Abstract
Background
Helicobacter pylori increases reactive oxygen species (ROS) and induces oxidative DNA damage and apoptosis in gastric epithelial cells. DNA damage activates DNA damage response (DDR) which includes ataxia-telangiectasia-mutated (ATM) activation. ATM increases alternative reading frame (ARF) but decreases mouse double minute 2 (Mdm2). Because p53 interacts with Mdm2, H. pylori–induced loss of Mdm2 stabilizes p53 and induces apoptosis. Previous study showed that Korean Red Ginseng extract (KRG) reduces ROS and prevents cell death in H. pylori–infected gastric epithelial cells.
Methods
We determined whether KRG inhibits apoptosis by suppressing DDRs and apoptotic indices in H. pylori–infected gastric epithelial AGS cells. The infected cells were treated with or without KRG or an ATM kinase inhibitor KU-55933. ROS levels, apoptotic indices (cell death, DNA fragmentation, Bax/Bcl-2 ratio, caspase-3 activity) and DDRs (activation and levels of ATM, checkpoint kinase 2, Mdm2, ARF, and p53) were determined.
Results
H. pylori induced apoptosis by increasing apoptotic indices and ROS levels. H. pylori activated DDRs (increased p-ATM, p-checkpoint kinase 2, ARF, p-p53, and p53, but decreased Mdm2) in gastric epithelial cells. KRG reduced ROS and inhibited increase in apoptotic indices and DDRs in H. pylori–infected gastric epithelial cells. KU-55933 suppressed DDRs and apoptosis in H. pylori–infected gastric epithelial cells, similar to KRG.
Conclusion
KRG suppressed ATM-mediated DDRs and apoptosis by reducing ROS in H. pylori–infected gastric epithelial cells. Supplementation with KRG may prevent the oxidative stress-mediated gastric impairment associated with H. pylori infection.
Keywords: Apoptosis, Gastric epithelial cells, Helicobacter pylori, Panax ginseng
1. Introduction
Infection of Helicobacter pylori, a gram-negative bacterium, is associated with various gastric diseases, including peptic ulcer and gastric cancer [1], [2], [3]. H. pylori infection disorganizes the gastric epithelial architecture by inducing apoptosis and accumulating DNA-damaged cells [4], [5]. Previously, we showed that H. pylori infection alters the gastric oxidative balance by activating nicotinamide adenine dinucleotide phosphate oxidase and producing reactive oxygen species (ROS) [6], which mediate oxidative DNA damage and apoptosis. DNA damage activates DNA damage responses (DDRs) such as ataxia-telangiectasia-mutated (ATM), checkpoint kinase 2 (Chk2), and p53 in gastric epithelial cells [7].
ATM is activated in response to DNA double-strand breaks, and it activates its downstream kinase Chk2, which subsequently phosphorylates p53. Activation of p53 weakens its interaction with mouse double minute 2 (Mdm2), which stabilizes p53 [8], [9], [10]. The proapoptotic and tumor suppressor molecule p53 is activated by exposure to a wide range of cellular insults, from oncogene activation to DNA damage [11], [12]. The activation of p53 causes cell cycle arrest and induces apoptosis, which mediates tumor suppression [13], [14]. The degradation of p53 is largely mediated by Mdm2. Binding of Mdm2 with p53 leads to the degradation of p53 and suppression of the transcriptional activity of its target genes [15], [16]. Therefore, interaction of Mdm2 and p53 induces degradation of p53 and thus, inhibits apoptosis.
The tumor suppressor alternative reading frame (ARF), p14ARF in human and p19ARF in murine systems, stabilizes p53 in response to oncogenic signals and ROS [17]. ARF directly interacts with Mdm2 and increases the p53 transcriptional response [18], [19], [20]. Ectopically expressed ARF activates ATM and ATM-dependent p53 phosphorylation [21]. Therefore, both ARF expression and ATM activation enhance p53 function [22]. Therefore, increased ARF and ATM activation may be associated with p53-dependent apoptosis. Because H. pylori increases ROS levels, H. pylori may induce ARF expression and ATM activation in gastric epithelial cells.
Korean Red Ginseng is the steamed root of 6-year-old Korean ginseng (Panax ginseng Meyer). Korean Red Ginseng has antioxidant, anti-inflammatory, and antitumor activities [23], [24], [25], [26]. The active components of Korean Red Ginseng are ginsenosides, which are triterpene glycosides. Korean Red Ginseng extract (KRG) prevented oxidative damage by inhibiting the activity of 5-lipoxygenase in H. pylori–infected gastric epithelial cells [27]. Moreover, KRG inhibited the expression of inducible nitric oxide synthase by reducing ROS in gastric epithelial cells infected with H. pylori [28]. KRG protected Mongolian gerbils from H. pylori–induced inflammation by inhibiting the oxidative stress–mediated expression of inducible nitric oxide synthase and inflammatory cytokines in gastric mucosal tissues [29]. These studies suggest that KRG may inhibit oxidative DNA damage and apoptosis in H. pylori–infected gastric epithelial cells.
