Abstract
Date (Phoenix dactylifera L.) seeds are seen as good drug to cure rheumatoid arthritis and asthma in Moroccan traditional medicine. The present research aimed to study the anti-inflammatory effect, of methanol extract of different date seed varieties using membrane stabilizing effect, nitric oxide radical scavenging activity, inhibition of protein denaturation, carrageenan-induced paw edema and croton oil induced ear edema. The polyphenolic profile was examined using HPLC-DAD. Rutin, quercetin, p-coumaric and caffeic acids were the main among the analysed phenolic compounds. Concerning the anti-inflammatory activity, the analysed date seed were significantly effective in scavenging nitric oxide free radical, in stabilisation of erythrocyte membrane and possessed a high anti denaturation effect. In agreement with this finding, date seed exhibited a profound ability to reduce paw and ear swelling induced by carrageenan and croton oil respectively. The biochemical parameters showed that date seed are able to reduce the erythrocyte sedimentation rate (ERS) and C-reactive protein (CRP) concentration in rats used in Carrageenan-induced paw edema model. The predominant phenolic compounds are the potential candidates that drive these activities and the differences observed among varieties are related to their chemical composition. These data suggest that date seeds can be explored as a therapeutic agent for the treatment of inflammatory diseases.
Keywords: Toxicology, Inflammation, Natural product, Polyphenol, Oxidative stress, Antioxidant, Lipid peroxidation, Pathophysiology, Immunology, Immune disorder, Alternative medicine, Date seed, Phoenix dactylifera, Anti-Inflammatory, Phenolic compounds, Carrageenan
Toxicology; Inflammation; Natural product; Polyphenol; Oxidative stress; Antioxidant; Lipid peroxidation; Pathophysiology; Immunology; Immune disorder; Alternative medicine; Date seed; Phoenix dactyliferaAnti-Inflammatory; Phenolic compounds; Carrageenan.
1. Introduction
Inflammation is a physiologic reaction that happens in response to unsafe stimuli like irritants, damaged cells or infection (Debnath et al., 2013). This inflammation can be chronic or acute, systemic or localized forms (Zhang and Tsao, 2016). A wide range of mediators, including prostaglandins, cytokines and various reactive oxygen species (ROS), such as hydroxyl radical (OH•), nitric oxide (NO), and superoxide anion (O2•-) are produced, by different types of immune cells or respiratory burst of neutrophils, produced to protect cells and tissues, throughout the process of acute inflammation (Zhang and Tsao, 2016). Nevertheless, the persistent immune reactions can lead to an overproduction of ROS, the key exogenous source of oxidative stress, oxidative stress, which can cause several diseases, including chronic inflammation-associated disorders (Tungmunnithum et al., 2018). Flavonoids and phenolic compounds, which are produced by plants to protect themselves or to boost growth during adverse conditions, scavenge reactive species and thus stop the chain reaction before the viability of the cell is seriously affected (Hussain et al., 2016). Moreover, polyphenols may have an anti-inflammatory effect by regulating cell activity in inflammatory cells and by modulating the activities of enzymes implicated in the metabolism of arachidonic acid (phospholipase A2, cyclooxygenase (COX), lipoxygenase (LOX)), arginine metabolism (NOS), modulating the secretion of other pro-inflammatory molecules (Hussain et al., 2016).
Synthetic drugs, widely used to treat inflammation, are not safe anymore as they are associated with drug-related toxicity, iatrogenic reactions, and harmful adverse reactions that complicate the treatment progress on long-term use (Elisha et al., 2016). These secondary effects include gastrointestinal bleeding and peptic ulcers, renal and hepatic failure, osteoporosis, cataracts and skin rashes (Beg et al., 2011). Therefore, safer and more effective anti-inflammatory agents need to be developed from traditional medicine known for their effectiveness in treating multiple human illnesses in recent decades.
Date palm (Phoenix dactylifera L.) represents the most important arboriculture crop in the oasis of the Middle East and North Africa (Bouhlali et al., 2018). Furthermore, date seeds are the waste product of many date processing plants producing pitted dates, date syrup and date confectionery (Al-Farsi and Lee., 2008).
Traditionally, date (Phoenix dactylifera L.) seed powder is used for treating liver problems, diabetes, cancer, gastrointestinal disorders, toothaches, pulmonary and throat diseases, diarrhea and various infectious diseases (Bnouham et al., 2002). In Ayurvedic medicine, seed powder is applied to wounds in order to reduce inflammation (Shanmugapriya and Patwardhan, 2012). Besides, date seeds contain a great amount of polyphenols which are known for their numerus pharmacological activities according to several studies (Bouhlali et al., 2017).
The aim of this research is to investigate the chemical composition and anti-inflammatory properties of the aqueous methanol extracts of date seeds, as a new possible source of bioactive compounds.
2. Materials and methods
2.1. Preparation of date seed powder
The seeds of four date fruit varieties, named locally Boufgous, Bousthammi, Jihl and Majhoul were washed, dried and ground separately using Cutting Mill to form a fine powder.
2.2. Preparation of rich polyphenol extracts
The preparation of date seed extracts was performed as described previously Bouhlali et al. (2017). Briefly, 150 ml of methanol–water was added to a 250 mL flask containing 30 g of the pulverized date seeds and extracted using an orbital shaker-incubator. After 12 h at 35 °C, the mixture filtered, and the solvent was evaporated at 40 C under reduced pressure using a rotary evaporator. The result dried extracts were kept in dark glass bottles at - 20 °C for downstream use. The extract of each date seed variety has been prepared in triplicate. The extracts were redissolved into a pre-established concentration known dilution to determine phenolic and flavonoidic contents as well as their anti-inflammatory capacity.
2.3. Animals
Thirty-six rats of Wistar strain (150–180 g) and thirty Swiss albino mice weighing about 25–30 g were obtained from the animal house, Faculty of sciences and techniques, Errachidia (FSTE), Morocco. Animals were housed under a light/dark period of (12h/12h) and controlled room temperature (24 ± 2 °C) in spacious hygienic plastic cages, with ad libitum access to water and food. The protocols for present research were agreed by the Animal Research Ethics Committee of the Faculty of Sciences and techniques (AREC) (AREC-FSTE-08/2017) and conducted in line with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
2.4. Phenolic and flavonoid compound identification and quantification
The phenolics and flavonoids profiling of seeds from studied date fruit varieties were performed as described previously in Bouhlali et al. (2018) using an Shimadzu HPLC system coupled to a diode array detector (SPD-M10A) with a LC-20AB model dual pump, SIL-20A autosampler, DGU-20A degasser and connected to a Shimadzu SCL-10A model system controller. A C18 analytical column of 150 mm × 50 mm and 5μm particle size (Restek, Bellefonte, USA) was used. The column oven temperature was set at 40 °C. The elution flow rate was maintained at 1 mL/min. The acquisition wavelengths were set at 280, 320, and 350 nm. A binary gradient solvent system of water-acetic acid (97:3, v/v) (A) and acetonitrile (B) was used as follows: 0–8% of B between 0 and 5 min (linear gradient); 8–25% of B between 5 and 25 min (linear gradient); 25% of B between 25 and 30 min (isocratic elution) and 25–90% of B between 30 to 50 min (linear gradient). Standard stock solutions (100 μg/mL of three flavonoids: rutin, quercetin, luteolin and seven phenolic acids: chlorogenic, caffeic, ferulic, p-coumaric, gallic, syringic and vanillic acids were used to prepare calibration curves. The sample was prepared by dissolving one gram on date seed extracts in 25 mL acidified methanol solution (1 N HCl/methanol/water, 1/80/19, v/v/v) using an ultrasonic homogenizer for 30 min and 20 μL of filtrate was injected in HPLC-DAD system. The peaks were identified based on phenolic standard retention time and UV spectra, and their quantity was determined using a calibration curve. The results were recorded as milligrams per 100 g dried weight of date seeds.
