Abstract
Background
Panax ginseng Meyer (Araliaceae) is a highly valued medicinal plant in Asian regions, especially in Korea, China, and Japan. Chemical and biological studies on P. ginseng have focused primarily on its roots, whereas the seeds remain poorly understood. This study explores the phytochemical and biological properties of compounds from P. ginseng seeds.
Methods
P. ginseng seeds were extracted with methanol, and 16 compounds were isolated using various chromatographic methods. The chemical structures of the isolates were determined by spectroscopic data. Antiinflammatory activities were evaluated for triterpene and steroidal saponins using lipopolysaccharide-stimulated RAW264.7 macrophages and THP-1 monocyte leukemia cells.
Results
Phytochemical investigation of P. ginseng seeds led to the isolation of a novel triterpene saponin, pseudoginsenoside RT8, along with 15 known compounds. Pseudoginsenoside RT8 exhibited more potent antiinflammatory activity than the other saponins, attenuating lipopolysaccharide-mediated induction of proinflammatory genes such as interleukin-1β, interleukin-6, inducible nitric oxide synthase, cyclooxygenase-2, and matrix metalloproteinase-9, and suppressed reactive oxygen species and nitric oxide generation in a dose-dependent manner.
Conclusion
These findings indicate that pseudoginsenoside RT8 has a pharmaceutical potential as an antiinflammatory agent and that P. ginseng seeds are a good natural source for discovering novel bioactive molecules.
Keywords: Antiinflammatory activity, Panax ginseng seeds, Pseudoginsenoside RT8
1. Introduction
Panax ginseng Meyer (Araliaceae) is a representative medicinal plant that is highly valued in several Asian countries, including Korea, China, and Japan, and has been traditionally used to prevent or treat a wide range of ailments and diseases [1]. A number of studies have shown that P. ginseng has various beneficial biological effects on human health and relieves many chronic diseases, including Alzheimer's disease, atherosclerosis, cancer, fatty liver, hyperlipidemia, inflammatory disease, insulin resistance, obesity, Parkinson's disease, and pulmonary disease [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]. It is thought that the biological effects of P. ginseng derive from a combination of diverse compounds, such as saponins, polysaccharides, peptides, phytosterols, polyacetylenes, and fatty acids [1], [13]. Ginseng saponins, called ginsenosides, are characteristic constituents of P. ginseng which are responsible for many of its pharmacological effects [14]. More than 100 ginsenosides have been identified in P. ginseng, and vigorous research efforts are still underway to discover novel ginsenosides with unique pharmacological activities.
Inflammation is a complex immune response to defend living organism against harmful stimuli such as mechanical injury, pathogens, or irritants [15]. Once such inducers of inflammation initiate inflammatory process, stimulated inflammatory cells generate higher level of proinflammatory cytokines [e.g., interleukin (IL)-1β, IL-6, and tumor necrosis factor-α] and inflammatory mediators including nitric oxide (NO), inducible nitric oxide synthase (iNOS), and cyclooxygenase (COX)-2 to modulate the functionality of tissues and organs. However, abnormal regulation of the inflammatory response can be triggered by nonpathogenic means [15], [16]. For example, it has been proposed that free fatty acids can bind to toll-like receptor 4 to trigger a proinflammatory response [17]. Low-grade, chronic inflammation is closely related to the development of various metabolic disorders, including atherosclerosis, cancer, fatty liver disease, insulin resistance, rheumatoid arthritis, type-2 diabetes, and vascular diseases [15], [16]. Therefore, it is important to maintain a balanced inflammatory status to remain healthy.
Most of the phytochemical research on P. ginseng has focused on roots, whereas other parts of the plant remain relatively poorly understood. Recently, the fruits of P. ginseng, called ginseng berries, have been used as dietary supplements because they also exert multiple beneficial effects on metabolic disorders, such as hyperglycemia, hyperlipidemia, diabetes, and insulin resistance [18], [19], [20]. It is feasible that other parts of P. ginseng, e.g., the stems, flowers, and seeds, may also contain functional components.
In this study, phytochemical evaluation of ginseng seeds led to the determination of a novel ginsenoside and six known ginsenosides, three steroidal saponins, three phenolics, and three primary metabolites. In addition, the antiinflammatory activities of triterpene and steroidal saponins were evaluated by estimating proinflammatory genes expression [IL-1β, IL-6, iNOS, COX-2, and matrix metalloproteinase (MMP)-9] in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages and the production of reactive oxygen species (ROS) and nitric oxide (NO).
2. Material and methods
2.1. Instrumentation
Semipreparative-scale high-performance countercurrent chromatography (HPCCC) was conducted with a Spectrum HPCCC (Dynamic Extractions, Berkshire, UK) consisting of a 1525 binary high-performance liquid chromatography (HPLC) pump (Waters, Milford, MA, USA), Sedex 75 ELSD (Sedere, Olivet, France) and a Foxy R2 fraction collector (Teledyne Isco, Lincoln, NE, USA). A CCA-1111 circulatory temperature regulator (Eyela, Tokyo, Japan) was used to control the inner temperature of the HPCCC at 30°C. Preparative HPLC was conducted with a Gilson HPLC system (Middleton, WI, USA) composed of a liquid handler, ultraviolet–visible (UV/VIS) detector, binary pumps, and a Luna C18 column (21.2 × 250 mm I.D., 5 μm; Phenomenex, Torrance, CA, USA). Chemical structures of the isolated compounds were identified using one-dimensional and two-dimensional nuclear magnetic resonance (NMR) data collected using an AVANCE 500 spectrometer (Bruker, Karlsruhe, Germany). Optical rotation was recorded on a Jasco P-2000 polarimeter (Tokyo, Japan). Electrospray ionization–quadrupole–time-of-flight–mass spectrometry (ESI-Q-TOF-MS) spectra were measured on a 6460 QTOF-MS spectrometer (Agilent Technologies, Santa Clara, CA, USA).
