Abstract
Background
Ginsenoside Rb1 (Rb1), one of the most abundant protopanaxadiol-type ginsenosides, exerts excellent neuroprotective effects even though it has low intracephalic exposure.
Purpose
The present study aimed to elucidate the apparent contradiction between the pharmacokinetics and pharmacodynamics of Rb1 by studying the mechanisms underlying neuroprotective effects of Rb1 based on regulation of microflora.
Methods
A pseudo germ-free (PGF) rat model was established, and neuroprotective effects of Rb1 were compared between conventional and PGF rats. The relative abundances of common probiotics were quantified to reveal the authentic probiotics that dominate in the neuroprotection of Rb1. The expressions of the gamma-aminobutyric acid (GABA) receptors, including GABAA receptors (α2, β2, and γ2) and GABAB receptors (1b and 2), in the normal, ischemia/reperfusion (I/R), and I/R+Rb1 rat hippocampus and striatum were assessed to reveal the neuroprotective mechanism of Rb1.
Results
The results showed that microbiota plays a key role in neuroprotection of Rb1. The relative abundance of Lactobacillus helveticus (Lac.H) increased 15.26 fold after pretreatment with Rb1. I/R surgery induced effects on infarct size, neurological deficit score, and proinflammatory cytokines (IL-1β, IL-6, and TNF-α) were prevented by colonizing the rat gastrointestinal tract with Lac.H (1 × 109 CFU) by gavage 15 d before I/R surgery. Both Rb1 and Lac.H upregulated expression of GABA receptors in I/R rats. Coadministration of a GABAA receptor antagonist significantly attenuated neuroprotective effects of Rb1 and Lac.H.
Conclusion
In sum, Rb1 exerts neuroprotective effects by regulating Lac.H and GABA receptors rather than through direct distribution to the target sites.
Keywords: Gamma-aminobutyric acid receptor, Ginsenoside Rb1, Lactobacillus helveticus, Microbiota, Neuroprotective effects
1. Introduction
The human body is considered a super-complex ecosystem, and the human gastrointestinal tract is inhabited by 1013–1014 microorganisms, which is thought to be 10 times greater than the number of cells [1]. The microbiome in the gastrointestinal tract is mainly defined by two bacterial phylotypes, namely Bacteroidetes and Firmicutes. Other bacterial phylotypes, including Actinobacteria, Fusobacteria, Proteobacteria, and Verrucomicrobia, are present in relatively low abundance [2]. Recent studies have provided compelling evidence that gut microbiota play crucial roles in shaping the metabolic and regulatory networks that define good health and a spectrum of disease states [3]. For instance, it has been found that gut microbiota can supply the host with multiple functions (e.g., by contributing to food digestion, drug biotransformation, vitamin supplementation, regulating expression of genes involved in utilization of carbohydrates and lipids, and providing defense against pathogenic strains) by interacting with the host organism through direct contact or various indirect soluble molecules [4], [5].
Apart from autonomic regulation of digestion by the central nervous system (CNS) and neuroendocrine factors, increasing evidence has suggested that gut microbiota are closely involved with brain functions, including mood, cognitive function, and stress-associated anxiety or depression in humans [6], [7]. The brain affects the gut function; similarly, the gut can induce changes in the CNS. Bidirectional communication between enteric microbiota and brain function has been defined as the “gut–brain axis” which may have profound effects on CNS development and most aspects of behavior relevant to pathological cognitive function [8], [9], [10], [11]. Probiotics, defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host,” were found to play a crucial role in the gut–brain axis [12]. For example, germ-free mice displayed exaggerated stress and anxiety-like behaviors compared with conventional specific pathogen–free mice, but the germ-free animals exhibited a complete normalization of behavior after being treated with Bifidobacterium (Bif.) infantis [12]. Ingestion of some Lactobacillus (Lac.) strains was demonstrated to not only treat certain disorders but also attenuate emotional behavior and impairment of cognition [13], [14]. To date, multiple probiotic bacteria with psychotropic potential have been reported, including Bif. bifidum, Bif. breve, Bif. longum (Bif.L), Bif. lactis, Lac. acidophilus, Lac. casei, Lac. plantarum, Lac. reuteri, Lac. rhamnosus (Lac.R), Lac. salivarius, and Enterococcus sp. [15], [16], [17].
Gamma-aminobutyric acid (GABA), one of the main inhibitory neurotransmitters in the adult brain, plays a central role in synaptic plasticity by modulating the inhibitory–excitatory balance necessary for proper brain function in adult brains [7], [18]. In general, the physiological effects of GABA are mediated by two major classes of receptors: ionotropic GABAA receptors, which are formed by coassembly of different subunits (α, β, and γ subunits), and GABAB receptors, which are G protein–coupled receptors, composed of two types of subunits (1b and 2) [19], [20], [21]. GABA receptors are found in a wide range of immune cells, such as dendritic cells, mast cells, and T cells, and are involved in regulating various immunological processes [4], [22]. Panax notoginsenoside extract (PNE), which is an extract from the traditional Chinese herb Panax notoginseng, has been commonly used to treat cardio-cerebrovascular diseases for thousands of years [23], [24]. However, cerebral exposure levels to components of PNE generally are extremely low [25]. In our previous study, we demonstrated that PNE could exert neuroprotective effects by enhancing Bif.L levels in rats subjected to ischemia/reperfusion (I/R) and upregulating expression of GABAB receptors [26]. However, the active ingredients of PNE are still poorly understood. In the past two decades, identification of ginsenosides in PNE has made notable progress. Ginsenosides can be classified into two major groups: protopanaxadiol (PPD) and protopanaxatriol [27]. A total of 13 PPD-type ginsenosides and 30 PPT-type ginsenosides were efficiently identified in our previous study [28]. Rb1, one of the most abundant PPD ginsenosides in PNE, exhibits various pharmacological activities including neuroprotective, antitumor, cardiovascular-protective, acute renal injury–protective, lung injury–protective, and antiaging effects, but its bioavailability is low because of its poor absorption [29], [30], [31], [32], [33]. Until now, the apparent contradiction between the pharmacokinetics and pharmacodynamics of Rb1 has not been well understood.
