Abstract
Background
Salting-out extraction (SOE) had been developed as a special branch of aqueous two-phase system recently. So far as we know, few reports involved in extracting ginsenosides with SOE because of the lower recovery caused by the unique solubility and surface activity of ginsenosides. A new SOE method for rapid pretreatment of ginsenosides from the enzymatic hydrolysates of Panax quinquefolium was established in this article.
Methods
The SOE system comprising ethanol and sodium carbonate was selected to extract ginsenosides from the enzymatic hydrolysates of Panax quinquefolium, and HPLC was applied to analyze the ginsenosides.
Results
The optimized extraction conditions were as follows: the aqueous two-phase extraction system comprising ethanol, sodium carbonate, ethanol concentration of 41.51%, and the mass percent of sodium carbonate of 7.9% in the extraction system under the experimental condition. Extraction time had minor influence on extraction efficiency of ginsenosides. The results also showed that the extraction efficiencies of three ginsenosides were all more than 90.0% only in a single step.
Conclusion
The proposed method had been successfully applied to determine ginsenosides in enzymatic hydrolysate and demonstrated as a powerful technique for separating and purifying ginsenosides in complex samples.
Keywords: enzymatic hydrolysate, ethanol, ginsenosides, salting-out extraction, sodium carbonate
Highlights
-
•
Salting-out extraction of ginsenosides was realized using ethanol/salt aqueous two-phase system.
-
•
The factors influencing the extraction efficiency of ginsenosides were investigated.
-
•
The high recovery of ginsenosides was obtained only in a single step.
-
•
The extraction was successfully applied to determine ginsenosides in enzymatic hydrolysate.
1. Introduction
Ginsenosides are the primary bioactive components in Panax Quinquefolii Radix (Panax quinquefolius L.) and usually used as therapeutic agents, dietary supplements, and raw materials of healthy food [1], [2]. Owing to their significant biological activities, the extraction, separation, and analysis of ginsenosides are always the focus of people's attention [3], [4], [5], [6]. As far as we know, more than 70 ginsenosides have been identified, isolated, and characterized [7]. Recently amylase-assisted extraction for preparing ginsenosides has attracted people's concern [8]. However, the composition of the enzyme hydrolysate is very complex. Meanwhile, the complex matrices could interfere with the analysis of effective components in enzymatic hydrolysate severely.
Aqueous two-phase extraction (ATPE) was an effective technique to pretreat complexed samples, and the extraction system usually comprised polyethylene glycol (PEG)/salt or PEG/dextran. Recently, a special branch of aqueous two-phase system (ATPS), namely salting-out extraction (SOE), which based on low molecular organic solvents (e.g., methanol, ethanol, acetonitrile, and n-propanol) and inorganic salts, had been developed. Owing to the obvious advantages such as being easier to scale up, quicker phase separation, lower cost, and lower toxicity [9], [10], the extraction mechanism and the application of SOE attracted broad attention in the separation field. Some works had been performed by the technique of extracting bioactive compounds from natural plants resources, such as anthocyanins from grape juice [11], phenylethanoid glycosides from Cistanche deserticola Y. C. Ma stems [12], alkaloids from Sophora flavescens Ait. [13], phenolic components from Ficus carica L. [14] and grape seeds [15], polysaccharides from Semen Cassiae [16], lignans from Zanthoxylum armatum [17], polyphenols and anthraquinones from Polygonum cuspidatum [18], and diosgenin and steroidal saponins from fermentation [19]. So far as we know, few reports involved in extracting ginsenosides with SOE because of the lower recovery caused by the unique solubility and surface activity of ginsenosides. Ionic liquid-salt ATPE had been proved as a reliable, rapid, and sensitive method for extracting rare ginsenosides from Xue-Sai-Tong injection. However, the method must use large amounts of ionic liquid such as [Bmim]Br [20]. In this article, a new SOE method was established without the use of ionic liquid and, meanwhile, the extraction conditions of ginsenosides were investigated. In addition, the method was successfully applied to determine ginsenosides in the enzymolysis liquid of Panacis Quinquefolii Radix.