2.2. Chemicals and plant materials
Deionized water was produced using a Milli-Q water purification system (Millipore, Billerica, MA, USA). Solvent for HPCCC, HPLC, and medium pressure liquid chromatography (MPLC) were provided by Daejung Chemical and Metals Co. Ltd. (Kyeonggi-Do, Korea). LPS, N-acetyl-L-cysteine (NAC), and Nω-nitro-L-arginine methyl ester were obtained from Sigma-Aldrich (St. Louis, MO, USA). H2-2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA) was purchased from Invitrogen (Carlsbad, CA, USA). Seeds of P. ginseng were provided by Amorepacific Co. (Yongin, Korea), and the voucher specimen (CU-PGS 20160824) was deposited in the herbarium of the College of Pharmacy at the Catholic University of Korea.
2.3. Cell culture
RAW264.7 mouse macrophage and THP-1 human monocyte leukemia cell lines were provided from the American Type Culture Collection (Manassas, VA, USA). RAW264.7 cells were grown in Dulbecco's modified Eagle's medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (HyClone, Logan UT, USA). THP-1 cells were maintained with Roswell Park Memorial Institute 1640 medium containing 10% fetal bovine serum. All media included 100 units/mL of penicillin and 100 mg/mL of streptomycin (Sigma-Aldrich). Cells were grown at 37°C in a humidified atmosphere containing 5% CO2.
2.4. Preparation of cDNA and quantitative real-time polymerase chain reaction
RNA was isolated using TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's protocol. Each RNA sample (1 μg) was subjected to cDNA synthesis with a RevertAid 1st Strand cDNA Synthesis kit (Thermo Fisher Scientific). Relative mRNA levels were evaluated by quantitative real-time polymerase chain reaction (Bio-Rad CFX96; Bio-Rad, Hercules, CA, USA) with a QuantiSpeed SyBR One-step kit (PhileKorea, Seoul, South Korea) and appropriate quantitative real-time polymerase chain reaction primers (Bioneer, Daejeon, South Korea).
2.5. Measurement of ROS and NO concentrations
ROS was measured using DCF-DA reagent. Briefly, after being treated with saponins and LPS, cells were washed with phosphate-buffered saline (Sigma-Aldrich) and incubated in 5 μM H2DCF-DA for 30 minutes at 37°C in the dark condition. After two additional washes with phosphate-buffered saline, the fluorescence intensity was measured with a Tecan Infinite 200 Pro multi-plate reader (Tecan trading AG, Männedorf, Switzerland; excitation wavelength: 492 nm, emission wavelength: 530 nm).
NO production was analyzed using Griess reagent (Promega, Madison, WI, USA) according to the manufacturer's protocol. In brief, 50 μL of sulfanilamide solution was added to 50 μL of each sample, followed by 10 minutes of incubation at room temperature (RT), protected from light. Then, a solution of N-1-napthylethylenediamine (50 μL) was dispensed into the mixture and incubated for an additional 10 minutes at RT without light. The colorimetric intensity of NO was estimated with a Tecan Infinite 200 Pro multi-plate reader at a wavelength of 540 nm. Sodium nitrite (NaNO2) was used to generate a standard curve.
2.6. Statistical analysis
All data points represent the average of at least triplicate samples, and standard deviation was represented as error bar. The p values were calculated by a one-way analysis of variance and Dunnett's test. p values less than <0.05 were regarded to be statistically significant.
2.7. Extraction and isolation of compounds 1–10
Seeds of P. ginseng (5.5 Kg) were ground to make a fine powder. The powder was extracted with methanol to yield a methanol extract (481 g). The methanol extract was suspended in H2O and partitioned sequentially with n-hexane and n-butanol to give n-hexane–soluble (322 g), n-butanol–soluble (114 g), and water-soluble (45 g) extracts. Further isolation process for compounds 1–16 were described in Supplementary Information S1.
2.8. HPLC-ESI-Q-TOF-MS analysis of P. ginseng extracts
HPLC-ESI-Q-TOF-MS analysis was conducted on compounds 1–9 from methanol extract and compound 10 from n-butanol–soluble extracts of P. ginseng seeds. The isolated compounds 1–10 were used as authentic samples. For mobile phases of HPLC, the gradient elution of methanol (0.1% formic acid) (solvent A) and water (0.1% formic acid) (solvent B) mixture was used (50% A in 0–5 min, 50–100% A in 5–30 min, and 100% A in 30–50 min). The flow rate was 1.0 mL/min, and the injection volume was 5 μL. The effluent of HPLC was divided by a split valve to flow one-tenth of the effluent to ESI-Q-TOF-MS. HPLC column Eclipse XDB-C18 (Agilent Technologies, Santa Clara, CA, USA). Positive ion mode ESI-Q-TOF-MS parameters are as follows: flow rate and temperature of drying gas: 13.0 mL/min and 350°C, respectively, nebulizer gas pressure: 35 psig, capillary voltage: 3500 V, nozzle voltage: 1000 V, OCT RFV voltage: 750 V, fragmentor voltage: 50 V, and skimmer voltage: 65 V. The data acquisition was performed using a MassHunter Workstation Software with a version of B.05.00.