2.5. Nitric oxide radical scavenging activity
The ability of date seeds extract to scavenge nitric oxide radicals was examined using a Griess reaction based on Boora et al. (2014). Griess reagent has been prepared by combining the same amount of 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride and 1% sulphanilamide, both prepared in 2.5% phosphoric acid. For the experiment, 500 μL of 10 mM sodium nitroprusside in phosphate buffered saline (pH 7.4), mixed with 1 mL of date seed extract at different concentrations (100–1500 μg/mL) were incubated for 150 min at 25 °C. After the incubation period, 1.5 mL of freshly prepared Griess reagent was added to the resulting mixture. Then, the absorbance of the mixture was measured at 546 nm. The control and standard were prepared in a similar manner as was done for the test samples using buffer and Trolox instead of extract. The nitric oxide scavenging capacity of each extract was calculated as follows:
| % NOsa = (Abs.control –Abs.sample)x 100)/(Abs.control) |
Where Abs control and Abs sample are the absorbance of buffer and the absorbance of sample extract/standard, respectively. Then IC50 value that represents the concentration of drug or extracts required to scavenge 50% of nitric oxide radicals was determined.
2.6. Inhibition of protein denaturation
The effect of date seeds extracts on protein denaturation was evaluated based on the method described by Hmidani et al. (2019). The reaction mix made of 1 mL date seed extracts at different doses (20–1000 μg/mL) and 1 mL of 1% bovine albumin prepared in phosphate-buffered saline (PBS, pH 6.4). The reaction mixtures were left at 37 °C for 20 min and then heated in a shaking water bath at 70 °C for 5 min. After cooling, the reaction mixtures turbidity was determined at 660 nm. The control and standard were prepared in a similar manner as was done for the test samples using buffer and diclofenac sodium (20–1000 μg/mL) instead of extract. The percent inhibition of protein denaturation (%IPD) was calculated as follows:
| % IPD = (Abs.control –Abs.sample) x 100)/(Abs.control) |
Abs control and Abs sample are the absorbance buffer and the absorbance of sample extract/standard, respectively. Then IC50 value that represents the concentration of drug or extracts required to inhibit protein denaturation by 50% was determined.
2.7. Membrane stabilizing effect
The membrane stabilizing effect was assessed as described previously by Hmidani et al. (2019). A volume of human blood collected from healthy volunteers who did not receive non-steroidal anti-inflammatory drugs (NSAIDs) within the fifteen days before to study, was mixed with the of Alsever sterilized solution which has been prepared in distilled water by combining 0.42% sodium chloride, 0.05% citric acid and 2% dextrose. The cell pellet obtained after centrifugation of blood solution at 3000 rpm was washed twice with normal saline (9 g/L) and then to make a 10 % cell pellet suspension. For the experiment, 500 μL of extract mixed with 1000 μL of PBS and 2000 μL of hypotonic saline (0.36 %) were added to 500 μL of cell pellet suspension (10%) and incubated for 30 min at 37 °C. After the incubation period, the solution mixture was centrifuged (5 min at 3000 rpm) and using a spectrophotometer at 560 nm the supernatant haemoglobin content was determined. Different concentrations (20–1000 μg/mL) of each extract have been tested. The control and standard were prepared in a similar manner as was done for the test samples using buffer and diclofenac sodium (20–1000 μg/mL) instead of extract. The membrane stabilisation rate was calculated as follows:
| %MS = (Abs.control –Abs.sample) x 100)/(Abs.control) |
Abs control and Abs sample are the absorbances of buffer and sample (extract or standard), respectively. Then IC50 value that represents the concentration of drug or extracts required to inhibit erythrocytes hemolysis by 50% was determined.
2.8. Croton oil-induced ear edema in mice
The ability of date seed extracts to reduce croton oil -induced ear edema was investigated as described in our previous research (Bouhlali et al., 2018). Swiss albino mice of either sex were split into five groups of 6 mice. The ear edema was induced by topical application of 10 μL of 5% freshly prepared Croton oil in acetone on the anterior and posterior surface of the right ear. The left ear received the vehicle and served as a control. Ten minutes after Croton oil treatment, the animals of group I, II, III, IV, V were given topically of Indomethacin, seeds extract of Boufgous, Bousthammi, Majhoul and Jihl respectively in the same place of the first treatment. The standard drug and date seed extracts were freshly prepared by dissolving 500 μg of each one in 20 μL of acetone. The difference of thickness between the right and left ear, determined using a digital vernier caliper was used to evaluate ear swelling degree 4 h after the application of the irritant. The anti-inflammatory activity of exract expressed as the percentage of ear edema inhibition was estimated as follows:
| Ear edema= (Vr – Vl) x 100/Vl |
| % Edema inhibition = (Et-Ec) x 100/Et |
where Vr and Vl are the right and left ear volumes respectively; Ec and Et are the rate of ear edema of control and treated groups respectively.
2.9. Carrageenan-induced rat paw edema
Carrageenan-induced rat paw edema was performed as described previously (Bouhlali et al., 2018). The rats were divided into six groups containing three rats of either sex (n = 6) and were subjected to fasting period (12h) with free access to drinking water. Prior to experiment the initial volume of the right hind paw for each rat was measured. The rats of group 1 (saline control: carrageenan induced group) received normal saline, the second group was given the indomethacin (10 mg/kg BW). The groups III, IV, V and VI received seed extracts of Boufgous, Bousthammi, Jihl and Majhoul at (30 mg/kg BW) respectively. One hour after the above administration, the paw oedema was induced by injection of 0.1 ml of 1% freshly prepared carrageenan in normal saline into the plantar surface of the right hind paw. The paw volume was measured once an hour for 6 h after carrageenan injection by means of a plethysmometer (Ugo Basile, Italy). The percentage inhibition of paw swelling was determined for each hour of the experience with the following formulas
| % Paw swelling = (V sample – V normal) x 100/ V normal |
| % IPS = (PS saline –PS sample) x 100/ PS sample |
where Vsample is the paw volume of treated rat and Vnormal is the volume of the paw of untreated rats; PS saline is the % of paw swelling of saline control (carrageenan induced group) and PS sample is the % of paw swelling of sample (treated rats by extract or drug).
2.9.1. C-reactive protein (CRP) determination
After 5hr of carrageenan administration, blood was collected by puncturing retro-orbital plexus and serum was separated. The serum levels of C reactive protein (CRP) was examined using a highly sensitive immune-turbidimetric method using Konelab 60i (Thermo Fisher Scientific Inc. MA, USA) automatic analyser, on the basis of the principle of particle-enhanced immunologic agglutination.
2.9.2. Erythrocyte sedimentation rate (ESR)
Animal ESR was determined 5hr after the carrageenan administration using the automatic instrument Ves-Matic 20 (DIESSE—DiagnosticaSenese, Siena, Italy). Blood samples were collected by puncturing retro-all orbital plexus into trisodium citrate tubes.
2.10. Ethics approval
All experiments carried out in this research paper, including human material (blood) were in line with the institutional and research committee's ethical standards and with the Helsinki Declaration of 1964 and its later modifications or comparable ethical rules and written informed consent for publication has been obtained from the participants.
2.11. Statistical analysis
The statistical software R (version 3.2.3) with ggplot2 package (version 2.1.0) used to plot the graphs was applied for statistical analysis. One-way ANOVA test was used to compare groups. The post hoc Bonferroni test was to determine the two-group differences in the levels of p < 0.01.
