Abstract
Atopic dermatitis (AD) is a chronic and relapsing inflammatory disease that affects 1%–20% of people worldwide. Despite affecting many people, AD current treatments, such as corticosteroids and calcineurin inhibitors, have not only harmful secondary effects but are also often ineffective. Therefore, natural nontoxic compounds are on high demand for developing new effective AD treatments. Panax ginseng Meyer has been used traditionally for its promising healing and restorative properties to treat many diseases including skin disorders, reason why in this review we want to explore the research performed with AD and P. ginseng as well as determining its potential for new drug development. Previous researches have shown that P. ginseng has positive effects in AD patients such as lower eczema area and severity index, transepidermal water loss, and immunoglobulin E levels and better quality of sleep. In vivo animal models, as well, have shown positive results to P. ginseng and derived ginsenosides, such as the decrease of transepidermal water loss, immunoglobulin E levels in serum, allergy-related cytokines, and downregulation of NF-κB, MAPK, and Ikaros pathways. All of these previous data suggest that P. ginseng and its derived ginsenosides are undoubtedly a nontoxic effective option to treat AD.
Keywords: Alternative medicine, Atopic dermatitis, Filaggrin, Ginsenosides, Panax ginseng
Abbreviations: AD, atopic dermatitis; ATX, plasma autotaxin; CG, cultivated ginseng; CCL2, Chemokine ligand 2; COX-2, Cyclooxygenase-2; DNFB, 1-fluoro-2,4-dinitrobenzene; DFE, Dermatophagoides farinae body extract; EASY, eczema area and severity index; FLG, filaggrin; GDP, 20-O-β-d-glucopyranosyl-20(S)-protopanaxadiol; GMCSF, granulocyte macrophage colony-stimulating factor; HMC-1, human mast cell line; IL, interleukin; IFN, interferon; KRG, Korean Red Ginseng; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; MDC, macrophage-derived chemokine; MIP-1alpha, macrophage inflammatory protein-1alpha; MIP-1beta, macrophage inflammatory protein-1beta; NO, Nitric oxide; PMA, phorbol-myristate acetate; RANTES, regulated on activation normal T cell expressed and secreted; RGE, red ginseng extract; TARC, thymus and activation-regulated chemokine; TH cell, lymphocyte T helper cell; TEWL, trans epidermal water loss; TNCB, 2,4,6-trinitro-1-chlorobenzene; TNF-α, tumor necrosis factor-alpha; TSLP, thymic stromal lymphopoietin
1. Introduction
Atopic dermatitis (AD) is a chronic and relapsing inflammatory skin disease characterized by pruritus, erythema, scaling, edema, and inflammatory eczematous eruptions that usually begin early in life [1]. AD is a major global public health problem, affecting 1%–20% of people worldwide, with a prevalence of about 1%–3% in adults and 10%–20% in children [2]. Instead of having one specific cause, AD is considered to be triggered by the interaction of many pathological mechanisms such as genetic background, impaired skin barrier function, impaired immunity, and environmental factors acting synergistically [3]. Despite affecting a great amount of people around the world, effective therapeutic strategies are yet to be established [4]. Panax ginseng has been extensively used in Asian traditional medicine because of its healing, restorative, and anti-inflammatory properties [5]. It has also been used in traditional Chinese medicine to treat skin disorders including atopic suppurative dermatitis, but the modern knowledge in this area continues to be lacking [6]. Nowadays, many studies focus on purified individual ginsenosides, which are ginseng's most important constituents and study their specific mechanism of action, so diseases' treatment can be more accurate [6]. Because of its traditional use in the treatment of skin disorders, in this review, we aim to examine the research performed with ginseng and determine its potential as a more natural nontoxic alternative for treating AD.
2. AD pathology and mechanism of action
Atopy is defined as an inherited tendency to produce immunoglobulin E (IgE) antibodies in response to minute amounts of common environmental proteins such as pollen, house dust mites, and food allergens [7]. Dermatitis derives from the Greek word ‘derma,’ which means skin and ‘itis,’ which means inflammation, therefore skin inflammation [3]. The occurrence of AD has been associated with two anomalies: the first one corresponding to an imbalance of the adaptive immune system [8] and the second one being the presence of a defective skin barrier [9]. Because AD is strongly correlated to inflammation, several arguments support that AD is primarily an immune disease [8].