In the present study, we aimed to elucidate the apparent contradiction between the pharmacokinetics and pharmacodynamics of Rb1 by studying neuroprotective mechanisms of Rb1 in rats subjected to I/R-induced focal cerebral injury.
2. Materials and methods
2.1. Chemicals and standards
Rb1 was purchased from Nanjing Sart Science & Technology Development Co., Ltd (Nanjing, Jiangsu, China). Saclofen (Sac; a GABAB receptor antagonist) and streptomycin (a GABAA receptor antagonist) were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). Rabbit polyclonal anti-GABAA (α2, β2, and γ2 subunits) and anti-GABAB (1b and 2 subunits) antibodies were also purchased from Sigma-Aldrich Corporation. Neomycin sulfate, streptomycin, and 2, 3, 5-triphenyltetrazolium hydrochloride (TTC) were also purchased from Sigma-Aldrich Corporation. Lactobacillus helveticus (CICC 20275) was purchased from China Center of Industrial Culture Collection (Beijing, China). A Bacterial Genomic DNA Extraction Kit was purchased from Takara Bio., Inc. (Nojihigashi, Kusatsu, Shiga, Japan). All the ExCell enzyme-linked immunosorbent assay (ELISA) kits were purchased from ExCell Biotechnology Corp., Ltd. (Shanghai, China). Anti-GABA receptor antibodies were purchased from Abcam (Ann Arbor, MI, USA).
2.2. Animals and treatments
Animals: All the animal experiments were approved by the Ethical Committee of Animal Experiments of China Pharmaceutical University. Healthy Sprague–Dawley rats (220 ± 10 g) were provided by Shanghai Super-B&K Laboratory Animal Corp., Ltd. (Shanghai, China). Ethical procedures were conducted by following the principles of Reduction, Replacement, and Refinement (the 3 Rs rule). All animals were kept in an environmentally controlled breeding room (temperature: 20–24°C, humidity: 40–70%, and a 12-h dark/light cycle) and fed with standard laboratory food and water for about 5 days before starting the experiments. Before each experiment, all the rats were fasted for 12 h with free access to water. In addition, the I/R rat model was prepared by a middle cerebral artery occlusion method as per previous reports [26]. The pseudo germ-free (PGF) rat model was established by intragastrical administration of neomycin sulfate combined with streptomycin [26].
Drug administration: Rb1 was dissolved in saline and intragastrically administered to rats at a dose of 50 mg/kg once a day. Pretreatment lasted for 6 d, and the rats were given a single administration (50 mg/kg) after reperfusion on the 7th day. Rats in the vehicle group were administered with saline using the same protocol mentioned previously.
Infarct volume analysis: Twenty-six hours after cerebral infarction (2-h ischemia and 24-h reperfusion), the rats were anesthetized and killed by rapid decapitation. Brains were removed and sectioned into standard coronal slices (2-mm thick). The sections were immediately immersed in TTC medium, which was prepared by dissolving TTC (0.125% w:v) in a buffer solution containing 62.5 mM tris-HCl, 13 mM MgCl2, and 1.5% dimethylformamide. Infarct size was analyzed using ImageJ software (an open source image processing program designed by National Institutes of Health) based on the following equation: [(VC-VL)/VC] × 100, where VC is the volume of the total area and VL is the volume of the noninfarcted area.
Neurological deficit evaluation: Neurological deficits were examined 26 h after reperfusion. Neurological findings were scored on a 5-point modified scale based on a previous study [34] as follows: no neurological deficit (0 point), failure to fully extend the left forepaw (1 point), turning to the left (2 points), circling to the left (3 points), unable to walk spontaneously (4 points), and stroke-related death (5 points).
2.3. Measurement of proinflammatory cytokine levels using ELISA
Tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) levels on the experimental stroke side were quantified using ExCell ELISA kits (ExCell Biology) as per the manufacturer's instructions. In brief, the brain tissue was homogenized in phosphate-buffered solution (PBS, pH 7.4). The protein concentration of each sample was determined using a BCA Protein Assay Kit (Beyotime, Shanghai, China).
2.4. Lac.H cultivation and colonization
Lac.H (CICC 20275, Lac) was inoculated into de Man, Rogosa, and Sharpe medium containing 8 g/L of beef extract, 4 g/L of yeast extract, 10 g/L of proteose peptone, 20 g/L of glucose, 5 g/L of sodium acetate, 2 g/L of triammonium citrate, 0.2 g/L of magnesium sulfate, 0.05 g/L of manganese sulfate, 2 g/L of dipotassium hydrogen phosphate, and 1 g/L of polysorbate 80. After incubating at 37°C for 12 h, Lac.H was collected by centrifuging at 5000 ×g (4°C) for 15 min and resuspending at a concentration of 1 × 109 colony-forming unit (CFU)/mL in de Man, Rogosa, and Sharpe medium. Lac.H was then stored as frozen aliquots at −70 °C until use. Lac.H–colonized rats were prepared by intragastrically administering 1 × 109 CFU of Lac.H once per day for 15 consecutive days before I/R surgery, followed by one dose after reperfusion.
2.5. The influence of GABA receptor antagonists on the neuroprotective effects of Rb1 and Lac.H
To investigate the effects of GABA receptors on the neuroprotective effect of Rb1 and Lac.H, 0.2 mg kg−1 of bicuculline (Bic; a GABAA receptor antagonist) was intraperitoneally administrated to rats, whereas 0.1 mg kg−1 of Sac (a GABAB receptor antagonist) was intravenously administrated to rats 30 min before I/R surgery. The rats in the vehicle group were treated with an isometric medium.