3. Results and discussion
3.1. Structure elucidation of compounds 1–16
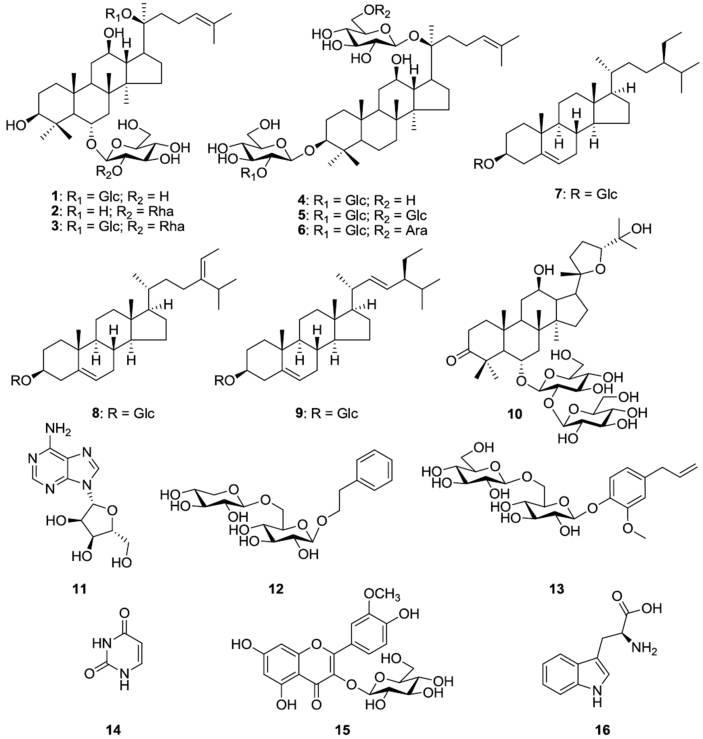
Phytochemical work of ginseng seeds led to the determination of a new saponin (10), three known protopanaxatriol saponins (1–3) [21], [22], three protopanaxadiol saponins (4–6) [23], three sterol glycosides (7–9) [24], [25], two phenolic glycosides (12–13) [26], [27], a flavonoid (15) [28], and three primary metabolites (11, 14, and 16) [29], [30], [31]. Fifteen known molecules were identified as ginsenoside (G)-Rg1 (1), G-Rg2 (2), G-Re (3), G-Rd (4), G-Rb1 (5), G-Rb2 (6), β-sitosterol 3-O-β-D-glucopyranoside (7), daucosterol (8), stigmasterol-3-O-β-D-glucopyranoside (9), adenosine (11), phenethyl alcohol β-D-xylopyranosyl(1→6)-β-D-glucopyranoside (12), eugenyl β-gentiobioside (13), uracil (14), isorhamnetin 3-O-β-D-glucopyranoside (15), and L-tryptophan (16). Spectroscopic evidence and structures for 1–9 and 11–16 are provided in the Fig. 1 and Supplementary Information S2.
Fig. 1.
Chemical structures of compounds 1–16 from P. ginseng seeds.
Compound 10 was isolated as a white amorphous powder showing a molecular formula of C42H70O15, based on a sodiated pseudomolecular ion peak at m/z 837.4617 [(M+Na)+, calcd. 837.4612] in its positive ion ESI-Q-TOF-MS spectrum. The 1H NMR spectrum of 10 contained eight methyl resonances at [δH 1.86 (3H, s, H-28), 1.69 (3H, s, H-29), 1.47 (3H, s, H-27), 1.25 (6H, s, H-21, 26), 1.10 (3H, s, H-18), 0.81 (3H, s, H-30), 0.75 (3H, s, H-19)]. In addition, two pairs of signals corresponding to anomeric proton and carbon atoms in two sugar moieties were detected at δH 6.02 (1H, d, J = 7.8, H-2″)/δC 104.08 (C-1′) and δH 4.91 (1H, d, J = 7.7, H-1′)/δC 104.32 (C-1″). 13C NMR and heteronuclear single quantum correlation spectra revealed 42 carbon signals. Apart from the two sugar moieties, the aglycone of 10 possessed eight methylenes, four methines, three oxygen-bearing methines [δC 79.79 (C-6), 71.40 (C-12), and 86.09 (C-24)], five quaternary carbon atoms, two oxygenated quaternary carbon atoms [δC 87.15 (C-20) and 70.78 (C-25)], eight methyl groups, and a carbonyl carbon [δC 218.85 (C-3)]. Exhaustive interpretation of 1H and 13C NMR data revealed that the aglycone of 10 was superimposed on pseudoginsengenin R1 [(20S,24R)-dammar-3-one-20,24-epoxy-6α,12β,25-triol] [32], [33], [34]. The absolute configuration of C-20 in 10 was deduced to be S from the chemical shift of C-21 (δC 27.67), and the 24R configuration was determined by the chemical shift of C-24 (δC 86.09), in accordance with previously published evidence [32], [33], [34]. The identities of the two sugar units were elucidated as β-D-glucopyranosyl moieties from their coupling constants of anomeric protons in the 1H NMR spectrum and 12 carbon resonances, along with acid hydrolysis data and gas chromatography analysis results. The glycosidic linkages were determined by heteronuclear multiple bond correlation, which showed cross peaks at δH 6.02 (H-1″)/δC 79.49 (C-2′) and δH 4.91 (H-1′)/δC 79.79 (C-6), demonstrating that the 2-O-(β-D-glucopyranosyl)-β-D-glucopyranosyl moiety was linked to C-6 of the aglycone in pseudoginsengenin R1 (Supplementary Information S3–S9). Therefore, the chemical structure of 10 was determined as (20S,24R)-6-O-[2-O-(β-D-glycophyrnosyl)-β-D-glucopyranosyl]-dammar-3-one-20,24-epoxy-6α,12β,25-triol, namely pseudoginsenoside RT8 (PG-RT8). The spectroscopic data of compound 10 are as follows:
Compound 10: C42H70O15; ESI-Q-TOF-MS: m/z 837.4617 [M+Na]+; [α] −4.82 (c 0.30, MeOH); 1H-NMR (500 MHz, pyridine-d5): δ 6.02 (1H, d, J = 7.8 Hz, H-1″), 4.91 (1H, d, J = 7.7 Hz, H-1′), 4.54 (1H, H-6′a)∗, 4.54 (1H, H-6″a)∗, 4.48 (1H, H-2′)∗, 4.38 (1H, H-3′)∗, 4.32 (1H, H-6′b)∗, 4.32 (1H, H-6″b)∗, 4.27 (1H, H-5″)∗, 4.18 (1H, H-2″)∗, 4.16 (1H, H-4′)∗, 4.15 (1H, H-5′)*, 4.15 (1H, H-6)∗, 4.12 (1H, H-4″)∗, 3.99 (1H, H-3″)∗, 3.94 (1H, t, J = 7.5 Hz, H-24), 3.68 (1H, td, J = 10.6, 4.5 Hz, H-12), 2.57 (1H, H-7a)∗, 2.23(1H, H-2a)∗, 2.22 (1H, H-11a)∗, 2.21 (1H, H-17)∗, 2.17 (1H, H-16a)∗, 2.06 (1H, d, J = 10.6 Hz, H-5), 1.87 (1H, H-16b)*, 1.86 (3H, s, H-28), 1.82 (1H, H-7b)∗, 1.82 (1H, H-23a)∗, 1.81 (1H, H-13)∗, 1.78 (1H, H-2b)∗, 1.69 (3H, s, H-29), 1.67 (1H, H-1a)∗, 1.64 (1H, H-15a)∗, 1.60 (1H, H-9)∗, 1.60 (1H, H-22a)∗, 1.49 (1H, H-1b)∗, 1.45 (3H, s, H-27), 1.37 (1H, H-22b)∗, 1.32 (1H, H-11b)∗, 1.26 (1H, H-15b)∗, 1.25 (3H, s, H-26), 1.25 (3H, s, H-21), 1.25 (1H, H-23b)∗, 1.10 (3H, s, H-18), 0.81 (3H, s, H-30), 0.75 (3H, s, H-19) (∗peak overlapped); 13C-NMR (125 MHz, pyridine-d5): δ 218.85 (C-3), 104.32 (C-1″), 104.08 (C-1′), 87.15 (C-20), 86.09 (C-24), 80.55 (C-3′), 79.94 (C-5′), 79.79 (C-6), 79.49 (C-2′), 79.11 (C-5″), 78.64 (C-3″), 76.34 (C-2″), 73.05 (C-4′), 72.34 (C-4″), 71.40 (C-12), 70.78 (C-25), 63.93 (C-6″), 63.53 (C-6′), 58.35 (C-5), 52.76 (C-14), 49.97 (C-13), 49.47 (C-9), 48.75 (C-17), 48.58 (C-4), 43.47 (C-7), 40.63 (C-1), 40.47 (C-8), 38.82 (C-10), 33.61 (C-2), 33.47 (C-11), 33.19 (C-15), 32.95 (C-28), 32.09 (C-22), 29.25 (C-23), 28.18 (C-27), 27.67 (C-21), 27.43 (C-26), 25.94 (C-16), 20.42 (C-29), 18.52 (C-30), 18.48 (C-19), 16.13 (C-18)
3.2. HPLC-ESI-Q-TOF-MS analysis of P. ginseng extracts
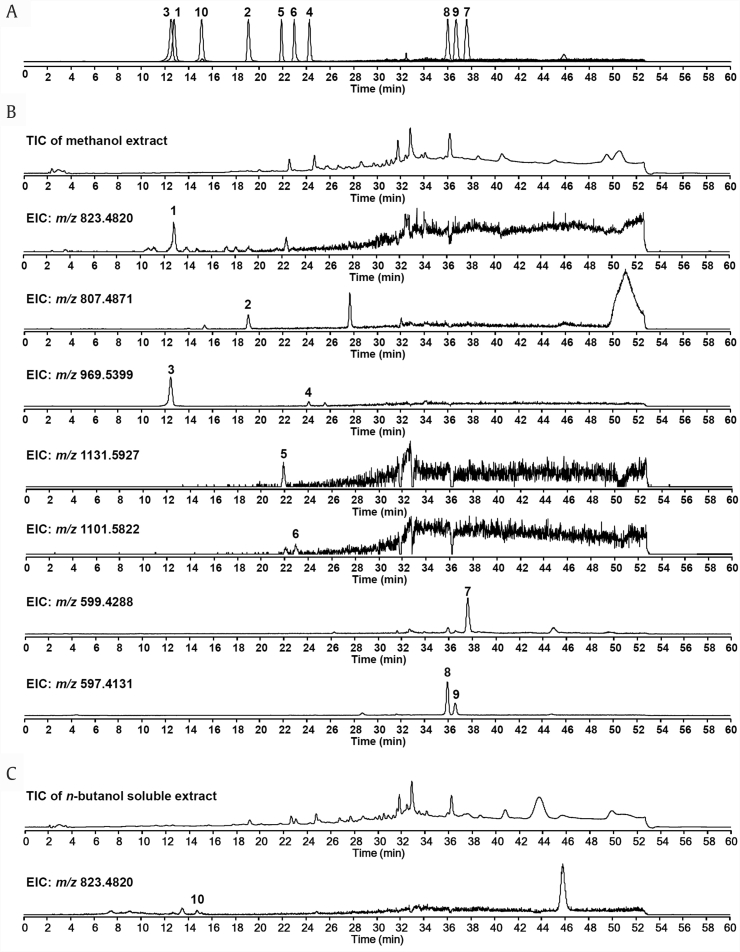
HPLC-ESI-Q-TOF-MS analysis was performed to confirm the presence of compounds 1–10 from the extracts of P. ginseng seeds. The identification of compounds 1–10 in ginseng extracts was achieved by comparison with retention times and extracted ion chromatograms (EICs) established by theoretical m/z values of sodiated pseudomolecular ion [M+Na]+ of authentic samples [m/z 823.4820 (G-Rg1); m/z 807.4871 (G-Rg2); m/z 969.5399 (G-Re and G-Rd); m/z 1131.5927 (G-Rb1); m/z 1101.5822 (G-Rb2); m/z 599.4288 (β-sitosterol 3-O-β-D-glucopyranoside); m/z 597.4131 (daucosterol and stigmasterol-3-O-β-D-glucopyranoside); 823.4820 (PG-RT8)]. As shown in Figs. 2A, 2B, all saponins were well detected in methanol extract of P. ginseng except for compound 10, which indicated that the content of compound 10 was relatively lower than that of the other ginseng saponins. However, compound 10 was observed in n-butanol–soluble extract because saponins are well concentrated in n-butanol (Fig. 2C).
Fig. 2.