3. Results and discussion
3.1. Phenolic and flavonoid compound identification and quantification
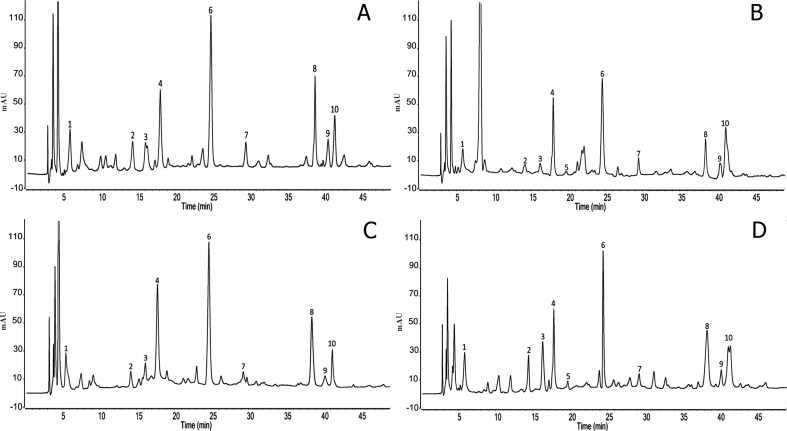
HPLC chromatogram showing the phenolic profiling of analysed date seed is displayed in Figure 1, and the results are illustrated in Table 1. Statistically significant differences (p < 0.05) were detected between the studied date seed varieties for analysed phenolic compounds. Date seeds of all varieties contained examined phenolic and flavonoid compound at different concentrations except syringic acid which was absent in Boufgous and Jihl seeds. p-coumaric acid was found to be the main phenolic acid in studied date seed ranging between 116.96 ± 1.64 and 143.60 ± 1.81 mg/100g followed by caffeic acid (59.40 ± 0.95–88.64 ± 0.82 mg/100g), gallic acid (10.35 ± 0.10–17.62 ± 0.18 mg/100g), ferulic acid (5.51 ± 0.21–12.16 ± 0.22 mg/100g), chlorogenic acid (4.55 ± 0.11–10.34 ± 0.31 mg/100g), vanillic acid (5.26 ± 0.16–10.81 ± 0.22 mg/100g) and syringic acid (0–1.43 ± 0.03 mg/100g) was the least abundant phenolic acid. Among the analyzed flavonoids compounds, rutin (39.46 ± 0.75–80.26 ± 0.94 mg/100g) was the predominant compound, followed by quercetin (21.93 ± 0.14–36.39 ± 0.32 mg/100g) and luteolin (7.62 ± 0.26–12.68 ± 0.31 mg/100g). The greatest amount of ferulic acid, caffeic acid, vanillic acid, chlorogenic acid, luteolin and rutin was found in Jihl seeds while the highest amount of gallic acid and p-coumaric acid was revealed in Boufgous seeds. The highest content of quercetin and syringic acid was established in Bousthammi seeds. The present findings are in agreement with those found by Al-Farsi and Lee. (2008) studied phenolic acids in seeds of Omanian date seed varieties and found nine phenolic acids included four hydroxylated benzoic acid derivatives (protocatechuic acid, gallic acid, vanillic acid and ρ-hydroxybenzoic acid) and five cinnamic acid derivatives (ferulic acid, caffeic acid, p-coumaric acid, o-coumaric acid and m-coumaric acid). The same phenolic profile has been reported also by El-Rahman and Al-Mulhem (2017) found mainly cinnamic acid, ferulic acid, syringic acid, protocatechuic acid, caffeic acid, ρ-coumaric acid, salicylic acid, benzoic acid and a high amount of kaempferol. Moreover, Messaoudi et al. (2013) studied phenolic acids in pits of seven Algerian date fruit varieties and found that catechin or epi catechin derivatives, sinapic, cinnamic, and coumaric acid derivatives are the main compounds of all varieties. Also, El-Mergawi et al. (2016) studied phenolic profiles of seeds of ten Saudi Arabian date fruits varieties and found all varieties contained four phenolic acids (protocatechuic acid, p-hydroxybezoic acid, caffeic acid and coumaric acid) and five flavonoid compounds (quercetin, kaempferol, naringenin, luteolin and isorhamnetin) with different concentrations. Although the present research displayed a similar phenolic profile, the concentration of each compound was different to those reported earlier. These differences among date seeds varieties in terms of seed phenolic profiles may be due to many factors such as inter-variety genetic diversity, growing conditions, water availability, diseases, maturity, harvesting times, storage conditions, geographic origin, light, temperature, fertilizer and soil type as well as extraction system and method of analysis (Bouhlali et al., 2018). Our findings suggest that date seeds serve as an important phenolic and flavonoid source known as natural anti-oxidative compounds that could potentially be used in food and nutraceutical formulation.
Figure 1.
HPLC-DAD chromatograms of different date seed varieties. Boufgous (A); Bousthammi (B); Jihl (C); Majhoul (D). Peak numbers: gallic acid (1); chlorogenic acid (2); vanillic acid (3); caffeic acid (4); syringic acid (5); p-coumaric acid (6); ferulic acid (7); rutin (8); luteolin (9); quercetin (10).
Table 1.
Phenolic acids and flavonoid profiles determined by HPLC in four Moroccan date seed cultivars extract (mg/100g DW).
| Boufgous | Bousthammi | Jihl | Majhoul | |
|---|---|---|---|---|
| Phenolic acid | ||||
| caffeic acid | 59.40 ± 0.95a | 76.62 ± 0.85b | 88.64 ± 0.82c | 66.39 ± 0.96d |
| chlorogenic acid | 8.39 ± 0.31d | 4.55 ± 0.11c | 10.34 ± 0.31b | 7.23 ± 0.20a |
| p-coumaric acid | 143.60 ± 1.81b | 122.36 ± 1.91a | 133.68 ± 1.73d | 116.96 ± 1.64c |
| ferulic acid | 6.46 ± 0.19c | 8.62 ± 0.19d | 12.16 ± 0.22a | 5.51 ± 0.21b |
| gallic acid | 17.62 ± 0.18a | 15.39 ± 0.20b | 13.80 ± 0.23c | 10.35 ± 0.10d |
| syringic acid | Nd | 1.43 ± 0.03d | Nd | 0.70 ± 0.02a |
| vanillic acid |
8.46 ± 0.17d |
5.26 ± 0.16c |
10.81 ± 0.22b |
9.67 ± 0.11a |
| Flavonoids | ||||
| luteolin | 8.73 ± 0.27a | 10.99 ± 0.29b | 12.68 ± 0.31c | 7.62 ± 0.26d |
| quercetin | 27.24 ± 0.15c | 36.39 ± 0.32d | 31.74 ± 0.41b | 21.93 ± 0.14a |
| rutin | 64.07 ± 0.87b | 73.29 ± 0.60a | 80.26 ± 0.94d | 39.45 ± 0.75c |
Values are means of triplicate determinations ± standard deviation. Nd, not determined.
Means in the same line followed by different letters are significantly different from each other according to post hoc Bonferroni tests (p < 0.01).