2.6. Quantitative analysis of Rb1 based on LC–MS/MS
Sample collection and preparation: Both I/R model and normal rats were administrated with Rb1 intragastrically at a dose of 50 mg/kg. The rat plasma, striatum, and hippocampus were collected at 1, 6, and 24 h after intragastrical administration of Rb1 at a dose of 50 mg/kg. All the tissues (∼0.1 g) were homogenized in 1 mL of water in an ice bath. The tissue homogenates were extracted using n-butanol as previously described [26].
Instruments, parameters, and conditions: The LC–MS/MS 8050 system (Shimadzu; Tokyo, Japan) was used to analyze Rb1 in a biological matrix. Chromatographic separation was performed on a C18 reversed-phase LC column (Thermo Hypersil GOLD ODS 5 m; 50 mm × 2.1 mm I.D., Thermo Scientific, USA). The optimized MS-operating parameters were as follows: 3 L/min of nebulizing gas, 10 L/min of heating gas, and 250°C desolvation temperature. Quantification was performed using multiple reaction monitoring acquisition mode by monitoring the precursor ion to product ion transitions of m/z 1143.7→945.6 for Rb1 and m/z 815.5→779.4 for digoxin (IS). The collision energy values for Rb1 and IS were 53 and 30, respectively. The present assay was fully validated in our previous study with respect to linearity, sensitivity, intraassay and interassay precision and accuracy, recovery, and matrix effect [35].
2.7. Western blot analysis of GABA receptors
The hippocampus and striatum were homogenized in radioimmunoprecipitation assay lysis and extraction buffers containing 1 mmol/L of the protease inhibitor phenylmethanesulfonyl fluoride(Beyotime). Protein concentration was determined using a BCA Protein Assay Kit (Beyotime). The proteins were separated on a 4–14% gel using tris-glycine sodium dodecyl sulfate polyacrylamide gelelectrophoresis (SDS-PAGE) and then transferred onto a nitrocellulose membrane. The blots were washed with tris-buffered saline with Tween 20 (TBST) buffer and then blocked in TBST buffer supplemented with 5% nonfat milk powder for 1 h at room temperature. The blots were then incubated with either rabbit polyclonal anti-GABAA α2 subunit antibody (1:1000, ab72445), rabbit anti-GABAA β2 subunit antibody (1:30000, ab16213), rabbit anti-GABAA γ2 subunit antibody (1:1000, ab16213), rabbit anti-GABAB 1b subunit antibody (1:1000, ab166604), or rabbit anti-GABAB 2 subunit antibody (1:1000, ab52248) overnight at 4°C. After washing the blots thrice with TBST, the membranes were incubated with horseradish peroxidase–conjugated goat anti-rabbit IgG antibody (1:10000) for 60 min and then washed again. Membrane-bound secondary antibodies were detected using chemiluminescence (ECL) reagents (Bio-Rad, Hercules, CA, USA). Integrative optical density readings were obtained using Gel-Pro 32-bit image software (Media Cybernetics, Rockville, MD, USA).
2.8. Real-time Polymerase Chain Reaction (PCR) analysis of GABA receptors
The rat hippocampus and striatum were dissected and isolated under cold conditions. RNA was extracted and isolated as per standard procedures using TRIzol reagent (Takara, Kyoto, Japan). Purity and concentration of RNA were determined using a dual-beam UV–Vis spectrophotometer (BioTek, Winooski, VT, USA). Total RNA (1 μg) was reverse transcribed to cDNA using the PrimeScript RT Reagent Kit (Takara). Real-time PCR was performed using a Real-Time PCR detection system (Bio-Rad) with SYBR Green Real-Time PCR Master Mix (Bio-Rad) as per the manufacturer's instructions. Specific primers for rat GABAA receptor subunits (α2, β2, and γ2), GABAB receptor subunits (1b and 2), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are listed in Supplemental Table 1.
2.9. Real-time PCR analysis of gut microbiota
Total genomic DNA of intestinal microbiota was extracted using the Bacterial Genomic DNA Extraction Kit, Ver. 3.0 (Takara). Real-time PCR for microbiota genomic DNA was performed using a thermal cycler with the following parameters: initial denaturation at 94°C for 5 min; 40 cycles of denaturation at 94°C for 15 s, annealing at 59°C for 30 s, and elongation at 72°C for 30 s. The primers are shown in Supplemental Table 1.
3. Results
3.1. Pharmacokinetics of Rb1 in control and I/R rats
As illustrated in Fig. 1, Rb1 concentrations in the rat hippocampus and striatum were significantly lower than those in plasma. In the hippocampus and striatum collected at 1 h, there was no significant difference in concentrations of Rb1 between the control and I/R groups (p > 0.05). At 6 h, Rb1 concentrations in the hippocampus and striatum of I/R model rats tended to be higher than those of the control rats, but the differences were not significant (p > 0.05). At 24 h, the difference of the Rb1 concentrations in the hippocampus and striatum between the control and I/R groups was also not significant (p > 0.05). In rat plasma, concentrations of Rb1 in the I/R model rats were significantly higher than those in control rats at 6 h and 24 h after drug administration. Ratios of brain to plasma concentrations were calculated to compare exposure of Rb1 in plasma and the brain. As shown in Supplemental Table 2, intracephalic exposure to Rb1 was very low because all ratios were much lower than 0.1.
Fig. 1.
Concentrations of Rb1 in the rat hippocampus, striatum, and plasma collected at 1, 6, and 24 h (n = 5). (A) Hippocampus concentrations. (B) Striatum concentrations. (C) Plasma concentrations. *, p < 0.05; **, p < 0.01. I/R, ischemia/reperfusion.