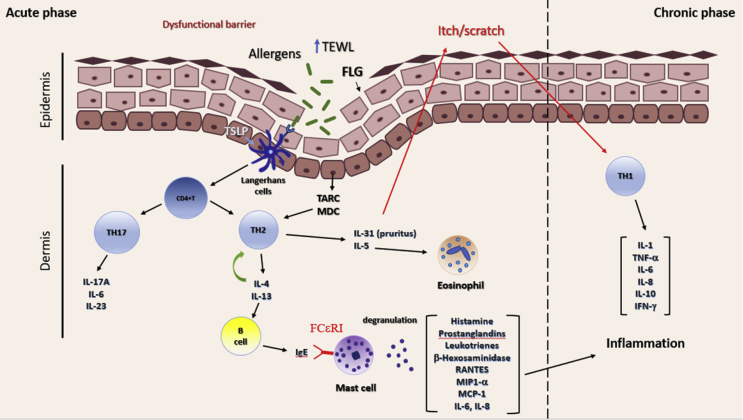
The theory of immunological imbalance argues that AD results from an imbalance of T cells, particularly T helper cell types 1 and 2, the latest being the predominant type in AD's acute phase and the second one predominating in an inflammatory chronic phase [3] (Fig. 1). Antigen-activated TH2 cells cause an increased production of interleukins (ILs), primarily IL-4, IL-5, IL-13, IL-31, and tumor necrosis factor-alpha (TNF-α) [10]. IL-5 induces eosinophil recruitment [11], whereas IL-31 is related to pruritus development [12] and IL-4 enhances B cells to start producing IgE antibodies. When IgE antibodies interact with the specific receptor FCεRI on mast cells, it activates a signaling cascade that ends up with an increase in the intracellular Ca2+, mast cell degranulation, and the release of allergic mediators (histamine, prostaglandins, β-hexosaminidase, and leukotrienes) [13]. Mast cell activation is also associated with an increase in Th17-associated cytokines (IL-17A, IL-6, IL-23) [14] and the production of proinflammatory cytokines (IL-1β, IL-6, IL-8) [15], [16], and chemokines (macrophage inflammatory protein [MIP]-1α, MIP-1β, regulated on activation normal T cell expressed and secreted, monocyte chemoattractant protein-1) [15], which together with TH1-derived mediators (IL-1β, IL-6, IL-8, IL-10, interferon [IFN]-γ) induce the chronic inflammatory phase of AD (Fig. 1).
Fig. 1.
AD patients have a dysfunctional epidermis due to a mutation in the filaggrin gene (FLG) that allows transepidermal water loss (TEWL) and easy entrance of allergens in the skin. Allergens induce the production of the thymus and activation-regulated chemokine (TARC), macrophage-derived chemokine (MDC), and thymic stromal lymphopoietin (TSLP) in the keratinocytes. TLSP activates Langerhans cells (dendritic cells), which induce the differentiation of CD4+T cells into T helper type 2 cells (TH2), whose infiltration into tissue is mediated by TARC and MDC. TH2 cells produce IL-4, IL-5, IL-13, and IL-31, among others. IL-31 induces pruritus response in the epidermis, which causes the change into the inflammatory chronic phase. IL-5 is related to eosinophil recruitment to the damaged tissue, whereas IL-4 further induces TH2 polarization and IgE production by B cells. IgE crosslinks with specific receptor FCεRI on mast cells, causing mast cell degranulation and release of allergic mediators (histamine, prostaglandins, leukotrienes, MIP-α, MCP-1, IL-6, and IL-8), which in cooperation with T helper cells type 1 (TH1) released mediators (IL-1, IL-6, IL-8, IL-10, TNF-α, and IFN-γ) enhance the inflammatory phase of AD. Recently, TH17 secreted mediators (IL-17A, IL-6, IL-23) have been shown to play a role in the development of AD.
IL, interleukin; AD, atopic dermatitis; IFN, interferon; MCP-1, monocyte chemoattractant protein-1; TNF-α, tumor necrosis factor-alpha; MIP, macrophage inflammatory protein; IgE, immunoglobulin E.
The allergic inflammatory response has been associated mainly with the activation of the mitogen-activated protein kinases (MAPKs), which include the extracellular signal–regulated kinase, c-Jun N-terminal kinase, and p38 MAPK [17]. MAPKs are involved in the activation of NF-κB pathway, whose translocation into the nucleus initiates the transcription of inflammatory and allergy-related mediators, reason why regulation of MAPK and NF-κB pathways is considered vital for AD prevention [15]. Another transcription factor involved in the allergic reaction is Ikaros, which has been related to Th2 activation and IL-4 production, therefore playing an important role in AD progression [17].
Barrier function has long been known to be reduced in the skin of patients with AD [9]. Previous studies showed that AD patients had increased transepidermal water loss and that this was due to a loss of function of the filaggrin (FLG) protein [18]. FLG facilitates not only the terminal differentiation of the epidermis but also the formation of the skin barrier, thus having an important role in maintaining the epidermis structure and hydration [19]. Because a defective skin barrier allows allergens to penetrate the epidermis more easily, patients with FLG mutation are more prone to develop AD [20]. Keratinocytes, the main cells in the epidermis, also play an important role in the progression of AD, because, when exposed to allergens or microbes, they are able to secrete Thymus and activation-regulated chemokine (TARC) and macrophage-derived chemokine (C-C motif chemokine ligand 2), which mediate the inflammatory tissue Th2 cells infiltration and thymic stromal lymphopoietin (TSLP), which activates Langerhans cells (dendritic cells) to induce TH2 differentiation [15] (Fig. 1).