HPLC-ESI-Q-TOF-MS analysis of P. ginseng seeds extracts. (A) EIC chromatogram of authentic samples of compounds 1–10. (B) TIC and EIC chromatograms of methanol extract of P. ginseng seeds. (C) TIC and EIC chromatograms of n-butanol–soluble extract of P. ginseng seeds. Peaks: G-Rg1 (1), G-Rg2 (2), G-Re (3), G-Rd (4), G-Rb1 (5), G-Rb2 (6), β-sitosterol 3-O-β-D-glucopyranoside (7), daucosterol (8), stigmasterol-3-O-β-D-glucopyranoside (9), and pseudoginsenoside RT8 (10). EIC, extracted ion chromatogram; HPLC-ESI-Q-TOF-MS, high-performance liquid chromatography–electrospray ionization–quadrupole–time-of-flight–mass spectrometry; TIC, total ion chromatogram.
The results of the present phytochemical work are comparable with those of a previously published study by Sugimoto et al [26], which identified 11 compounds from P. ginseng seeds: a dammarane-type triterpene ketone, two dammarane- and lupine-type triterpenes, an aromatic oligo-glycoside, three sterol glycosides, G-Rd, G-Re, and G-Rg2. The previous study revealed that the content of G-Re was the highest among the ginsenosides. In the present phytochemical work, the isolation yield of G-Re was the highest among the saponins. Therefore, we carefully assume that G-Re is a characteristic ginsenoside in P. ginseng seeds.
3.3. Inhibition of proinflammatory genes expression of compounds 1–10
Many studies have revealed that ginsenosides exert antiinflammatory effects of P. ginseng [12], [35], [36], [37], [38]. In this study, seven triterpene saponins (1–6 and 10) and three steroidal saponins (7–9) were tested to evaluate and compare their inhibition level of proinflammatory genes expression (IL-1β, IL-6, and iNOS) using LPS-stimulated RAW264.7 macrophages. The primary metabolites and phenolic compounds were excluded from the experiments.
Cytokines, IL-1β, and IL-6 are produced from diverse cell types, most importantly macrophages and mast cells. Both cytokines possess important homeostatic functions in normal condition. In most inflammatory states, they are overproduced, giving rise to pathophysiological changes in the human body and inducing many inflammatory diseases [39], [40]. iNOS is one of the nitric oxide synthase isoforms, which generates high levels of NO in inflammatory events, causing cellular death and tissue destruction [41], [42].
As shown in Fig. 3, nine of the tested saponins (1–9) showed moderate inhibition of LPS-induced proinflammatory genes expression at a concentration of 10 μM. Remarkably, compound 10, PG-RT8, showed the most potent inhibitory activities against IL-1β, IL-6, and iNOS genes expression compared with those of compounds 1–9 at the same concentration. To rule out the possibility that the saponins may be affecting inflammatory gene expression through cytotoxic activity, MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assays were conducted to assess cell growth in the presence of 10 saponins (Supplementary Information S10). None of the tested saponins showed any cytotoxicity up to 50 μM. These results indicate that all saponins negatively regulate the expression of proinflammatory genes without harmful effects on cell viability.
Fig. 3.
P. ginseng seed–derived saponins exhibit antiinflammatory properties. RAW264.7 macrophages were treated for 2 h with 10 μM of each ginseng seed–derived triterpene and steroidal saponins (1–10) and then with lipopolysaccharide (LPS) (10 ng/mL) for an additional 6 h. mRNA expression of inflammatory genes was measured by quantitative polymerase chain reaction analyses and normalized by cyclophilin. *P < 0.05 vs. LPS, **P < 0.01 vs. LPS. ***P < 0.001 vs. LPS. IL-1β, interleukin-1β; IL-6, interleukin-6; iNOS, inducible nitric oxide synthase.
The antiinflammatory properties of G-Rg1 (1), G-Re (3), G-Rd (4), G-Rb1 (5), and G-Rb2 (6) have been reviewed previously [12], which described that such ginsenosides exert antiinflammatory activities of Korean White Ginseng and Korean Red Ginseng by suppressing the generation of proinflammatory cytokines and regulating inflammatory signaling pathways. It is notable that a novel saponin, PG-RT8, showed most potent antiinflammatory properties than well-known ginsenosides and steroidal saponins. Thus, detailed antiinflammatory experiments were performed on PG-RT8.
3.4. Pseudoginsenoside RT8 relieved proinflammatory responses of LPS-stimulated RAW264.7 macrophages and THP-1 monocytes leukemia cells in a dose-dependent manner
As described previously, PG-RT8 decreased the mRNA expression of IL-1β, IL-6, and iNOS at a concentration of 10 μM. To confirm the inhibitory effects of PG-RT8 against antiinflammatory genes expression, further experiments were performed to measure the relative amounts of transcripts of COX-2 and MMP-9, as well as IL-1β, IL-6, and iNOS, in four concentration levels (1, 5, 10, and 20 μM) of PG-RT8.
COX-2 is an inducible form of prostaglandin synthetase enzyme that is involved in prostaglandin production pathway and closely related to generating prostaglandin E2 (PGE2) from arachidonic acid. The expression of COX-2 is elevated in inflammatory and malignant states by proinflammatory cytokines such as tumor necrosis factor-α, IL-1β, and epidermal growth factor (EGF) [43], [44].
MMP-9 is one of the MMPs of gelatinase family which cleave extracellular matrix and mediate immune cells migration into tissues. MMP-9 is mainly found in monocytes, macrophages, and neutrophils and highly induced by chemokines and cytokines during inflammatory events. The enhanced level of MMP-9 from immune cells is related to diverse inflammatory pathologies, including autoimmunity, arthritis, and transplant rejection [45], [46].
