory mechanisms that downregulate the host immune response against malignant cells. In 2018, the Food and Drug Administration approved cemiplimab as the first systemic therapy to treat patients with advanced cutaneous SCC, based on a phase 2 study that reported an overall response rate of 47%.1
Patients who receive immune checkpoint inhibitors sometimes experience immune-related adverse events, such as thyroiditis, pneumonitis, hepatitis, colitis, dermatitis, and other organ toxicities.6 Cutaneous immune-related adverse events appear to be a class effect among checkpoint inhibitors and can lead to pruritus, maculopapular rash, pigmentary changes, eczematous eruptions, lichenoid dermatitis, and other inflammatory skin diseases. Autoimmune blistering disorders represent approximately 1% of cutaneous immune-related adverse events3,7,8 In this report, we present the first reported case, to our knowledge, of bullous pemphigoid resulting after cemiplimab treatment. Many questions remain unanswered in regard to why checkpoint blockade triggers autoimmune blistering disease in some patients and not in others, as well as in regard to understanding the differences in timing, severity, and subtypes. Our case report offers further evidence that cutaneous immune-related adverse events are a classwide effect observed among anti–programmed cell death protein 1 therapy. Future studies are needed to better understand the pathogenesis, prognostic factors, and therapeutic algorithms for patients affected by immune checkpoint–related bullous pemphigoid. Identifying predictive factors, such as biomarkers inherent to the tumor or characteristics unique to the patient, may help predict which patients will develop specific toxicities.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Modi is a paid consultant and a member of the speakers bureau for Regeneron and Sanofi Genzyme. Drs Virgen, Nguyen, Di Raimondo, Amini, Margolin, Parekh, and Abdulla have no conflicts of interest to declare.
Presented at the Pacific Dermatologic Association 2019 Annual Meeting, August 17, 2019.
References
- 1.Migden M.R., Rischin D., Schmults C.D. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 2.Naidoo J., Schindler K., Querfeld C. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4:383–389. doi: 10.1158/2326-6066.CIR-15-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel J., Totonchy M., Damsky W. Bullous disorders associated with anti-PD-1 and anti-PD-L1 therapy: a retrospective analysis evaluating the clinical and histopathologic features, frequency, and impact on cancer therapy. J Am Acad Dermatol. 2018;79:1081–1088. doi: 10.1016/j.jaad.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Polansky M., Eisenstadt R., DeGrazia T., Zhao X., Liu Y., Feldman R. Rituximab therapy in patients with bullous pemphigoid: a retrospective study of 20 patients. J Am Acad Dermatol. 2019;81:179–186. doi: 10.1016/j.jaad.2019.03.049. [DOI] [PubMed] [Google Scholar]
- 5.Sowerby L., Dewan A.K., Granter S., Gandhi L., LeBoeuf N.R. Rituximab treatment of nivolumab-induced bullous pemphigoid. JAMA Dermatol. 2017;153:603–605. doi: 10.1001/jamadermatol.2017.0091. [DOI] [PubMed] [Google Scholar]
- 6.Weber J.S., Yang J.C., Atkins M.B., Disis M.L. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33:2092–2099. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacouture M., Sibaud V. Toxic side effects of targeted therapies and immunotherapies affecting the skin, oral mucosa, hair, and nails. Am J Clin Dermatol. 2018;19:31–39. doi: 10.1007/s40257-018-0384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damsky W., Kole L., Tomayko M.M. Development of bullous pemphigoid during nivolumab therapy. JAAD Case Rep. 2016;2:442–444. doi: 10.1016/j.jdcr.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]