3. Commonly used AD treatments
As previously mentioned, it has been generally established that AD patients suffer either a skin barrier dysfunction, skin inflammation, or both, reason why it is difficult to find an adequate treatment and a combined treatment is often recommended [21]. An important feature of AD treatment is the maintenance of skin function; thus, typical AD treatments have included the use of emollients for improving skin hydration and barrier repair [22], [23], as well as the elimination of factors (including allergens, irritants, and emotional triggers) that might exacerbate the scratch-itch cycle [24].
Even though emollients are vital for maintaining skin hydration, they cannot be used, for example, against Staphylococcus aureus infection and there is also no definite evidence to prove that their use diminishes AD's severity [23]. Because chronic and severe pruritus reduces the quality of life in patients and scratching damages the skin barrier and worsens inflammation of the skin, the regulation of both symptoms has become one of the most important aims for the treatment of AD [25]. Previous pruritus' treatment has included the use of antihistamines and antiallergic drugs [26], which have been shown to help in the pruritus-related insomnia; however, further studies are needed to prove their true efficacy [27]. Previous treatments for managing AD-related inflammation include corticosteroids and calcineurin inhibitors. Corticosteroids act on a variety of immune cells, including T lymphocytes, monocytes, macrophages, and dendritic cells, interfering with antigen processing and suppressing the release of proinflammatory cytokines. However, if used for long terms, they can lead to skin atrophy or the development of rosacea, striae, and hypothalamic–pituitary–adrenal axis alteration, among other secondary effects [28]. Calcineurin inhibitors, even though less potent than steroids, are also used with anti-inflammatory purposes [29]. Calcineurin inhibitors' common side effects include burning, redness, and pruritus that may appear depending on each patient [7].
On the other hand, several studies have shown that the narrowband UVB (311 nm) and high dose UVA1 (340–400 nm) can act as moderately potent topical steroids for acute, severe atopic eczema. However, special irradiation devices, which are only available in specialist centers, are needed for this type of treatment [30], and depending on the patient, unwanted side effects such as erythema, blistering, hyperpigmentation, and eczema, among others, might appear [31]. In addition, specific AD mediator's inhibition treatments have been developed such as cyclosporine A [32] and azathioprine [33] (Tcell inhibitors), infliximab [34] (TNF-α inhibitor), omalizumab [35] (IgE inhibitor), mepolizumab (IL-5 inhibitor) [11], and dupilumab (IL-4 and IL-13 inhibitors) [36]. Exempting dupilumab, which is currently on medical trial, all remaining treatments have proved to have either low efficacy on AD's treatment or undesired secondary effects (Table 1).
Table 1.
Commonly used treatments for AD
| Treatment | Mode of action | Benefits | Limitations/side effects | References |
|---|---|---|---|---|
| Emollients | Hydration, moisturizing | (↓) TEWL (↑) hydration |
Not effective against Staphylococcusaureus colonization or AD severity reduction. | [23] |
| TCS | Immune cells blocking | (↓) proinflammatory cytokines | skin atrophy, hypothalamic–pituitary–adrenal axis alteration, rosacea | [28] |
| TCI | Calcineurin-dependent T- cell activation | (↓) proinflammatory cytokines | Burning, stinging and pruritus | [7], [29] |
| Phototherapy | NbUVB (311 nm) | Steroid-like effect | Erythema | [30], [31] |
| UVA1 (340–400 nm) | Eczema, blisters, hyperpigmentation, skin aging | |||
| Cyclosporine A | Inhibits Th1 and Th2 | (↓) inflammation (↓) pruritus |
Nausea, headache, hypertension, renal impairment, chronic immunosuppression | [7], [32] |
| Azathioprine | TH cell proliferation inhibition | (↓) inflammation | Nausea, vomiting, diarrhea, bone marrow suppression | [33] |
| Infliximab | Antagonist against TNF-α | (↓) inflammation | Risk of infection | [34] |
| Omalizumab | Monoclonal antibody that blocks IgE function | (↓) IgE in serum | Slight differences against the placebo High cost |
[35], [48] |
| Mepolizumab | IL-5 monoclonal antibody | (↓) eosinophil recruitment | No significant differences in AD | [11] |
| Dupilumab | Blocks IL-4 and IL-13 signaling | (↓) pruritus | No apparent adverse effects | [36] |
AD, atopic dermatitis; TEWL, trans epidermal water loss; TCS, topical corticosteroids; TCI, topical calcineurin inhibitors; UV, ultraviolet light; NbUVB, narrowband UVB; IL, interleukin; TH, T helper cell; TNF-α, tumor necrosis factor-alpha.