2. Experiment
2.1. Materials and reagents
Panacis Quinquefolii Radix samples were bought from a local drug store in Jinan (Shandong, China), powdered in a mill, and passed through a 40-mesh sieve. Cellulase (60000 u/ml, Jinan, China) was used. The standard ginsenoside Rg1, Re, and Rb1 were purchased from National Institute for Food and Drug Control (Beijing, China). Acetonitrile and methanol were HPLC grade (Tedia company, Inc., Fairfield, USA). Dipotassium phosphate (K2HPO4), sodium sulfate (Na2SO4), ammonium sulfate ((NH4)2SO4), and sodium carbonate (Na2CO3) were purchased from Chinese Pharmaceutical Group Shanghai Chemical Reagent Company. Phosphoric acid, n-butanol, and ethanol were procured from Tianjin Bodi Chemical Ltd. Other chemicals used were of analytical grade. The water used in the mobile phase was double distilled.
2.2. Apparatus
The analysis was carried out on a Shimadzu HPLC equipped with an LC-10ATvp pump, an injector with 20-μL loop and an SPD-10Avp UV-VIS detector. The chromatograms were integrated with the EZChrom Elite chromatographic software. UV spectrophotometer (Spectronic UV-160A, Shimadzu, Japan) and Mettler AE 240 electronic balance (0.01 mg/20g, Switzerland) were used.
2.3. Analysis of ginsenosides
2.3.1. Preparation of the standard solution
Ginsenoside Rg1 (0.1 mg mL−1), Re (0.4 mg mL−1), Rb1 (1.0mg mL−1), and mixed stock standard solution were prepared in methanol. From the obtained solution, a series of standard solution with different concentrations were prepared.
2.3.2. Chromatographic conditions
A Venusil XBP C18 column (250 mm × 4.6 mm i.d., 5μm, theoretical plate number > 5000) was used for determining ginsenosides in a binary solvent that consisted of acetonitrile (A) and 0.1% phosphoric acid solution (B) with a gradient elution: 0.01–25 min, 19–20% A, 81–80% B; 25.01–60 min, 20–40% A, 80–60% B; 60.01–80 min, 40.1–100% A, 59.9–0% B.
The mixture was first filtered through a 0.45-μm membrane filter and then degassed. The flow rate of mobile phase was 1.0 mL min−1. The column temperature was kept at 30°C. The UV–VIS detector was set at 203 nm.
2.3.3. HPLC determination
The top phase was taken and evaporated to dryness. Then 3.0 ml of water was added to resolve and transferred to a test tube, extracted with 6.0 ml of water-saturated n-butanol twice, evaporated to dryness. The residue was dissolved in 1.0 ml of 50% methanol and filtrated. The filtrate was kept for HPLC analysis.
2.4. Amylase-assisted extraction
Fifty milligram of drug power of Panacis Quinquefolii Radix (40 meshes) was taken, weighed precisely, and put into 50-ml round bottom flask. Twenty-five milliliter of water and enzyme solution was then added; the pH value of the mixture was adjusted to 4.0 with 0.12 mol L−1 HCl and extracted for 75 min at 47°C using a water bath.
2.5. SOE procedure
Ethanol was used to prepare SOE systems with different salt solution in water, such as sodium sulfate (0.585g→6ml), potassium dihydrogen phosphate (1.615g→7ml), ammonium sulfate (0.915g→5ml), and sodium carbonate (0.873g→7ml), while the total volume of the SOE systems was maintained at 10ml. Among them, ethanol and sodium carbonate (Na2CO3) were selected to prepare the ATPS according to a certain mass ratio of the solution. 7.0 mL of Na2CO3 solution containing 25-mg ginsenosides was mixed with 3.0 mL of ethanol in a 25-mL centrifuge tube. The mixed liquid was treated ultrasonically for 5 min and allowed to stand for the phase separation. Then the phase volume ratio was recorded and the ginsenosides were extracted into the top phase. In addition, the top and bottom phases were collected and analyzed for the ginsenosides concentration. All the experiments were performed trice and the average values had been taken.
