Abstract
Cytoglobin is an evolutionary ancient hemoglobin with poor functional annotation. Rather than constrained to penta coordination, cytoglobin's heme iron may exist either as a penta or hexacoordinated arrangement when exposed to different intracellular environments. Two cysteine residues at the surface of the protein form an intramolecular disulfide bond that regulates iron coordination, ligand binding, and peroxidase activity. Overall, biochemical results do not support a role for cytoglobin as a direct antioxidant enzyme that scavenges hydrogen peroxide because the rate of the reaction of cytoglobin with hydrogen peroxide is several orders of magnitude slower than metal and thiol-based peroxidases. Thus, alternative substrates such as fatty acids have been suggested and regulation of nitric oxide bioavailability through nitric oxide dioxygenase and nitrite reductase activities has received experimental support. Cytoglobin is broadly expressed in connective, muscle, and nervous tissues. Rational for differential cellular distribution is poorly understood but inducibility in response to hypoxia is one of the most established features of cytoglobin expression with regulation through the transcription factor hypoxia-inducible factor (HIF). Phenotypic characterization of cytoglobin deletion in the mouse have indicated broad changes that include a heightened inflammatory response and fibrosis, increase tumor burden, cardiovascular dysfunction, and hallmarks of senescence. Some of these changes might be reversed upon inhibition of nitric oxide synthase. However, subcellular and molecular interactions have been seldom characterized. In addition, specific molecular mechanisms of action are still lacking. We speculate that cytoglobin functionality will extend beyond nitric oxide handling and will have to encompass indirect regulatory antioxidant and redox sensing functions.
Keywords: Cancer, Cardiovascular disease, Cytoglobin, Fibrosis, Hemoglobin, Hydrogen peroxide, Hypoxia, Myoglobin, Nitric oxide, Oxidative stress
Abbreviations
- ADGB
androglobin
- AP-1
activator protein 1
- cGMP
cyclic guanosine monophosphate
- CYGB
cytoglobin
- ETS-1
protein C-ets-1
- FGF2
fibroblast growth factor 2
- HB
hemoglobin
- HIF
hypoxia-inducible factor
- IL-1ß
interleukin-1ß
- LEF-1
lymphoid enhancer binding factor 1
- MB
myoglobin
- NGB
neuroglobin
- NFAT
nuclear factor of activated T-cells
- Sp1
specificity protein 1
- SRF
serum responsive factor
- Wnt
wingless-related integration site.
1. Introduction
Mammalian globins including hemoglobin (gene abbreviation, HB) and myoglobin (MB) have served as important examples of molecular evolution and as model systems for the study of protein structure-function from which clinical research and molecular medicine have benefited [1]. The discovery over the past 20 years of neuroglobin (NGB), cytoglobin (CYGB), and androglobin (ADGB) has opened new functional paradigms for mammalian globins, beyond their role in regulating molecular oxygen transport and storage. The first description of cytoglobin in liver stellate cells in 2001 [2] was rapidly followed by two other independent studies indicating expression of cytoglobin in many tissues and providing initial biochemical characterization [3,4]. Detailed biochemical and biophysical studies followed and outlined important structural features for cytoglobin including reversible hexa to penta heme coordination, and conformational changes in response to diatomic gas binding. Based on these initial studies, several functional hypotheses for cytoglobin have been proposed and cover many possibilities. While there is good indication that cytoglobin expression is regulated by oxygen in cell culture systems and possibly in vivo, probably one of the most persistent interpretation is that cytoglobin function is related to oxidative stress. The intent of this review is to delineate functional phenotypes and – when possible - extrapolate these observations to biochemical features of cytoglobin. One objective for this review will be to evaluate critically the tenet that cytoglobin regulates oxidative stress and link it to possible functions.
2. Biochemical properties of cytoglobin
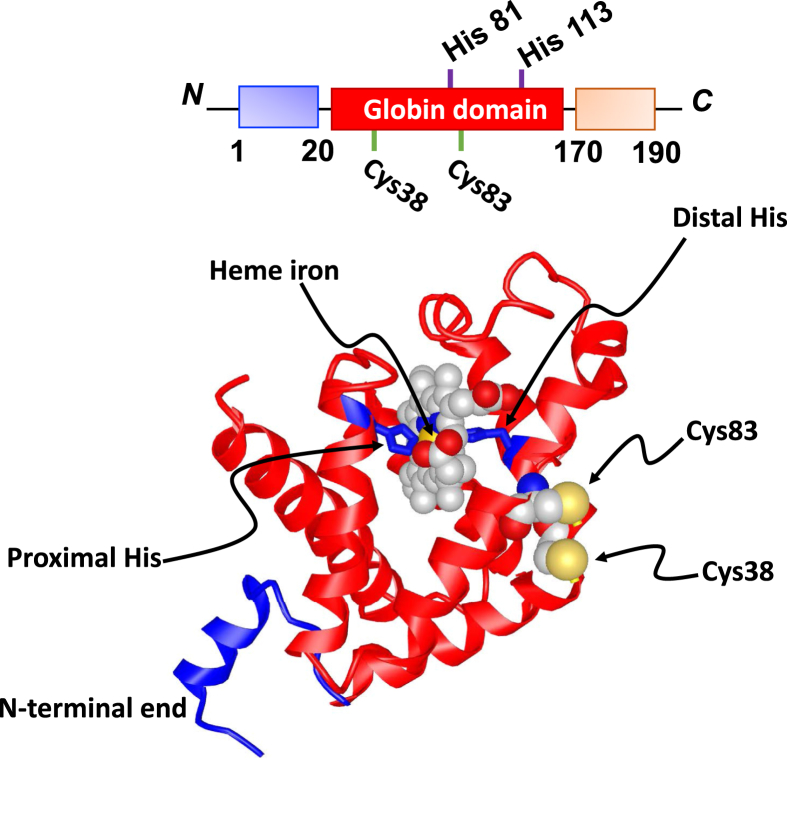
Cytoglobin belongs to the globin protein family, which numbers 5 members in mammals. Its 190 amino acid primary structure consists of a globin domain flanked by two distinct 20 amino acids N- and C-terminal ends that are unique to cytoglobin (Fig. 1). Protein sequence conservation is high across humans, rats, and mice. The protein exhibits a canonical 3-on-3 globin fold composed of eight alpha helices that hosts the heme porphyrin group. The heme is hexacoordinated in both the ferrous and ferric state with the imidazoles from His113 (F8; proximal histidine) and His81 (E7; distal histidine) providing the fifth and sixth axial coordinations, respectively. This configuration is different from pentacoordinated hemoglobin and myoglobin in which the distal histidine residue does not contact the iron but stabilizes the interaction of ligands with the heme group through hydrogen bonding. In the case of cytoglobin (and neuroglobin), the distal histidine residue can still undergo reversible dissociation, allowing for binding of diatomic gases classically associated with hemoglobin and myoglobin including molecular oxygen (O2), carbon monoxide (CO) and nitric oxide (NO) [[4], [5], [6]]. Upon ligand binding, the distal histidine residue in cytoglobin rotates out of the heme pocket, similar to the repositioning observed for myoglobin but different from the heme sliding mechanism for neuroglobin [7]. Thus, the ability of cytoglobin to undergo hexa to penta coordination configurations allows for reversible ligand binding but with redox properties different from pentacoordinated hemoglobins.