4. Use of P. ginseng extract and ginsenosides in AD treatment
Ginseng refers to the root and rhizome of P. ginseng (Araliaceae), an herb extensively used in Asia because of its anti-inflammatory, anticancerous, antidiabetic, and antiallergic properties [37]. Ginsenosides, ginseng's major active pharmacological components, are steroid-like saponins which can only be found in the ginseng species [37]. Besides being used in the treatment of many inflammatory diseases, P. ginseng and derived ginsenosides have shown to be effective in the treatment of many skin diseases [38]. Ginseng roots have been used in Chinese medicine to treat skin ailments such as wounds, psoriasis, skin inflammation, and suppurative AD. Nevertheless, relatively few studies have been performed regarding the use of P. ginseng in the treatment of AD [6].
P. ginseng has been proven to be a good candidate in the treatment of AD because Korean Red Ginseng (KRG) extract trials in AD patients resulted not only in a decrease of eczema area and severity index but also a decrease in the transepidermal water loss [39], IgE serum levels, and skin squamation, while improving the patients sleep disturbance and aiding the stratum corneum recovery [40]. In addition, KRG treatment in 1-fluoro-2,4-dinitrobenzene (DNFB)–induced NC/Nga showed a decrease in ear thickness, TEWL, IgE contents in serum, and AD-related cytokines such as TSLP, TNF-α, IL-4, IL-17, and IFN-γ [41], whereas, when induced with 2,4,6-trinitro-1-chlorobenzene, it not only showed decrease in ear thickness and IgE contents but also downregulated the expression of TSLP, TNF-α, IFN-γ, and IL-31 (Table 2) [42].
Table 2.
Effects of P. ginseng and derived ginsenosides on AD
| Treatment | Experimental model | Effects | References |
|---|---|---|---|
| KRG | 41 AD patients (KRG consumption for 8 weeks) | (↓) EASI, (↓) TEWL, (↓) pruritus (↓) sleep disturbance | [39] |
| 30 AD patients (KRG consumption for 16 weeks) | (↓) Skin squamation (↓)TEWL (↓) IgE in serum (↑) Stratum corneum recovery | [40] | |
| TNCB-treated NC/Nga mice | (↓) ear thickness (↓)TEWL (↓) IgE in serum (↓) TSLP (↓) TNF-α (↓)IL-4, IL-17, IFN-γ | [41] | |
| Compound 48/80–induced anaphylactic shock and DNFB-induced skin lesion in Balb/c mice TNF-α– and IFN--γ–induced HaCaT cells |
(↓) AD-like skin lesions and anaphylaxis (↓) IL-1β, IL-6, and IL-8 (↓) IgE in serum (↓) MAPK and NF-κB pathway | [15] | |
| (↓) IL-1β, IL-6, and IL-8 (↓) MAPK and NF-κB pathway (↓) TARC, MDC | [15] | ||
| PMA/A23187-induced HMC-1 cells | (↓) MIP-1a, MIP-1b, RANTES, MCP-1 | [15] | |
| (↓) IL-1β, IL-6, and IL-8 (↓) MAPK and NF-κB pathway | |||
| TNCB-induced NC/Nga mice | (↓) scratching (↓) ear thickness (↓) TEWL (↓) IgE in serum (↓) IL-31, TNF-α, IFN-γ, TSLP | [42] | |
| DNCB-induced Balb/c mice | (↓) IL-4, IL-10 (↓) scratching (↓) IgE in serum (↓) MAPK, NF-κB, Ikaros | [17] | |
| DNFB-induced Wistar rats, Balb/c and ICR mice | (↓) scratching (↓) ear thickness (↓) substance P (pruritus related) | [43] | |
| KRG, Rh2, Rg3 | TNCB-treated NC/Nga mice | (↓) TNF-α, IL-4, IFN-γ scratching (↓) IgE in serum |
[10] |
| Gintonin | DNFB-induced NC/Nga mice | (↓) ear thickness, (↓) IL-4, IFN-γ (↓) ear thickness histamine (↓) IgE in serum (↓) plasma ATX | [45] |
| CG | DNCB-induced NC/Nga mice TNF-α– and IFN--γ–induced HaCaT cells |
(↓) IgE in serum (↓) IL-4, IL-5, IL-13, TNF-α, IFN-γ (↓) ear thickness (↓) immune cells infiltration | [44] |
| (↓) TARC | [44] | ||
| GDP | DFE-induced AD-like symptoms in NC/Nga mice | (↓) dermatitis score (↓) ear thickness, (↓) scratching (↓) skin lesion (↓) IL-12, IL-4, IL-5, IL-10, IFN-γ, GM-CSF (↓) eosinophils and mast cell infiltration | [46] |
| RGE, Rb1, Rg1, Rg3, and Rh1 | IgE crosslinking induced–KU812 cells | (↓) IFN-γ | [47] |
| IFN-γ–induced human epidermal keratinocyte NHEK (NB) | (↓) CCL2 | ||