3. Results and discussion
3.1. Figures of merit
The analytical parameters of the ginsenosides were determined under the optimized experimental conditions. Results indicated that the ideal linearity relationships, satisfactory repeatability, and high sensitivity were obtained, such as repeatability for peak area (relative standard deviation (RSD) = 1.24 ~2.77%, n = 6), correlation coefficient (more than 0.9990), and limit of detection (less than 0.40 μg mL−1)for the three ginsenosides.
Ginsenoside Rg1, Re, and Rb1 were separated according to the baseline, and no interference was observed from the complex sample matrix under the optimized experimental conditions. The severe interference caused by the impurities was significantly reduced. The impurity peaks, especially the water-soluble impurities, decreased significantly. The accuracy of the method had been studied by adding the three ginsenosides into the herbal material and the mixed solution to assess matrix effects, respectively. The contents of ginsenosides in the aforementioned samples were detected and calculated by the external standard method. Results in Table 1 showed that the average recoveries of ginsenoside Rg1, Re, and Rb1 (at spiking level of 0.210, 1.227, 2.430 mg in 2.0 ml of sample solution, respectively) were 101.40, 92.50, and 99.61% (RSD <6.1, n = 5) for the herbal material and 101.02, 92.57, and 101.57 (RSD <6.5, n = 5) for the mixed solution, respectively.
Table 1.
Recovery test of three ginsenosides (n = 5)
| Analyte | Sample | Added (mg) | Found (mg) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Ginsenoside Rg1 | Herbal material | — | 0.1955 | — | 1.3 |
| 0.21 | 0.4084 | 101.40 | 2.1 | ||
| Mixed solution | — | 0.1955 | — | 2.7 | |
| 0.21 | 0.4076 | 101.02 | 2.0 | ||
| Ginsenoside Re | Herbal material | — | 1.2130 | — | 2.2 |
| 1.227 | 2.3480 | 92.50 | 6.1 | ||
| Mixed solution | — | 1.2129 | — | 2.8 | |
| 1.227 | 2.3488 | 92.57 | 6.5 | ||
| Ginsenoside Rb1 | Herbal material | — | 2.2788 | — | 1.5 |
| 2.43 | 4.6994 | 99.61 | 2.0 | ||
| Mixed solution | — | 2.2791 | — | 1.9 | |
| 2.43 | 4.7472 | 101.57 | 4.4 |
3.2. Optimization of SOE conditions
3.2.1. The ATPS for extracting ginsenosides
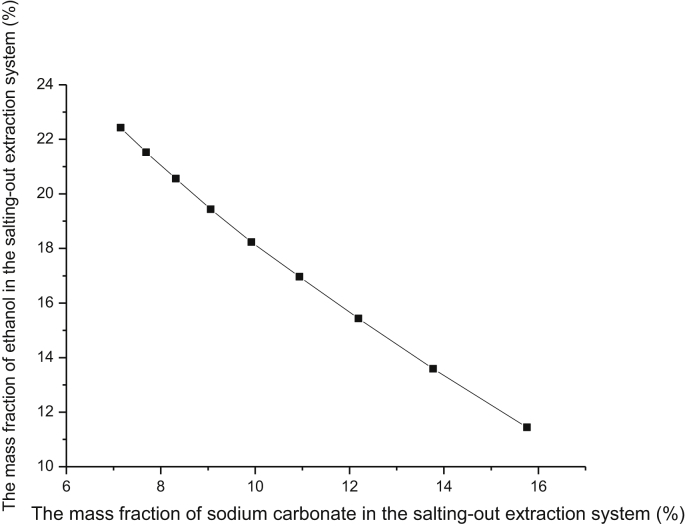
The extraction system comprising organic solvent and inorganic salt was used owing to its many advantages, and ethanol was selected as phase-forming organic solvent because of its cheapness, nontoxicity, harmlessness, and the ideal solubility for ginsenosides. To select the proper salt for extracting ginsenosides, a series of salts such as sodium sulfate, potassium dihydrogen phosphate, ammonium sulfate, and sodium carbonate were used as phase forming salts to prepare ATPS with ethanol. Fig. 1 showed the ATPS phase diagram for ethanol/Na2CO3 system, which was plotted according to the turbidity point method. With the addition of ethanol to the concentrated salt aqueous solution, the ATPS was formed after ultrasonic treatment and phase separation. Then a top ethanol-rich phase and a bottom salt-rich phase appeared. The ginsenosides were maintained at 2.5 mg in the total phase system, and all the experiments were at room temperature. Results in Fig. 2 showed that the ginsenosides were easier to partition to the top phase except the ethanol-ammonium