Abstract
Arsenic (As) is naturally occurring toxic metalloid which is considered as a serious environmental and health concern. Red blood cells are the prime target for any toxicants as their population is higher in systemic circulation. High prevalence of anaemia too has been reported from arsenic contaminated area, suggesting possible linkage between arsenic and the damaging effects on RBCs. The exact mechanism for these effects is still not clear, however, oxidative/nitrosative stress might be one of the causative factors to play a key role. The present study was planned to evaluate the protective effects of a metal chelator, MiADMSA either alone or in combination with a natural antioxidant (gallic acid) for the reversal of arsenic induced oxidative damage in red blood cells. We collected rat RBCs and cultured them in appropriate medium. They were incubated with MiADMSA and gallic acid and then treated with sodium arsenite at 37 °C. Hemolysates were prepared and assayed for various biochemical parameters such as oxidative/nitrosative variables, osmotic fragility, acetylcholinesterase activity, and cellular metal accumulation. We found there was reversibility of oxidative/nitrosative stress variables, elevated cellular antioxidant power, and decreased osmotic fragility of red blood cells both in MiADMSA alone as well as in combination with gallic acid treated group compared with arsenic treated group. In conclusion, MiADMSA efficiently participated in the reversal of arsenic induced oxidative/nitrosative damage in red blood cells where as Gallic acid improved its reversal when given in combination with MiADMSA.
Keywords: Sodium arsenite, MiADMSA, Gallic acid, Red blood cells, Oxidative/nitrosative stress, Reactive oxygen species, Antioxidant, Behavioral neuroscience, Developmental biology, Flavonoid, Nervous system, Neurotoxicology, Oxidative stress, Pathophysiology, Photochemical, Toxicology
Sodium arsenite, MiADMSA, Gallic acid, Red blood cells, Oxidative/nitrosative stress, Reactive oxygen species; Antioxidant; Behavioral neuroscience; Developmental biology; Flavonoid; Nervous system; Neurotoxicology; Oxidative stress; Pathophysiology; Photochemical; Toxicology.
1. Introduction
Water resources are continuously getting contaminated by various toxic elements such as lead, mercury, cadmium and arsenic etc. due to industrial or agricultural wastes. Out of these contaminants, the major contaminant is arsenic (As), which is contaminating the water resources due to anthropogenic activities and is considered as serious health concern resulting in various manifestations such as lung toxicity, nephrotoxicity, liver diseases like non cirrhotic portal fibrosis; polyneuropathy; peripheral vascular disease; conjunctival congestion; carcinogenicity, weakness, and anemic conditions [1]. It is naturally occurring toxic metalloid present in air, water and soil [2]. After lead (Pb), As has drawn the considerable attention of researchers towards its toxicity [3]. According to WHO guidelines the maximum allowable value of arsenic concentration is 10 ppb. But recent survey reports depicted that, its concentration in drinking water is 80 times higher than its maximum permissible value in several places of world like Indo-Bangladesh region [1, 3]. This finding was even supported by epidemiological data which revealed that, out of 200 million people who get affected by arsenic intoxication, approximately 38 million were from the Indo-Bangladesh region only [1, 4, 5]. Naturally, arsenic is found in organic as well as inorganic forms among which the inorganic form is likely to be more toxic. Inorganic As exists in two major valence forms: trivalent arsenite (As3+) and pentavalent arsenate (As5+) [3].
During detoxification, arsenic undergoes a series of reduction and oxidative methylation processes in liver to form toxic metabolites [6]. Most of pentavalent arsenate is reduced to the trivalent arsenite form, a form which is considered biologically active and major source of arsenic toxicity [7]. Although, the exact underlying mechanism of arsenic toxicity is not well elucidated till now, but oxidative stress is known to play key role in arsenic induced toxicity [1, 8, 9, 10]. It has been assumed that, toxic metabolites that are generated as outcome of arsenic detoxification process are the main responsible factors for generation of oxidative/nitrosative stress which eventually leads to imbalance between generation and elimination of reactive oxygen species (ROS) and reactive nitrogen species (RNS) [11]. Inside cellular environment, arsenic heavily produces ROS/RNS which includes superoxide anions (O2•-), peroxy radicals (ROO•), singlet oxygen (1O2), hydroxyl radicals (OH•) via the Fenton reaction, hydrogen peroxide (H2O2) [12], nitric oxide (NO•) [13], dimethyl arsenic radical [(CH3)2As•] and dimethylarsenic peroxy radical [(CH3)2AsOO•] [14]. Arsenic is thought to produce ROS majorly by three or four pathways, firstly, cycling between oxidation state of various metals or interaction with various antioxidants such as thiol group having Glutathione (GSH) and other thiol group possessing enzymes [9, 15, 16]. Secondly, production of intermediary arsine species [14]; thirdly, methylated arsenic species induced release of iron from ferritin which could play a role in generating ROS by the production of OH• via Fenton reaction [17] and fourthly, oxidation of arsenite to arsenate [18]. Finally, the generated oxidative/nitrosative stress scatters to various organs and tissues and associated with various adverse health effects [11, 19]. In compensation of markedly generated ROS, body expresses counter defence mechanism by altering the level of some antioxidants such as glutathione (GSH), superoxide dismutase (SOD) and catalase [20].
Currently, chelation therapy has been found to be highly effective in metal intoxication and shows promising results for prevention of metal poisoning. Chelators form insoluble complex with arsenic and excrete it out successfully. A number of chelators such as Sodium 2, 3-dimercaptopropane 1-sulfonate (DMPS), dimercaptosuccinic acid (DMSA), and one of its analogues, monoisoamyl-DMSA (MiADMSA) have been found effective against arsenic poisoning [21]. Among these, MiADMSA is a lipophilic chelating agent and an alkyl monoester analogue of DMSA. It is currently under phase 2 clinical trial. Its thiol group serves as metal chelator and free radical scavenger and eventually prevents the depletion of cellular antioxidants. It is reported that MiADMSA efficiently chelates arsenic and reduces oxidative stress in experimental rodent models [9, 22]. It has been confirmed that MiADMSA treatment does not affect cellular biochemical variables even after repeated administration in rats [23, 24].
Recent in vivo studies reveal the better efficacy of antioxidants in combination with chelation therapy on metal intoxication [25, 26]. Gallic acid (3, 4, 5- trihydroxybenzoic acid), a naturally occurring polyphenolic antioxidant is a key component of beverages and food. It is a promising pharmacological lead molecule having a wide spectrum of activities [27, 28]. However, our main focus in the present study is to evaluate the antioxidant profile of gallic acid in combination with chelating agent in an ex-vivo antioxidant model of RBCs.
