Figure 2.
HP Activation Ameliorates Protein Aggregation in Mammalian PolyQ Models
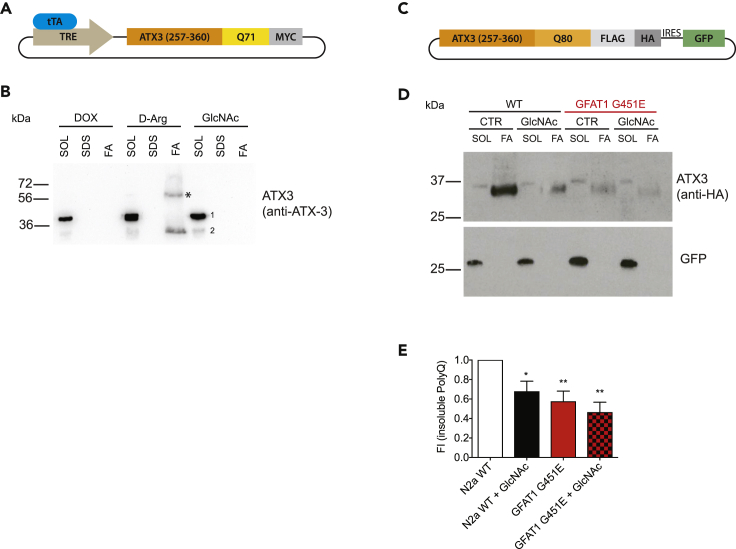
(A) Schematic representation of a vector expressing an aggregation-prone ATX3 fragment. A C-terminal fragment of ATX3 cDNA corresponding to amino acid residues 257–360 in the full-length protein was cloned under the control of a tetracycline responsive element (TRE) and tagged with a C-terminal c-myc epitope as described elsewhere (Haacke et al., 2006).
(B) N2a cells stably expressing a Tet-Off operator (tTA) were transiently transfected with the construct described above and treated as indicated (10 mM D-Arg, 10 mM GlcNAc, 1 μg/mL doxycycline). Cells were lysed and processed for fractionation 24 h after treatment and transfection. * Partially formic acid-insoluble material detected by the ATX3 antibody; 1, endogenous ATX3; 2, exogenous ATX3 fragment.
(C) Schematic representation of a vector expressing an aggregation-prone ATX3 fragment including an internal ribosomal entry site (IRES) followed by GFP.
(D) Fractionation of cell lysates from WT, GFAT1 G451E engineered N2a cells, and both treated with 10 mM GlcNAc 48 h after transfection with the ATX3-PolyQ construct (Figure 2C). SOL, SDS-soluble fraction; SDS, SDS wash fraction; FA, SDS-insoluble formic acid fraction.
(E) Quantification of fractionation experiments from Figure 2D. Insoluble fraction was normalized to GFP expression. Mean + SD (n ≥ 4), *p < 0.05, **p < 0.01 (ANOVA).