Fig. 1.
Cytoglobin structure. A) Primary structure of cytoglobin. A globin domain (red) is flanked with distinct N (blue, amino acids 1 to 20) and C (orange, amino acids 170 to 190) terminal ends. Position of the proximal (His113) and distal (His81) histidine residues and surface cysteine residues (Cys38 and Cys83) are shown B) Ribbon view of the tertiary structure of cytoglobin. The globin domain consists of 8 alpha helices that folds on each other to receive the heme porphyrin group. Heme in yellow with iron in purple is shown with proximal (His113) and distal (His81) residues heme-iron coordinating residues. Only one monomer is shown from PDB structure 2DC3 with the surface cysteine residues (Cys38 and Cys83). No structure for the monomeric form exists that would also include the intramolecular disulfide bridge. The C-terminal end is missing due to the lack of interpretable electron density [9]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Characterization of the quaternary structure of cytoglobin indicates possible homodimerization and cooperativity between the two heme centers with respect to oxygen binding with a Hill coefficient of 0.63–1.16 [8]. High resolution crystallography studies have also indicated alternate dimeric arrangements that may underline differences in ligand binding properties and suggest that the N-terminal end of the monomer may form an additional helix while the C-terminal end may exist as an ordered random coil (Fig. 1) [9]. These extensions do not regulate any known biochemical and functional features characterized thus far [10]. In cells -in which cytoglobin concentrations maybe in the low micromolar range - the monomeric form should be predominant unless other interactions exist that would facilitate dimerization [8].
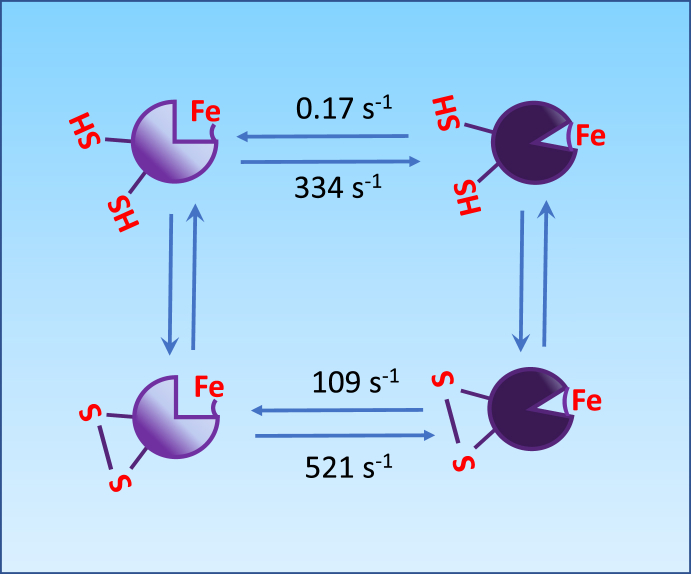
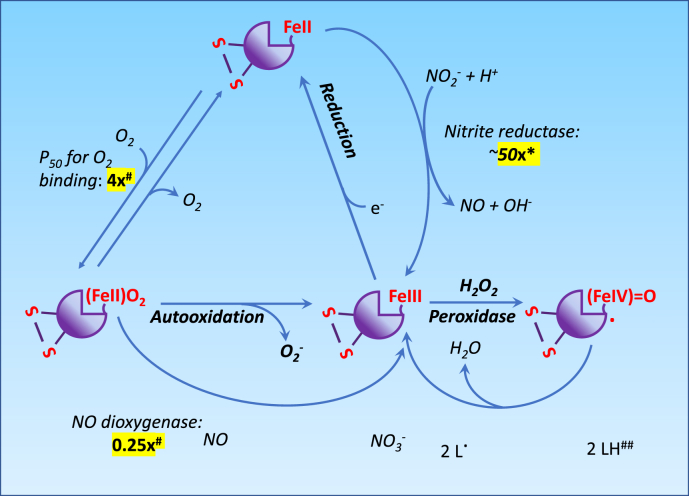
The apparent P50 of cytoglobin for O2 increases from approximately 0.1 to 1 Torr following reversible formation of a disulfide bridge between two surface cysteine residues at positions C38 (B2) and C83 (E9) [8,11] (Fig. 2, Fig. 3 The presence of an intramolecular disulfide bridge modifies the position of the distal histidine residue relative to the heme, increases its off rates from the iron, thereby producing an increase in rates of ligand binding to the heme center [5,11,12]. In fact, a number of studies have shown that the activities of the monomer with reduced disulfide bond and those of the dimeric protein with an intermolecular disulfide bond are usually similar, with the monomer with the intramolecular disulfide generally dissimilar [[13], [14], [15]]. In the case of O2, this cysteine redox switch could provide a mechanism through which cytoglobin O2 binding and release could be tuned to local changes in redox state. The physiological significance of these observations is however unclear. Although the O2 affinity of 1 Torr is comparable to myoglobin, cytoglobin intracellular concentration is usually considered to be too low to alter O2 storage or diffusion [16]. Instead, the cysteine redox switch and conformational changes from hexa to pentacoordination might provide additional functionalities (Fig. 2, Fig. 3). For example, NO dioxygenation by ferrous (FeIIO2) cytoglobin to yield nitrate (NO3-) and ferric (FeIII) cytoglobin is rapid enough to suggest significant NO deactivation functions at physiological NO concentrations [6,17] (Fig. 3). The rate limiting step is the reduction of ferric cytoglobin back to its ferrous form [6], which might be achieved in vivo through reaction with ascorbate, the cytochrome b5/cytochrome b5 reductase/NADH system, or other reductants [6,17,18].