Findings suggest that people living in high arsenic content zone are highly susceptible to develop anaemia and other cardiovascular disorders [29, 30, 31]. Being the major cells in blood circulation, red blood cells (RBC) are main target of attack by arsenic, after its absorption into systemic circulation. Arsenic itself and its metabolites possess high haemoglobin (Hb) binding affinity and thus eventually accumulate in blood [32]. Arsenic causes membrane and cytoplasmic damage to RBC resulting in morphological changes in RBC from discoid shape to stomatocytes, decrease in deformability, increase in osmotic fragility and cell agreeability [33]. The above all factors contribute for the elimination of damaged cells from blood circulation and impairs oxygen delivery. Furthermore, arsenic triggers generation of ROS and lipid peroxidation finally leading to oxidative stress and depletion of cellular antioxidant machinery. In response to elevated ROS level, RBCs elicit the action of their antioxidant system. There are some key differences between rat and human RBCs which make rat RBCs more suitable as a model of oxidative stress. Percentage aggregation in rat RBCs is less than human RBCs, thus surface of rat RBCs are highly exposed to toxicants. Beside this, rat RBCs are more susceptible to osmotic fragility and demonstrates high diffusion permeability as compared to human RBCs [34]. These all key differences suggest that rat RBCs may be considered as an emerging model to understand the molecular mechanism of oxidative stress imparted by toxicants [35].
There have been several reports concerning the effect of As on RBC [33, 35] but corresponding studies accounting the role of novel thiol chelator MiADMSA against toxic effects of As on RBC are lacking. Even no study revealed the effect of gallic acid alone or in combination with thiol chelator upon reversal of oxidative/nitrosative damage to red blood cells. Hence the aim of current study is to evaluate potential oxidative stress mediated mechanism in rat blood cell damage imparted by arsenic and chelating efficiency of MiADMSA alone and in combination with gallic acid for reversal of arsenic induced oxidative damage.
2. Materials and methods
2.1. Chemicals
Sodium (meta) arsenite (SA) was procured from Sigma-Aldrich (St. Louis, MO, USA), while all other chemicals were of Analytical or Extra pure grade and purchased from Merck (Darmstadt, Germany) or Sigma (St. Louis, MO, USA).
2.2. Animals
The experimental protocols were approved by the Institutional Animal Ethics Committee (IAEC) containing approval number NIPER/RBL/IAEC/09/Aug2017. Six male wistar rats (weighing about 180–200 g, ~7–8 weeks) were obtained from the CSIR-Central Drug Research Institute, Lucknow. Out of six rats three animals were taken for the purpose of erythrocyte experiments and three animals were taken for the Measurement of blood arsenic concentration at different time points. Blood was collected in animal house of NIPER-Raebareli, UP. All animal husbandry procedures were maintained as per the Standard Operating Procedures (SOPs) followed in the test facility. All experimental animals were housed in standard polypropylene cages (3rats/cage) and were maintained at standard conditions of temperature (22 ± 2 °C), humidity (55 ± 5%) and light (12 h light and 12 h dark cycle). They were fed with standard pellet feed (purchased from Lipton's India Ltd) and water (aqua pure) ad libitum. The CPCSEA guidelines for care and use of laboratory animals were carefully followed. Animals were allowed to acclimatize to the experimental conditions for a period of 1 week prior to the blood withdrawal. After administration of sodium (meta) arsenite blood drawn from the saphenous vein repeatedly at different time point (0 time point, 30 min, 1hr, 2hr, 4hr, 8hr, 12hr, 24hr) without anaesthesia. In each time point 100μl of samples were collected.
2.3. Measurement of blood arsenic concentration at different time points
In order to determine the concentration of arsenic in blood, we administered the dose containing 200 ppm and measured arsenic concentration at different time points. The selection of dose based on an established dose equivalent equation that adjusts for surface area differences between rodents and humans [36] and the use of similar exposures in studies addressing arsenic cytotoxicity in murine model [37]. In short, our approach was designed to evaluate arsenic toxicity in red blood cells in the context of high, acute exposure, so that the actual scenario of arsenic toxicity to RBCs can be mimicked. To perform this study, three healthy male rats were subjected to arsenic (200ppm) by oral route and blood samples were collected at different time intervals (30 min, 1 h, 2 h, 4 h, 8 h, 16 h and 24 h). After that, estimation of the level of arsenic in whole blood was done by atomic absorption spectroscopy.
2.4. Isolation of RBCs
Blood samples taken from healthy rats were collected in heparinised tubes and immediately subjected to centrifugation at 1700 rpm, 4 °C for 10 min Supernatant (plasma) was removed and the packed RBCs were washed with phosphate buffered saline (PBS, 10 mM sodium phosphate buffer in 0.9% NaCl, pH 7.4). The RBCs were then suspended in DMEM media to prepare a cell suspension of 2 × 108 cells per 100 μL.
2.5. Experimental design
RBC cell suspension was divided into 7 groups, each group having N = 3.2 × 108 cells and subjected to exposure and treatment in each group. The groups were as follows:
Group 1: Control (treated as normal and received PBS).
Group 2: MiADMSA (0.1mM).
Group 3: Gallic acid (1mM).
Group 4: Inorganic Arsenic as sodium (meta) arsenite (2.5 ppm).
Group 5: Arsenic + MiADMSA.
Group 6: Arsenic + Gallic acid.
Group 7: Arsenic + MiADMSA + Gallic acid.
Stock solution of SA was prepared in PBS while MiADMSA was prepared in phosphate buffer pH = 8. RBC suspensions were acclimatized at 37 °C, 5% CO2 and 85% humidity for 2 h. Nutrients to cell culture were supplied by DMEM media along with 5% FBS. RBCs were first incubated with protective agents (MiADMSA and gallic acid) in respective groups for 2 h prior to arsenic exposure. Then cell suspensions were exposed to arsenic overnight. Untreated (control) RBC were also incubated overnight at 37 °C under identical conditions. After these incubations, samples were centrifuged at 2600 rpm for 10 min at 4 °C and the RBC pellets were washed with PBS. Packed RBCs were lysed by addition of lysis/hypotonic buffer (5 mM sodium phosphate buffer, pH 7.4) followed by centrifugation at 3000 rpm for 10 min to remove cell debris. The supernatant (hemolysates) were used immediately or stored at 4 °C for later use.
2.6. Element analysis
Arsenic estimation in hemolysates was performed by Atomic Absorption spectrophotometer. Initially the hemolysates were subjected to digestion using a conventional acid digestion method. Arsenic level was determined after wet acid digestion by using microwave digestion system (CEM, USA, model MDS- 2100). Arsenic was estimated using a hydride vapour generation system (Perkin Elmer model MHS-10) fitted with an atomic absorption spectrophotometer (AAS, Perkin Elmer model Analyst 100).
2.7. Osmotic fragility
Osmotic fragility of erythrocytes was determined according to the protocol described by Veena et al., [38]. In brief, 0.05 mL of RBC suspension was incubated with 5 mL of hypotonic NaCl (0.2%) at 37 °C for 30 min followed by centrifugation at 2500 rpm for 10 min. The absorbance of supernatants was recorded at 540 nm in order to estimate the release of Hb in the extracellular medium. The absorbance of sample cell suspensions was compared with the absorbance of untreated RBC lysed with lysis buffer (5 mM sodium phosphate buffer, pH 7.4) at the test conditions (corresponds to 100% lysis) [38].