sulfate system. This phenomenon would be helpful for the enrichment of ginsenosides. It could also be seen that the extraction efficiency of ginsenosides obtained by the ethanol-sodium carbonate system was higher than that obtained by the other three systems. The ginsenosides showed higher selectivity in the ethanol-sodium carbonate system. So Na2CO3 was selected as the phase-forming salt. The reason that the ginsenosides could not partition to the top phase of the ethanol-ammonium sulfate system was worth further discussion.
Fig. 1.
Phase diagram of the salting-out extraction system composed of ethanol + sodium carbonate + water at 18 ± 2°C. The mass fraction (% = the weight of phase-forming substance/the total weight of the salting-out extraction system × 100%.
Fig. 2.
(A) Chromatograms of ginsenosides from enzyme hydrolysis solution of Panacis Quinquefolii Radix (equivalent raw material 5 mg ml−1) obtained by the salting-out extraction method. (a) Ethanol-ammonium sulfate system; (b) ethanol-sodium sulfate system; (c) ethanol-sodium carbonate system; (d) the crude enzyme solution; (e) reference; (f) ethanol-potassium phosphate system. (B) Extraction efficiency of ginsenosides from enzyme hydrolysis solution of Panacis Quinquefolii Radix (equivalent raw material 5 mg ml−1) obtained by the salting-out extraction method.
3.2.2. Effect of ATPS composition
The tie lines and tie line length were the important parameters of the ATPS and could be calculated from the ATPS composition. To explore the effect of ATPS composition on extraction efficiency, the extraction efficiency of ginsenosides was investigated by changing the amount of ethanol and forming salts in ATPS. First, different volumes of ethanol were added to the concentrated salt solution containing 0.873g Na2CO3 to form the ATPS. The total volume of system was adjusted to 10.0 ml while other conditions were constant. The ATPS was prepared to extract ginsenosides with the constant salt amount. The top phase was taken after the phase separation, and the ginsenosides were pretreated and analyzed as procedure 2.3 described. Results showed that the extraction efficiency of ginsenosides was significantly influenced by the ethanol volume in ATPS, and the maximum concentration for three ginsenosides in the top phase was observed at the ethanol concentration of 41.51% as Fig. 3(a) showed. Second, the inequable amount of salt was used to prepare the concentrated Na2CO3 solution. The ATPS was prepared to extract ginsenosides with the constant ethanol concentration. Results showed that the extraction efficiency of ginsenosides was also significantly influenced by the salt amount in ATPS. The maximum ginsenoside concentration in the top phase was observed at the mass percent of sodium carbonate of 7.9% in the extraction system as Fig. 3(b) showed. In addition, the extraction conditions showed good ability of removing impurity. The three components of ginsenosides could be all separated according to the baseline, so the aforementioned extraction conditions were selected for further study.
Fig. 3.
Effect of the composition of salting-out extraction system on the extraction efficiency of ginsenosides. (A) Ethanol concentration in the extraction system, (B) the mass percent of sodium carbonate.