3.2. Nitric oxide (NO) radical scavenging activity
The activation of macrophages, neutrophil granulocytes and many other immune cells by lipopolysaccharide (LPS) during bacterial infection or by cytokines being IL-1, TNF-α or IFN-γ, induces overexpression of iNOS that enhances overproduction of NO (Coleman, 2001). Nitric oxide is a signalling agent that possesses a crucial role in the pathogenesis of inflammation (Boora et al., 2014). Using in-vitroassays, Asanuma et al. (2001) have revealed that the non-steroidal anti-inflammatory medicines are able to block inducible nitric oxide synthase (iNOS) and to scavenge nitric oxide. Numerous studies have shown that NO boosts the expression of COX-2 leading to increased prostaglandin formation (González-Gallego et al., 2007). Therefore, NO quenching ability represents an important therapeutic advance in the inflammatory diseases management. The statistical analysis of NO scavenging capacity results showed a significant difference (p < 0.05) between the studied date seed varieties (Table 2). Jihl variety seeds (IC50 = 108.57 μg/mL) showed the strongest antioxidant ability, whereas Majhoul seeds exhibited the weakest capacity (IC50 = 163.63 μg/mL). These results are similar to those obtained by Trolox (84.87 μg/mL). Date seeds have the property of quenching NO and thus might be of great importance in avoiding the detrimental effects of excessive NO production in the human body. Additionally, the scavenging activity may help interfere with the chain of reactions induced by NO overproduction that are harmful to human health. These results corroborate our previous study in which we found that date seeds possessed an important antioxidant activity based on three antioxidant assays. In the same study, we showed that flavonoids compounds are key contributor of antioxidant activity (Bouhlali et al., 2018). Hence, the observed variation among the analysed date seed varieties may be attributed to the differences in their phenolic and flavonoids content.
Table 2.
Effect of date fruit varieties on membrane stabilization activity and protein denaturation inhibition (μg/mL).
| Membrane stabilization activity (IC50) (μg/mL) |
Inhibition of protein denaturation (IC50) (μg/mL) |
Nitric oxide radical scavenging activity (IC50) (μg/mL) |
|
|---|---|---|---|
| Boufgous seeds | 241.65 ± 6.69a | 167.32 ± 5.82a | 144.45 ± 7.63a |
| Bousthammi seeds | 138.04 ± 7.83b | 110.87 ± 6.45b | 118.36 ± 5.92b |
| Jihl seeds | 116.63 ± 6.75c | 90.34 ± 4.62c | 108.57 ± 5.15b |
| Majhoul seeds | 209.38 ± 9.01d | 193.71 ± 7.25d | 163.63 ± 6.39c |
| Standards | 304.87 ± 7.54#e | 225.04 ± 5.96#e | 84.87 ± 4.66##d |
Values in average (n = 6) ± SE.#: Diclofenac sodium; ##: Trolox.
Means in the same column followed by different letters are significantly different from each other according to post hoc Bonferroni tests (p < 0.01).
3.3. Inhibition of protein denaturation
Protein denaturation in tissue is one of the clearly established causes of arthritic and inflammatory diseases (Bouhlali et al., 2018). Indeed, many disorders like serum disease, rheumatoid arthritis, glomerulonephritis and systemic lupus erythematosus result from hypersensitive reaction a type III, which, in turn is related to the antigens produced during protein denaturation (Elisha et al., 2016). Thus, substances that can avoid proteins denaturation would be useful for developing new anti-inflammatory drugs. As displayed in Table 2, all analysed date seed extracts are able to inhibit protein denaturation in a dose-dependent manner and significant difference (p < 0.05) has been shown between studied date seed varieties. The most potent anti-denaturation effect was observed in Jihl seeds (IC50 = 90.34 μg/mL) and the least impactful effect was found in Majhoul seeds (IC50 = 193.71 μg/mL). The anti-denaturation effect of date seed is greater compared to that of the diclofenac sodium (IC50 = 225.04 μg/mL). Several studies have shown that the interaction with polyphenolic compounds improved the thermal stability of proteins (Czubinski and Dwiecki, 2017; Ozdal et al., 2013). Indeed, using bovine serum albumin (BSA), bovine p-Iactoglobulin, and soybean glycinin with gallic acid, caffeic acid and courmaric acid, Ali et al. (2012) have shown that the thermal stability of these proteins improves compared to these proteins alone. Moreover, Ojha et al. (2012) reported that the interaction with ferulic acid increased the thermal stability of the BSA. Indeed, strong correlations (R2 > 0.760) were found between the inhibition of protein denaturation effect and caffeic acid, ferulic acid, luteolin, rutin and quercetin (Table 3). Phenol interactions with proteins caused changes in protein secondary structure (Ali et al., 2012). Therefore, the differences in phytochemical composition of the analysed date seed varieties may explain the differences in their anti-denaturation effect.
Table 3.
Correlation between phenolic and flavonoid compounds with the anti-inflammatory assays.
| caffeic. A | chlorogenic. A | p-coumaric.A | ferulic.A | syringic.A | gallic.A | vanillic.A | luteolin | rutin | quercetin | |
|---|---|---|---|---|---|---|---|---|---|---|
| EEI | 0.517 | 0.045 | 0.004 | 0.579 | 0.161 | 0.118 | 0.214 | 0.773 | 0.783 | 0.973 |
| IPS | 0.866 | 0.017 | 0.003 | 0.906 | 0.025 | 0.008 | 0.005 | 0.976 | 0.763 | 0.693 |
| IPD | 0.766 | 0.017 | 0.028 | 0.886 | 0.085 | 0.003 | 0.014 | 0.982 | 0.867 | 0.763 |
| MSE | 0.940 | 0.000 | 0.064 | 0.764 | 0.019 | 0.070 | 0.003 | 0.806 | 0.460 | 0.530 |
| NoSA | 0.688 | 0.018 | 0.060 | 0.858 | 0.143 | 0.001 | 0.021 | 0.967 | 0.923 | 0.790 |
| CRP | 0.942 | 0.009 | 0.149 | 0.668 | 0.137 | 0.051 | 0.014 | 0.640 | 0.252 | 0.289 |
| ESR | 0.967 | 0.179 | 0.004 | 0.883 | 0.050 | 0.017 | 0.129 | 0.774 | 0.393 | 0.227 |
NoSa: Nitric oxide scavenging activity; MSE: Membrane stabilization activity; IPD: Inhibition of protein denaturation; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; EEI: Ear edem inhibition; IPS: inhibition of paw swelling.
3.4. Membrane stabilizing effect
During an inflammatory response, lysosomal components, including bactericidal enzymes and proteases are released by activated neutrophils release and result in further inflammation of tissue and a wide range of disorders (Bouhlali et al., 2018). Several anti-inflammatory drugs limit the inflammatory reaction by stabilizing the lysosomal membranes and thereafter inhibiting the release of their constituent (Mounnissamy et al., 2008). The resemblance between lysosomal and erythrocyte membrane suggest that the capacity of date seed extracts to stabilize membrane erythrocyte may also protect the lysosomal membranes (Hmidani et al., 2019). Based on data presented in Table 2, all analysed date seeds stabilise erythrocyte membrane in a dose-dependent way. Jihl seeds exhibited the highest membrane stabilizing activity by IC50 = 116.63 μg/mL and the lowest was found in Boufgous seeds by IC50 = 241.65 μg/mL. Date seed membrane stabilizing capacity reported here is higher compared to that of diclofenac sodium (IC50 = 304.87 μg/mL). Significant difference (p < 0.05) has been revealed between date seed varieties for the membrane stabilizing effect. Flavonoids may interact at the water lipid interface with the polar head of phospholipids and raised the rigidity of membrane, reduced fluidity and increased mechanical lipid bilayers stability (Tarahovsky et al., 2014). In addition, Oteiza et al. (2005) proposed that polyphenol interactions at the bilayer surface by hydrogen bonding could diminish access to harmful molecules (i.e., oxidants), thereby preserving membrane structure and function. High correlations between the membrane stabilizing effect and caffeic acid (R2 = 0.94), ferulic acid (R2 = 0.76) and luteolin (R2 = 0.81) were observed at a 95% confidence level. Moderation correlation (R2 = 0.53) was also found between quercetin and the membrane stabilizing effect (Table 3). Hence, the dissimilarity observed between the studied date seed varieties for the membrane stabilisation effect may be attributed to the differences their phenolic composition especially luteolin, quercetin, ferulic and caffeic acids.