| Rg3, Rf, Rh2 | IgE crosslinking induced–RBL-2H3 cells anti-DNP IgE/DNP-HSA induced–ICR mouse |
(↓) β-hexosaminidase | [16] |
| LPS induced RAW264.7 cells | (↓) TNF-α, IL-1β, COX-2, IL-4, IFN-γ (↓) ear thickness (↓) COX-2 NO |
[16] |
KRG, Korean red Ginseng; EASI, eczema area and severity index; TNCB, 2,4,6-trinitro-1-chlorobenzene; TEWL, transepidermal water loss; TSLP, thymic stromal lymphopoietin; TNF-α, tumor necrosis factor; IL, interleukin; IFN, interferon; DNFB, 1-fluoro-2,4-dinitrobenzene; TARC, thymus and activation-regulated chemokine; MDC, macrophage-derived chemokine; MIP-1a, macrophage inflammatory protein-1a; MIP-1b, macrophage inflammatory protein-1b; RANTES, regulated on activation normal T cell expressed and secreted; MCP-1, monocyte chemoattractant protein-1; PMA, phorbol-myristate acetate; HMC-1, human mast cell line; ATX, plasma autotaxin; CG, cultivated ginseng; GDP, 20-O-β-d-glucopyranosyl-20(S)-protopanaxadiol; DFE, Dermatophagoides farinae body extract; GM-CSF, granulocyte macrophage colony–stimulating factor; RGE, red ginseng extract; CCL2, chemokine ligand 2; LPS, lipopolysaccharide; COX-2, cyclooxygenase-2; NO, nitric oxide.
In Balb/c mice, when induced with DNFB, KRG managed to decrease ear thickness, IgE contents in serum, AD-like skin lesions, anaphylaxis, AD-related cytokines such as IL-1β, IL-6, and IL-8 and MAPK and NF-κB pathways [15]. When induced with 2,4-dinitrochlorobenzene (DNCB), other AD-related cytokines such as IL-4 and IL-10 were downregulated, as well as IgE serum contents and MAPK and NF-κB pathways, as well as Ikaros transcription factor [17]. Likewise, DNFB-induced Wistar rats, Balb/c, and Institute of Cancer Research (ICR) mice showed an improvement in the scratching behavior, ear thickness, and substance P (pruritus-related) [43]. KRG also decreased the levels of IL-1β, IL-6, IL-8, TARC, macrophage-derived chemokine, MAPK, and NF-κB pathways in TNF-α– and IFN-γ–induced HaCaT cells and downregulation of IL-1β, IL-6, IL-8, MIP-1α, MIP-1β, regulated on activation normal T cell expressed and secreted, and monocyte chemoattractant protein-1, as well as MAPK and NF-κB pathway in phorbol-myristate acetate/A23187-induced HMC-1 cells [15]. Cultivated ginseng could not only downregulate IgE contents in serum, IL-4, IL-5, IL-13, TNF-α, and IFN-γ expression, ear thickness, and immune cells infiltration but also downregulated TARC pathway in TNF-α/IFN-γ–induced HaCaT cells [44].
Ginsenosides have also been proven to be effective in AD treatment. Gintonin, for example, could decrease ear thickness, IgE levels in serum, plasma autotaxin levels in plasma, histamine release, and IL-4 and IFN-γ levels in DNFB-induced NC/Nga mice (Table 2) [45]. 20-O-β-d-glucopyranosyl-20(S)-protopanaxadiol, ginseng's main metabolite, had also a good effect in Dermatophagoides farinae body extract–induced NC/Nga mice, reducing the dermatitis score, ear thickness, scratching behavior, skin lesion, cytokines including IL-12, IL-4, IL-5, IL-10, IFN-γ, and GM-CSF, as well as diminishing eosinophils and mast cell infiltration [46]. Rh2- and Rg3-treated 2,4,6-trinitro-1-chlorobenzene–induced NC/Nga mice also showed improvement in the scratching behavior and decrease in both the IgE in serum and TNF-α, IL-4, and IFN-γ levels [10]. Red ginseng extract, Rb1, Rg1, Rg3, and Rh1 treatment in IgE-induced KU812 cells decreased the levels of IFN-γ and CCL-2 in induced human epidermal keratinocytes NHEK (NB) [47]. Treatment with Rg3, Rf, and Rh2 decreased the β-hexosaminidase release in IgE-sensitized RBL-2H3 cells and the levels of TNF-α, IL-1β, cyclooxygenase-2, IL-4, and IFN-γ in anti-DNP IgE/DNP-HSA–induced ICR mouse, managing to also decrease lipopolysaccharide-induced inflammatory response including the production of cyclooxygenase-2 and nitric oxide in RAW264.7 cells [16].