3.2.3. Effect of extraction time
Effect of the extraction time on the extraction efficiency was also investigated because the partitioning equilibrium of the targets could be influenced significantly by the extraction time. The sample solution was extracted under the optimized conditions in the range of 5–30 min and then pretreated and analyzed as procedure 2.3 described. Results showed that the extraction efficiency of ginsenosides was not affected obviously when the extraction time exceeded 5 min. SOE kept the advantages of conventional ATPE, such as the rapid mass transfer equilibrium at the phase surface. So, the most time-consuming step for preparing ginsenosides by the conventional method was improved greatly.
3.2.4. Effect of extraction times
The conventional liquid–liquid extraction (LLE) for ginsenosides usually needs extract repeatedly to obtain the ideal recovery. Thus, it was very necessary to investigate the effect of extraction times on the extraction efficiency. Results in Fig. 4 showed that the one time extraction rate of ginsenoside Rg1, Re, and Rb1 was 92.8%, 92.4%, and 90.0%, respectively. As a result, the high recoveries of the three ginsenosides could be obtained only in a single-step extraction.
Fig. 4.
Effect of extraction number on the extraction efficiency of ginsenosides. SOE conditions: ethanol concentration, 41.51% (ethanol/water,v/v); the mass percent of sodium carbonate, 7.9%; salting-out extraction time, 10 min; and the loaded sample solution containing 25-mg ginsenosides.
3.3. Effect of the enzyme amounts on the extraction efficiency of ginsenosides
Chromatograms for the separation of the enzyme hydrolysis solution were given in Fig. 5, which displayed well-resolved peaks for the three ginsenosides. With the addition of enzyme, the extraction amount of different ginsenosides increased at first and then decreased when the enzyme further increased. Fig. 5 (d) showed that peak 3 disappeared because the component hydrolyzed under the experimental conditions with the pH value of 4.0 and a new component formed, so a new peak thereafter appeared. For extracting ginsenoside Rb1, the optimum volume of enzyme solution was 2.0 mL, while for extracting ginsenoside Re, the optimum volume of enzyme solution was 4.0 mL. The reasons for this phenomenon were properly that the enzyme contained β-glucosidase whose bonds could be hydrolyzed and that the stability differences of β-glucoside bonds in different ginsenoside molecular could exist because of the different steric hindrance. So some ginsenosides could be hydrolyzed because of the existence of β-glucoside bonds.
Fig. 5.
Chromatograms of ginsenosides from complexed sample solution (equivalent raw material 20 mg ml−1) obtained with SOE methods under the optimized conditions. (a) Panacis Quinquefolii Radix raw material (25 mg ml−1) determined according to the method in Chinese Pharmacopoeia (2015 edition); (b) moderate enzyme hydrolysis solution with 2.0 mL of enzyme solution; (c) partial hydrolysis of ginsenoside Rb1 with excessive enzyme hydrolysis solution; (d) complete hydrolysis of ginsenoside Rb1 with excessive enzyme hydrolysis solution; (e) reference (1-ginsenoside Rg1 0.1 mg ml−1, 2-Re 0.4 mg ml−1, 3-Rb1 1.0 mgml−1).
The enzyme-assisted extraction was an ideal method with high extraction efficiency because enzymes could damage the cell membranes and walls, render cells more permeable, and cause the release of intracellular products under moderate conditions easily. However, the structure characteristic of the target components should be paid much attention because the ginsenosides were easily destroyed. If the hydrolysates, such as aglycons, were the target components to extract, enzyme-assisted extraction could be regarded as an ideal extraction method for the existence of glycosides in their key structure. In addition, ATPE could make the herb residue fall into the bottom phase and the ginsenosides distributed into the top phase at the same time. Moreover, there were many other obvious advantages, such as high capacity, rapid transfer equilibrium, convenient phase separation, and so on.