In the present study, we determined whether KRG inhibits apoptosis by reducing ROS and suppressing DDRs and apoptotic indices in H. pylori–infected gastric epithelial cells. ROS levels, apoptotic indices (cell death, DNA fragmentation, Bax/Bcl-2 ratio, and caspase-3 activity), and DDRs (activation and levels of ATM, Chk2, Mdm2, ARF, and p53) were determined. To investigate the role of ATM on apoptosis, the infected cells were treated with or without an ATM kinase selective inhibitor KU-55933 instead of KRG, and DDRs and apoptosis were measured.
2. Materials and methods
2.1. Reagents
A standardized water extract of Korean Red Ginseng (KRG) of P. ginseng Meyer was supplied by Korea Ginseng Corporation (Daejeon, Korea). KRG contains 7% ginsenosides containing the ginsenoside Rb, Rb2, Rc, Rd, Re, Rf, Rg1, (20S)-Rg2, (20S)-Rg3, and Rh1 [28].
2.2. Cell culture and H. pylori stimulation
AGS human gastric epithelial cells were stimulated with H. pylori, strain NCTC 11637 (virulence factor cagA+, vac A+) at bacterium/cell ratio of 300:1 [6], [30]. Cells were treated with KRG (0.01, 0.1, and 1 μg/mL) or KU-55933 (5 and 10 μM) 2 h before H. pylori infection, based on our previous study [28]. Furthermore, the infected cells were cultured for 1 h (for ROS determination), 6 h (for determination of Bax, Bcl-2, caspase-3 activity, levels of DDR proteins, and nuclear p53 level), and 24 h (viable cell numbers and DNA fragmentation).
2.3. Cell viability and ROS levels
Viable cells were counted by trypan blue exclusion test. Levels of ROS were determined using 2′,7′-dichlorodihydrofluorescein (DCF) diacetate (Molecular Probes, Eugene, OR, USA) as described previously [7]. Briefly, the cells were loaded with 10 μM DCF diacetate for 30 min, washed, and scraped off into 1 mL of phosphate-buffered saline (PBS). The fluorescent DCF was measured (excitation at 495 nm and emission at 535 nm) with VICTOR2 multi-label counter (PerkinElmer Life and Analytical Sciences, Boston, MA, USA).
2.4. Caspase-3 activity and DNA fragmentation
Caspase-3 activity was determined in whole-cell extracts using a specific caspase-3 substrate N-acetyl-Asp-Glu-Val-Asp-7-amino-4-trifluoromethylcoumarin based on our previous study [30]. DNA fragmentation was determined by measuring oligonucleosome-bound DNA level in whole-cell extracts as described previously [7].
2.5. Immunofluorescence staining
The cells were fixed with methanol and treated with 0.1% Triton X-100 in PBS for 15 min, followed by blocking in phosphate-buffered saline (PBS) (1% bovine serum albumin). After incubation with a primary antibody p53 (sc-1311, Santa Cruz Biotechnology, Dallas, TX, USA) for 1 h, rhodamine-labeled mouse anti-rabbi IgG antibody (sc-2492, Santa Cruz Biotechnology) was treated for 1 h. The cells were washed and stained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich, St. Louis, MO, USA) for 30 min. Images were obtained using a confocal laser scanning microscope (Zeiss LSM 700; Carl Zeiss Inc., Thornwood, NY, USA). Fluorescence images were expressed as the ratio of the fluorescence densities of p53 and the nuclei stain DAPI using NIH ImageJ 5.0 software (National institutes of Health, Bethesda, MD, USA).
2.6. Western blot analysis
Whole-cell extracts, prepared by the method previously described [31], were separated using 6–14% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) by the method previously described [7]. By electroblotting, the proteins were transferred onto nitrocellulose membranes (Amersham, Inc., Arlington Heights, IL, USA). The membranes were reacted with antibodies for Bax (sc-526, Santa Cruz Biotechnology), Bcl-2 (sc-492, Santa Cruz Biotechnology), p-ATM (#4526, Cell Signaling Technology, Danvers, MA, USA), ATM (07-1286, Millipore, Darmstadt, Germany), p-Chk2 (#2661, Cell Signaling Technology), Chk2 (#2662, Cell Signaling Technology), Mdm2 (sc-965, Santa Cruz Biotechnology), p14ARF (ab470, Abcam, Cambridge, UK), p-p53 (#9284, Cell Signaling Technology), p53 (sc-1311, Santa Cruz Biotechnology), and actin (sc-1615, Santa Cruz Biotechnology). The proteins were visualized using goat anti-mouse secondary antibodies conjugated with horseradish peroxidase. Actin was used as a loading control. For the ratio of Bax/Bcl-2 as a parameter of apoptosis, the protein bands of Bax and Bcl-2 were scanned using a Bio-Rad scanner (GS-700, Bio-Rad Laboratories, Hercules, CA, USA) driven by volume analysis software and quantified with Scion Image Software (National Institutes of Health, Bethesda, MD, USA).