2.8. Biochemical assays
2.8.1. Estimation of protein
Total protein content of RBCs was measured according to the method of Lowry et al. [39]. Briefly, 5μl of cell hemolysate was mixed with 1 ml of lowery mixture/solution D (0.5% CuSO4 in 1% Na+/K+ tartarate and 2% Na2CO3 in 0.1N NaOH), and incubated for 10 min. After incubation 0.1ml of Folin-Ciocalteau reagent was added to the previous mixture and again kept on incubation for 30 min at room temperature. Blue colour developed was read at 660 nm. Protein concentration in samples was calculated using a standard reference curve plotted using bovine serum albumin as a standard. The results were expressed as mg protein/ml [39].
2.8.2. Measurement of ROS level
The level of reactive oxygen species (ROS) in samples was estimated according to the protocol described by Keller et al. [40], with slight modifications. Briefly, the reaction mixture contained 5 μL of cell hemolysates along with 5 μL of DCFDA (5μM) and 990 μL of PBS. Mixture was incubated for 30 min, and the product fluorescence was measured on multimode plate reader at excitation and emission wavelengths of 485 and 529 nm, respectively. Results were expressed as fluorescence units/mg protein [40].
2.8.3. DPPH assay
The antioxidant power in samples was estimated by DPPH (α, α-diphenyl-β-picrylhydrazyl) assay developed by Blois, [41]. DPPH is a stable free radical with characteristic violet colour at 520 nm. The reaction mixture consisted of 300 μL sample, 300 μL DPPH (0.3mM) and 300 μL methanol while blank contained 300 μL DPPH and 600 μL methanol. The reaction mixtures were then incubated for 10 min and colour change from violet to yellow was read spectrophotometrically at 520 nm [41].
2.8.4. Measurement of malondialdehyde (MDA) level
MDA, which is a measure of lipid peroxidation, was measured according to the protocol of Okhawa et al., [42]. The assay mixture consisted of acetic acid 1.5 ml (20%), pH 3.5, 1.5 ml of thiobarbituric acid (0.8%), 0.2 ml of sodium dodecyl sulfate (8.1%) and 0.1 ml of processed cell hemolysate. The mixture was then heated at 100 °C for 60 min and allowed to cool. The reaction mixture was then centrifuged at 4000 rpm for 10 min. Supernatant was collected and absorbance was measured at 532 nm. MDA levels was calculated from the standard curve plotted using the 1, 1, 3, 3-tetramethoxy propane (97 %) and expressed as μM MDA/mg protein [42].
2.8.5. Estimation of reduced glutathione level
Reduced glutathione GSH(r) was estimated by following the protocol of Ellman et al. [43], with some modifications. The RBC hemolysate prepared as indicated above was mixed with 5 % sulfosalicylic acid and vortexed. The mixture was then kept for 30 min in ice bath followed by centrifugation at 30,000 rpm for 10 min at 4 °C. After centrifugation, the supernatant was collected and GSH content was estimated by using Ellman's reagent i.e. 5, 5-dithiobis (2-nitrobenzoic acid) (DTNB) solution. GSH level in samples was calculated using a standard reference curve plotted using reduced glutathione as a standard. Results were expressed in μM GSH/mg protein [43].
2.8.6. Measurement of catalase activity
Catalase activity in hemolysates was estimated in accordance with the protocol of Goth, [44]. Ammonium molybdate develops a yellow colored complex with H2O2 and is suitable for measuring catalase activity in hemolysates. To assess the catalase activity, 0.2 ml of cell hemolysate was incubated with 1 ml of 65 μM H2O2 in 6.0 mM sodium potassium phosphate buffer, pH 7.4 for 60 s (sample 1). Control reactions were prepared with 1 ml H2O2 along with 0.2 ml buffer (no enzyme control; blank 2) and 1.2 ml buffer (no enzyme/no substrate, blank 3). At last, the reactions were stopped by adding 1.0 ml of 32.4 mM ammonium molybdate to sample and both the blank reactions followed by determination of absorbance at 405 nm [44].
2.8.7. Measurement of SOD activity
Superoxide dismutase (SOD) activity was estimated following the protocol of Kakkar et al., [45]. Briefly, the sample reaction mixture consisted of 1.2 ml of sodium pyrophosphate, 0.3 ml of PMS, and 0.3 ml of NBT, 0.2 ml of hemolysate, 0.8 ml of distilled water and 0.2 ml of NADH while in the control reaction mixture instead of hemolysate 0.2 ml of distilled water was added. Both mixtures were incubated at 37 °C for 90 s and then 1 ml of acetic acid was added in order to stop the reaction. The assay mixtures were allowed to stand for 10 min and then absorbance was taken at 560 nm [45].
2.8.8. Measurement of nitrite level
Nitrite Content in the hemolysates was measured according to the method previously described by Giustarini et al. [46], with some modifications. For the determination of nitrite content, equal volumes of Griess reagent and hemolysates were added in a 96 well plate. The plate was then incubated at 37 °C for 10 min in dark and pink colour thus developed was read at 540 nm. Nitrite levels were calculated by a standard curve plotted using sodium nitrite as a reference standard and expressed as μM/mg protein [46].
2.8.9. Estimation of acetylcholinesterase activity
The Acetylcholinesterase (AChE) activity in hemolysates was determined following the method previously described by Ellman et al. [47], with some modifications. Briefly, 10 μl of hemolysate was mixed with 272 μl of mixture containing 10mM DTNB, 75mM ATCI and 50 mM phosphate buffer pH 7.4 in 96 well micro plate. The absorbance of the reaction mixture was measured at 412 nm in kinetic loop using Multimode plate Reader [47].
2.9. Statistical analysis
All results are expressed as the mean ± standard error of the mean (SEM). The statistical differences between groups were analyzed by one-way ANOVA using Graph Pad (Prism 6) software. Post hoc testing for inter-group comparisons was performed with student's T test. A probability level of P < 0.05 was considered as statistically significant.
3. Results
3.1. Effect of arsenic concentration in blood at different time points
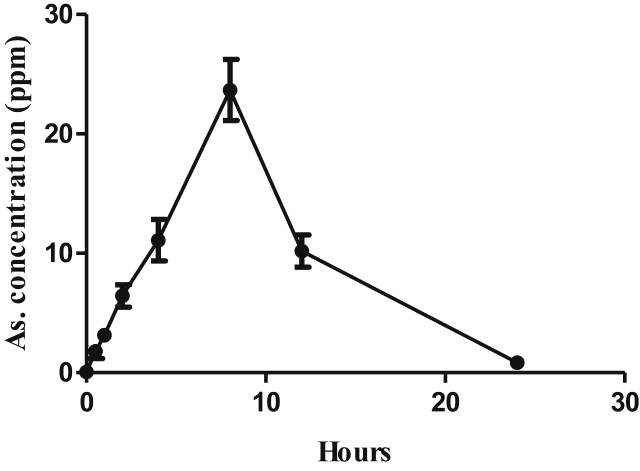
In order to determine the concentration of arsenic in whole blood, we administered the dose containing 200 ppm and measured arsenic concentration at different time intervals (30 min, 1 h, 2 h, 4 h, 8 h, 16 h and 24 h) by using hydride vapour generation system fitted with an atomic absorption spectrophotometer. From the result of metal estimation, we found the highest concentration (~25 ppm) of arsenic at 8 h as shown in Figure 1.