Fig. 2.
A cysteine switch in cytoglobin. The formation of an intramolecular disulfide bridge (-S-S-) between the surface cysteines Cys 38 and Cys 83 regulates the dissociation of the distal histidine residue from the heme iron to alternate between hexa and pentacoordinated cytoglobin. Dissociation and association rate constants are those reported by Beckerson et al. [14]; units for rate constants are s-1. The disulfide bond formation increases the rate of dissociation of the distal histidine from the heme iron by approximately 1000 folds.
Fig. 3.
Effect of the disulfide bridge on the oxygen binding, nitric oxide dioxygenase, nitrite reductase, and peroxidase activity of cytoglobin. Values highlighted in yellow show fold-change in reaction rates upon formation of the disulfide bridge. For simplicity, not all reactions are shown on this schematic. For example, the binding of NO or CO to cytoglobin are not shown. *, this denotes the fold-increase for the monomeric form [23]; # according to Ref. [22]. ##, cytoglobin – in its ferric (FeIII) state - react with hydrogen peroxide (H2O2) to yield an oxoferryl (FeIV O) and a protein centered free radical. Thesecan react with several electron donors such as lipids. There is a 4-fold increase in lipid peroxidase activity between the monomer lacking the disulfide bridge (C38R mutant) and monomer with disulfide bridge, based on the oxidation of soya bean phospholipid liposomes [15]. Significantly, some anionic phospholipids might serve as direct activator of cytoglobin peroxidase activity independent of the disulfide bridge formation [10]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The reduction of nitrite (NO2-) to NO by ferrous cytoglobin is also possible and is increased by acidic and anaerobic conditions, consistent with a reaction where nitrous acid (HNO2) formed from the protonation of NO2- reacts with oxyferrous cytoglobin to yield NO [19,20] (Fig. 3). Similar to NO deoxygenation, the nitrite reductase activity of cytoglobin requires coupling to some reductive capacity, possibly provided by the cytochrome b5/cytochrome b5 reductase/NADH system [21]. While the rate of NO consumption in the presence of O2 would seem to be only minimally affected by the cysteine redox switch [[22], [23], [24]], the nitrite reductase activity is increased by as much as 50-fold upon formation of the disulfide bridge and decreased upon reduction [23].
Another distinctive feature of cytoglobin is its peroxidase activity. The reaction of hydrogen peroxide (H2O2) with ferrous (FeII) and ferric (FeIII) iron of globins is well characterized for other globins such as myoglobin and proceeds to form an oxoferryl (FeIV O) heme and transient protein radicals when starting from the ferric heme [25] (Fig. 3). Hexacoordinated globins including cytoglobin and neuroglobin have higher rates of autoxidation than pentacoordinated globins with comparable O2 binding affinities [24]. In contrast to neuroglobin however, the monomeric ferric cytoglobin also reacts with H2O2 to produce redox active ferryl species [[26], [27], [28]]. The distal histidine is still required for the formation of ferryl species stable enough to sustain the oxidation of external substrates such as lipids and limit heme degradation [28]. Early studies indicated that significant peroxidase activity also requires oxidation of the cysteine redox switch to its disulfide form with the binding of fatty acids as co-activators to facilitate the transition from hexa to penta-coordination [15,29]. However, Tejero et al. more recently showed that some anionic phospholipids could increase the peroxidase activity of cytoglobin on their own, independent of disulfide bridge formation [10]. Overall, the effects of thiol oxidation and lipid binding may amount to a 5-fold increase in peroxidase activity, well below those achieved by more conventional metal and thiol-based peroxidases [30]. Still, it is likely that the disulfide bridge may serve important regulatory functions during conditions associated with increased oxidant load and points to a functional role for the pentacoordinated structure under these conditions. It is also possible that lipid binding, coupled to disulfide bond formation, could trigger translocation of cytoglobin to a membrane bilayer, thereby providing an additional modality for regulating its function [10].
3. Cytoglobin expression and molecular controls
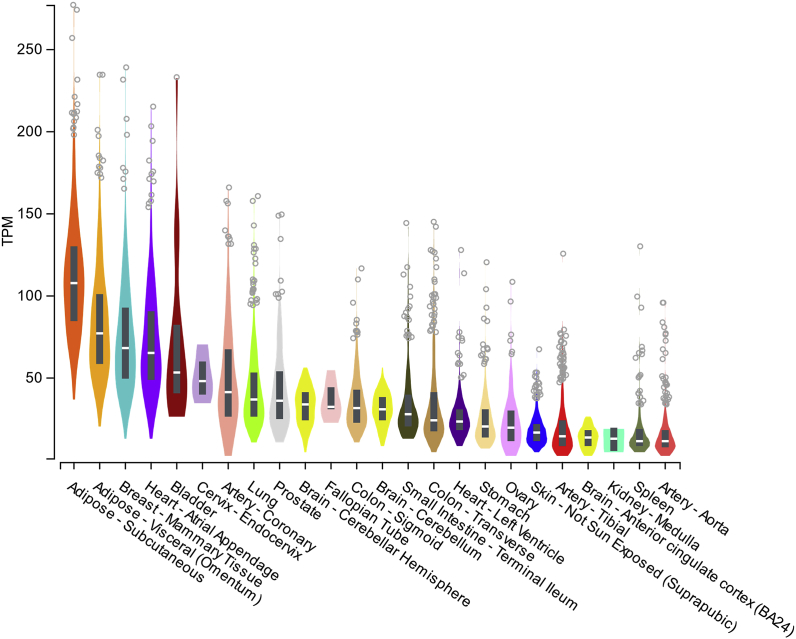
The gene coding for cytoglobin is located on chromosome regions 17q25.3 in humans and 11qE2 in the mouse; and in the case of humans overlaps with the gene for photoreceptor disc component (gene code, PRCD). Although several mRNA variants covering 4 exons are described in both species, only one cytoglobin isoform has been reported at the protein level. In the mouse, cytoglobin transcript levels are low in the early stages of embryogenesis and have been shown to increase in the latter stages, at least in the brain [31]. In adult humans and mice, cytoglobin is ubiquitously expressed at variable levels with no evident tissue specificity. For example, the Genotype Tissue Expression portal shows that – among 53 human tissues and cell types - the highest cytoglobin mRNA transcript levels are found in adipose tissues, thyroid, breast mammary tissue, heart, cervix, and coronary artery (Fig. 4). The presence of mRNA transcripts for cytoglobin in many human tissues is most likely related to its expression in fibroblasts and other stromal cells. In agreement, single-cell RNA sequencing studies that define cellular composition of some mouse tissues indicate highest expression of cytoglobin at the mRNA level in myofibroblasts, fibroblasts, and mesenchymal cells from various sites including the heart, bladder, and adipose tissue (Table 1) [32]. However, the low resolution achieved in some of these studies might not allow for detailed assignment of specific cell-type and limit interpretation, but the rapid increase in tissue-targeted single cell analyses -at least in the mouse - should provide such resolution [33,34].