2.7. Statistical analysis
A one-way analysis of variance and Newman-Keuls test were used. All values were expressed as mean ± standard error of the mean of three independent experiments. p < 0.05 was considered significantly different.
3. Results
3.1. KRG decreases ROS levels and inhibits apoptotic indices in H. pylori–infected AGS cells
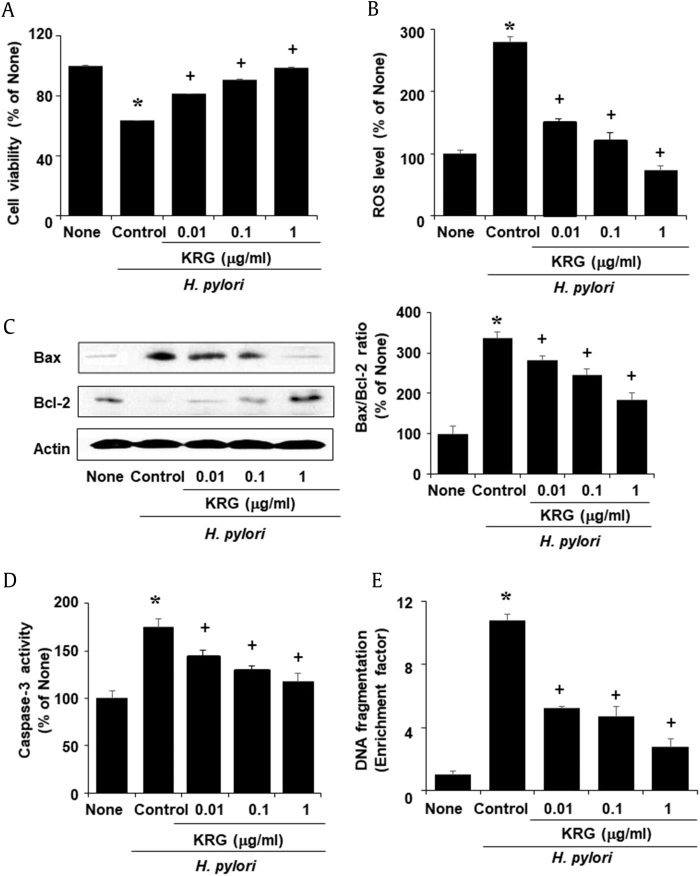
KRG inhibited cell death at 24-h culture and reduced ROS levels at 1-h culture in H. pylori–infected AGS cells (Fig. 1A, 1B). At 24-h culture, H. pylori increased Bax level and caspase-3 activity but decreased Bcl-2 level in the cells. Increased ratio of Bax/Bcl-2 and caspase-3 activation, which were induced by H. pylori infection, were inhibited by KRG treatment (Fig. 1C, 1D). KRG suppressed increase in nucleosome-bound DNA, an indicator of DNA fragmentation, in H. pylori–infected cells dose-dependently (Fig. 1E).
Fig. 1.
Cell viability, ROS levels, Bax/Bcl-2 ratio, caspase-3 activity, and DNA fragmentation in H. pylori–infected cells treated with or without KRG. KRG was treated to the cells 2 h before H. pylori stimulation. 24 h- (A, cell viability), 1 h- (B, ROS levels), and 6 h- (C, protein levels of Bax and Bcl-2; D, caspase-3 activity; E, DNA fragmentation) cultures were used. Protein band intensity of Bax/Bcl-2 in the untreated, uninfected group (none) was set as 100%. Values are mean ± SEM. *p < 0.05 vs. untreated, uninfected group (none) and +p < 0.05 vs. untreated, H. pylori–infected group (control).
KRG, Korean Red Ginseng extract; ROS, reactive oxygen species; SEM, standard error of the mean.