3.2. Neuroprotective effects of Rb1 mediated by intestinal microflora
As shown in Figs. 2A, 2B, cerebral infarcts were pronounced after I/R surgery, and infarct volumes were significantly reduced by pretreatment with 50 mg/kg of Rb1 for 7 consecutive days. Compared with the I/R group, infarct size was significantly decreased from 22.83 ± 7.33% to 5.95 ± 2.47% by Rb1. Neurological deficits were evaluated by scoring specific behaviors; as shown in Fig. 2C, the mean neurological deficit score of the control group was significantly lower than that of the I/R group. Furthermore, the increase in the neurological deficit score caused by I/R surgery could be greatly reduced by pretreatment with Rb1 (p < 0.001). In addition, levels of IL-1β, IL-6, and TNF-α in the stroke region were much higher in the I/R group than in the control group (p < 0.05). However, after pretreatment of the I/R group with Rb1, levels of IL-1β and IL-6 remained normal, and levels of TNF-α were significantly decreased when compared with untreated I/R model rats (p < 0.05) (Figs. 2D–2F). Taken together, these results demonstrate that Rb1 exerts remarkable neuroprotective effects during cerebral I/R injury in rats.
Fig. 2.
Neuroprotective effects of Rb1 mediated by intestinal microflora. (A) Coronal sections of TTC-stained brains. (B) Infarct volume. (C) Neurology deficit score. (D) IL-6 level. (E) IL-1β level. (F) TNF-α level. (n = 5 per group). *, p < 0.05; **, p < 0.01. I/R, ischemia/reperfusion; PGF, pseudo germ-free; TTC; 2, 3, 5-triphenyltetrazolium hydrochloride.
In our previous study, it was found that the intestinal microflora, which is a complex ecosystem consisting of a wide range of microorganisms, plays a major role in metabolism and disposition of ginsenosides [36]. Moreover, the influence of intestinal microflora on occurrence and treatment of brain diseases has received much attention in the past decade [37], [38]. Herein, a PGF rat model was established to investigate the role of gut microbiota in neuroprotective effects of Rb1. As shown in Fig. 2A, cerebral infarcts were prominent after establishing the I/R model in PGF rats. Cerebral infarct volume was 20.86 ± 1.72% in the PGF+ I/R group. However, the infarct volume was reduced to 15.67 ± 4.48% by pretreatment with Rb1 (p < 0.05) (Fig. 2B). The mean neurological deficit scores of the PGF+I/R and PGF+I/R+Rb1 treatment groups were 2.4 ± 0.42 and 1.9 ± 0.42, respectively (Fig. 2C), with no significant difference between the two groups. Similarly, levels of IL-1β, IL-6, and TNF-α in the stroke region of the PGF+I/R group were much higher than those of the control group, and pretreatment with Rb1 tended to decrease the levels of IL-1, IL-6, and TNF-α. However, differences between the PGF+I/R and PGF+I/R+Rb1 groups were not significant (p > 0.05) (Figs. 2D–2F).
3.3. Regulation of gut microbiota by Rb1
Increasing evidence suggests that probiotic bacteria, including Bif.L, Bif. dentium (Bif.D), Lac. brevis (Lac.B), Lac.H, and Lac.R, play important roles in the bidirectional gut–brain axis by regulating expression of central GABA receptors, stress-induced behavioral deficits, immune function, and gut dysbiosis [6], [19], [39], [40]. To identify the species of probiotics involved in Rb1 neuroprotection, the abundances of Bif.L, Bif.D, Lac.B, Lac.H, and Lac.R in the intestinal microflora of the control, I/R, I/R+Rb1, PGF+I/R, and PGF+I/R+Rb1 groups were measured by real-time PCR technique. Then the ratios of the probiotics abundances in I/R, I/R+Rb1, PGF+I/R, and PGF+I/R+Rb1 rats to those of the corresponding control group rats were calculated to express their relative populations. As shown in Fig. 3A, the ratios of Bif.L, Lac.B, Lac.H, and Lac.R in the I/R rats to those in the control group rats were much lower than 1, and the ratios of Lac.B, Lac.H, and Lac.R in I/R+Rb1 rats to those in the control group rats were much greater than 1. Thus, the relative populations of Bif.L, Lac.B, Lac.H, and Lac.R were downregulated by I/R surgery, and Rb1 treatment could significantly upregulate the populations of Bif.L and Lac.H [one-way analysis of variance (ANOVA), p < 0.05]. After pretreatment with Rb1 for 7 consecutive days, the abundances of Bif.L, Lac.B, Lac.H, and Lac.R increased by 2.13, 5.18, 15.26, and 3.30 folds relative to the control group, respectively. Hence, Rb1 had the most significant effect on Lac.H in I/R rats. Then, populations of Lac.H in the control, I/R, I/R+Rb1, PGF+I/R, and PGF+I/R+Rb1 groups were compared to further investigate the role of Lac.H in Rb1 neuroprotection. The relative populations of Lac.H were obtained by calculating the ratios of the probiotics abundances in I/R, I/R+Rb1, PGF+I/R, or PGF+I/R+Rb1 rats to those of the corresponding control group rats. Clearly, the relative populations of Lac.H in the I/R, PGF+I/R, and PGF+I/R+Rb1 group rats were lower than those in the control group rats, yet the relative populations of Lac.H in the I/R+Rb1 group rats were significantly higher than those in the control group rats (one-way ANOVA, p < 0.05) (Fig. 3B). Thus, Rb1 treatment could significantly reverse the reduction of Lac.H caused by I/R surgery (one-way ANOVA, p < 0.05). The lack of increase of Lac.H. in PGF mice treated with Rb1 could be related to the action of antibiotics neomycin sulfate and streptomycin.
Fig. 3.
The ratios (relative populations) of the probiotics abundances in I/R, I/R+Rb1, PGF+I/R, and PGF+I/R+Rb1 rats to those of control rats. (A) The relative populations in the I/R and I/R+Rb1 group rats. (B) The relative populations of Lac.H and Lac.R in the I/R, I/R+Rb1, PGF+I/R and PGF+I/R+Rb1 group rats. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Bif.D, Bifidobacterium dentium; Bif.L, Bifidobacterium longum; I/R, ischemia/reperfusion; Lac.B, Lactobacillus brevis; Lac.H, Lactobacillus helveticus; Lac. R, Lactobacillus rhamnosus; PGF, pseudo germ-free.