Fig. 4.
Gene expression for human cytoglobin in selected tissues. Gene expression are shown in Transcripts Per Million (TPM) for the top 20 tissues and cells expressing cytoglobin found in the Genotype-Tissue Expression (GTEx) Project portal. GTEx was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS. The data used for the analyses described in this manuscript were obtained from the GTEx Portal on 01/03/2020.
Table 1.
Cellular specificity of cytoglobin expression from single cell transcriptome analysis in the mouse. Gene expression are shown as ln (1 + CPM) for the top 10 cell types expressing cytoglobin and were obtained from Tabula Muris [32]. Results shown are those obtained from the FACS-based full length transcript analysis. CPM = counts per millions.
| Tissue | Median>0 | # cells | % 0 | % > 0 |
|---|---|---|---|---|
| Myofibroblast (heart) | 6.42 | 178 | 4.49 | 95.51 |
| Bladder cell (bladder) | 5.80 | 695 | 1.44 | 98.56 |
| Fibroblast (heart) | 5.97 | 2189 | 7.45 | 92.55 |
| Pancreatic stellate cell (pancreas) | 5.78 | 49 | 14.29 | 85.71 |
| Mesenchymal stem cell of adipose (fat) | 5.56 | 2107 | 14.95 | 85.05 |
| Mesenchymal stem cell (limb_muscle) | 5.09 | 499 | 16.03 | 83.97 |
| Stromal cell (mammary gland) | 4.83 | 440 | 32.73 | 67.27 |
| Stromal cell (lung) | 5.71 | 423 | 46.34 | 53.66 |
| Mesenchymal cell (trachea) | 5.04 | 830 | 46.87 | 53.13 |
| Neuron (brain non myeloid) | 4.25 | 281 | 52.67 | 47.33 |
| Smooth muscle (heart) | 4.96 | 42 | 57.14 | 42.86 |
In tissue-focused studies, the Kawada laboratory demonstrated that in the liver in rodents stellate cells and myofibroblasts but not hepatocytes, Kupfer cells, or bile duct epithelial cells express cytoglobin at the protein level [2]. Cytoglobin is expressed in reticulocytes in the spleen, and mesenchymal cells in the gastrointestinal tract [35], in the brain in neurons but not glial cells or the local micro-vasculature [31]. Its expression is also found in the eye, in neurons of the retinal ganglion cell layer and inner nuclear layer [[36], [37], [38]]. In the rat kidney, stromal cells along the proximal and distal tubule, renal cortical interstitial cells, and glomerular mesangial cells but not tubular epithelial cells express cytoglobin [[39], [40], [41]]. Strong expression is associated with the heart in adult epicardial cells and cardiomyocytes [42] and in human cardiac progenitor cells [43]. Other studies have shown that in the vasculature, at least large conduit arteries and veins may express cytoglobin in contractile medial smooth muscle cells with limited expression in the intima and adventitia [17,44,45]. Overall, commonality in tissue and cell specificity is difficult to delineate and understand.
The molecular mechanisms that may lead to cell-type specific expression of cytoglobin are not understood. Notch2 and 3 signaling derived from endothelial cells drives the expression of cytoglobin in human aortic vascular smooth muscle cells in vitro [46], consistent with the role of Notch signaling in smooth muscle differentiation [47,48] and association of cytoglobin with differentiated smooth muscle. In a different setting, cytoglobin was found to be a downstream effector of lymphoid enhancer-binding factor 1 (LEF-1, Fig. 5), a transcription factor that regulates the cell response to Wnt signaling [49]. Epigenetic regulation is another important mechanism to regulate cell fate and there are strong indications that cytoglobin expression is regulated epigenetically through methylation/demethylation of its promoter region. Inspection of the gene sequence shows a CpG island starting in the promoter region (nt -1009 relative to the TSS) and covering the rest of exon 1 to end in intron 1, 335 bp after the splice junction). Thus, increase in promoter methylation at several CpG sites of the cytoglobin gene was first demonstrated in samples obtained from patients with oral squamous cell carcinoma [50]. This was also documented in non-small cell lung carcinoma [51] and sporadic head and neck squamous cell carcinoma [52]. In some of these studies, hypermethylation of the gene promoter region correlated with decrease cytoglobin expression and pharmacological inhibition of DNA cytosine methylation is associated with increased cytoglobin protein expression [51].
Fig. 5.