3.2. KRG inhibits H. pylori–induced DDRs in AGS cells
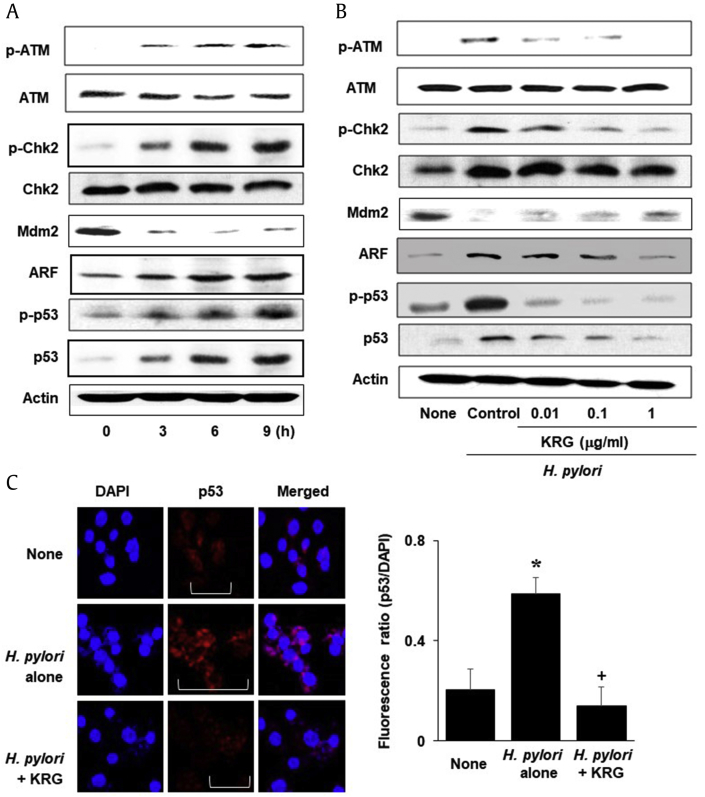
Both ATM and Chk2 were phosphorylated by H. pylori infection. However, total levels of ATM and Chk2 were not changed by H. pylori infection (Fig. 2A). The levels of ARF, p-p53, and p53 increased but Mdm2 level decreased by H. pylori infection in AGS cells. The results suggest that H. pylori–induced apoptosis may be mediated by the activation of ATM, Chk2, and p53 and induction of ARF and p53 with the loss of Mdm2 in AGS cells.
Fig. 2.
DDR protein and nuclear p53 levels in H. pylori–infected cells treated with or without KRG. (A) Various culture periods were used to determine DDR protein levels (p-ATM, ATM, p-Chk2, Chk2, Mdm2, ARF, p-p53, and p53). (B) Indicated concentration of KRG was treated to the cells 2 h before H. pylori stimulation. 6 h-culture was used to determine DDR protein levels. (C) For determination of nuclear p53 level, 1 μg/mL of KRG was treated to the cells 2 h before H. pylori infection and the cells were cultured for 6 h. Images show immunofluorescence staining for p53 (red) and DNA counterstaining with DAPI (blue). Nuclear p53 (shown by brackets) was found in small areas in uninfected (none) group and KRG-treated and H. pylori–infected group (H. pylori + KRG), while nuclear p53 was found in relatively large areas in untreated, H. pylori–infected group (H. pylori alone). (D) Fluorescence images expressed the ratio of the fluorescence densities of p53 and the nuclei stain DAPI. Values are mean ± SEM. *p < 0.05 vs. untreated, uninfected group (none) and +p < 0.05 vs. untreated, H. pylori–infected group (H. pylori alone).
ARF, alternative reading frame; ATM, ataxia-telangiectasia-mutated; Chk2, checkpoint kinase 2; DAPI, 4ʹ,6-diamidino-2-phenylindole; DDR, DNA damage response; KRG, Korean Red Ginseng extract; Mdm2, mouse double minute 2; SEM, standard error of the mean.
As shown in Fig. 2B, KRG suppressed DDRs (phosphorylation of ATM and Chk2; increase in ARF, p-p53, and p53; and a decrease in Mdm2) induced by H. pylori infection. To assess the effect of KRG on p53 activation in H. pylori–infected cells, the nuclear level of p53 was analyzed using immunofluorescence staining (Fig. 2C). Nuclear p53 (shown by brackets) was found in small areas in uninfected (none) group and KRG-treated and H. pylori–infected group (H. pylori + KRG), while nuclear p53 was found in relatively large areas in untreated, H. pylori–infected group (H. pylori alone). DNA counterstaining with DAPI was not affected by any treatment. Fig. 2D shows ratio of the fluorescence densities of p53 and the nuclei stain DAPI. The result demonstrates that KRG suppressed H. pylori–induced nuclear localization of p53 in the cells.