3.5. C-reactive protein (CRP) determination
C-reactive protein (CRP) is an acute phase protein secreted by hepatocytes in response to circulating interleukin-6 (IL-6), and to a lesser extent in response to IL-1β and TNF-α during acute and chronic inflammatory conditions (González-Gallego et al., 2007). Significant (p < 0.05) anti-inflammatory effect monitored by the decrease in serum level of C reactive protein (CRP) was noticed in rat treated with date seeds compared to the saline control (carrageenan induced group) (14.37 mg/L). As displayed in Table 4, C reactive protein amount ranged from 7.14 mg/L for Jihl seeds to 12.39 mg/L for Boufgous seeds, both were greater than the C reactive protein level of indomethacin (5.32 mg/L) and normal control (2.24 mg/L). The interleukin-6 stimulates the replication of CRP mRNA by hepatocytes, which in turn is induced by tumor necrosis factor (TNF-α) and interleukin-1 (Sakande et al., 2013). Nile et al. (2016) have found that gallic acid, ferulic acid, caffeic acid and p-coumaric acid, the main polyphenolic molecules in analysed date seeds, revealed promising inhibitory effect of TNF-α and IL-6. Moreover, González-Gallego et al. (2007), found that high intake of flavonoid-rich foods are negatively correlated with CRP levels. Hence, the disparity between the studied date seed extracts to regulate the CRP level depends the quantity and chemical diversity of phenolic compounds.
Table 4.
Effect of date seed cultivars on erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP).
| CRP (mg/L) | ESR (mm/h) | |
|---|---|---|
| Boufgous seeds | 12.39 ± 0.32a | 6.07 ± 0.23a |
| Bousthammi seeds | 8.56 ± 0.40b | 5.39 ± 0.17b |
| Jihl seeds | 7.14 ± 0.33c | 4.47 ± 0.18c |
| Majhoul seeds | 10.04 ± 0.27d | 5.72 ± 0.20ab |
| Normal control | 2.24 ± 0.18e | 1.82 ± 0.13d |
| Indomethacin | 5.32 ± 0.28f | 3.54 ± 0.23e |
| Saline | 14.37 ± 0.31g | 6.72 ± 0.20f |
Values expressed in average (n = 6) ± SE.
Means in the same column followed by different letters are significantly different from each other according to post hoc Bonferroni tests (p < 0.01).
3.6. Erythrocyte sedimentation rate (ESR)
The rate of erythrocyte sedimentation (ESR) remains a useful method for diagnosing inflammatory diseases, especially, rheumatoid arthritis (Wolfe et Pincus, 2001). During the inflammatory process, great amount of fibrinogen is released in blood (Esmon, 2005) which causes red cells to stick to each other, resulting in raising ESR (Harrison, 2015). The results of this test are given in Table 4. All extracts demonstrated significant (p < 0.01) reduction of erythrocyte sedimentation rate as compared to saline control (6.72 mm/h). Significant differences (p < 0.01) were established among the studied date seeds varieties which may depend the quantity and chemical diversity of their phenolic compounds. The strongest reduction of erythrocyte sedimentation rate was observed for Jihl by (4.47 mm/h) and the least potent effect was found in Boufgous seeds (6.07 mm/h). These results still lower compared to that found for the indomethacin (3.54 mm/h). The study of Kleemann et al. (2011), conducted using the humanized atherosclerosis model, ApoE3L mice, has revealed that quercetin supplementation reduced significantly fibrinogen level during atherogenic process. Moreover, Dallas et al. (2014) found that the consumption of a citrus polyphenolic for 12-week reduced significantly circulating level of CRP and fibrinogen based on study conducted on volunteers. Wannamethee et al. (2011) observed low concentrations of CRP and fibrinogen in men taking vitamin C supplements.
3.7. Croton oil-induced inflammation
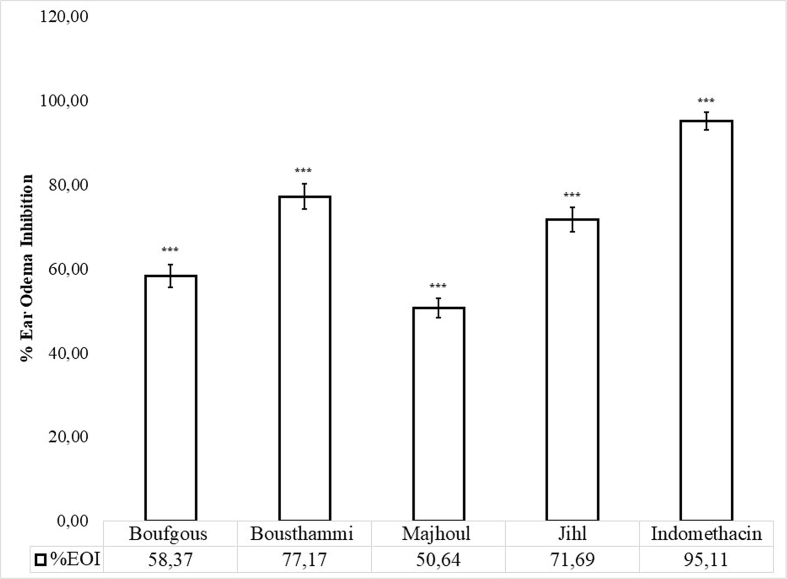
Inflammatory response caused by Croton oil is a commonly used model for evaluating the topical anti-inflammatory efficacy of various substances (Cabrini et al., 2011). Topical use of Croton oil or its predominant phorbol ester, the 12-O-tetradecanoylphorbol-13-acetate (TPA), provokes an intense dermatitis characterized by vasodilatation, leukocytes migration and edema establishment (Cabrini et al., 2011). These inflammatory reactions are mediated by protein kinase C that stimulates a rise in phospholipase A2, cyclooxygenase and lipoxygenase activities resulting in the release of arachidonic acid, prostaglandins and leukotrienes (Otuki et al., 2005). Other inflammation mediators such as histamine 5-hydroxitriptamine, bradykinin have been implicated in these inflammatory processes (Junior et al., 2004). The topical application of date seed extracts mitigated significantly ear thickness compared to the control mice (Figure 2). Significant variations (p < 0.01) in ear edema inhibition were observed between the studied date seeds varieties. Bousthammi seeds possessed the greatest diminution of ear edema (77.17%), while Majhoul seeds presented the least potent effect on ear edema (50.64%). The inhibition of ear edema obtained by indomethacin was 95.11%. These findings suggest that date seeds extract may regulate at least one step of the inflammatory pathwaycaused by the croton oil. In fact, rutin, quercetin and luteolin efficiently inhibited lipoxygenase, cyclooxygenase and phospholipase (PLA2) pathway (Lee and Kim, 2010; Kumar et al., 2014; Yarla et al., 2015). Moreover, the study of Nile et al. (2016) has revealed that p-coumaric, ferulic, caffeic and gallic acids have demonstrated potential inhibition of cyclooxygenase-2. The strong relationships were found between the inhibition of ear edema and luteolin (R2 = 0.77), rutin (R2 = 0.78) and quercetin (R2 = 0.97). While moderate correlations were found between this effect and caffeic acid (R2 = 0.52) and ferulic acid (R2 = 0.58) (Table 3). Hence, the differences in ear thickness inhibition capacity among date seeds varieties might depend the quantity and chemical diversity of their phenolic compounds.
Figure 2.
Inhibition of croton oil induced mice ear edema by date seed cultivars. Values expressed in average (n = 6) ± SE. *** indicates a significant difference (p < 0.001) vs. negative control group.