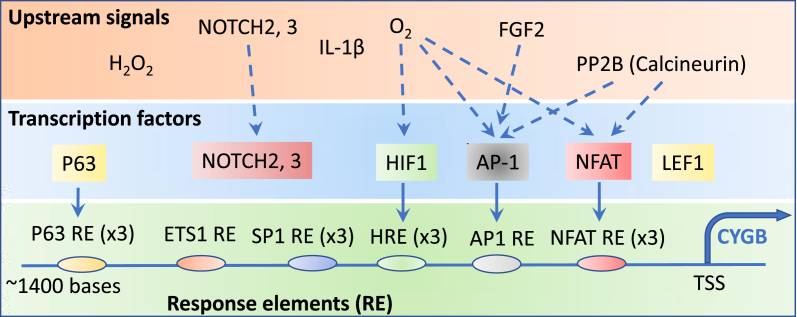
Transcriptional regulation of cytoglobin expression. Upstream signals that regulate cytoglobin expression include oxidants (hydrogen peroxide, H2O2), oxygen (O2) tension, growth and inflammatory factors (IL-1ß, FGF2), and cell-cell interactions (NOTCH 2 and 3) regulate the expression of cytoglobin. NOTCH is a membrane-bound transcription factor that is activated in response to ligand binding through cell-cell interactions and has been shown to promote cytoglobin expression in smooth muscle [46]. The effects of oxygen, FGF2, and the phosphatase calcineurin (PP2B) have been linked experimentally to the regulation of specific transcription factors (blue dotted arrows) including HIF1, AP-1, and NFAT. These transcription factors bind to response elements (RE) located within 1400 bases of the translation start sites (TSS) of the cytoglobin gene. Although binding motifs for ETS1, SP1, and LEF1 have been identified, direct evidence of these transcription factors binding to promoter elements has not been demonstrated [49,59]. In contrast, p63 has been shown to interact with response elements but upstream regulators are unknown [60]. The numbers in parentheses associated with each response elements indicate the number of sequences detected in the promoter region of the cytoglobin gene. See Text for details. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In addition to constitutive levels of cytoglobin expression in some tissues, it is now evident that cytoglobin can also be induced. This was first demonstrated in an experimental model of fibrotic liver in the adult rat [2]. Inducibility was later confirmed in many different settings but is most evident during hypoxia – for example, in the mouse heart and brain and in vitro in multiple cell types including human-derived lines [40,42,44,[52], [53], [54], [55]] (Fig. 5). Response elements for HIF-1, AP-1, and NFAT have been located within the upstream region of the cytoglobin gene, all of which are sensitive to hypoxia and anoxia [42,56] (Fig. 5); and direct binding of these transcription factors to their response elements on the cytoglobin promoter region has been demonstrated. Singh et al. have argued that AP-1 represents a primary trans-activator of cytoglobin transcription during conditions associated with hypoxia [42]. In other settings, we have found that cytokines such as interleukin-1β that usually rely on AP-1 activation for signaling are robust inducers of cytoglobin in rodents, especially upon hypoxic co-stimulation [44]. Significantly, fibroblast growth factor 2 (FGF2) enhances cytoglobin expression through phosphorylation of c-Jun N-terminal kinase (JNK) and c-JUN and binding of phospho-c-JUN to the AP-1 response element in the cytoglobin promoter region [57]. There are also some indications of cross-talk between cytoglobin expression and the pro-inflammatory transcription factor NF-kB, although these effects would seem to be indirect and in some cases require nitric oxide [43,58].
The transcription factors ETS1 and SP1 may have a role in regulating human cytoglobin expression [59] (Fig. 5), but direct protein-DNA interactions have not been demonstrated. Recent studies have also identified the transcription factor p63 (gene code, TP63, Fig. 5) as an important transcriptional regulator of cytoglobin expression in keratinocytes. In this case, ΔNp63 – a p63 isoform lacking an N-terminal end transactivation domain – physically interacts with three p63-responsive elements located upstream of the start codon for the human cytoglobin gene [60]. Silencing of ΔNp63 decreased cytoglobin expression at the mRNA and protein levels in human keratinocytes and lung cancer cells [60]. The same p63 isoform also positively regulates the expression of inactive rhomboid 2 (iRHOM2). This protein is a member of the seven transmembrane family of rhomboid serine proteases that controls the trafficking of TNFα converting enzyme (TACE/ADAM17) to regulate the proteolytic processing of several cytokines. Significantly, iRHOM2 deletion or silencing increases cytoglobin mRNA and protein expression [61]. Evidence for the formation of a protein complex between cytoglobin and iRHOM2 was also provided. The relationship between this interaction and control of cytoglobin expression was not detailed. Thus, TP63 may regulate multiple aspects of cytoglobin expression through interaction at the cytoglobin gene promoter region and through additional protein signals such as iRHOM2. The gene coding for iRHOM2 is rhomboid family member 2 (gene code, RHBDF2) located on chromosome 17 in humans, in the same loci as CYGB. Missense mutations in RHBDF2 cause a rare autosomal dominant proliferative skin disease called tylosis with esophageal cancer syndrome (TOC). The gain-of-function phenotype afforded by the missense mutations causing TOC is consistent with the decrease in cytoglobin expression associated with iRHOM2 [61] and the trans-allele repression of cytoglobin expression that had been previously observed in TOC [62]. Whether this contributes to the disease is unknown.
4. Functional significance of cytoglobin
4.1. Nitric oxide and nitrite signaling
In addition to molecular oxygen, nitric oxide (NO) binds and reacts with hemoproteins in general and there is now good evidence to indicate that cytoglobin regulates NO bioactivity. Nitric oxide is a membrane permeable, freely diffusible, paracrine transmitter that functions throughout the cardiovascular system. In blood vessels, NO is primarily generated by an endothelial isoform of nitric oxide synthase (eNOS, NOS3) in response to rise in intracellular calcium that is initiated by stretch and chemical agonists [63]. Nitric oxide acts in target cells by binding to soluble guanylate cyclase, an intracellular NO receptor that propagates signaling through the generation of cGMP [64]. A cellular target of NO in the vasculature are medial smooth muscle cells where it promotes vasorelaxation through regulation of cGMP-regulated protein kinases and phosphatases [65]. Nitric oxide synthase 3 (NOS3) is expressed in endothelial cells to respond to physical and chemical changes in the lumen of the vessel and the signal, once propagated in smooth muscle, is turned off through timely activation of cGMP phosphodiesterases [[66], [67], [68]].
Apart from guanylate cyclase, other hemoproteins including hemoglobin bind NO, or in their oxyferrous form react with NO to form nitrate [69]. Some endothelial cell populations at myo-endothelial junctions express NO dioxygenases composed of alpha hemoglobin and cytochrome b5 reductase [45]. Estimation of bioactive NO concentrations have been the topic of many studies and the characterization of any new NO consuming activities must be interpreted in the context of reasonable physiological ranges [70]. Thus, reaction of NO with millimolar concentrations of hemoglobin in erythrocytes imposes a basal component to NO inactivation and it has been calculated that in the presence of circulating red blood cells, vascular endothelial cells donate only 20 to 100 pM NO to the underlying smooth muscle [71,72].