3.3. KU-55933 inhibits DDRs and apoptosis in H. pylori–infected AGS cells
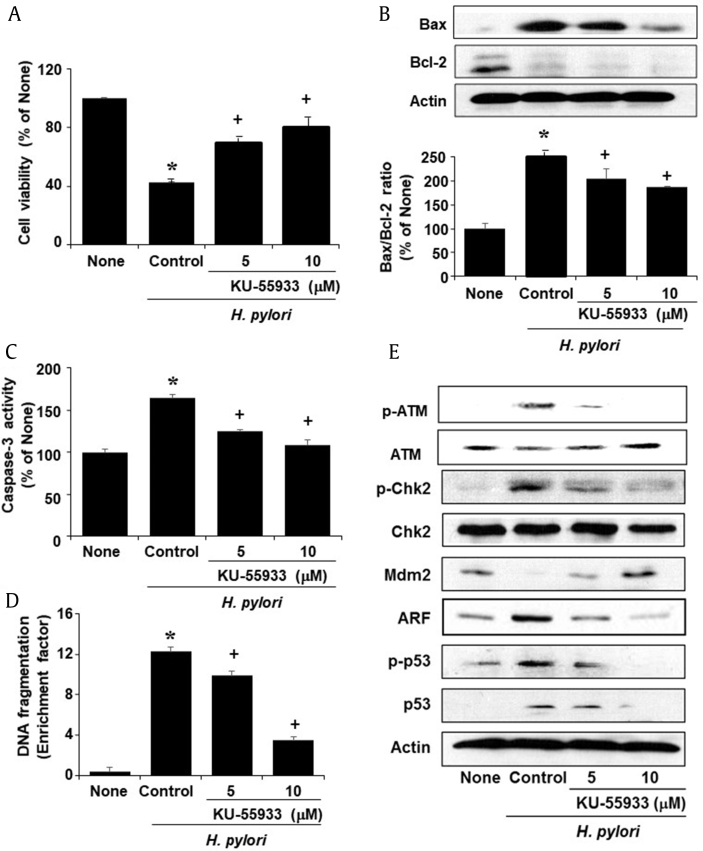
For determining the role of ATM on apoptosis, H. pylori–infected cells were treated with an ATM kinase inhibitor KU-55933. KU-55933 inhibited H. pylori–induced cell death dose-dependently (Fig. 3A). KU-55933 suppressed the increment of Bax/Bcl-2 ratio, caspase-3 activation, and nucleosome-bound DNA level in H. pylori–infected cells (Fig. 3B–3D).
Fig. 3.
Cell viability, Bax/Bcl-2 ratio, caspase-3 activity, DNA fragmentation, and DDR protein levels in H. pylori–infected cells treated with or without KU-55933. KU-55933 was treated to the cells 2 h before H. pylori stimulation. 24 h- (A, cell viability; D, DNA fragmentation) and 6 h-(B, protein levels of Bax and Bcl-2; C, caspase-3 activity; E, DDR proteins) cultures were used. (B) Protein band intensity of Bax/Bcl-2 in the untreated, uninfected group (none) was set as 100%. Values are mean ± SEM. *p < 0.05 vs. untreated, uninfected group (none) and +p < 0.05 vs. untreated, H. pylori–infected group (control).
ARF, alternative reading frame; ATM, ataxia-telangiectasia-mutated; Chk2, checkpoint kinase 2; DDR, DNA damage response; Mdm2, mouse double minute 2; SEM, standard error of the mean.
H. pylori induced activation of ATM, which was suppressed by KU-55933. ATM activation was determined by the protein levels of p-ATM and ATM (Fig. 3E). Furthermore, KU-5593 suppressed the increased p-Chk2, ARF, p-p53, and p-53 and the decreased Mdm2 levels in H. pylori–infected cells. These results show that ATM mediates the activation of Chk2 and p53 as well as the decrease in Mdm2 and increase in ARF. Therefore, H. pylori–induced apoptosis may be mediated by ATM activation that leads to the upregulation of ARF and loss of Mdm2, which subsequently activates p53 in AGS cells.
4. Discussion
The present study shows that H. pylori infection increased the levels of ARF and p53 and p53 activation but decreased Mdm2 level in AGS cells. The results are in parallel with the increase in apoptotic indices (cell death, Bax/Bcl-2 ratio, caspase-3 activity, and DNA fragmentation) and DDRs (activation of ATM and Chk2) in H. pylori–infected cells. KRG inhibited DDRs (increased p-ATM, p-Chk2, ARF, p-p53, and p53, but decreased Mdm2) and apoptosis in the cells infected with H. pylori. In addition, the antiapoptotic effect of KRG was similar to that of a selective ATM kinase inhibitor, KU-55933, determined by the apoptotic indices and DDRs (activation of Chk2 and p53, induction of ARF, and a decrease in Mdm2) in the cells infected with H. pylori. The results show that ATM has a critical role in apoptosis in H. pylori–infected cells. KRG may inhibit ATM-dependent DDRs and apoptosis in H. pylori–infected cells.