5. Concluding remarks and future perspectives
Despite affecting a major part of the population around the world, effective and nonharmful treatments for AD have yet to be developed. As per the research performed with P. ginseng, we can conclude that, besides its promising healing and restorative properties to treat many skin disorders, it has shown to be also a promising treatment for AD. Compared with the currently used treatments, P. ginseng and its derived ginsenosides might prove to be not only a nontoxic but also less expensive, natural, and effective AD treatment. Therefore, more research related with P. ginseng as well as its derived ginsenosides are further needed for new drug development.
Conflicts of interest
The authors report no conflicts of interest.
Acknowledgments
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1A6A1A03015642) from South Korea.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2018.12.012.
Contributor Information
Mi-Yeon Kim, Email: kimmy@ssu.ac.kr.
Jae Youl Cho, Email: jaecho@skku.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lee J.H., Son S.W., Cho S.H. A comprehensive review of the treatment of atopic eczema. Allergy Asthma Immunol Res. 2016;8:181–190. doi: 10.4168/aair.2016.8.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odhiambo J.A., Williams H.C., Clayton T.O., Robertson C.F., Asher M.I. Global variations in prevalence of eczema symptoms in children from ISAAC Phase three. J Allergy Clin Immunol. 2009;124 doi: 10.1016/j.jaci.2009.10.009. 1251-1258.e1223. [DOI] [PubMed] [Google Scholar]
- 3.Thomsen S.F. Atopic dermatitis: natural history, diagnosis, and treatment. ISRN Allergy. 2014;2014 doi: 10.1155/2014/354250. 354250-354250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boguniewicz M., Alexis A.F., Beck L.A., Block J., Eichenfield L.F., Fonacier L., Guttman-Yassky E., Paller A.S., Pariser D., Silverberg J.I. Expert perspectives on management of moderate-to-severe atopic dermatitis: a multidisciplinary consensus addressing current and emerging therapies. J Allergy Clin Immunol. 2017;5:1519–1531. doi: 10.1016/j.jaip.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Park H.J., Kim D.H., Park S.J., Kim J.M., Ryu J.H. Ginseng in traditional herbal prescriptions. J Ginseng Res. 2012;36:225–241. doi: 10.5142/jgr.2012.36.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura Y., Sumiyoshi M., Sakanaka M. Effects of ginsenoside Rb₁ on skin changes. J Biomed Biotechnol. 2012;2012 doi: 10.1155/2012/946242. 946242-946242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowicki R., Nowicki R., Trzeciak M., Wilkowska A., sokolowska-wojdylo M., Ługowska-Umer H., Baranska-Rybak W., Kaczmarski M., Kowalewski C., Kruszewski J. Polish Society of Allergology, and the Allergology Section, Polish Society of Dermatology; 2015. Special paper atopic dermatitis: current treatment guidelines. Statement of the experts of the Dermatological Section. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eyerich K., Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy. 2013;68:974–982. doi: 10.1111/all.12184. [DOI] [PubMed] [Google Scholar]
- 9.Egawa G., Kabashima K. Barrier dysfunction in the skin allergy. Allergol Int. 2018;67:3–11. doi: 10.1016/j.alit.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Brandt E.B., Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol. 2011;2:110. doi: 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oldhoff J.M., Darsow U., Werfel T., Katzer K., Wulf A., Laifaoui J., Hijnen D.J., Plötz S., Knol E.F., Kapp A. Anti-IL-5 recombinant humanized monoclonal antibody (Mepolizumab) for the treatment of atopic dermatitis. Allergy. 2005;60:693–696. doi: 10.1111/j.1398-9995.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 12.Furue M., Yamamura K., Kido-Nakahara M., Nakahara T., Fukui Y. Emerging role of interleukin-31 and interleukin-31 receptor in pruritus in atopic dermatitis. Allergy. 2018;73:29–36. doi: 10.1111/all.13239. [DOI] [PubMed] [Google Scholar]
- 13.De Benedetto A., Agnihothri R., McGirt L.Y., Bankova L.G., Beck L.A. Atopic dermatitis: a disease caused by innate immune defects? J Invest Dermatol. 2009;129:14–30. doi: 10.1038/jid.2008.259. [DOI] [PubMed] [Google Scholar]
- 14.Cesare A.D., Meglio P.D., Nestle F.O. A role for Th17 cells in the immunopathogenesis of atopic dermatitis? J Invest Dermatol. 2008;128:2569–2571. doi: 10.1038/jid.2008.283. [DOI] [PubMed] [Google Scholar]