In the cardiovascular system, cytoglobin expression was first demonstrated to be concurrent with myoglobin in cardiomyocytes and an approximately 3-fold increase in protein expression in the heart was observed in mice exposed to hypoxia for two weeks [42]. Soon after, it was shown that cytoglobin is expressed in vascular smooth muscles [73], an observation latter confirmed by others [19,45]. We also provided some evidence that cytoglobin is expressed in medial VSM cells in vessels in rodents and humans including the aorta, carotids, and renal arteries, and some veins. Surprisingly, two recent studies using single-cell RNA-seq analysis associated cytoglobin expression not with smooth muscle cells but with fibroblasts in the mouse aortic root and aorta [33,34]. The discrepancy between these studies and earlier ones is unclear but could suggest mRNA levels that don't reflect protein levels or differential expression of splice variants. Similarly, it is yet to be determined whether cytoglobin protein expression extends to smaller vessels that contain smooth muscle or at precapillary sphincters in the microcirculation. Because of its NO dioxygenase activity and expression in the vascular wall, it has been postulated that cytoglobin directly regulates NO function in the circulation. In agreement, Liu et al. observed a profound depression of cardiovascular functions associated with the loss of cytoglobin in the mouse that included a decrease in blood pressure and systemic vascular resistance, and increase in cardiac output; all of which could be normalized to wild-type levels upon pharmacological inhibition of nitric oxide synthase [17] (Table 2). Combined with evidence that changes in cytoglobin expression alter NO decay, these results strongly support a role for cytoglobin in regulating vascular reactivity through direct regulation of NO levels [74]. Thus, in addition to hemoglobin and myoglobin, cytoglobin may impose an additional sink for NO, in this case located in smooth muscle.
Table 2.
Cytoglobin mouse lines and phenotypic analysis.
| Title | Mouse line | Primary phenotype | References |
|---|---|---|---|
| Gain of function | |||
| Selective overexpression of cytoglobin in stellate cells attenuates thioacetamide-induced liver fibrosis in mice. | Cygb-2A-mCherry (transgenic) | Decrease liver fibrosis Decrease liver inflammation |
[90] |
| Loss of function | |||
| The anti-oxidative role of cytoglobin in podocytes: implications for a role in chronic kidney disease | B6.Cygbtm1Nka (global ko) | Mild reduction in renal function | [41] |
| Cytoglobin regulates blood pressure and vascular tone through nitric oxide metabolism in the vascular wall. | B6.Cygbtm1Nka (global ko) | Decrease systemic blood pressure Decrease NO handling Increased response to angiotensin II |
[17] |
| The Hemoglobin Homolog Cytoglobin in Smooth Muscle Inhibits Apoptosis and Regulates Vascular Remodeling. | B6.Cygbtm1Nka (global ko) | Inhibition of neointima formation after carotid ligation | [44] |
| Possible Involvement of Nitric Oxide in Enhanced Liver Injury and Fibrogenesis during Cholestasis in Cytoglobin-deficient Mice. | B6.Cygbtm1Nka (global ko) | Increase liver fibrosis Increase liver inflammation Some reversal of effect after nitric oxide synthase inhibition |
[76] |
| Absence of cytoglobin promotes multiple organ abnormalities in aged mice. | B6.Cygbtm1Nka (global ko) | Multiple organ dysfunction Increase inflammation Senescence Some sensitivity to nitric oxide inhibition |
[78] |
| Cytoglobin Deficiency Promotes Liver Cancer Development from Hepatosteatosis through Activation of the Oxidative Stress Pathway. | B6.Cygbtm1Nka (global ko) | Increase liver tumorigenesis | [78] |
| Involvement of hepatic stellate cell cytoglobin in acute hepatocyte damage through the regulation of CYP2E1-mediated xenobiotic metabolism. | B6.Cygbtm1Nka (global ko) | Protection against acetaminophen-induced hepatotoxicity Reduced acetaminophen liver metabolism |
[104] |
| Cytoglobin modulates myogenic progenitor cell viability and muscle regeneration. | (129 x C57BL/6) Cygbtm1Ppam | Abnormal myoblast differentiation Skeletal muscle degeneration Impaired muscle regeneration Increased apoptosis |
[88] |
| Promotion of Liver and Lung Tumorigenesis in DEN-Treated Cytoglobin-Deficient Mice. | B6.Cygbtm1Nka (global ko) | Increase tumorigenesis | [98] |
| - | C57BL/6 N.Cygbtm1b(EUCOMM)Wtsi/TCP (global ko) | Multiple organ dysfunction | Informatics.jax.org |
Pro-inflammatory conditions are often associated with an increase output of NO due to increase expression of inducible nitric oxide synthase (gene abbreviation, NOS2), at least in rodent models [75]. Remarkably, cytoglobin deficiency in aged mice, and mouse models of bile duct ligation and nonalcoholic steatohepatitis increase NOS2 expression [[76], [77], [78]] (Table 2). In the case of the aged and bile duct ligation mouse models, pharmacological inhibition of NOS decreased the inflammatory burden including a decrease in neutrophil and macrophage tissue infiltration. Because cytoglobin possess a dioxygenase activity, the increase in NO metabolites in these models is usually interpreted to be due to the decrease in NO dioxygenase activity associated with the loss of cytoglobin. However, it is also possible that cytoglobin regulates some aspects of the immune response which upon its loss leads to inflammation and increases NO production, in addition of the loss of NO dioxygenase activity. It is notable that Liu et al. used 9–12 months old mice to examine the role of cytoglobin in the regulation of vascular tone, an age that might be already associated with an increase in NO production through NOS2 and might have complicated the interpretation of some of the results [77].
Over the past three decades, nitrite has been recognized as an alternative source of NO in many different settings [79,80]. In this case, hemoglobin and myoglobin functions as mammalian nitrite reductase controlled by ambient oxygen levels [81]. As already described, the deoxy form of cytoglobin also produces NO from nitrite through reaction with its deoxy ferrous state. Thus, in conjunction with its O2-dependent dioxygenase activity and efficient reduction by the cytochrome b5/cytochrome b5 reductase/NADH system, cytoglobin could clearly regulate NO bioavailability in response to differential gradients of oxygen. However, the physio- and pathophysiological importance of cytoglobin nitrite reductase activity has yet to be examined in vivo [82]. In addition to cardiomyocyte signaling during ischemia [83], myoglobin expressed in vessels contributes to nitrite-dependent vasorelaxation [84] and the relative contribution of cytoglobin in these settings would be important to establish. Considering also the association of cytoglobin loss with some forms of cancer, it is possible that cytoglobin expression in the hypoxic environment of a tumor might provide added functionality related to its reaction with oxidants at cysteine sites to increase its nitrite reductase activity [23], or through antioxidant functions [85].