3.8. Carrageenan induced rat paw edema
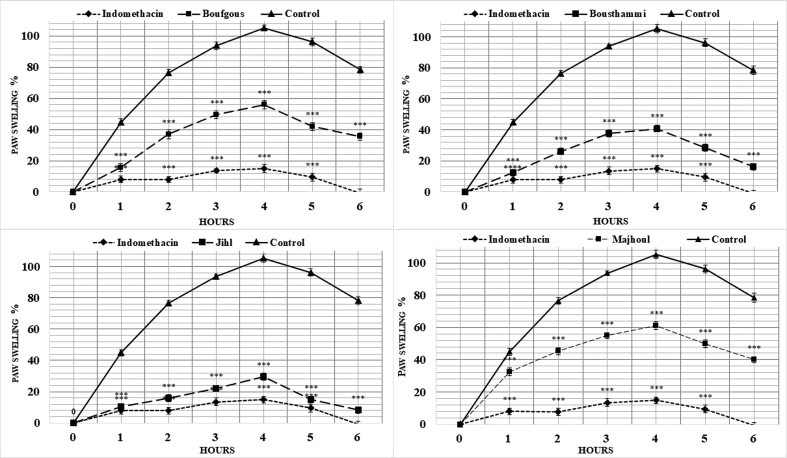
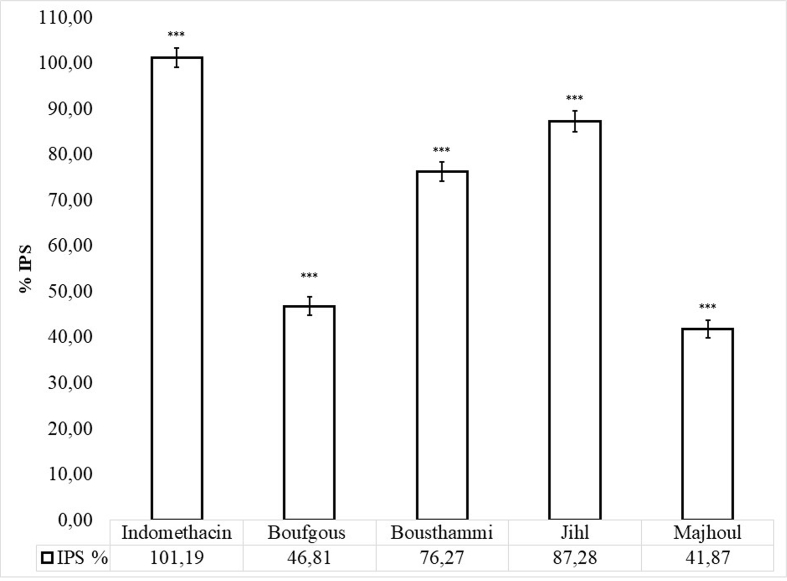
Carrageenan-induced paw edema assay has been widely used to determine anti-inflammatory effect and is supposed to be biphasic (Tsai et al., 2015). The early stage (0–1 h) is characterised with the secretion of histamine, serotonin, bradykinin, and overproduction of prostaglandins in surrounding damaged tissue (Begum et al., 2015). The later stage (1–6 h) is the target of the most clinically effective anti-inflammatory drugs due to an overproduction of pro-inflammatory mediators like bradykinin, leukotrienes, prostaglandins, platelet-activating factor, nitric oxide and proteolytic enzymes by neutrophil in the inflamed tissues (Tsai et al., 2015; Kuedo et al., 2016).The effect of date seed extracts on carrageenan-induced swelling of rat paw was monotored for up to 6 h (Figure 4). For all tested extracts, paw swelling reaches the maximum level on the 4th hour and ranged from 29.54% for Jihl seed to 61.10 % for Majhoul seed (p < 0.05). In the treated rats, date seed extracts, given at a dose of 30 mg/kg, caused a significant diminution in paw swelling levels at 6 h of treatment and varied significantly (p < 0.01) between 41.87% for Majhoul seed and 87.28 % for Jihl seed as compared to the saline control (Figure 3). Indomethacin was more effective than date seed extracts (p < 0.01) as it caused a 101.19% reduction in paw swelling levels. Saline had no effect (0%). The low anti-edematogenic effect observed for indomethacin during the early stage looks to be normal since the non-steroidal anti-inflammatory remedies like aspirin or indomethacin are unable to inhibit the early stage of swelling as reported by Salvemini et al. (1996). The fact that the oral administration of date seed extracts inhibited significantly (p < 0.05) paw edema formation during all phases of inflammation (Figure 4), suggests that date seed compounds inhibit diverse facets and chemical mediators of inflammation. Therefore, we hypothesize that these compounds might be acting through the inhibition of histamine release, cyclooxygenase enzymes that produced prostaglandin, lysosomal enzymes as well as scavenging ability the free radical produced by polymorphonuclear leucocytes that would lead to tissue damage in the site of inflammation. According to Kimata et al. (2000), histamine, prostaglandin and leukotrienes release is inhibited by luteolin and quercetin. Chlorogenic and caffeic acids also inhibited histamine and leukotriene production (Kimura et al., 1985). Jayaprakasam et al. (2006) have found that ferulic and caffeic acids inhibited the enzymes COX-1 and COX-2. Moreover, using in-vitro assay, Kim et al. (2005) have found the production of histamine and pro-inflammatory cytokines such as TNF-α and IL-6 from human activated mast cells was inhibited by gallic acid. Indeed, there was a strong correlation between paw swelling inhibition with caffeic acid (R2 = 0.87), ferulic acid (R2 = 0.91), luteolin (R2 = 0.97), rutin (R2 = 0.76) and quercetin (R2 = 0.70) (Table 3). Therefore, the synergistic effect of these phenolic compounds identified in date seed may be responsible of their potent anti-inflammatory activity. These variations of reduction in paw swelling levels among date seeds varieties may be related to the quantity and chemical diversity of their phenolic moleculesmainly, luteolin, rutin, quercetin, caffeic and ferulic acids.
Figure 4.
Evolution of edema formation in rat paws. The animals (n = 6) were treated with aqueous extract of four date seed varieties at 30 mg/kg: Indomethacin was used as a positive control (10 mg/kg). Data obtained from animal experiments were expressed as mean ± SE. ∗∗∗: points out the significant differences (p < 0.001) from the negative control: carrageenan induced group.
Figure 3.
Inhibition of carrageenan induced paw swilling by date seed cultivars at 6th hour. Results are the mean values (n = 6) ±SE. ∗∗∗: points out the significant differences (p < 0.001) from the saline control: carrageenan induced group.
The strong linear relationships (R2 ≥ 0.736) of carrageenan-induced rat paw edema with both in-vitro inflammatory assays, hematological and biochemical parameters (Table 5) show that the stabilization of the lysosomal membranes, the inhibition of protein denaturation, the ability to scavenge nitric oxide free radical and the inhibition of c-reactive protein and fibrinogen production may be the action mechanisms of date seed to attenuate the paw thickness induced by carrageenan. High correlations between Croton oil induced ear edema reduction and nitric scavenging activity (R2 = 0.882), inhibition of protein denaturation (R2 = 0.874) and membrane stabilizing effect (R2 = 0.684), however moderate to low associations have been established between Croton oil induced ear edema and C-reactive protein level (R2 = 0.438) and erythrocyte sedimentation rate (R2 = 0.377). In line with these findings, Tatiya et al. (2017) have reported significant correlations between inflammation developments assessed using adjuvant-induced arthritis in rats assay and membrane stabilizing effect, inhibition of protein denaturation, ESR and CRP in the other hand. Moreover, the study of Garg et al. (2017) has revealed that the inflammatory disease is associated with augmented concentration of serum nitrite (NO) concentration CRP and ESR. Also, Vazquez et al. (2015) studying the systemic changes in rats treated by carrageenan have found wide modifications in some blood parameters, such as fibrinogen, CRP and nitric oxide (NO) levels. By comparing the correlation coefficients (R-value), it is possible to suggest that nitric scavenging activity, inhibition of protein denaturation and membrane stabilizing effect of date seed extracts are highly responsible for the anti-inflammatory activity determined using the croton oil induced ear edema assay.