Figure 1.
Concentration of arsenic in whole blood at different time interval after single dose oral administration in male Wistar rats.
3.2. Concentration of arsenic in RBCs post exposure
The concentration of arsenic in arsenic exposed group and groups exposed with monotherapy of MiADMSA and gallic acid as well as in combination group is demonstrated in Figure 2. The graph depicted the enhanced concentration of metal in arsenic exposed group as compared to control, though it was not significantly enhanced. The concentration of arsenic was found to be high in MiADMSA monotherapy group as compared to arsenic exposed group. Furthermore, the concentration of arsenic was found to be significantly declined in group exposed to combination therapy as compared to monotherapy with MiADMSA as well as gallic acid.
Figure 2.
Concentration of arsenic in RBCs of various groups. All the values are expressed as mean ± SEM (n = 3), ****P < 0.0001 vs control, #P < 0.05, ##P < 0.01 vs arsenic and $$$$P < 0.0001 vs As + MiADMSA. [GA = gallic acid, MiADMSA = monoisoamyl DMSA, As = arsenic].
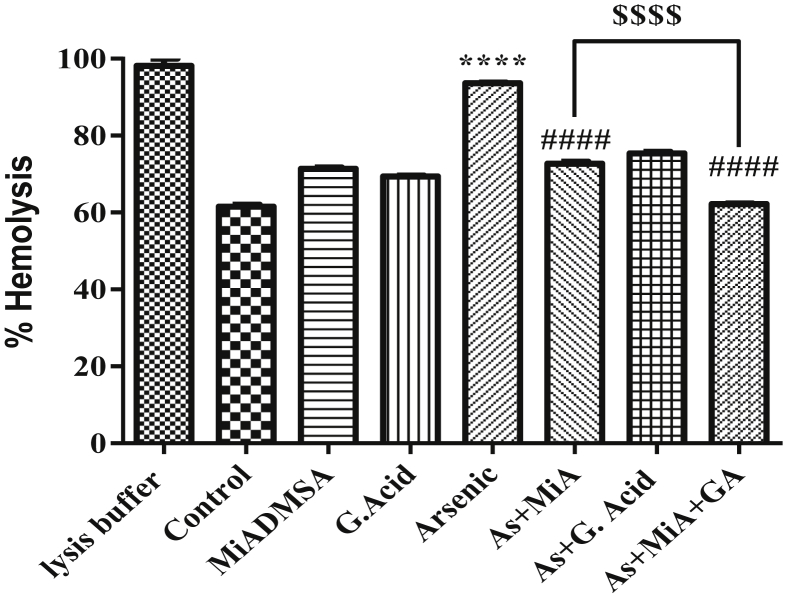
3.3. Effect of protective therapies on arsenic induced osmotic fragility
Figure 3 demonstrated the osmotic fragility pattern of various groups in hypotonic (0.2% saline) conditions. Osmotic fragility in terms of % hemolysis was found to be significantly high in arsenic exposed group as compared to control group. Prior treatment with MiADMSA alone or in combination with gallic acid significantly declined the % hemolysis. Furthermore, combination therapy significantly reduced the % hemolysis as compared to monotherapy with MiADMSA.
Figure 3.
Effect of arsenic exposure on osmotic fragility. All the values are expressed as mean ± SEM (n = 3), ****P < 0.0001 vs. Control and ####P < 0.0001 vs. Arsenic and $$$$P < 0.0001 vs. Arsenic + MiADMSA.
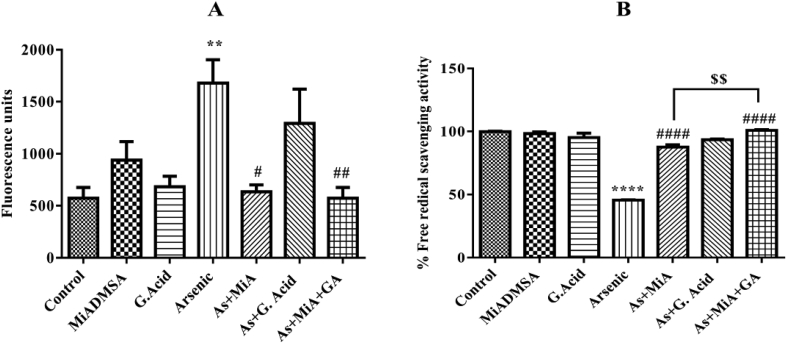
3.4. Effect of arsenic exposure and protective therapies on ROS level and antioxidant power
Figure 4 demonstrated the ROS level and free radical scavenging efficiency in exposed and treated groups. Arsenic exposure significantly elevated ROS level which was then significantly counteracted in MiADMSA and gallic acid treated group resulting in decreased level of ROS. In parallel, the free radical scavenging property was too significantly enhanced in combination group as well as in MiADMSA protective group as compared to arsenic exposed group. No significant difference was observed in ROS level in combination group as compared to monotherapy with MiADMSA while there was significant increase in antioxidant power in combination group as compared to monotherapy with MiADMSA.
Figure 4.
A) Effect of arsenic exposure and protective therapies on ROS level B) Effect of arsenic exposure and protective therapies on antioxidant power. All the values are expressed as mean ± SEM (n = 3), **P < 0.01, ****P < 0.0001 vs. Control and #P < 0.05, ##P < 0.01, ####P < 0.0001 vs. Arsenic $$P < 0.01 vs. Arsenic + MiADMSA.
3.5. Effect of arsenic exposure and protective therapies on various oxidative/nitrosative stress variables
Figure 5 demonstrated the effect of arsenic exposure and protective therapies on various oxidative/nitrosative stress variables upon overnight incubation. The results depicted the generation of oxidative as well as nitrosative stress along with the lipid peroxidation of cell membrane. Arsenic exposure significantly elevated the level of malondialdehyde (MDA), an indicative of membrane damage. MiADMSA treatment marginally decreased MDA level while combination therapy resulted in significant decrease in the level of MDA. Beside this, MDA level was further reduced significantly in combination therapy as compared to monotherapy with MiADMSA. A significant reduction in reduced GSH level was found in arsenic exposed group as compared to control. MiADMSA treatment marginally enhanced level of GSH while significant elevation of reduced GSH level in combination therapy was noticed. Though, no significant effect of either exposure or treatment was found on catalase and SOD activity, but there was decreased activity of these antioxidant enzymes in arsenic exposed group while increased activity in combination group. Significantly enhanced level of nitrite, an indicative of nitrosative stress, was found in arsenic exposed group. Though decreased level of nitrite was found in MiADMSA alone and combination therapy, but no significant effect was observed.