3.4. Comparison of the extraction methods
Liquid–liquid extraction was usually used to extract ginsenosides in sample solution with n-butanol as the solvent. The consumption of n-butanol was usually large because the extraction factor of ginsenosides was proportional to the ratio of the solvent volume and the sample solution, and the repeated extraction was usually required to improve the extraction efficiency of ginsenosides. In this article, the volume of the n-butanol used was half the volume of the sample solution, and the ginsenosides were extracted five times with the solvent. The recovery of ginsenoside Re was selected to compare the advantages of the SOE and LLE method because ginsenoside Re was stable at the enzyme-assisted extraction condition, and its content was higher than other components. Results showed that the extraction of ginsenoside Re was still incomplete after three times of extraction and small amount of ginsenoside Re could be detected even in the fifth extract using the LLE method. The main advantages of the present method were compared with the conventional extraction LLE methods as Table 2 summarized. Results showed that the sample extraction process was simple and quick, especially with the good reproducibility and high recovery. The results also suggested that the method could be applied to extract ginsenosides from other complex solutions as a powerful method.
Table 2.
The comparison of the advantages of the current method with conventional LLE method
| Extraction method | Extraction solvent | Phase separation time (min) | Extraction times | Recovery (%) |
|---|---|---|---|---|
| Salting-out extraction | Ethanol | <5 | 1–2 | >92.5 (in this article) |
| Conventional LLE method | Water-saturated n-butanol | >10 min, easily emulsifying | 3–5 | Usually unsatisfactory 86.4 (3 times) |
4. Conclusion
SOE could be effectively used for the pretreatment of the complex enzymatic hydrolysates of Panax quinquefolium, and the ethanol/sodium carbonate system was found to be the most suitable ATPS for extraction and purification of ginsenosides. The ATPS, its composition and extraction times were the main factors influencing the extraction efficiency of ginsenosides, while extraction time had slight influence on extraction efficiency of ginsenosides. Results also showed that the higher recoveries for extracting ginsenosides could be obtained only in a single step. This article also suggested that the hydrolytic destruction of glycosides should be noticed when the polysaccharide enzyme was used and that the application of enzyme extraction should be selected according to the structural characteristics of the target components.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
The work was supported by China Postdoctoral Science Foundation funded project (No. 201104073), A Project of Shandong Province Higher Educational Science and Technology Program (No. J15LM02).
References
- 1.Shen X.H., Ren Y.P., Chen Y. Detection of ginsenoside in healthy foods by HPLC. Chin J Health Lab Technol. 2003;5:600–601. [Google Scholar]
- 2.Wang Y.T., You J.Y., Yu Y., Qu C.L., Zhang H.R., Ding L. Analysis of ginsenosides in Panax ginseng in high pressure microwave-assisted extraction. Food Chem. 2008;110:161–167. doi: 10.1016/j.foodchem.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Quan K., Liu Q., Wan J.Y., Zhao Y.J., Guo R.Z., Alolga R.N., Li P., Qi L.W. Rapid preparation of rare ginsenosides by acid transformation and their structure-activity relationships against cancer cells. Sci Rep. 2015;5:8598–8605. doi: 10.1038/srep08598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang A., Wang C.Z., Wu J.A., Osinski J., Yuan C.S. Determination of major ginsenosides in Panax quinquefolius :American ginseng; using high performance liquid chromatography. Phytochem Analysis. 2005;16:272–277. doi: 10.1002/pca.838. [DOI] [PubMed] [Google Scholar]