- 15.Kee J.-Y., Jeon Y.-D., Kim D.-S., Han Y.-H., Park J., Youn D.-H., Kim S.-J., Ahn K.S., Um J.-Y., Hong S.-H. Korean red ginseng improves atopic dermatitis-like skin lesions by suppressing expression of proinflammatory cytokines and chemokines in vivo and in vitro. J Ginseng Res. 2017;41:134–143. doi: 10.1016/j.jgr.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae E.-A., Han M.J., Shin Y.-W., Kim D.-H. Inhibitory effects of Korean red ginseng and its genuine constituents ginsenosides Rg3, Rf, and Rh2 in mouse passive cutaneous anaphylaxis reaction and contact dermatitis models. Biol Pharmaceut Bull. 2006;29:1862–1867. doi: 10.1248/bpb.29.1862. [DOI] [PubMed] [Google Scholar]
- 17.Sohn E.-H., Jang S.-A., Lee C.-H., Jang K.-H., Kang S.-C., Park H.-J., Pyo S. Effects of Korean red ginseng extract for the treatment of atopic dermatitis-like skin lesions in mice. J Ginseng Res. 2011;35:479–486. doi: 10.5142/jgr.2011.35.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McPherson T. Current understanding in pathogenesis of atopic dermatitis. Indian J Dermatol. 2016;61:649–655. doi: 10.4103/0019-5154.193674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawasaki H, Kubo A, Sasaki T, Amagai M. Loss-of-function mutations within the Filaggrin gene and atopic dermatitis. [DOI] [PubMed]
- 20.Osawa R., Akiyama M., Shimizu H. Filaggrin gene defects and the risk of developing allergic disorders. Allergol Int. 2011;60:1–9. doi: 10.2332/allergolint.10-RAI-0270. [DOI] [PubMed] [Google Scholar]
- 21.Leung D.Y.M., Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014;134:769–779. doi: 10.1016/j.jaci.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horimukai K., Morita K., Narita M., Kondo M., Kitazawa H., Nozaki M., Shigematsu Y., Yoshida K., Niizeki H., Motomura K-i. Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol. 2014;134 doi: 10.1016/j.jaci.2014.07.060. 824-830.e826. [DOI] [PubMed] [Google Scholar]
- 23.Simpson E.L., Chalmers J.R., Hanifin J.M., Thomas K.S., Cork M.J., McLean W.H.I., Brown S.J., Chen Z., Chen Y., Williams H.C. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818–823. doi: 10.1016/j.jaci.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suárez A.L., Feramisco J.D., Koo J., Steinhoff M. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Derm Venereol. 2012;92:7–15. doi: 10.2340/00015555-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mollanazar N.K., Smith P.K., Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin Rev Allergy Immunol. 2016;51:263–292. doi: 10.1007/s12016-015-8488-5. [DOI] [PubMed] [Google Scholar]
- 26.Eichenfield L.F., Tom W.L., Berger T.G., Krol A., Paller A.S., Schwarzenberger K., Bergman J.N., Chamlin S.L., Cohen D.E., Cooper K.D. Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116–132. doi: 10.1016/j.jaad.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He A., Feldman S.R., Fleischer A.B. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92–96. doi: 10.1016/j.jaad.2017.12.077. [DOI] [PubMed] [Google Scholar]
- 28.Mooney E., Rademaker M., Dailey R., Daniel B.S., Drummond C., Fischer G., Foster R., Grills C., Halbert A., Hill S. Adverse effects of topical corticosteroids in paediatric eczema: australasian consensus statement. Australas J Dermatol. 2015;56:241–251. doi: 10.1111/ajd.12313. [DOI] [PubMed] [Google Scholar]
- 29.El-Batawy M.M.Y., Bosseila M.A.W., Mashaly H.M., Hafez V.S.G.A. Topical calcineurin inhibitors in atopic dermatitis: a systematic review and meta-analysis. J Dermatol Sci. 2009;54:76–87. doi: 10.1016/j.jdermsci.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Taylor K., Swan D.J., Affleck A., Flohr C., Reynolds N.J., Dermatology UKTRNi, the UKDCTN Treatment of moderate-to-severe atopic eczema in adults within the U.K.: results of a national survey of dermatologists. Br J Dermatol. 2017;176:1617–1623. doi: 10.1111/bjd.15235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds N.J., Franklin V., Gray J.C., Diffey B.L., Farr P.M. Narrow-band ultraviolet B and broad-band ultraviolet A phototherapy in adult atopic eczema: a randomised controlled trial. Lancet. 2001;357:2012–2016. doi: 10.1016/S0140-6736(00)05114-X. [DOI] [PubMed] [Google Scholar]
- 32.Goujon C., Viguier M., Staumont-Sallé D., Bernier C., Guillet G., Lahfa M., Ferrier Le Bouedec M.-C., Cambazard F., Bottigioli D., Grande S. Methotrexate versus cyclosporine in adults with moderate-to-severe atopic dermatitis: a Phase III randomized noninferiority trial. J Allergy Clin Immunol. 2018;6 doi: 10.1016/j.jaip.2017.07.007. 562-569.e563. [DOI] [PubMed] [Google Scholar]