Figure 5.
A) Effect of arsenic exposure and protective therapies on MDA level B) Effect of arsenic exposure and protective therapies on reduced GSH level. C) Effect of arsenic exposure and protective therapies on catalase activity. D) Effect of arsenic exposure and protective therapies on SOD activity. E) Effect of arsenic exposure and protective therapies on nitrite level. All the values are expressed as mean ± SEM (n = 3), ****P < 0.0001 vs. Control and #P < 0.05, ##P < 0.01, ####P < 0.0001 vs. Arsenic and $$$P < 0.0001 vs. Arsenic + MiADMSA.
3.6. Effect of arsenic exposure and protective therapies on acetylcholinesterase activity
Figure 6 demonstrated the AChE activity in different groups. The AChE activity was significantly reduced in arsenic exposed group while it was significantly enhanced in MiADMSA treatment and combination treated groups. Moreover, a significantly enhanced AChE activity was noticed in combination therapy as compared to monotherapy with MiADMSA.
Figure 6.
Effect of arsenic exposure and protective therapies on AChE activity. All the values are expressed as mean ± SEM (n = 3), ***P < 0.001, vs. Control and ####P < 0.0001 vs. Arsenic and $$$$P < 0.0001 vs. Arsenic + MiADMSA.
3.7. Morphological changes of RBCs on exposure with gallic acid, MiADMSA and arsenic alone and in combination
The protective effect of Gallic acid and chelating effect of MiADMSA against SA-induced oxidative damage was visualized by inverted microscope. The control (SA untreated) RBC showed the normal smooth biconcave shape (Figure 7A). No such damaging effect was observed in MiADMSA and Gallic acid treatment group (Figure 7B, C). However fragmentation, spindle shape and rupturation of RBCs upon treatment with SA was observed as shown in Figure 7D. Incubation of RBCs with SA in the presence of MiADMSA, gallic acid showed normal erythrocyte shape and only a minority of cells had slight irregularities on their surface (Figure 7E, F, G).
Figure 7.
Inverted microscopic images of RBCs. (A) Untreated control cells. RBCs treated with (B) MiADMSA alone (C) Gallic acid alone and (D) SA alone (E) combination of SA and MiADMSA (F) combination of Gallic acid and MiADMSA (G) combination of SA, MiADMSA and Gallic acid. Morphological view of all group have taken on 100X magnification. Contrast and brightness was adjusted by Microsoft picture manager 2007.
4. Discussion
Arsenic is currently a major health issues including skin lesions, cancer, neurological effects, and diabetes and lung diseases [48]. In view of omnipresence nature of arsenic in the environment and its harmful effects on almost every organ of the body, efforts to develop a suitable therapies having ability either to prevent or counteract the effects of arsenic need to be intensified.
Although the exact mechanism by which arsenic exerts harmful effects is still not clearly defined. The ability of arsenic to generate harmful reactive species (ROS/RNS) leading to oxidative stress in the biological system is most widely accepted hypothesis to explain mechanism behind its toxicity. Arsenites may alter the redox balance of the cell either by directly reacting with cellular components or by exerting their effect on the antioxidant system [49]. RBCs, being the most abundant cells are also more susceptible to oxidative damage due to toxicants. Ability of RBCs to deliver oxygen to body tissues, presence of large content of polyunsaturated fatty acids, redox sensitive Hb molecules, and transition metals are major characteristics which make them more susceptible to oxidative damage. To deal with elevated level of ROS, the RBCs possess an efficient and competent antioxidant defence system [50]. Thus, we selected RBCs as the target for studying arsenic induced oxidative stress.
Currently available modalities of combating arsenic induced toxicity include chelation therapy and antioxidant supplementation. Recent studies have been more inclined towards the use of chelating agents alone or in combination with natural antioxidants in order to achieve synergistic effects against toxicity [9]. In this study, we tried to figure out the combinatorial effect of MiADMSA and gallic acid on arsenic induced cytotoxicity in rat RBCs. The various enzymatic and non-enzymatic parameters of oxidative stress were analyzed, in addition to metal estimation and cell fragility study.
The result of metal concentration in exposed and intact RBCs suggests that there was a significant accumulation of arsenic inside the cell. From the previous finding [26] it was clear that, there was a pronounced decrease in the level of arsenic in MiADMSA treated group possibly due to chelating abilities imparted by MiADMSA. In the current study we noted a more accumulation of arsenic in the arsenic + MiADMSA treated group compared to arsenic treated group alone, which again confirmed the intrinsic arsenic chelation abilities imparted by MiADMSA in erythrocytes in vitro. Due to the presence of eliminatory system in living objects, the chelated arsenic gets eliminated from the body and thus decreases arsenic burden inside the cell but in the intact cell there was deprivation of eliminatory system and hence it is unable to eliminate after being chelated by MiADMSA. The data of metal concentration obtained in current study get credibility from earlier studies which suggested accumulation of arsenic in RBCs [51, 52].
Metal accumulation inside cells leads to a series of events generating ROS/RNS which causes damage to lipid bilayer of cell membrane as evident by high MDA level [8]. This results into decreased resistant power of cells against hemolysis and osmotic fragility. Thus, the alterations in membrane related parameters due to arsenic were greatly declined by protective interventions. Arsenic exposure to cells makes them more susceptible to the hemolysis as evident from large amount of Hb liberated in supernatant as well as increases their osmotic fragility making them to lyse at higher salt concentration. MiADMSA and gallic acid treated cells demonstrated markedly decreased hemolysis and osmotic fragility.
The augmentation in ROS level in RBCs exposed to SA, suggests that SA increases ROS production in cells which are liable to cause oxidative damage of cellular components. Significantly reduced ROS level in MiADMSA and gallic acid combination therapy as compared to SA exposed and individual (MiADMSA or gallic acid) treated groups provides conclusive evidence regarding synergistic effect of combination therapy against SA induced ROS production and thus oxidative stress. Oxidative damage to RBCs impairs their oxygen delivery capacity and reduces life span [53]. The harmful effects of ROS are not just limited to RBC itself but large quantity of ROS leaving the RBC exert enormous effects on other components of circulation [54]. In another assay, we estimated the antioxidant power in terms of free radical scavenging potency in various groups. SA significantly decreased antioxidant activity which may be correlated with elevated level of ROS. Enhanced free radical scavenging potency in MiADMSA pre-treated SA exposed group may be attributed due to chelating efficiency of thiol group in its structure. Furthermore, the antioxidant power was further enhanced in combination therapy as compared to monotherapy with MiADMSA and this enhancement may be due to synergism in antioxidant activity of gallic acid and chelation potency of MiADMSA.