4.2. Antioxidant functions
One of the most reported claims is that cytoglobin preserves cell viability by serving as an antioxidant to scavenge reactive oxygen species (ROS) produced by cells. The peroxidase activity associated with cytoglobin would point to hydrogen peroxide and lipids as possible substrates. However, the second order reaction rate constant for the reaction of hydrogen peroxide with monomeric cytoglobin (with the internal disulfide bond) was estimated to be 300 M-1.s-1 [28]. While this is consistent with reaction rates measured for pentacoordinated hemoglobins, this is several orders of magnitude lower than metal or cysteine-based peroxidases, potentially excluding cytoglobin as an antioxidant or redox-signaling intermediate (Table 3). In support, studies by McRonald et al. showed no difference in oxidant load whether cytoglobin was present or absent following treatment of cells with buthionine sulfoxamine to decrease intracellular glutathione levels [86]. Still, this needs to be reconciled with the many studies that have shown some redox sensitivity associated with the manipulation of cytoglobin [41,43,60,78,[86], [87], [88], [89], [90]]. It is possible for example that cytoglobin specificity might be slanted towards other substrates such as lipids. Significantly, some of the most potent lipid activators for cytoglobin peroxidase activity are phosphatidylinositolphosphates potentially relating cytoglobin to specific signaling networks in vivo [10].
Table 3.
Apparent second order rate constants for the reaction of hydrogen peroxide with some representative cysteine, selenocysteine, and heme peroxidases, and hemoglobins. See Text for discussion.
Some of the antioxidant effects could be interpreted within the context of NO/nitrite reactions with cytoglobin as already described in this review, especially when reversal might be achieved through nitric oxide synthase inhibition. It is also possible that the function of cytoglobin is not related to any direct biochemical antioxidant properties. For example, the loss of cytoglobin might sensitize cells to apoptosis due to alteration of a critical pathway or function, which in turn might trigger a pro-oxidant response. Alternatively, cytoglobin has been shown to localize to the nucleus [91] and gene regulation upon loss of cytoglobin has been observed including down-regulation of antioxidant enzymes or dysregulation of the Nrf2 system that is responsible for the antioxidant response during oxidative stress [41,43,88]. Overall, the role of cytoglobin in regulating the redox environment as a primary driver of its function merit additional attention.
4.3. Regulation of apoptosis
A role for cytoglobin in apoptosis might be inferred from its interaction with p63 since the latter has been shown to be required for the cellular response to DNA damage induced apoptosis [92]. Several studies also indicate that the loss of cytoglobin hyperactivates the downstream effector of apoptosis caspase-3 and promotes autonomous and redox-sensitive apoptosis. Tian et al. showed that adenoviral-mediated overexpression of cytoglobin in neonatal mouse brain before initiation of ischemia is protective [55]. This was based on assessment of necrotic region size, TUNEL assay, and long-term cognitive impairment. In this case, cytoglobin overexpression inhibited caspase-2 and –3 activation. In a mouse model of muscle injury induced by intramuscular injection of cardiotoxin, skeletal muscle specific deletion of cytoglobin impaired muscle regeneration through increase in skeletal muscle apoptosis as indicated by caspase-3 activation [88]. An increase in caspase-3 and decrease cell survival was also observed in a mouse model of retinal degeneration combined with loss of cytoglobin [36]. In a recent study, we evaluated whether cytoglobin is involved in the response of arteries to vascular remodeling [43]. The combination of denudation and stretching of the rat carotid through balloon angioplasty is usually associated with negative remodeling and the formation of a neointima that is contributed by de-differentiated vascular smooth muscle. In this model, we observed an inhibition of neointima formation upon site-specific silencing of cytoglobin achieved through adenoviral delivery of short hairpin RNAs. We also examined the effect of cessation of blood flow upon ligation of the left common carotid in wild-type and cytoglobin-deficient mice with similar results, i.e an inhibition of neointimal hyperplasia. Because NO has vasculoprotective functions in the context of these two models, it is possible that these results might be explained through an increase in NO bioavailability due to the loss of cytoglobin as shown by Liu et al. [17]. However, we failed to show a decrease in proliferating capacity early on in the rat balloon angioplasty model, a feature that is usually associated with increase in NO bioavailability. Instead, the inhibition of neointima formation was primarily associated with an increase in medial cell apoptosis and a dramatic increase in caspase-3 activation [34].
Several models may exist to account for the effect of cytoglobin on caspase-3. One simple and most direct one could involve the inhibition of caspase-3 activity through direct glutathionylation of cysteine residues [93,94]. Procaspase-3 exists in a glutathiolated (oxidized, -SSG) form that cannot be processed by upstream initiator caspases such as caspase-8 or -9 [93]. Upon activation of glutaredoxin (Grx), caspase-3 is de-glutathiolated, allowing for cleavage of procaspase-3 and cell death to proceed through apoptosis. If cytoglobin does interfere with or is sensitive to redox changes, we would expect a decrease in caspase-3 glutathiolation and reciprocal increase in caspase-3 activation upon depletion of cytoglobin. Another model is that the direct effect of cytoglobin on caspase oxidation state is not solely sufficient for caspase-3 inactivation but requires additional mediators that are also altered through depletion of cytoglobin. One possibility is that cytoglobin regulates the gene expression of survival pathways that would be in part required for regulation of procaspase-3 cleavage and caspase-3 activity by cytoglobin. Regulation of global gene expression by changing levels of cytoglobin has been shown in different settings. There are also some suggestions that cytoglobin localizes to the nucleus, although the extent, regulation, and functional significance of these observations are unclear [88,91,95].