3.4. Neuroprotective effects of Lac.H in I/R model rats
Lac.H was cultured and colonized in rats by intragastrically administrating 1 × 109 CFU of Lac.H once per day for 15 consecutive days, and then neuroprotective effects of Lac.H were evaluated. As shown in Figs. 4A–4C, Lac.H colonization greatly reduced the volume of infarcts caused by I/R surgery, and the increase in neurological deficit score caused by I/R surgery was significantly attenuated by colonization with Lac.H (p < 0.001). The levels of IL-1β, IL-6, and TNF-α in the I/R group were significantly downregulated by Lac.H (Figs. 4D–4F).
Fig. 4.
Investigation of the neuroprotective effects of Lac.H in I/R model rats. (A) Coronal sections of TTC-stained brains. (B) Infarct volume. (C) Neurology deficit score. (D) IL-6, (E) IL-1β, and (F) TNF-α levels of normal, I/R model, and I/R + Lac.H rats (n = 5 per group). *, p < 0.05; **, p < 0.01; ***, p < 0.001. I/R, ischemia/reperfusion; Lac.H, Lactobacillus helveticus; TTC, 2, 3, 5-triphenyltetrazolium hydrochloride.
3.5. Effects of Lac.H on GABA receptor expression in the hippocampus
Previous studies have revealed that lactic acid bacteria, such as Lac.R, could directly affect neurotransmitter receptors in normal, healthy animals [19]. However, changes in GABAA receptor subunit expression induced by I/R surgery and Lac.H colonization were not clearly elucidated. Herein, expression levels of GABAA receptor subunits (α2, β2, and γ2) and GABAB receptor subunits (1b and 2) in the hippocampus from control, I/R, and I/R+Lac.H rats were assessed using reverse transcription PCR and Western blotting techniques. As shown in Figs. 5A, 5B, protein expression levels of GABAA subunits (α2, β2, and γ2) and GABAB subunits (1b and 2) in the I/R model rats were significantly lower than those in the control group rats. However, colonization with Lac.H significantly upregulated protein expression of all GABA receptor subunits, especially α2 and γ2 (p < 0.001). Trends in changes of GABA receptor mRNA levels were similar to changes in protein levels, and Lac.H colonization greatly attenuated the downregulation of mRNA levels caused by I/R surgery (P < 0.05).
Fig. 5.
The expressions of GABA receptors in the rat hippocampus. (A) Protein band. (B) Gray-scale analysis. (C) RNA expression. **, p < 0.01 vs. control; ***, p < 0.001 vs. control; #, p < 0.05 vs. I/R; ##, p < 0.01 vs. I/R; ###, p < 0.01 vs. I/R. GABA, gamma-aminobutyric acid; I/R, ischemia/reperfusion; Lac.H, Lactobacillus helveticus.
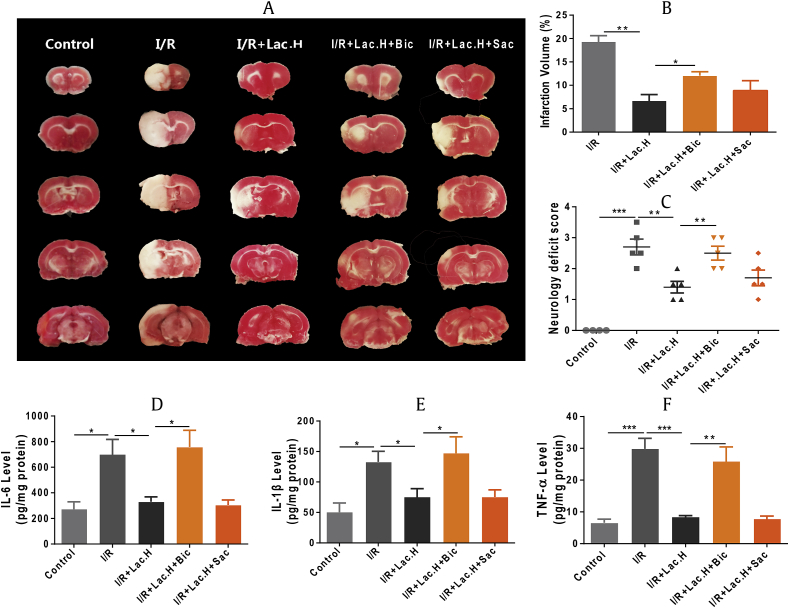
3.6. Roles of GABAA and GABAB receptors in neuroprotective effects of Lac.H
A GABAA receptor antagonist (bicuculline, Bic) and GABAB receptor antagonist (Sac) were used to inhibit GABA receptors by intraperitoneal injection before I/R surgery. As shown in Figs. 6A, 6B, Lac.H colonization greatly reduced infarct volumes caused by I/R surgery, and both Bic and Sac reduced the effect of Lac.H on the infarct volume. The mean cerebral infarct volumes in the I/R+Lac.H, I/R+Lac.H+Bic, and I/R+Lac.H+Sac groups were 7.38 ± 2.47%, 16.0 ± 2.30%, and 10.94 ± 3.63%, respectively. The mean neurological deficit scores of the I/R+Lac.H, I/R+Lac.H+Bic, and I/R+Lac.H+Sac groups were 1.00 ± 0.35, 2.38 ± 0.45, and 1.50 ± 0.42, respectively (Fig. 6C). Measurement of inflammatory factor levels demonstrated that IL-1β, IL-6, and TNF-α levels in the I/R+Lac.H+Bic group were significantly higher than those in the I/R+Lac.H group, whereas no significant difference was found between the I/R+Lac.H and I/R+Lac.H+Sac groups.
Fig. 6.
Effects of GABA receptor antagonists on efficacy of Lac.H (n = 5 per group). (A) Coronal sections of TTC-stained brains. (B) Infarct volume. (C) Neurology deficit score. (D) IL-6 levels. (E) IL-1β levels. (F) TNF-α levels. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Bic, bicuculline; GABA, gamma-aminobutyric acid; I/R, ischemia/reperfusion; Lac.H, Lactobacillus helveticus; Sac, saclofen; TTC, 2, 3, 5-triphenyltetrazolium hydrochloride.