Table 5.
Relationship between NoSa, MSE, IPD, CRP, ESR, EEI and IPS of date seed extract.
| NoSa | MSE | IPD | CRP | ESR | EEI | IPS | |
|---|---|---|---|---|---|---|---|
| NoSa | 1 | ||||||
| MSE | 0.732 | 1 | |||||
| IPD | 0.991 | 0.808 | 1 | ||||
| CRP | 0.516 | 0.938 | 0.608 | 1 | |||
| ESR | 0.611 | 0.826 | 0.683 | 0.870 | 1 | ||
| EEI | 0.882 | 0.684 | 0.874 | 0.438 | 0.377 | 1 | |
| IPS | 0.948 | 0.900 | 0.981 | 0.736 | 0.779 | 0.829 | 1 |
NoSa: Nitric oxide scavenging activity; MSE: Membrane stabilization activity; IPD: Inhibition of protein denaturation; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; EEI: Ear edem inhibition; IPS: inhibition of paw swelling.
4. Conclusion
This is the first study to investigate the anti-inflammatory propriety of date seed extracts. Our findings suggest that these extracts mediate anti-inflammatory activities through the inhibition of protein denaturation, stabilisation of the lysosomal membranes, nitric oxide free radical scavenging ability and inhibition of C-reactive protein and fibrinogen production. These findings may be connected with considerable quantity of phenolic compounds as rutin, quercetin, p-coumaric and caffeic acids present in these date seed varieties, especially Jihl seeds which showed the most important anti-inflammatory activity among analysed date seed varieties. Further investigations are underway in our lab to address the precise mechanisms involved for effective and safe usage.
Declarations
Author contribution statement
Eimad dine Tariq BOUHLALI: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Abdelbasset HMIDANI, Tarik KHOUYA: Performed the experiments; Analyzed and interpreted the data.
Bouchra BOURKHIS: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mhamed RAMCHOUN: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Younes FILALI-ZEGZOUTI, Chakib ALEM: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank Pr. Harnafi, Pr. Amrani and Dr. Adil Essarioui for their valuable guidance that helped improve the quality of the research.
References
- Al-Farsi M.A., Lee C.Y. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008;108(3):977–985. doi: 10.1016/j.foodchem.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Ali H., Alli I., Ismail A., Kermasha S. Protein-phenolic interactions in food. Eurasian. J. Anal. Chem. 2012;7(3):123–133. [Google Scholar]
- Asanuma M., Nishibayashi-Asanuma S., Miyazaki I., Kohno M., Ogawa N. Neuroprotective effects of non-steroidal anti-inflammatory drugs by direct scavenging of nitric oxide radicals. J. Neurochem. 2001;76(6):1895–1904. doi: 10.1046/j.1471-4159.2001.00205.x. [DOI] [PubMed] [Google Scholar]
- Beg S., Swain S., Hasan H., Barkat M.A., Hussain M.S. Systematic review of herbals as potential anti-inflammatory agents: recent advances, current clinical status and future perspectives. Pharmacogn. Rev. 2011;5(10):120. doi: 10.4103/0973-7847.91102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum R., Sharma M., Pillai K.K., Aeri V., Sheliya M.A. Inhibitory effect of Careyaarborea on inflammatory biomarkers in carrageenan-induced inflammation. Pharm. Biol. 2015;53(3):437–445. doi: 10.3109/13880209.2014.923005. [DOI] [PubMed] [Google Scholar]
- Bnouham M., Mekhfi H., Legssyer A., Ziyyat A. Ethnopharmacology Forum Medicinal plants used in the treatment of diabetes in Morocco. Int. J. Diabetes. Metab. 2002;10:33–50. [Google Scholar]
- Boora F., Chirisa E., Mukanganyama S. Evaluation of nitrite radical scavenging properties of selected Zimbabwean plant extracts and their phytoconstituents. J. Food Process. 2014 [Google Scholar]
- Bouhlali E.D.T., Alem C., Ennassir J., Benlyas M., Mbark A.N., Zegzouti Y.F. Phytochemical compositions and antioxidant capacity of three date (Phoenix dactylifera L.) seeds varieties grown in the South East Morocco. J. Saudi. Soc. Agric. Sci. 2017;16(4):350–357. [Google Scholar]
- Bouhlali E.D.T., El Hilaly J., Ennassir J., Benlyas M., Alem C., Amarouch M.Y., Filali-Zegzouti Y. Anti-inflammatory properties and phenolic profile of six Moroccan date fruit (Phoenix dactylifera L.) varieties. J. King Saud Univ. Sci. 2018;30(4):519–526. [Google Scholar]
- Cabrini D.A., Moresco H.H., Imazu P., Silva C.D.D., Pietrovski E.F., Mendes D.A.G.B., Otuki M.F. Analysis of the potential topical anti-inflammatory activity of Averrhoa carambola L. in mice. Evid. Based. Complement. Alternat. Med. 2011 doi: 10.1093/ecam/neq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001;1(8):1397–1406. doi: 10.1016/s1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- Czubinski J., Dwiecki K. A review of methods used for investigation of protein–phenolic compound interactions. Int. J. Food Sci. Technol. 2017;52(3):573–585. [Google Scholar]
- Dallas C., Gerbi A., Elbez Y., Caillard P., Zamaria N., Cloarec M. Clinical study to assess the efficacy and safety of a citrus polyphenolic extract of red orange, grapefruit, and orange (Sinetrol-XPur) on weight management and metabolic parameters in healthy overweight individuals. Phytother Res. 2014;28(2):212–218. doi: 10.1002/ptr.4981. [DOI] [PubMed] [Google Scholar]
- Debnath T., Park S.R., Kim D.H., Jo J.E., Lim B.O. Anti-oxidant and anti-inflammatory activities of Inonotus obliquus and germinated brown rice extracts. Molecules. 2013;18(8):9293–9304. doi: 10.3390/molecules18089293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisha I.L., Dzoyem J.P., McGaw L.J., Botha F.S., Eloff J.N. The anti-arthritic, anti-inflammatory, antioxidant activity and relationships with total phenolics and total flavonoids of nine South African plants used traditionally to treat arthritis. BMC Compl. Alternative Med. 2016;16(1):307. doi: 10.1186/s12906-016-1301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mergawi R., Al-Humaid A., El-Rayes D. Phenolic profiles and antioxidant activity in seeds of ten date cultivars from Saudi Arabia. J. Food Agric. Environ. 2016;14(2):38–43. [Google Scholar]
- El-Rahman S.N.A., Al-Mulhem S.I. Characteristic analysis, antioxidant components and antioxidant activity of date fruits, DateSeeds and palm shell. Clin. Med. Case Rep. 2017;9(10) [Google Scholar]
- Esmon C.T. The interactions between inflammation and coagulation. Br. J. Haematol. 2005;131(4):417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- Garg N., Syngle A., Krishan P. Nitric oxide: link between inflammation and Endothelial dysfunction in rheumatoid arthritis. Int. J. Angiol. 2017;26(3):165–169. doi: 10.1055/s-0036-1597577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Gallego J., Sánchez-Campos S., Tuñón M.J. Anti-inflammatory properties of dietary flavonoids. Nutr. Hosp. 2007;22(3):287–293. [PubMed] [Google Scholar]
- Harrison M. Erythrocyte sedimentation rate and C-reactive protein. Aust. Prescr. 2015;38(3):93–94. doi: 10.18773/austprescr.2015.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmidani A., Bouhlali E.D.T., Khouya T., Ramchoun M., Filali-Zegzouti Y., Alem C., Benlyas M. Antioxidant, anti-inflammatory and anticoagulant activities of three Thymus species grown in southeastern Morocco. Future. J. Pharm. Sci. 2019;5(1):4. [Google Scholar]
- Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C., Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid. Med. Cell. Longev. 2016 doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaprakasam B., Vanisree M., Zhang Y., Dewitt D.L., Nair M.G. Impact of alkyl esters of caffeic and ferulic acids on tumor cell proliferation, cyclooxygenase enzyme, and lipid peroxidation. J. Agric. Food Chem. 2006;54(15):5375–5381. doi: 10.1021/jf060899p. [DOI] [PubMed] [Google Scholar]
- Junior O.D., Andreucci V.C., Da Silva Cunha I.B., Araujo C.E.P., De Oliveira F., Marcucci M.C. Investigation of the anti-inflammatory and analgesic activities of a sample of Brazilian propolis. Acta Farm. Bonaerense. 2004;23(3):285–291. [Google Scholar]
- Kim S.H., Jun C.D., Suk K., Choi B.J., Lim H., Park S., Shin T.Y. Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol. Sci. 2005;91(1):123–131. doi: 10.1093/toxsci/kfj063. [DOI] [PubMed] [Google Scholar]
- Kimata M., Shichijo M., Miura T., Serizawa I., Inagaki N., Nagai H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin. Exp. Allergy. 2000;30(4):501–508. doi: 10.1046/j.1365-2222.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Okuda H., Okuda T., Hatano T., Agata I., Arichi S. Studies on the activities of tannins and related compounds from medicinal plants and drugs. VI. Inhibitory effects of caffeoylquinic acids on histamine release from rat peritoneal mast cells. Chem. Pharm. Bull. 1985;33(2):690–696. doi: 10.1248/cpb.33.690. [DOI] [PubMed] [Google Scholar]
- Kleemann R., Verschuren L., Morrison M., Zadelaar S., van Erk M.J., Wielinga P.Y., Kooistra T. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis. 2011;218(1):44–52. doi: 10.1016/j.atherosclerosis.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Kuedo Z., Sangsuriyawong A., Klaypradit W., Tipmanee V., Chonpathompikunlert P. Effects of astaxanthin from Litopenaeusvannamei on carrageenan-induced edema and pain behavior in mice. Molecules. 2016;21(3):382. doi: 10.3390/molecules21030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Singh M., Rawat J.K., Gautam S., Saraf S.A., Kaithwas G. Effect of rutin against gastric esophageal reflux in experimental animals. Toxicol. Mech. Methods. 2014;24(9):666–671. doi: 10.3109/15376516.2014.961215. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Kim G.H. Evaluation of antioxidant and inhibitory activities for different subclasses flavonoids on enzymes for rheumatoid arthritis. J. Food Sci. 2010;75(7) doi: 10.1111/j.1750-3841.2010.01755.x. [DOI] [PubMed] [Google Scholar]
- Messaoudi R., Abbeddou S., Mansouri A., Calokerinos A.C., Kefalas P. Phenolic profile and antioxidant activity of date-pits of seven Algerian date palm fruit varieties. Int. J. Food Prop. 2013;16(5):1037–1047. [Google Scholar]
- Mounnissamy V.M., Kavimani S., Balu V., Quine S.D. Evaluation of anti-inflammatory and membrane stabilizing property of Ethanol extract of cansjerarheedii J. Gmelin (Opiliaceae). Iran. J. Pharmacol. Ther. 2008;6(2):235–237. [Google Scholar]
- Nile S.H., Ko E.Y., Kim D.H., Keum Y.S. Screening of ferulic acid related compounds as inhibitors of xanthine oxidase and cyclooxygenase-2 with anti-inflammatory activity. Rev. Bras. Farmacogn. 2016;26(1):50–55. [Google Scholar]
- Ojha H., Mishra K., Hassan M.I., Chaudhury N.K. Spectroscopic and isothermal titration calorimetry studies of binding interaction of ferulic acid with bovine serum albumin. Thermochim. Acta. 2012;548:56–64. [Google Scholar]
- Oteiza P.I., Erlejman A.G., Verstraeten S.V., Keen C.L., Fraga C.G. Flavonoid-membrane interactions: a protective role of flavonoids at the membrane surface? J. Immunol. Res. 2005;12(1):19–25. doi: 10.1080/10446670410001722168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otuki M.F., Vieira-Lima F., Malheiros A., Yunes R.A., Calixto J.B. Topical antiinflammatory effects of the ether extract from Protiumkleinii and α-amyrinpentacyclictriterpene. Eur. J. Pharmacol. 2005;507(1):253–259. doi: 10.1016/j.ejphar.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Ozdal T., Capanoglu E., Altay F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013;51(2):954–970. [Google Scholar]
- Sakande J., Kabre E., Lompo M., Pale E., Nikiema J.B., Nacoulma O.G., Guissou I.P. Anti-inflammatory and antioxidant activities of a fraction I1 of male inflorescences of Borassusaethiopum Mart.(Arecaceae) Am. J. Biochem. Mol. Biol. 2013;3:101–109. [Google Scholar]
- Salvemini D., Wang Z.Q., Wyatt P.S., Bourdon D.M., Marino M.H., Manning P.T., Currie M.G. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1996;118(4):829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugapriya M., Patwardhan K. Uses of date palm in Ayurveda. In: Manickavasagan A., Essa M.M., Sukumar E., editors. Dates: Production, Processing, Food, and Medicinal Values. CRC Press; Boca Raton: 2012. pp. 377–385. [Google Scholar]
- Tarahovsky Y.S., Kim Y.A., Yagolnik E.A., Muzafarov E.N. Flavonoid–membrane interactions: involvement of flavonoid–metal complexes in raft signaling. Biochim. Biophys. Acta Biomembr. 2014;1838(5):1235–1246. doi: 10.1016/j.bbamem.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Tatiya A.U., Saluja A.K., Kalaskar M.G., Surana S.J., Patil P.H. Evaluation of analgesic and anti-inflammatory activity of Brideliaretusa (Spreng) bark. J. Tradit. Complement Med. 2017;7(4):441–451. doi: 10.1016/j.jtcme.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai D.S., Huang M.H., Tsai J.C., Chang Y.S., Chiu Y.J., Lin Y.C., Peng W.H. Analgesic and anti-Inflammatory activities of rosataiwanensisnakai in mice. J. Med. Food. 2015;18(5):592–600. doi: 10.1089/jmf.2014.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tungmunnithum D., Thongboonyou A., Pholboon A., Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines. 2018;5(3):93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez E., Navarro M., Salazar Y., Crespo G., Bruges G., Osorio C., López M. Systemic changes following carrageenan-induced paw inflammation in rats. Inflamm. Res. 2015;64(5):333–342. doi: 10.1007/s00011-015-0814-0. [DOI] [PubMed] [Google Scholar]
- Wannamethee S.G., Lowe G.D., Rumley A., Bruckdorfer K.R., Whincup P.H. Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am. J. Clin. Nutr. 2006;83(3):567–574. doi: 10.1093/ajcn.83.3.567. [DOI] [PubMed] [Google Scholar]
- Wolfe F., Pincus T. The level of inflammation in rheumatoid arthritis is determined early and remains stable over the longterm course of the illness. J. Rheumatol. 2001;28(8):1817–1824. [PubMed] [Google Scholar]
- Yarla N.S., Satyakumar K., Srinivasu D., DSVGK K., Aliev G., Dharmapuri G., Putta G.R.S.P.S., Jagarlapoodi S., Bheeram V., Sadu S.P., Duddukuri G.R. Phospholipase A2: a potential therapeutic target in inflammation and cancer (In silico, In vitro, In vivo and clinical approach) J. Cancer Sci. Ther. 2015;7:8. [Google Scholar]
- Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food. Sci. 2016;8:33–42. [Google Scholar]