A tumor suppressor ARF inhibits Mdm2 and thus, increases p53 activity [31]. Because Mdm2 induces ubiquitinylation and proteasomal degradation of p53, Mdm2 is considered as a regulator of p53 [32]. Loss of Mdm2 activates p53 function, which induces apoptosis by arresting cell cycle on DNA damage. Chk2 mediates the stabilization and activation of p53 by phosphorylating the N-terminal serine residues of p53 [33], [34]. We previously showed that H. pylori activates ATM, Chk2, and p53 in gastric epithelial cells [7]. In the present study, we demonstrate that H. pylori increased alternative reading frame (ARF) and decreased Mdm2, while it decreased the activation of p53 in gastric epithelial AGS cells. The results suggest that H. pylori inhibited the interaction between Mdm2 and p53 by increasing ARF in the infected cells.
p53 activation is mediated by ATM and its substrate kinase Chk2 [33], [34], [35]. In response to oncogenes such as c-myc, ARF enhances the phosphorylation of p53 in NIH3T3, IMR90, and MCF7 cells [18]. Downregulation of ATM using RNA interference techniques attenuated ARF-induced phosphorylation of p53. In addition, ectopically expressed ARF induced ATM nuclear foci and activated ATM-dependent phosphorylation of p53 in NIH3T3 cells [18]. ATM activation is related with increased ARF levels in the studies using ATM-null mouse embryo fibroblasts and mice [36], ARF-null mouse embryo fibroblasts and mice [37], and human cancer cells and tissue culture models [38]. These studies demonstrate the inter-regulation of ATM–ARF and functional role of ATM in ARF-mediated tumor suppression.
In the present study, the ATM inhibitor KU-55933 inhibited H. pylori–induced ARF expression and apoptosis. Therefore, ATM activation by H. pylori infection may induce ARF expression and decreases Mdm2, thereby activating p53. Similarly, KRG inhibited H. pylori–induced ATM activation, ARF induction, p53 activation, and apoptosis. Therefore, KRF may act as an ATM inhibitor in H. pylori–infected cells.
ROS lead to DDRs including activation of ATM and p53 in H. pylori–infected AGS cells [7]. Yang et al [39] reported that ROS-producing shikonin, an active naphthoquinone isolated from traditional Chinese herb, decreased Mdm2, which was restored by treatment of an antioxidant N-acetylcysteine. The loss of Mdm2 increased the stability and activity of p53 and its downstream target Bax in thyroid cancer cells. This study supports the present results showing that the decrease in Mdm2 may have been caused by ROS in H. pylori–infected gastric epithelial cells.
Recently, Elias [40] showed that in the presence of DNA damage, ATM is activated which phosphorylates Mdm2, which forms a complex with p53 mRNA at the DNA sites, and this complex moves from the nucleus to the cytoplasm. Then, phosphorylated Mdm2 increases p53 translation and acts as a positive regulator of p53. Therefore, decrease in Mdm2 by KRG treatment may prevent p53-mediated apoptosis in H. pylori–infected cells.
A limitation of this study is that we only used apoptotic indices (cell death, DNA fragmentation, Bax/Bcl-2 ratio, caspase-3 activity) of the cells to investigate the effect of KRG on H. pylori–induced apoptosis. Therefore, additive experiments including flow cytometry–based apoptosis detection findings should be performed for further study to prove antiapoptotic effect of KRG on H. pylori–infected cells. In addition, KRG contains various ginsenosides. Thus, it is difficult to find which ginsenoside is responsible for protection against DNA damage and apoptosis of gastric epithelial cells due to H. pylori infection. For the future study, each ginsenoside should be treated in this system to find the preventive efficacy of each ginsenoside against H. pylori–induced apoptosis of the gastric epithelial cells.
The present study suggests that H. pylori induces the activation of ATM and p53 and expression of ARF, while it decreases Mdm2 in the cells. Therefore, H. pylori infection leads to increased p53 stability, which induces apoptosis. Because the ATM kinase inhibitor suppresses H. pylori–induced ARF expression and p53 activation, ATM has a main role in orchestrating the activities of apoptotic pathways mediated by ARF-Mdm2-p53 in H. pylori–infected cells. KRG inhibited ATM-mediated apoptosis by reducing ROS levels and suppressing the ARF-Mdm2-p53 interaction (increased ARF and p53 with the loss of Mdm2) in H. pylori–infected cells.
In summary, H. pylori induces apoptosis by increasing apoptotic indices and ROS levels. H. pylori increases p-ATM, p-Chk2, ARF, p-p53, and p53 but decreases Mdm2. KRG inhibits H. pylori–induced apoptosis by decreasing ROS and by suppressing DDRs (increased ARF and p53 with decreased Mdm2). KRG inhibits H. pylori–induced nuclear translocation of p53. KU-55933 suppresses apoptosis and DDRs in H. pylori–infected AGS cells, which was similar to the effects of KRG. In conclusion, KRG suppresses H. pylori–induced DDRs and apoptosis by reducing ROS levels in gastric epithelial cells. Supplementation with KRG may be beneficial for preventing the oxidative stress–mediated gastric disorders associated with H. pylori infection.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the BK21 Plus Project for Bioactive Nutrition, Yonsei University, Seoul 03722, Republic of Korea.