3.7. Effects of Rb1 on GABA receptor expression
The effects of Rb1 on expression of GABA receptors were investigated by measuring the protein and RNA expression levels of GABAA receptors (α2, β2, and γ2) and GABAB receptors (R1b and R2) in the rat hippocampus using Western blot real-time PCR analysis. As shown in Figs. 7A, 7B, pretreatment with Rb1 significantly attenuated decreases in protein expression levels of GABAA subunits (α2, β2, and γ2) in two brain regions of the I/R group (p < 0.05). Although protein expression of the GABAB subunits was also regulated by Rb1, differences between the I/R and I/R+Rb1 groups were not significant (p > 0.05). Besides, the results of the determination of relative RNA expression indicated that the variation trend of RNA expression of GABA receptors was in accordance with that of proteins (Fig. 7C).
Fig. 7.
The protein and RNA expression of GABA receptors in the rat hippocampus. (A) Protein band. (B) Gray-scale analysis. (C) Relative RNA expression. **, p < 0.01 vs. control; ***, p < 0.001 vs. control; #, p < 0.05 vs. I/R; ##, p < 0.01 vs. I/R; ###, p < 0.01 vs. I/R. GABA, gamma-aminobutyric acid; I/R, ischemia/reperfusion.
The effects of Rb1 and Lac.H on expression of GABA receptors were also investigated by measuring the protein and RNA expression levels of GABAA receptors (α2, β2, and γ2) and GABAB receptors (R1b and R2) in the rat striatum. As shown in Fig. S1, both Rb1 and Lac.H could upregulate expression of the GABAA subunits (α2, β2, and γ2), and the regulation extent of Lac.H was significantly greater than that of Rb1. Although protein and RNA expression of the GABAB receptor subunits could also be upregulated by Rb1 or Lac.H, the regulation extent was much less than that on the GABAA receptors.
3.8. Roles of GABAA and GABAB receptors in neuroprotective effects of Rb1
The effects of GABA receptor antagonists on neuroprotective effects of Rb1 were investigated. As shown in Figs. 8A, 8B, mean cerebral infarct volumes in the I/R, I/R+Rb1, I/R+Rb1+Bic, and I/R+Rb1+Sac groups were 19.29 ± 2.31%, 5.95 ± 2.47%, 16.64 ± 2.30%, and 8.85 ± 3.62%, respectively. The mean neurological deficit score was also significantly affected by Bic. The neurological deficit scores in the I/R, I/R+Rb1, I/R+Rb1+Bic, and I/R+ Rb1+Sac groups were 2.70 ± 0.57, 1.00 ± 0.35, 2.30 ± 0.45, and 1.40 ± 0.42 (Fig. 8C), respectively. Moreover, IL-1β, IL-6, and TNF-α levels in the I/R+Rb1+Bic group were significantly higher than those in the I/R+Rb1 group, whereas no significant difference was found between the I/R+Rb1 and I/R+Rb1+Sac groups.
Fig. 8.
Effects of GABA receptor antagonist. (A) Coronal sections of TTC-stained brains. (B) Infarct volume. (C) Neurology deficit score.on efficacy of Rb1 (n = 5). (D) IL-1β6 levels. (E) IL-61β levels. (F) TNF-α levels. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Bic, bicuculline; GABA, gamma-aminobutyric acid; I/R, ischemia/reperfusion; Sac, saclofen; TTC, 2, 3, 5-triphenyltetrazolium hydrochloride.
4. Discussion
Ginsenosides are the major bioactive component in Panax and other types of ginseng, including American ginseng Panax quinquefolius, Korean ginseng Panax ginseng, and Chinese ginseng Panax notoginseng [28], [36]. Rb1, one of the most abundant PPD ginsenosides, exhibits various pharmacological activities, including neuroprotective, antitumor, cardiovascular-protective, acute renal injury–protective, lung injury–protective, and antiaging effects, in many in vitro and in vivo models [29], [30], [31], [32], [33]. In the present study, all concentrations at 1 h, 6 h, and 24 h were found to be much lower than 100 ng/g, and the Rb1 concentration ratios of brain to plasma were far lower than 0.1. To date, the apparent contradiction between the pharmacokinetics and pharmacodynamics of Rb1 has not been elucidated fully. In our previous study, we found that ginsenosides could promote various metabolic processes, such as oxidation, dehydrogenation, demethylation, and deglycosylation, in an intestinal microflora incubation system, and metabolism of ginsenosides was greatly affected by the intestinal microflora [36]. Given that intestinal microflora can significantly influence the pharmacokinetics of Rb1 in vitro and in vivo, we hypothesized that intestinal microflora can also affect the pharmacology of Rb1. As shown in Fig. 2, neuroprotective effects of Rb1 differed greatly between conventional and PGF rats. Effects of Rb1 on cerebral infarct volume; neurological deficit score; and levels of IL-1, IL-6, and TNF-α in I/R rats were attenuated by coadministering an antibiotic cocktail. Thus, the gut microbiota plays a key role in mediating neuroprotective effects of Rb1.
Research using probiotics to improve CNS function has increased significantly over the last 15 years. Both the vagus and enteric nerves are thought to be involved in gut–brain interactions and can be affected by certain probiotics including Bif.L, Bif.D, Lac.B, Lac.H, and Lac.R [41]. In the present study, the relative abundances of Bif.L, Bif.D, Lac.B, Lac.H, and Lac.R were quantified to determine which probiotics dominate neuroprotective effects of Rb1. As shown in Fig. 3, the effect on Lac.H was the most prominent one, with Rb1 increasing abundance of Lac.H by 15.26 folds. Therefore, in the present study, Lac.H was chosen as the most promising therapeutic probiotic for cerebral I/R. In the past few years, Lac.R (JB-1) was found to exert significant effects on associated behavioral and physiological responses by altering the expression of specific GABA receptors in certain areas of the brain. In addition, Lac.R was shown to change levels of neurometabolites including glutamate and glutamine, total N-acetyl aspartate and N-acetyl aspartyl glutamic acid, and GABA [19]. However, neuroprotective effects of Lac.H remained unexplored. To investigate these effects, we colonized Lac.H in rats by intragastrically administrating 1 × 109 CFU of Lac.H once per day for 15 consecutive days. As shown in Fig. 4, cerebral infarct volume; neurological deficit score; and levels of IL-1, IL-6, and TNF-α in I/R rats were greatly improved by Lac.H.