- 5.Ha J., Shim Y.S., Seo D., Kim K., Ito M., Nakagawa H. Determination of 22 ginsenosides in ginseng products using ultra-high-performance liquid chromatography. J Chromatogr Sci. 2013;51:355–360. doi: 10.1093/chromsci/bms148. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y.J., Zhang D.B., Yang D.C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol Adv. 2015;33:717–735. doi: 10.1016/j.biotechadv.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Jee H.S., Chang K.H., Park S.H., Kim K.T., Paik H.D. Morphological characterization, chemical components, and biofunctional activities of panax ginseng, panax quinquefolium, and panax notoginseng roots: a comparative study. Food Rev Intern. 2014;30:91–111. [Google Scholar]
- 8.Sun L., Wu D., Ning X., Yang G., Lin Z., Tian M. α-amylase-assisted extraction of polysaccharides from panax ginseng. Int. J. Biol. Macromol. 2015;75:152–157. doi: 10.1016/j.ijbiomac.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 9.Liu D., Zou X., Gao M., Gu M., Xiao H. Hydrophilic organic/salt containing aqueous two-phase solvent system for counter-current chromatography: a novel technique for separation of polar compounds. J Chromatogr A. 2014;1356:157–162. doi: 10.1016/j.chroma.2014.06.050. [DOI] [PubMed] [Google Scholar]
- 10.Fu H., Yang S.T., Xiu Z. Phase separation in a salting-out extraction system of ethanol-ammonium sulfate. Sep. Purif. Technol. 2015;148:32–37. [Google Scholar]
- 11.Wu Y., Wang Y., Zhang W., Han J., Liu Y., Hu Y., Ni L. Extraction and preliminary purification of anthocyanins from grape juice in aqueous twophase system. Sep. Purif. Technol. 2014;124:170–178. [Google Scholar]
- 12.Dong B., Yuan X., Zhao Q., Feng Q., Liu B., Guo Y. Ultrasound-assisted aqueous two-phase extraction of phenylethanoid glycosides from Cistanche deserticola Y. C. Ma stems. J Chromatogr Sci. 2015;38:1194–1203. doi: 10.1002/jssc.201401410. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W., Zhu D., Fan H., Liu X., Wan Q., Wu X., Liu P., Tang J.Z. Simultaneous extraction and purification of alkaloids from Sophora flavescens Ait. by microwave-assisted aqueous two-phase extraction with ethanol/ammonia sulfate system. Sep. Purif. Technol. 2015;141:113–123. [Google Scholar]
- 14.Feng Y.C., Li W.L., He F.M., Kong T.T., Huang X.W., Gao Z.H., Lu N.H., Li H.L. Aqueous two-phase system as an effective tool for purification of phenolic compounds from fig fruits :Ficus carica L. Sep. Purif. Technol. 2015;50:1785–1793. [Google Scholar]
- 15.Dang Y.Y., Zhang H., Xiu Z.L. Microwave-assisted aqueous two-phase extraction of phenolics from grape (Vitis vinifera) seed. J Chem Technol Biot. 2014;89:1576–1581. [Google Scholar]
- 16.Chen Z., Zhang W., Tang X., Fan H., Xie X., Wan Q., Wu X., Tang J.Z. Extraction and characterization of polysaccharides from Semen Cassiae by microwave-assisted aqueous two-phase extraction coupled with spectroscopy and HPLC. Carbohyd Polym. 2016;144:263–270. doi: 10.1016/j.carbpol.2016.02.063. [DOI] [PubMed] [Google Scholar]
- 17.Guo T., Su D., Huang Y., Wang Y., Li Y.-H. Ultrasound-assisted aqueous two-phase system for extraction and enrichment of Zanthoxylum armatum lignans. Molecules. 2015;20:15273–15286. doi: 10.3390/molecules200815273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H., Dong Y.S., Xiu Z.L. Microwave-assisted aqueous two-phase extraction of piceid, resveratrol and emodin from Polygonum cuspidatum by ethanol/ammonium sulphate system. Biotechnol. Lett. 2008;30:2079–2084. doi: 10.1007/s10529-008-9815-1. [DOI] [PubMed] [Google Scholar]
- 19.Liu L., Dong Y.S., Xiu Z.L. Three-liquid-phase extraction of diosgenin and steroidal saponins from fermentation of Dioscorea zingibernsis, C. H. Wright. Process Biochem. 2010;45(5):752–756. [Google Scholar]
- 20.Li L.J., Jin Y.R., Wang X.Z., Liu Y., Wu Q., Shi X.L. Ionic liquid and aqueous two-phase extraction based on salting-out coupled with high-performance liquid chromatography for the determination of seven rare ginsenosides in Xue-Sai-Tong injection. J Chromatogr Sci. 2015;38:3055–3062. doi: 10.1002/jssc.201500363. [DOI] [PubMed] [Google Scholar]