References
- 1.Brown L.M. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;22:283–297. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 2.Marshall B.J. Helicobacter pylori. Am J Gastroenterol. 1994;89:S116–S128. [PubMed] [Google Scholar]
- 3.Covacci A., Telford J.L., Del Giudice G., Parsonnet J., Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 4.Misiewsicz J. Management of Helicobacter pylori-related disorders. Eur J Gastroenterol Hepatol. 2012;9:S17–S20. [PubMed] [Google Scholar]
- 5.Polk D.B., Peek R.M., Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cha B., Lim J.W., Kim K.H., Kim H. 15-Deoxy-delta 12, 14-prostaglandin J2, NADPH oxidase, and RANTES expression in Helicobacter pylori-infected gastric epithelial cells. J Physiol Pharmacol. 2011;62:167–174. [PubMed] [Google Scholar]
- 7.Jang S.H., Lim J.W., Morio T., Kim H. Lycopene inhibits Helicobacter pylori-induced ATM/ATR-dependent DNA damage response in gastric epithelial AGS cells. Free Radic Biol Med. 2012;52:607–615. doi: 10.1016/j.freeradbiomed.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 9.Shieh S.Y., Ahn J., Tamai K., Taya Y., Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 10.Chehab N.H., Malikzay A., Appel M., Halazonetis T.D. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 2000;14:278–288. [PMC free article] [PubMed] [Google Scholar]
- 11.Canman C.E., Lim D.S., Cimprich K.A., Taya Y., Tamai K., Sakaguchi K., Appella E., Kastan M.B., Siliciano J.D. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 12.Banin S., Moyal L., Shieh S., Taya Y., Anderson C.W., Chessa L., Smorodinsky N.I., Prives C., Reiss Y., Shiloh Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 13.Miyashita T., Reed J.C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 14.Lahiry L., Saha B., Chakraborty J., Bhattacharyya S., Chattopadhyay S., Banerjee S., Choudhuri T., Mandal D., Bhattacharyya A., Sa G. Contribution of p53-mediated Bax transactivation in theaflavin-induced mammary epithelial carcinoma cell apoptosis. Apoptosis. 2008;13:771–781. doi: 10.1007/s10495-008-0213-x. [DOI] [PubMed] [Google Scholar]
- 15.Haupt Y., Maya R., Kazaz A., Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 16.Kubbutat M.H., Jones S.N., Vousden K.H. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 17.Bieging-Rolett K.T., Johnson T.M., Brady C.A., Beaudry V.G., Pathak N., Han S., Attardi L.D. P19Arf is required for the cellular response to chronic DNA damage. Oncogene. 2016;35:4414–4421. doi: 10.1038/onc.2015.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pomerantz J., Schreiber-Agus N., Liégeois N.J., Silverman A., Alland L., Chin L., Potes J., Chen K., Orlow I., Lee H.W. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., Xiong Y. Mutations in human ARF exon 2 disrupt its nucleolar localization and impair its ability to block nuclear export of MDM2 and p53. Mol Cell. 1999;3:579–591. doi: 10.1016/s1097-2765(00)80351-2. [DOI] [PubMed] [Google Scholar]
- 20.Weber J.D., Taylor L.J., Roussel M.F., Sherr C.J., Bar-Sagi D. Nucleolar Arf sequesters Mdm2 and activates p53. Nat Cell Biol. 1999;1:20–26. doi: 10.1038/8991. [DOI] [PubMed] [Google Scholar]
- 21.Li Y., Wu D., Chen B., Ingram A., He L., Liu L., Zhu D., Kapoor A., Tang D. ATM activity contributes to the tumor-suppressing functions of p14ARF. Oncogene. 2004;23:7355–7365. doi: 10.1038/sj.onc.1207957. [DOI] [PubMed] [Google Scholar]
- 22.Pauklin S., Kristjuhan A., Maimets T., Jaks V. ARF and ATM/ATR cooperate in p53-mediated apoptosis upon oncogenic stress. Biochem Biophys Res Commun. 2005;334:386–394. doi: 10.1016/j.bbrc.2005.06.097. [DOI] [PubMed] [Google Scholar]
- 23.Wu J.Y., Gardner B.H., Murphy C.I., Seals J.R., Kensil C.R., Recchia J., Beltz G.A., Newman G.W., Newman M.J. Saponin adjuvant enhancement of antigen-specific immune responses to an experimental HIV-1 vaccine. J Immunol. 1992;148:1519–1525. [PubMed] [Google Scholar]