GABA is the primary inhibitory neurotransmitter of the CNS. We previously demonstrated that PNE could enhance Bif.L levels in I/R rats. Herein, expression levels of GABAA receptor subunits (α2, β2, and γ2) and GABAB receptor subunits (1b and 2) in the hippocampus and striatum of control, I/R, I/R+Lac.H, and I/R+Rb1 rats were assessed to investigate neuroprotective mechanisms of Lac.H and Rb1. As shown in Fig. 5, Fig. 7 and S1, pretreatment with Rb1 and Lac.H could attenuate decreases in protein and mRNA levels of GABAA (α2, β2, and γ2) and GABAB (1b and 2) receptor subunits caused by I/R surgery. However, Rb1 effects on the GABAB receptor subunits were not as significant as Rb1 effects on the GABAA receptor subunits, especially α2 and γ2.
Overall, the findings of the present study suggest that I/R surgery downregulates the population of certain probiotics (Bif.L, Bif.D, Lac.B, Lac.H, and Lac.R). After pretreatment with Rb1, the relative abundance of specific probiotics can be significantly enhanced, and Lac.H is upregulated far more that the other studied probiotics. Enhanced Lac.H levels can then upregulate the expression of GABAA (α2, β2, and γ2) and GABAB (1b and 2) receptor subunits in the rat hippocampus and striatum. Upregulation of GABAA receptors may play a crucial role in mediating neuroprotective effects of Rb1 and Lac.H.
Acknowledgments
This study was supported by the National Nature Science Foundation of China (81374054, 81573559, 81530098), the Nature Science Foundation of Jiangsu Province (BK20171395), and the National Key Special Project of Science and Technology for Innovation Drugs of China (2017ZX09301013).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2018.09.002.
Contributor Information
Guangji Wang, Email: guangjiwang@hotmail.com.
Yan Liang, Email: liangyan0679@163.com.
Conflicts of interest
All authors have no conflicts of interest to declare.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.McFall-Ngai M., Hadfield M.G., Bosch T.C.G., Carey H.V., Domazet-Lošo T., Douglas A.E., Dubilier N., Eberl G., Fukami T., Gilbert S.F. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci USA. 2013;110:3229–3236. doi: 10.1073/pnas.1218525110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M., Gill S.R., Nelson K.E., Relman D.A. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin S.C., Kim S.H., You H., Kim B., Kim A.C., Lee K.A., Yoon J.H., Ryu J.H., Lee W.J. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science. 2011;334:670–674. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- 4.Mazzoli R., Pessione E. The neuro-endocrinological role of microbial glutamate and GABA signaling. Front Microbiol. 2016;7:1934. doi: 10.3389/fmicb.2016.01934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chimerel C., Emery E., Summers D.K., Keyser U., Gribble F.M., Reimann F. Reimann. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014;9:1202–1208. doi: 10.1016/j.celrep.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cryan J.F., O'Mahony S.M. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 7.Al-Asmakh M., Anuar F., Zadjali F., Rafter J., Pettersson S. Gut microbial communities modulating brain development and function. Gut Microbes. 2012;3:366–373. doi: 10.4161/gmic.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee S.H., Pothoulakis C., Mayer E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyte M. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003726. e1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maqsood R., Stone T.W. The gut-brain axis, BDNF, NMDA and CNS disorders. Neurochem Res. 2016;41:2819–2835. doi: 10.1007/s11064-016-2039-1. [DOI] [PubMed] [Google Scholar]
- 11.Tse J.K.Y. Gut microbiota, nitric oxide and microglia as pre-requisites for neurodegenerative disorders. ACS Chem Neurosci. 2017;8:1438–1447. doi: 10.1021/acschemneuro.7b00176. [DOI] [PubMed] [Google Scholar]
- 12.Liang S., Wang T., Hu X., Luo J., Li W., Wu X., Duan Y., Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 13.Arseneault-Bréard J., Rondeau I., Gilbert K., Girard S.A., Tompkins T.A., Godbout R., Rousseau G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br J Nutr. 2012;107:1793–1799. doi: 10.1017/S0007114511005137. [DOI] [PubMed] [Google Scholar]
- 14.Davari S., Talaei S.A., Alaei H., Salami M. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience. 2013;240:287–296. doi: 10.1016/j.neuroscience.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 15.Lee N.K., Paik H.D. Bioconversion using lactic acid bacteria: ginsenosides, GABA, and phenolic compounds. J Microbiol Biotechnol. 2017;27:869–877. doi: 10.4014/jmb.1612.12005. [DOI] [PubMed] [Google Scholar]
- 16.Yunes R.A., Poluektova E.U., Dyachkova M.S., Klimina K.M., Kovtun A.S., Averina O.V., Orlova V.S., Danilenko V.N. GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe. 2016;42:197–204. doi: 10.1016/j.anaerobe.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Borrelli L., Aceto S., Agnisola C., Paolo S.D., Dipineto L., Stilling R.M., Dinan T.G., Cryan J.F., Menna L.F., Fioretti A. Probiotic modulation of the microbiota-gut-brain axis and behaviour in zebrafish. Sci Rep. 2016;6:30046. doi: 10.1038/srep30046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C., Sun D. GABA receptors in brain development, function, and injury. Metabolic Brain Disease. 2015;30:367–379. doi: 10.1007/s11011-014-9560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cryan J.F., Kelly P.H., Chaperon F., Gentsch C., Mombereau C., Lingenhoehl K., Froestl W., Bettler B., Kaupmann K., Spooren W.P. Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N'-dicyclopentyl- 2-methylsulfanyl- 5-nitro -pyrimidine -4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther. 2004;310:952–963. doi: 10.1124/jpet.104.066753. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson L.H., Cryan J.F. Evaluation of the anxiolytic-like profile of the GABAB receptor positive modulator CGP7930 in rodents. Neuropharmacology. 2008;54:854–862. doi: 10.1016/j.neuropharm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Auteri M., Zizzo M.G., Serio R. GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol Res. 2015;93:11–21. doi: 10.1016/j.phrs.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Lee M.R., Yun B.S., Sung C.K. Comparative study of white and steamed BlackPanax ginseng, P. Quinquefolium, and P. Notoginsengon cholinesterase inhibitory and antioxidative activity. J Ginseng Res. 2012;36:93–101. doi: 10.5142/jgr.2012.36.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y.K., Miao C.P., Chen H.H., Huang F.F., Xia Y.M., Chen Y.W., Zhao L.X. Endophytic fungi harbored in Panax notoginseng: diversity and potential as biological control agents against host plant pathogens of root-rot disease. J Ginseng Res. 2016;41:353–360. doi: 10.1016/j.jgr.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang X.P., Ding H., Lu J.D., Tang Y.H., Deng B.X., Deng C.Q. Effects of the combination of the main active components of Astragalus and Panax notoginseng on inflammation and apoptosis of nerve cell after cerebral ischemia-reperfusion. Am J Chin Med. 2015;43:1419–1438. doi: 10.1142/S0192415X15500809. [DOI] [PubMed] [Google Scholar]
- 26.Li H., Xiao J., Li X., Chen H., Kang D., Shao Y., Shen B., Zhu Z., Yin X., Xie L. Low cerebral exposure cannot hinder the neuroprotective effects of panax notoginsenosides. Drug Metab Dispos. 2017;117 doi: 10.1124/dmd.117.078436. 078436. [DOI] [PubMed] [Google Scholar]
- 27.Qi L.W., Wang H.Y., Zhang H., Wang C.Z., Li P., Yuan C.S., Yuan Diagnostic ion filtering to characterize ginseng saponins by rapid liquid chromatography with time-of-flight mass spectrometry. J Chromatogr A. 2012;1230:93–99. doi: 10.1016/j.chroma.2012.01.079. [DOI] [PubMed] [Google Scholar]
- 28.Xing R., Zhou L., Xie L., Hao K., Rao T., Wang Q., Ye W., Fu H., Wang X., Wang G. Development of a systematic approach to rapid classification and identification of notoginsenosides and metabolites in rat feces based on liquid chromatography coupled triple time-of-flight mass spectrometry. Analytica Chimica Acta. 2015;867:56–66. doi: 10.1016/j.aca.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 29.Liu C., Hu M., Guo H., Zhang M., Zhang J., Li F., Zhong Z., Chen Y., Li Y., Xu P. Combined contribution of increased intestinal permeability and inhibited deglycosylation of ginsenoside Rb1 in the intestinal tract to the enhancement of ginsenoside Rb1 exposure in diabetic rats after oral administration. Drug Metab Dispos. 2015;43:1702–1710. doi: 10.1124/dmd.115.064881. [DOI] [PubMed] [Google Scholar]
- 30.Shen L., Xiong Y., Wang D.Q., Howles P., Basford J.E., Wang J., Xiong Y.Q., Hui D.Y., Woods S.C., Liu M. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats. J Lipid Res. 2013;54:1430–1438. doi: 10.1194/jlr.M035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J., Qiao L., Li S., Yang G. Protective effect of ginsenoside Rb1 against lung injury induced by intestinal ischemia-reperfusion in rats. Molecules. 2013;18:1214–1226. doi: 10.3390/molecules18011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Q., Meng Q., Jiang Y., Liu H., Lei S., Su W., Duan W., Wu Y., Xia Z. Protective effect of ginsenoside Rb1 against intestinal ischemia-reperfusion induced acute renal injury in mice. Plos One. 2013;8 doi: 10.1371/journal.pone.0080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed T., Raza S.H., Maryam A., Setzer W., Braidy N., Nabavi S.F., de Oliveira M.R., Nabavi S.M. Ginsenoside Rb1 as neuroprotective agent: a review. Brain Res Bull. 2016;125:30–43. doi: 10.1016/j.brainresbull.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Swanson R.A., Morton M.T., Tsao-Wu G., Savalos R.A., Davidson C., Sharp F.R. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 35.Zhou L., Xing R., Xie L., Rao T., Wang Q., Ye W., Fu H., Xiao J., Shao Y., Kang D. Development and validation of an UFLC-MS/MS assay for the absolute quantitation of nine notoginsenosides in rat plasma: application to the pharmacokinetic study of Panax notoginseng extract. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;995–996:46–53. doi: 10.1016/j.jchromb.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Xiao J., Chen H., Kang D., Shao Y., Shen B., Li X., Yin X., Zhu Z., Li H., Rao T. Qualitatively and quantitatively investigating the regulation of intestinal microbiota on the metabolism of panax notoginseng saponins. J Ethnopharmacol. 2016;194:324–326. doi: 10.1016/j.jep.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 37.Carabotti M., Scirocco A., Maselli M.A., Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 38.Bienenstock J., Kunze W. Microbiota and the gut-brain axis. Nutr Rev. 2015;73(Suppl 1):28–31. doi: 10.1093/nutrit/nuv019. [DOI] [PubMed] [Google Scholar]
- 39.Barrett E., Ross R.P., O'Toole P.W., Fitzgerald G.F., Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2014;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 40.Bharwani A., Mian M.F., Surette M.G., Bienenstock J., Forsythe P. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 2017;15:7. doi: 10.1186/s12916-016-0771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H., Lee I.S., Braun C., Enck P. Effect of probiotics on central nervous system functions in animals and humans: a systematic review. J Neurogastroenterol Motil. 2016;22:589–605. doi: 10.5056/jnm16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.