As demonstrated in Fig. 4, PG-RT8 strongly attenuated the LPS-mediated induction of proinflammatory genes expression (IL-1β, IL-6, iNOS, COX-2, and MMP-9) in LPS-stimulated macrophages at 20 μM (P < 0.001). Moreover, transcripts of IL-1β, IL-6, and MMP-9 were also downregulated by PG-RT8 in LPS-stimulated THP-1 monocyte leukemia cells in a dose-dependent manner (Fig. 5). These results demonstrate that PG-RT8 possesses potent antiinflammatory activity. Because MMP-9 is also known to play an important role in cancer progression and metastasis, our results imply a therapeutic value of PG-RT8 in treating cancers and immune-related diseases.
Fig. 4.
PG-RT8 suppresses the expression of proinflammatory genes in a dose-dependent manner. RAW264.7 macrophages were treated with PG-RT8 (1, 5, 10, and 20 μM) for 2 h and then with LPS (10 ng/mL) for an additional 6 h. mRNA expression of inflammatory genes was measured by quantitative polymerase chain reaction analyses and normalized by cyclophilin. *P < 0.05 vs. LPS, **P < 0.01 vs. LPS. ***P < 0.001 vs. LPS. COX-2, cyclooxygenase-2; IL-1β, interleukin-1β; IL-6, interleukin-6; iNOS, inducible nitric oxide synthase, LPS, lipopolysaccharide; MMP-9, metalloproteinase-9; PG-RT8, pseudoginsenoside RT8.
Fig. 5.
PG-RT8 suppresses the LPS-stimulated induction of proinflammatory genes in THP-1 monocytes leukemia cells. THP-1 monocytes leukemia cells were treated with PG-RT8 (1, 5, 10, and 20 μM) for 2 h and then with LPS (10 ng/mL) for an additional 6 h. mRNA expression of inflammatory genes was measured by quantitative polymerase chain reaction and normalized by cyclophilin. *P < 0.05 vs. LPS, **P < 0.01 vs. LPS. ***P < 0.001 vs. LPS. IL-1β, interleukin-1β; IL-6, interleukin-6; LPS, lipopolysaccharide; MMP-9, metalloproteinase-9; PG-RT8, pseudoginsenoside RT8.
3.5. Pseudoginsenoside RT8 reduced the production of NO and ROS in a dose-dependent manner
Proinflammatory cytokines mediate the recruitment of activated immune cells to inflamed tissues. The effector immune cells build up a cytotoxic condition to get rid of invading pathogens by producing high levels of toxic molecules such as NO and ROS. Finally, such excessive reactive oxygen and nitrogen species lead to tissue damage [12]. As mentioned previously, NO is generated by the catalytic action of iNOS, and both are involved as proinflammatory mediators. Considering these factors, ROS and NO production was evaluated in the presence of PG-RT8 using LPS-stimulated RAW264.7 macrophages.
The production of ROS and NO in RAW264.7 macrophages decreased with increasing doses of PG-RT8 (Fig. 6), which was consistent with the iNOS gene expression pattern (Fig. 4). N-acetylcysteine (NAC) has been used as a positive control when measuring the antiinflammatory effects of PG-RT8. In this regard, it is noteworthy that 20 μM of PG-RT8 was as effective in inhibiting ROS production as 1 mM of NAC (P < 0.001). Furthermore, 20 μM of PG-RT8 inhibited NO production (P < 0.001) to a level comparable with Nω-nitro-L-arginine methyl ester (10 μM), a well-known nitric oxide synthase inhibitor. These results indicate that PG-RT8 possesses multiple and strong antiinflammatory properties and has a pharmaceutical potential as an antiinflammatory agent.
Fig. 6.
PG-RT8 decreases ROS and NO levels in LPS-stimulated RAW264.7 macrophages. (A) RAW264.7 macrophages were treated with N-acetyl-L-cysteine (NAC; 1 mM) or PG-RT8 (1, 5, 10, and 20 μM) for 1 h. Then, cells were treated with LPS (10 ng/ml) for 30 minutes. The relative levels of ROS were measured as described in the Section 2. (B) RAW264.7 macrophages were treated with Nω-nitro-L-arginine methyl ester (L-NAME; 10 μM) or PG-RT8 (1, 5, 10, and 20 μM) for 1 h. The cells were then treated with LPS (10 ng/mL) for an additional 1 h. NO accumulation in the medium was assessed by the Griess reaction, as described in the Section 2. ***P < 0.001 vs. LPS. LPS, lipopolysaccharide; NO, nitric oxide; PG-RT8, pseudoginsenoside RT8; ROS, reactive oxygen species.
4. Conclusion
A phytochemical study of P. ginseng seeds led to the isolation of a novel ginsenoside, namely pseudoginsenoside RT8, as well as 15 known compounds. The yield of G-Re was the highest among the isolated ginsenosides, which suggests that G-Re may be a characteristic ginsenoside in P. ginseng seeds. The seven isolated ginsenosides and three steroidal saponins were evaluated for their antiinflammatory activities using LPS-stimulated RAW264.7 macrophages and THP-1 human monocyte leukemia cells. A novel compound, PG-RT8, showed the most potent antiinflammatory activities, attenuating the LPS-mediated induction of proinflammatory genes (IL-1β, IL-6, iNOS COX2, and MMP-9) in a dose-dependent manner. In addition, PG-RT8 strongly suppressed ROS and NO production. These findings indicate that PG-RT8 has a pharmaceutical potential as an antiinflammatory agent and that P. ginseng seeds are also a good natural source for discovering novel bioactive molecules.
Conflicts of interests
None of authors declare conflicts of interest.
Acknowledgments
This work was supported by a research fund from Amorepacific Co., (2016) and grants from National Research Foundation of Korea (Grant No. NRF-2017R1A2B4003888 and 2018R1A6A1A03025108).