Elevated level of ROS causes lipid peroxidation in cell membrane eventually leading to damage as also evident by osmotic fragility and haemolytic studies. Increased level of MDA, a thiobarbituric acid reactive substance (TBARS) is considered as a biomarker of lipid peroxidation. Lipid peroxidation in cell membrane may adversely affect its fluidity, permeability and osmotic fragility. There are plentiful of evidences which suggest negative impact of arsenic on cell membrane [26, 55]. These evidences correlate with the levels of MDA estimated in our study where a significant increase was observed in arsenic exposed group as compared to control. Pre-treatment with MiADMSA protected the cells against SA effects and marginally declined the elevated MDA level, portraying improvement from oxidative burden. The combination therapy of MiADMSA and gallic acid is supposed to be more effective than monotherapy as also evident by the significantly reduced MDA level.
Free radical scavengers play crucial role in tackling oxidative stress. GSH, a main cysteine containing tripeptide biological antioxidant is one such scavenger which is responsible for maintaining reducing environment inside cell. ROS are well known to react with thiol groups and consequently there was depletion in total GSH content in SA exposed group. GSH being a nucleophile plays a critical role in protecting RBC against free radicals. Due to presence of thiol groups in MiADMSA, it chelates free radicals and thus protects the biological antioxidants from depletion while gallic acid being an antioxidant facilitates the normal level of GSH in cells. Thus, these characteristics of MiADMSA and gallic acid may be elucidative for significant response in combination therapy.
Moreover, other antioxidants like SOD and CAT were also unfavourably affected by arsenicosis. The impact of arsenic obtained in our study on these antioxidants level was in accordance with the previously published study [26]. Significant drop in the level of these antioxidants was observed in arsenic exposed erythrocytes. As soon as oxidative stress develops in body, antioxidant enzymes come in front in order to fight against elevated level of ROS and thus are considered as the first line defense system against oxidative damage. Decrease in SOD and CAT activity in erythrocytes may be attributed to the enhanced level of superoxide and peroxide radicals respectively [56, 57]. In the current study, therapeutic regimens were found to be efficacious, though not significant effect, in reducing oxidative stress burden as evident by the data of SOD and CAT. Enhanced affectivity of MiADMSA in combination with gallic acid may be attributed to the antioxidant property of gallic acid.
Arsenicosis is not only responsible for generation of reactive oxygen species but also involved in production of reactive nitrogenous species (RNS) resulting in nitrosative stress too in addition to oxidative stress [58, 59]. Reactive nitrogen species tends to exert more deleterious effects inside cells than ROS. They are considered to be short-living and thus are difficult to measure. Therefore, they are quantified in terms of nitrite content of cell. In present study, arsenic enhanced RNS level and therapeutic regimens were found to exert beneficial effects, though non-significant, on elevated nitrosative stress.
AChE level in RBCs may be correlated with cell aging. There are evidences supporting the role of AChE in erythrocytes aging [60, 61]. In our study, arsenic was found to have adverse effects on erythrocyte AChE level. AChE activity was significantly decreased upon arsenic exposure which may be correlated with cell membrane damage and aging process of RBCs. The impact of arsenic exposure and protective regimens on AChE level is related to that obtained on osmotic fragility and suggests that as AChE activity decreases, the RBCs tends to become more susceptible to osmotic fragility. Prior treatment with MiADMSA and combination therapy successfully recovered the enzyme activity. Data obtained in our study further get credibility from earlier study done by Maheshwari et al [35].
A change in morphology with respect to any exposed toxicant is a key feature of cells. Being dangerous toxicant arsenic changed morphology of most abundant cells in circulation which may result into various life threatening disorders like anemia. In our microscopic observation we found that, there was normal smooth biconcave shape RBCs in control (SA untreated) group however fragmentation of cells, spindle shape and rupturation of RBCs was observed in SA treatment group which may possibly cause anemia. Chemically induced intravascular hemolysis is not limited to anemia it may also cause kidney damage and severe cases disseminated intravascular coagulation (DIC syndrome) [62]. According to Lee et al., there is link between arsenic and hemolytic anemia [63]. Besides these, it has been reported that arsenic alters erythrocyte morphology and induces erythrocyte death [64, 65]. Moreover, incubation of RBCs with SA in the presence of MiADMSA, gallic acid showed normal erythrocyte shape and only minority of cells had slight irregularities on their surface signifies the protective sign by the following agents.
In our study we found that arsenic resulted into oxidative stress either due to increase in ROS level or depletion of natural antioxidant stores of cell. There was reversibility of oxidative/nitrosative stress variables, elevated cellular antioxidant power, and decreased osmotic fragility of red blood cells both in MiADMSA alone as well as in combination with gallic acid treated group as compared with arsenic treated group. Previous in-vivo study suggested that MiADMSA in combination with gallic acid possess limited beneficial effects over MiADMSA against arsenic-induced oxidative stress [26]. In our results, we found that combination of gallic acid and MiADMSA is more efficacious than monotherapy with either MiADMSA or gallic acid in handling arsenic-induced oxidative stress.
5. Conclusion
In conclusion, our present study illustrated that there is a strong link between arsenic and oxidative/nitrosative stress as confirmed by high metal accumulation, elevated level of ROS and depleted cellular antioxidants level. Oxidative stress may be a possible link between arsenic induced RBC damage as mostly observed in people of high arsenic contaminated area. Despite of the fact that current study gives concrete experimental support for possible linkage between arsenic, oxidative/nitrosative stress and RBCs damage yet further studies focused on deep mechanisms need to be conducted. From the data, we concluded that novel metal chelator MiADMSA efficiently participated for reversal of arsenic induced oxidative/nitrosative damage in red blood cells where as gallic acid improve its reversal when given in combination with MiADMSA.
Declarations
Author contribution statement
S. Flora: Conceived and designed the experiments; Wrote the paper.
A. Panghal: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
K.B. Sathua: Performed the experiments; Analyzed and interpreted the data.