- 24.Sato K., Mochizuki M., Saiki I., Yoo Y.C., Samukawa K., Azuma I. Inhibition of tumor angiogenesis and metastasis by a saponin of Panax ginseng, ginsenoside-Rb2. Biol Pharm Bull. 1994;17:635–639. doi: 10.1248/bpb.17.635. [DOI] [PubMed] [Google Scholar]
- 25.Kaneko H., Nakanishi K. Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, Korean red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004;95:158–162. doi: 10.1254/jphs.fmj04001x5. [DOI] [PubMed] [Google Scholar]
- 26.Maffei Facino R., Carini M., Aldini G., Berti F., Rossoni G. Panax ginseng administration in the rat prevents myocardial ischemia-reperfusion damage induced by hyperbaric oxygen: evidence for an antioxidant intervention. Planta Med. 1999;65:614–619. doi: 10.1055/s-1999-14034. [DOI] [PubMed] [Google Scholar]
- 27.Park S., Yeo M., Jin J.H., Lee K.M., Kim S.S., Choi S.Y., Hahm K.B. Inhibitory activities and attenuated expressions of 5-LOX with red ginseng in Helicobacter pylori-infected gastric epithelial cells. Dig Dis Sci. 2007;52:973–982. doi: 10.1007/s10620-006-9440-6. [DOI] [PubMed] [Google Scholar]
- 28.Cho S.O., Lim J.W., Kim H. Red ginseng extract inhibits the expression of MCP-1 and iNOS in Helicobacter pylori-infected gastric epithelial cells by suppressing the activation of NADPH oxidase and Jak2/Stat3. J Ethnopharmacol. 2013;150:761–764. doi: 10.1016/j.jep.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Bae M., Jang S., Lim J.W., Kang J., Bak E.J., Cha J.H., Kim H. Protective effect of Korean Red Ginseng extract against Helicobacter pylori-induced gastric inflammation in Mongolian gerbils. J Ginseng Res. 2014;38:8–15. doi: 10.1016/j.jgr.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho S.O., Lim J.W., Kim H. Diphenyleneiodonium inhibits apoptotic cell death of gastric epithelial cells infected with H. pylori in a Korean isolate. Yonsei Med J. 2015;56:1150–1154. doi: 10.3349/ymj.2015.56.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honda R., Yasuda H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 1999;18:22–27. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Momand J., Zambetti G.P., Olson D.C., George D., Levine A.J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 33.Appella E., Anderson C.W. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268:2764–2772. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa K., Taya Y., Tamai K., Yamaizumi M. Requirement of ATM in phosphorylation of the human p53 protein at serine 15 following DNA double-strand breaks. Mol Cell Biol. 1999;19:2828–2834. doi: 10.1128/mcb.19.4.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tibbetts R.S., Brumbaugh K.M., Williams J.M., Sarkaria J.N., Cliby W.A., Shieh S.Y., Taya Y., Prives C., Abraham R.T. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 1999;13:152–157. doi: 10.1101/gad.13.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamijo T., van de Kamp E., Chong M.J., Zindy F., Diehl J.A., Sherr C.J., McKinnon P.J. Loss of the ARF tumor suppressor reverses premature replicative arrest but not radiation hypersensitivity arising from disabled atm function. Cancer Res. 1999;59:2464–2469. [PubMed] [Google Scholar]
- 37.Sherr C.J. The INK4a/ARF network in tumour suppression. Nat Rev Mol Cell Biol. 2001;2:731–737. doi: 10.1038/35096061. [DOI] [PubMed] [Google Scholar]
- 38.Velimezi G., Liontos M., Vougas K., Roumeliotis T., Bartkova J., Sideridou M., Dereli-Oz A., Kocylowski M., Pateras I.S., Evangelou K. Functional interplay between the DNA-damage-response kinase ATM and ARF tumour suppressor protein in human cancer. Nat Cell Biol. 2013;15:967–977. doi: 10.1038/ncb2795. [DOI] [PubMed] [Google Scholar]
- 39.Yang Q., Ji M., Guan H., Shi B., Hou P. Shikonin inhibits thyroid cancer cell growth and invasiveness through targeting major signaling pathways. Clin Endocrinol Metab. 2013;98:E1909–E1917. doi: 10.1210/jc.2013-2583. [DOI] [PubMed] [Google Scholar]
- 40.Elias J. Positive effect of Mdm2 on p53 expression explains excitability of p53 in response to DNA damage. J Theor Biol. 2017;418:94–104. doi: 10.1016/j.jtbi.2017.01.038. [DOI] [PubMed] [Google Scholar]