Footnotes
Supplementary data to this article can be found online at http://doi.org/10.1016/j.jgr.2018.11.001.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.The Compilation Committee of Pharmacognosy Textbook . 2nd ed. Dong Meyoung Press; Geyong-gi: 2015. Pharmacognosy; pp. 196–203. [Google Scholar]
- 2.Shergis J.L., Di Y.M., Zhang A.L., Vlahos R., Helliwell R., Ye J.M., Xue C.C. Therapeutic potential of Panax ginseng and ginsenosides in the treatment of chronic obstructive pulmonary disease. Complement Ther Med. 2014;22:944–953. doi: 10.1016/j.ctim.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Van Kampen J.M., Baranowski D.B., Shaw C.A., Kay D.G. Panax ginseng is neuroprotective in a novel progressive model of Parkinson's disease. Exp Gerontol. 2014;50:95–105. doi: 10.1016/j.exger.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Hong S.H., Suk K.T., Choi S.H., Lee J.W., Sung H.T., Kim C.H., Kim E.J., Kim M.J., Han S.H., Kim M.Y. Anti-oxidant and natural killer cell activity of Korean red ginseng (Panax ginseng) and urushiol (Rhus vernicifera Stokes) on non-alcoholic fatty liver disease of rat. Food Chem Toxicol. 2013;55:586–591. doi: 10.1016/j.fct.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Xue C.C., Shergis J.L., Zhang A.L., Worsnop C., Fong H., Story D., Da Costa C., Thien F.C. Panax ginseng C.A Meyer root extract for moderate chronic obstructive pulmonary disease (COPD): study protocol for a randomised controlled trial. Trials. 2011;12:164. doi: 10.1186/1745-6215-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S.T., Chu K., Sim J.Y., Heo J.H., Kim M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:222–226. doi: 10.1097/WAD.0b013e31816c92e6. [DOI] [PubMed] [Google Scholar]
- 7.Li J., Liu Y., Li W., Wang Z., Guo P., Li L., Li N. Metabolic profiling of the effects of ginsenoside Re in an Alzheimer's disease mouse model. Behav Brain Res. 2018;337:160–172. doi: 10.1016/j.bbr.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Gao Y., Yang M.F., Su Y.P., Jiang H.M., You X.J., Yang Y.J., Zhang H.L. Ginsenoside Re reduces insulin resistance through activation of PPAR-gamma pathway and inhibition of TNF-alpha production. J Ethnopharmacol. 2013;147:509–516. doi: 10.1016/j.jep.2013.03.057. [DOI] [PubMed] [Google Scholar]
- 9.Han D.H., Kim S.H., Higashida K., Jung S.R., Polonsky K.S., Klein S., Holloszy J.O. Ginsenoside Re rapidly reverses insulin resistance in muscles of high-fat diet fed rats. Metabolism. 2012;61:1615–1621. doi: 10.1016/j.metabol.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto M., Uemura T., Nakama S., Uemiya M., Kumagai A. Serum HDL-cholesterol-increasing and fatty liver-improving actions of Panax ginseng in high cholesterol diet-fed rats with clinical effect on hyperlipidemia in man. Am J Chin Med. 1983;11:96–101. doi: 10.1142/S0192415X83000161. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M., Kumagai A. Anti-atherogenic action of Panax ginseng in rats and in patients with hyperlipidemia. Planta Med. 1982;45:149. doi: 10.1055/s-2007-971317. [DOI] [PubMed] [Google Scholar]
- 12.Kim J.H., Yi Y.S., Kim M.Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attele A.S., Wu J.A., Yuan C.-S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa H. Proof of the myskoterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004;95:153–157. doi: 10.1254/jphs.fmj04001x4. [DOI] [PubMed] [Google Scholar]
- 15.Wellen K.E., Hotamisligil G.S. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoelson S.E., Lee J., Goldfine A.B. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J.Y., Sohn K.H., Rhee S.H., Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 18.Xie J.T., Zhou Y.P., Dey L., Attele A.S., Wu J.A., Gu M., Polonsky K.S., Yuan C.S. Ginseng berry reduces blood glucose and body weight in db/db mice. Phytomedicine. 2002;9:254–258. doi: 10.1078/0944-7113-00106. [DOI] [PubMed] [Google Scholar]
- 19.Attele A.S., Zhou Y.P., Xie J.T., Wu J.A., Zhang L., Dey L., Pugh W., Rue P.A., Polonsky K.S., Yuan C.S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–1858. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 20.Seo E., Kim S., Lee S.J., Oh B.C., Jun H.S. Ginseng berry extract supplementation improves age-related decline of insulin signaling in mice. Nutrients. 2015;7:3038–3053. doi: 10.3390/nu7043038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee D.Y., Cho J.G., Lee M.K., Lee J.W., Park H.J., Lee Y.H., Yang D.C., Baek N.I. Identification of NMR data for ginsenoside Rg1. J Ginseng Res. 2008;32:291–299. [Google Scholar]
- 22.Cho J.G., In S.J., Jung Y.J., Cha B.J., Lee D.Y., Kim Y.B., Yeom M., Baek N.I. Re-evaluation of physicochemical and NMR data of triol ginsenosides Re, Rf, Rg2, and 20-gluco-Rf from Panax ginseng roots. J Ginseng Res. 2014;38:116–122. doi: 10.1016/j.jgr.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho J.G., Lee M.K., Lee J.W., Park H.J., Lee D.Y., Lee Y.H., Yang D.C., Baek N.I. Physicochemical characterization and NMR assignments of ginsenosides Rb1, Rb2, Rc, and Rd isolated from Panax ginseng. J Ginseng Res. 2010;34:113–121. [Google Scholar]