Funding statement
We wish to acknowledge the financial assistance received from Department of Pharmaceutical, Ministry of Chemicals & Fertilizers, Government of India, for carrying out the present experimentation.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Flora S.J.S. Arsenic-induced oxidative stress and its reversibility. Free Radic. Biol. Med. 2011;51(2):257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Flora S.J.S., Flora G., Saxena G. Arsenicals: toxicity, their use as chemical warfare agents and possible remedial measures. In: Gupta R.C., editor. Handbook of the Toxicology of Chemical Warfare Agents. Academic Press; San Diego: 2009. pp. 109–133. [Google Scholar]
- 3.Valko M., Morris H., Cronin M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005;12(10):1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhury U.K., Biswas B.K. Groundwater arsenic contamination in Bangladesh and West Bengal, India. Environ. Health Perspect. 2000;108(5):393–397. doi: 10.1289/ehp.00108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazumder D.N.G., Haque R., Ghosh N., De B.K., Santra A., Chakraborty D., Smith A.H. Arsenic levels in drinking water and the prevalence of skin lesions in West Bengal, India. Int. J. Epidemiol. 1998;27:871–877. doi: 10.1093/ije/27.5.871. [DOI] [PubMed] [Google Scholar]
- 6.Aposhian H.V., Aposhian M.M. Arsenic toxicology: five questions. Chem. Res. Toxicol. 2006;19:1–15. doi: 10.1021/tx050106d. [DOI] [PubMed] [Google Scholar]
- 7.Thomas D.J., Li J. Arsenic (+3 oxidation state) methyltransferase and the methylation of arsenicals. Exp. Biol. Med. 2007;232:3–13. [PMC free article] [PubMed] [Google Scholar]
- 8.Flora S.J.S. “Arsenic-induced oxidative stress and its reversibility following combined administration of N-acetylcysteine and meso 2,3–dimercaptosuccinic acid in rats”. Clin. Exp. Pharmacol. Physiol. 1999;26:865–869. doi: 10.1046/j.1440-1681.1999.03157.x. [DOI] [PubMed] [Google Scholar]
- 9.Flora S.J.S., Bhadauria S. Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: a review. J. Environ. Biol. 2007;28(2):333–347. [PubMed] [Google Scholar]
- 10.Shi H., Shi X., Liu K.J. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol. Cell. Biochem. 2004;255:67–78. doi: 10.1023/b:mcbi.0000007262.26044.e8. [DOI] [PubMed] [Google Scholar]
- 11.Gebel T.W. Arsenic methylation is a process of detoxification through accelerated excretion. Int. J. Hyg Environ. Health. 2002;205(6):505–508. doi: 10.1078/1438-4639-00177. [DOI] [PubMed] [Google Scholar]
- 12.Jomova K., Jenisova Z. Arsenic: toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011;31:95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- 13.Pi J., Horiguchi S., Sun Y., Nikaido M., Shimojo N., Hayashi T. A potential mechanism for the impairment of nitric oxide formation caused by prolonged oral exposure to arsenate in rabbits. Free Radical Biol. Med. 2003;35:102–113. doi: 10.1016/s0891-5849(03)00269-7. [DOI] [PubMed] [Google Scholar]
- 14.Rin K., Kawaguchi K., Yamanaka K., Tezuka M., Oku N., Okada S. DNA-strand breaks induced by dimethylarsenic acid, a metabolite of inorganic arsenics, are strongly enhanced by superoxide anion radicals. Biol. Pharm. Bull. 1995;18:45–48. doi: 10.1248/bpb.18.45. [DOI] [PubMed] [Google Scholar]
- 15.Ercal N., Gurer-Orhan H., Aykin-Burns N. “Toxic metals and oxidative stress.” Part I. Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 16.Mishra D., Mehta A., Flora S.J.S. Reversal of hepatic apoptosis with combined administration of DMSA and its analogues in Guinea pigs: role of glutathione and linked enzymes. Chem. Res. Toxicol. 2008;21:400–407. doi: 10.1021/tx700315a. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad S., Kitchin K.T., Cullen W.R. Arsenic species that cause release of iron from ferritin and generation of activated oxygen. Arch. Biochem. Biophys. 2000;382:195–202. doi: 10.1006/abbi.2000.2023. [DOI] [PubMed] [Google Scholar]
- 18.Nemeti B., Gregus Z. Reduction of arsenate to arsenite in hepatic cytosol. Toxicol. Sci. 2002;70:4–12. doi: 10.1093/toxsci/70.1.4. [DOI] [PubMed] [Google Scholar]
- 19.Santra A., Chowdhury A. Arsenic induces apoptosis in mouse liver is mitochondria dependent and is abrogated by N-acetylcysteine. Toxicol. Appl. Pharmacol. 2007;220:146–155. doi: 10.1016/j.taap.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 20.Birben E., Sahiner U.M. Oxidative stress and antioxidant defense. World allergy organ J. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flora S.J.S., Pachauri V. Chelation in metal intoxication. Int. J. Environ. Res. Publ. Health. 2010;7:2745–2788. doi: 10.3390/ijerph7072745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar M.R., Flora S.J.S., Reddy G.R. Monoisoamyl 2,3-dimercaptosuccinic acid attenuates arsenic induced toxicity: Behavioral and neurochemical approach. Environ. Toxicol. Pharmacol. 2013;36:231–242. doi: 10.1016/j.etap.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Mehta A., Kannan G.M. Haematological, hepatic and renal alterations after repeated oral or intraperitoneal administration of monoisoamyl DMSA.I. Changes in male rats. J. Appl. Toxicol. 2002;22:359–369. doi: 10.1002/jat.871. [DOI] [PubMed] [Google Scholar]
- 24.Flora S.J.S., Mehta A. Haematological, hepatic and renal alterations after repeated oral and intraperitoneal administration of monoisoamyl DMSA. II. Changes in female rats. J. Appl. Toxicol. 2003;23:97–102. doi: 10.1002/jat.890. [DOI] [PubMed] [Google Scholar]
- 25.Flora S.J.S., Srivastava R., Mittal M. Chemistry and pharmacological properties of some natural and synthetic antioxidants for heavy metal toxicity. Curr. Med. Chem. 2013;20:4540–4574. doi: 10.2174/09298673113209990146. [DOI] [PubMed] [Google Scholar]
- 26.Pachauri V., Flora S.J.S. Combined efficacy of gallic acid and MiADMSA with limited beneficial effects over MiADMSA against arsenic-induced oxidative stress in mouse. Biochem. Insights. 2015;8:1–10. doi: 10.4137/BCI.S30505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel S.S., Goyal R.K. Cardioprotective effects of gallic acid in diabetes-induced myocardial dysfunction in rats. Pharmacogn. Res. 2011;3:239–245. doi: 10.4103/0974-8490.89743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh M.P., Gupta A., Sisodia S.S. Gallic acid: pharmacological promising lead molecule: a review. Int. J. Pharmacogn. Phytochem. Res. 2018;10(4):132–138. [Google Scholar]
- 29.Lee C.H., Chang H.R., Chen J.S. Defective adrenergic responses in patients with arsenic-induced peripheral vascular disease. Angiology. 2007;58(2):161–168. doi: 10.1177/0003319707300351. [DOI] [PubMed] [Google Scholar]
- 30.Yu H.S., Lee C.H., Chen G.S. Peripheral vascular diseases resulting from chronic arsenical poisoning. J. Dermatol. 2002;29(3):123–130. doi: 10.1111/j.1346-8138.2002.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 31.Sieradzki A., Skoczynska A., Andrzejak R. Effect of arsenic and its compounds on the circulatory system. Med. Pr. 2000;51(5):485–493. [PubMed] [Google Scholar]