- 33.Sidbury R., Davis D.M., Cohen D.E., Cordoro K.M., Berger T.G., Bergman J.N., Chamlin S.L., Cooper K.D., Feldman S.R., Hanifin J.M. Guidelines of care for the management of atopic dermatitis: Section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327–349. doi: 10.1016/j.jaad.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee H.-H., Song I.-H., Friedrich M., Gauliard A., Detert J., Röwert J., Audring H., Kary S., Burmester G.-R., Sterry W. Cutaneous side-effects in patients with rheumatic diseases during application of tumour necrosis factor-α antagonists. Br J Dermatol. 2007;156:486–491. doi: 10.1111/j.1365-2133.2007.07682.x. [DOI] [PubMed] [Google Scholar]
- 35.Fernández-Antón Martínez M.C., Leis-Dosil V., Alfageme-Roldán F., Paravisini A., Sánchez-Ramón S., Suárez Fernández R. Omalizumab for the treatment of atopic dermatitis. Actas Dermo-Sifiliográficas (English Edition) 2012;103:624–628. doi: 10.1016/j.ad.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Simpson E.L., Bieber T., Guttman-Yassky E., Beck L.A., Blauvelt A., Cork M.J., Silverberg J.I., Deleuran M., Kataoka Y., Lacour J.-P. Two phase 3 trials of Dupilumab versus placebo in atopic dermatitis. N Eng J Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 37.Kim E.H., Kim W. An insight into ginsenoside metabolite compound K as a potential tool for skin disorder. Evidence-based complementary and alternative medicine. eCAM. 2018;2018 doi: 10.1155/2018/8075870. 8075870-8075870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarvenaz S.-R., Sara S.-R., Amirhossein S., Zahra T.-N. Ginseng in dermatology: a review. Cur Pharmaceut Des. 2017;23:1649–1666. doi: 10.2174/1381612822666161021152322. [DOI] [PubMed] [Google Scholar]
- 39.Kim H., Park C.W., Cho S.H. The beneficial effect of Korean red ginseng extract on atopic dermatitis patients: an 8 weeks open, noncomparative clinical study. Ann Dermatol. 2018;30:304–308. doi: 10.5021/ad.2018.30.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee K.G., Son S.W. Efficacy of korean red ginseng in the treatment of atopic dermatitis. J Ginseng Res. 2011;35:149–154. doi: 10.5142/jgr.2011.35.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho E., Cho S.H. Effects of Korean red ginseng extract on the prevention of atopic dermatitis and its mechanism on early lesions in a murine model. J Ethnopharmacol. 2013;145:294–302. doi: 10.1016/j.jep.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Lee H.J., Cho S.H. Therapeutic effects of Korean red ginseng extract in a murine model of atopic dermatitis: anti-pruritic and anti-inflammatory mechanism. J Kor Med Sci. 2017;32:679–687. doi: 10.3346/jkms.2017.32.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samukawa K., Izumi Y., Shiota M., Nakao T., Osada-Oka M., Miura K., Iwao H. Red ginseng inhibits scratching behavior associated with atopic dermatitis in experimental animal models. J Pharmacol Sci. 2012;118:391–400. doi: 10.1254/jphs.11182fp. [DOI] [PubMed] [Google Scholar]
- 44.Choi J.H., Jin S.W., Park B.H., Kim H.G., Khanal T., Han H.J., Hwang Y.P., Choi J.M., Chung Y.C., Hwang S.K. Cultivated ginseng inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in NC/Nga mice and TNF-α/IFN-γ-induced TARC activation in HaCaT cells. Food Chem Toxicol. 2013;56:195–203. doi: 10.1016/j.fct.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 45.Lee B.-H., Kim H.-K., Jang M., Kim H.-J., Choi S.-H., Hwang S.-H., Kim H.-C., Rhim H., Cho I.-H., Nah S.-Y. Effects of gintonin-enriched fraction in an atopic dermatitis animal model: involvement of autotaxin regulation. Biol Pharmaceut Bull. 2017;40:1063–1070. doi: 10.1248/bpb.b17-00124. [DOI] [PubMed] [Google Scholar]
- 46.Kim J.R., Choi J., Kim J., Kim H., Kang H., Kim E.H., Chang J.-H., Kim Y.-E., Choi Y.J., Lee K.W. 20-O-β-d-glucopyranosyl-20(S)-protopanaxadiol-fortified ginseng extract attenuates the development of atopic dermatitis-like symptoms in NC/Nga mice. J Ethnopharmacol. 2014;151:365–371. doi: 10.1016/j.jep.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 47.Osada-Oka M., Hirai S., Izumi Y., Misumi K., Samukawa K., Tomita S., Miura K., Minamiyama Y., Iwao H. Red ginseng extracts attenuate skin inflammation in atopic dermatitis through p70 ribosomal protein S6 kinase activation. J Pharmacol Sci. 2018;136:9–15. doi: 10.1016/j.jphs.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Wang H.-H., Li Y.-C., Huang Y.-C. Efficacy of omalizumab in patients with atopic dermatitis: a systematic review and meta-analysis. J Allergy Clin Immunol. 2016;138 doi: 10.1016/j.jaci.2016.05.038. 1719-1722.e1711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.