- 24.De Rosa S., De Giulio A., Tommonaro G. Triterpene and sterol glucoside from cell cultures of Lycopersicon esculentum. Phytochemistry. 1997;44:861–864. doi: 10.1016/0031-9422(96)00083-0. [DOI] [PubMed] [Google Scholar]
- 25.Grishkovets V.I., Tolkacheva N.V., Shashkov A.S., Ya. Chirva V. Triterpene glycosides of Hedera taurica VII. Structures of taurosides A and D from the leaves of Crimean ivy. Chem Nat Compd. 1991;27:603–606. [Google Scholar]
- 26.Sugimoto S., Nakamura S., Matsuda H., Kitagawa N., Yoshikawa M. Chemical constituents from seeds of Panax ginseng: structure of new dammarane-type triterpene ketone, panaxadione, and HPLC comparisons of seeds and flesh. Chem Pharm Bull. 2009;57:283–287. doi: 10.1248/cpb.57.283. [DOI] [PubMed] [Google Scholar]
- 27.Orihara Y., Furuya T., Hashimoto N., Deguchi Y., Tokoro K., Kanisawa T. Biotransformation of isoeugenol and eugenol by cultured cells of Eucalyptus perriniana. Phytochemistry. 1992;31:827–831. doi: 10.1016/0031-9422(92)80022-7. [DOI] [PubMed] [Google Scholar]
- 28.Chang Y.C., Chang F.R., Khalil A.T., Hsieh P.W., Wu Y.C. Cytotoxic benzophenanthridine and benzylisoquinoline alkaloids from Argemone mexicana. Z Naturforsch C. 2003;58:521–526. doi: 10.1515/znc-2003-7-813. [DOI] [PubMed] [Google Scholar]
- 29.Park S.Y., Kim J.S., Lee S.Y., Bae K.H., Kang S.S. Chemical constituents of Lathyrus davidii. Nat Prod Sci. 2008;14:281–288. [Google Scholar]
- 30.Fang Z., Jeong S.Y., Choi J.S., Min B.S., Min B.K., Woo M.H. Cholinesterase inhibitory constituents from Capsosiphon fulvescens. Nat Prod Sci. 2012;18:233–238. [Google Scholar]
- 31.Rho T., Yoon K.D. Chemical constituents of Nelumbo nucifera seeds. Nat Prod Sci. 2017;23:253–257. [Google Scholar]
- 32.Liu J.P., Tan X., Liu H.Y., Zhang Q.H., Lu D., Li P.Y., Zhao C.F. Two novel dammarane-type compounds from the leaves and stems of Panax quinquefolium L. J Asian Nat Prod Res. 2012;15:974–978. doi: 10.1080/10286020.2013.794416. [DOI] [PubMed] [Google Scholar]
- 33.Fujita S., Kasai R., Ohtani K., Yamasaki K., Chiu M.H., Nie R.L., Tanaka O. Dammarane glycoside from aerial parts of Neoalsomitra integrifolia. Phytochemistry. 1995;38:465–472. doi: 10.1016/0031-9422(95)00020-8. [DOI] [PubMed] [Google Scholar]
- 34.Fusita S., Kasai R., Ohtani K., Yamasaki K., Chiu M.H., Nie R.L., Tanaka O. Dammarane glycoside from aerial parts of Neoalsomitra integrifolia. Phytochemistry. 1995;39:591–602. doi: 10.1016/0031-9422(95)00020-8. [DOI] [PubMed] [Google Scholar]
- 35.Wang M., Chen Y., Xiong Z., Yu S., Zhou B., Ling Y., Zheng Z., Shi G., Wu Y., Qian X. Ginsenoside Rb1 inhibits free fatty acids induced oxidative stress and inflammation in 3T3L1 adipocytes. Mol Med Rep. 2017;16:9165–9172. doi: 10.3892/mmr.2017.7710. [DOI] [PubMed] [Google Scholar]
- 36.Kim S.Oh MH., Kim B.S., Kim W.I., Cho H.S., Park B.Y., Park C., Shin G.W., Kwon J. Upregulation of heme oxygenase-1 by ginsenoside Ro attenuates lipopolysaccharide-induced inflammation in macrophage cells. J Ginseng Res. 2015;39:365–370. doi: 10.1016/j.jgr.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee I.A., Hyam S.R., Jang S.E., Han M.J., Kim D.H. Ginsenoside Re ameliorates inflammation by inhibiting the binding of lipopolysaccharide to TLR4 on macrophages. J Agric Food Chem. 2012;60:9595–9602. doi: 10.1021/jf301372g. [DOI] [PubMed] [Google Scholar]
- 38.Kim T.W., Joh E.H., Kim B., Kim D.H. Ginsenoside Rg5 ameliorates lung inflammation in mice by inhibiting the binding of LPS to toll-like receptor-4 on macrophages. Int Immunopharmacol. 2012;12:110–116. doi: 10.1016/j.intimp.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Sheller J., Chalaris A., Schmit-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 40.Ren K., Torres R. Role of interleukin-1β during pain and inflammation. Brain Res Rev. 2009;60:57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.García-Ortiz A., Serrador J.M. Nitric oxide signaling in T cell-mediated immunity. Trends Mol Med. 2018;24:412–427. doi: 10.1016/j.molmed.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Botta M., Distrutti E., Mencarelli A., Parlato M.C., Raffi F., Cipriani S., Fiorucci S. Anti-inflammatory activity of a new class of nitric oxide synthase inhibitors that release nitric oxide. ChemMedChem. 2008;3:1580–1588. doi: 10.1002/cmdc.200800201. [DOI] [PubMed] [Google Scholar]
- 43.Dubois R.N., Abramson S.B., Crofford L., Gupta R.A., Simon L.S., Van De Putte L.B., Lipsky P.E. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 44.Abdalla S.I., Sanderson I.R., Fitzgerald R.C. Effect of inflammation on cyclooxygenase (COX)-2 expression in benign and malignant oesophageal cells. Carcinogenesis. 2005;26:1627–1633. doi: 10.1093/carcin/bgi114. [DOI] [PubMed] [Google Scholar]
- 45.Moore B.A., Manthey C.L., Johnson D.L., Bauer A.J. Matrix metalloproteinase-9 inhibition reduces inflammation and improves motility in murine models of postoperative ileus. Gastroenterology. 2011;141:1283–1292. doi: 10.1053/j.gastro.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saja K., Babu M.S., Karunagaran D., Sudhakaran P.R. Anti-inflammatory effect of curcumin involves downregulation of MMP-9 in blood mononuclear cells. Int Immunopharmacol. 2007;7:1659–1667. doi: 10.1016/j.intimp.2007.08.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.