- 32.Lu M., Wang H., Li X.-F. Evidence of hemoglobin binding to arsenic as a basis for the accumulation of arsenic in rat blood. Chem. Res. Toxicol. 2004;17:1733–1742. doi: 10.1021/tx049756s. [DOI] [PubMed] [Google Scholar]
- 33.Bollini A., Huarte M. Arsenic intoxication, a hemorheologic view. Clin. Hemorheol. Microcirc. 2010;44(1):3–17. doi: 10.3233/CH-2010-1246. [DOI] [PubMed] [Google Scholar]
- 34.Da Silveira Cavalcante L., Acker J.P., Holovati J.L. Differences in rat and human erythrocytes following blood component manufacturing: the effect of additive solutions. Transfus. Med. Hemotherapy. 2015;42(3):150–157. doi: 10.1159/000371474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maheshwari N., Khan F.H., Mahmood R. 3,4-Dihydroxybenzaldehyde lowers ROS generation and protects human red blood cells from arsenic(III) induced oxidative damage. Environ. Toxicol. 2018:1–15. doi: 10.1002/tox.22572. [DOI] [PubMed] [Google Scholar]
- 36.Veena C.K., Josephine A., Preetha S.P., Varalakshmi P. Effect of sulphated polysaccharides on erythrocyte changes due to oxidative and nitrosative stress in experimental hyperoxaluria. Hum. Exp. Toxicol. 2007;26(12):923–932. doi: 10.1177/0960327107087792. [DOI] [PubMed] [Google Scholar]
- 37.Lowery O.H., Rosebrough N.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 38.Keller A., Mohamed A., Drose S. Analysis of dichlorodihydrofluorescein and dihydrocalcein as probes for the detection of intracellular reactive oxygen species. Free Radic. Res. 2004;38(12):1257–1267. doi: 10.1080/10715760400022145. [DOI] [PubMed] [Google Scholar]
- 39.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 40.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 41.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 42.Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta. 1991;196(2):143–151. doi: 10.1016/0009-8981(91)90067-m. [DOI] [PubMed] [Google Scholar]
- 43.Kakkar P., Das B., Viswanathan P. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 44.Giustarini D., Rossi R., Milzani A., Dalle-Donne I. Nitrite and nitrate measurement by Griess reagent in human plasma: evaluation of interferences and standardization. Methods Enzymol. 2008;440:361–380. doi: 10.1016/S0076-6879(07)00823-3. [DOI] [PubMed] [Google Scholar]
- 45.Ellman G.L., Courtney K.D., Anders V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 46.Hong Y.S., Song K.H., Chung J.Y. Health effects of chronic arsenic exposure. J. Prev. Med. Public Health. 2014;47(5):245–252. doi: 10.3961/jpmph.14.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaishankar M., Tseten T., Anbalagan N. Toxicity, mechanism and health effects of some heavy metals. Interdiscipl. Toxicol. 2014;7(2):60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuhn V., Diederich L., Keller T.C.S., IV Red blood cell function and dysfunction: redox regulation, nitric oxide metabolism, anaemia. Antioxidants Redox Signal. 2017;26(13):718–742. doi: 10.1089/ars.2016.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohanty J.G., Nagababu E., Rifkind J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014;5:84. doi: 10.3389/fphys.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen B., Lu X., Shen S., Arnold L.L., Cohen S.M., Le X.C. Arsenic speciation in the blood of arsenite-treated F344 rats. Chem. Res. Toxicol. 2013;26:952–962. doi: 10.1021/tx400123q. [DOI] [PubMed] [Google Scholar]
- 51.Guo M., Wang W., Hai X., Zhou J. HPLC-HG-AFS determination of arsenic species in acute promyelocytic leukemia (APL) plasma and blood cells. J. Pharmaceut. Biomed. 2017;145:356–363. doi: 10.1016/j.jpba.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Cimen M.Y. Free radical metabolism in human erythrocytes. Clin. Chim. Acta. 2008;390(1-2):1–11. doi: 10.1016/j.cca.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 53.Nandi D., Patra R.C., Swarup D. Oxidative stress indices and plasma biochemical parameters during oral exposure to arsenic in rats. Food Chem. Toxicol. 2006;44:1579–1584. doi: 10.1016/j.fct.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 54.Souza A.C.F., Marchesil S.C. Effects of arsenic compounds on microminerals content and antioxidant enzyme activities in ra liver. Biol. Trace Elem. Res. 2018;183(2):305–313. doi: 10.1007/s12011-017-1147-3. [DOI] [PubMed] [Google Scholar]
- 55.Rao M.V., Avani G. Arsenic induced free radical toxicity in brain of mice. Indian J. Exp. Biol. 2004;42:495–498. [PubMed] [Google Scholar]
- 56.Ma N., Sasoh M., Kawanishi S. Protection effects of taurine on nitrosative stress in the mice brain with chronic exposure to arsenic. J. Biomed. Sci. 2010;17(1):S7. doi: 10.1186/1423-0127-17-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gurr J.R., Yih L.H., Samikkannu T., Bau D.T., Lin S.Y., Jan K.Y. Nitric oxide production by arsenite. Mutat. Res. 2003;533(1):173–182. doi: 10.1016/j.mrfmmm.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 58.Leal J.K.F., Adjobo-Hermans M.J.W., Brock R. Acetylcholinesterase provides new insights into red blood cell ageing in vivo and in vitro. Blood Transfus. 2017;15(3):232–238. doi: 10.2450/2017.0370-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hajjawi O.S. Acetylcholinesterase in human red blood cells. Eur. J. Sci. Res. 2012;75(4):510–522. [Google Scholar]
- 60.Perry M.R., Wyllie S., Raab A., Feldmann J., Fairlamb A.H. Chronic exposure to arsenic in drinking water can lead to resistance to antimonial drugs in a mouse model of visceral leishmaniasis. Proc. Natl. Acad. Sci. U.S.A. 2013;110:19932–19937. doi: 10.1073/pnas.1311535110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arnold L.L., Suzuki S., Yokohira M., Kakiuchi-Kiyota S., Pennington K.L., Cohen S.M. Time course of urothelial changes in rats and mice orally administered arsenite. Toxicol. Pathol. 2014;42:855–862. doi: 10.1177/0192623313489778. [DOI] [PubMed] [Google Scholar]
- 62.Barcellini W., Fattizzo B. Clinical applications of hemolytic markers in the differential diagnosis and management of hemolytic anemia. Dis. Markers. 2015;2015:635670. doi: 10.1155/2015/635670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee J.J., Kim Y.K., Cho S.H., Park K.S., Chung I.J., Cho D. Hemolytic anemia as a sequela of arsenic intoxication following long-term ingestion of traditional Chinese medicine. J. Kor. Med. Sci. 2004;19(1):127–129. doi: 10.3346/jkms.2004.19.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winski S.L., Carter D.E. Arsenate toxicity in human erythrocytes: characterization of morphologic changes and determination of the mechanism of damage. J. Toxicol. Environ. Health. 1998;53:345–355. doi: 10.1080/009841098159213. [DOI] [PubMed] [Google Scholar]
- 65.Mahmud H., Foller M., Lang F. Arsenic-induced suicidal erythrocyte death. Arch. Toxicol. 2009;83:107–113. doi: 10.1007/s00204-008-0338-2. [DOI] [PubMed] [Google Scholar]