4.4. Antifibrotic functions
An association between cytoglobin expression and fibrosis was proposed early on because cytoglobin was discovered in the context of a proteomic analysis of a rat model of liver fibrosis [2]. A similar association of cytoglobin expression with tissue fibrosis was later shown in rodent models of kidney disease [39]. Immunodetection of cytoglobin expression in rodent kidneys has shown potential association with interstitial fibroblasts and mesangial cells based on exclusion of co-localization with endothelial cell and macrophage and monocyte markers [40]. These earlier studies also indicated up-regulation of cytoglobin protein expression after kidney injury, although the extent and localization were poorly reported or not established [39,40]. Significantly, transgenic overexpression of cytoglobin in the rat afforded protection and decreased fibrosis in a model of kidney nephrectomy [40]. Similar antifibrotic effects were characterized more recently in a transgenic mouse line overexpressing cytoglobin in a model of thioacetamide-induced liver fibrosis, although in this case liver toxicity was not alleviated by cytoglobin overexpression [90] (Table 2). In agreement, increase fibrosis was observed in global cytoglobin knockout mice in cholestatic livers [76] and in the liver and kidneys of 1 to 2-year-old mice [77], although a latter study indicated limited fibrosis in the same line but with altered kidney functions [41] (Table 2). There was also a strong pro-inflammatory phenotype associated with the absence of cytoglobin that included neutrophilic infiltration and activation of resident and infiltrated macrophages [76,77]. Finally, it is worth noting that one of the first hypothesis regarding the function of cytoglobin was to serve as an oxygen carrier for collagen synthesis [95]. The studies described above would indicate otherwise. Instead, they suggest a role in inhibiting injury-induced fibrosis, possibly through inhibition of pro-inflammatory signals and direct inhibition of synthetic phenotypes associated with trans-differentiation to myofibroblasts.
4.5. Cytoglobin and cancer
An association and potential role for cytoglobin in cancer was first established through promoter methylation studies from tissue samples obtained from human patients with oral squamous cell carcinoma [50]. Thus, promoter methylation of the cytoglobin gene at individual CpG sites was increased in tumor tissues in comparison to non-tumor control samples. Similar findings were obtained in head and neck and non-small cell lung cancers [52,96]. Overall, these studies suggest that a combination of promoter hypermethylation, loss of heterozygosity, and decrease expression is common for cytoglobin in several types of cancers including aggressive tumors [62,97]. However, the functional significance of these observations is unclear. In cytoglobin global knockout mice, tumor formation occurred in aging mice over 1 year old in the lung, liver, intestine and lymphoid system [77] (Table 2). This was associated with increased inflammatory burden and defects in cellular redox. In the same mouse line, earlier studies showed an increase in lung and liver tumorigenesis compared to wild-type controls upon treatment with the carcinogen diethylnitrosamine [98]. The molecular mechanisms that underline these observations is unknown but, pharmacological inhibition of nitric oxide synthase activity reversed some of the phenotypic changes. There are also several in vitro studies showing dysregulation of cytoglobin expression in several cancer cell lines and reversal of effect upon silencing or overexpression of cytoglobin [[99], [100], [101]]. For example, Brantley et al. indicated that the aryl hydrocarbon receptor (AhR) partial agonist 5F 203 might promotes the expression of cytoglobin in triple negative cancer cells and that silencing of cytoglobin partially inhibits the pro-apoptotic effect of 5F 203 [99]. However, a consensus related to the outcome of modifying cytoglobin levels in these models and the underlying molecular mechanisms are difficult to delineate. This is even more puzzling when considering a bimodal response associated with cytoglobin expression [102]. It has been suggested that upregulation of cytoglobin following hypoxia may convey a growth advantage to cancer cells to adapt and grow in hypoxic environments [103]. Lastly, in an interesting study, Pongsuchart et al. found that overexpression of cytoglobin in osteosarcoma cell lines increased cell extravasation whereas its knockdown had an opposing effect suggesting a role for cytoglobin in promoting metastasis in this case [49].
2. Conclusion
The study of hemoglobins has illuminated fundamental evolutionary, biochemical, and physiological processes and will continue to have important implications for medical research. The discovery of three additional mammalian globins including cytoglobin over the past two decades has reinvigorated this field and is poised to provide new knowledge and actionable strategies for therapeutics. The generation of cytoglobin deficient mouse models has provided important information on cytoglobin functionality related to increased inflammatory burden and oxidative and nitrosative stress. The challenge will be to relate these findings to biochemical and molecular mechanisms, which are still lacking. One the primary issue is that the peroxidase activity of cytoglobin and its concentration are in general too low to provide direct antioxidant functions.
Funding
This research was funded by grants from the American Heart Association (16GRNT31280002) and the National Institutes of Health (1RO1HL142807-01A1).
Declaration of competing interest
None.
Acknowledgements
Some of the materials presented in the figures was created using images from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
References
- 1.Schechter A.N. Hemoglobin research and the origins of molecular medicine. Blood. 2008;112(10):3927–3938. doi: 10.1182/blood-2008-04-078188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawada N., et al. Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells. J. Biol. Chem. 2001;276(27):25318–25323. doi: 10.1074/jbc.M102630200. [DOI] [PubMed] [Google Scholar]
- 3.Burmester T., et al. Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues. Mol. Biol. Evol. 2002;19(4):416–421. doi: 10.1093/oxfordjournals.molbev.a004096. [DOI] [PubMed] [Google Scholar]
- 4.Trent J.T., 3rd, Hargrove M.S. A ubiquitously expressed human hexacoordinate hemoglobin. J. Biol. Chem. 2002;277(22):19538–19545. doi: 10.1074/jbc.M201934200. [DOI] [PubMed] [Google Scholar]
- 5.Hamdane D., et al. The redox state of the cell regulates the ligand binding affinity of human neuroglobin and cytoglobin. J. Biol. Chem. 2003;278(51):51713–51721. doi: 10.1074/jbc.M309396200. [DOI] [PubMed] [Google Scholar]
- 6.Smagghe B.J., Trent J.T., 3rd, Hargrove M.S. NO dioxygenase activity in hemoglobins is ubiquitous in vitro, but limited by reduction in vivo. PloS One. 2008;3(4) doi: 10.1371/journal.pone.0002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makino M., et al. Crystal structure of the carbon monoxide complex of human cytoglobin. Proteins. 2011;79(4):1143–1153. doi: 10.1002/prot.22950. [DOI] [PubMed] [Google Scholar]
- 8.Fago A., et al. Allosteric regulation and temperature dependence of oxygen binding in human neuroglobin and cytoglobin. Molecular mechanisms and physiological significance. J. Biol. Chem. 2004;279(43):44417–44426. doi: 10.1074/jbc.M407126200. [DOI] [PubMed] [Google Scholar]
- 9.Makino M., et al. High-resolution structure of human cytoglobin: identification of extra N- and C-termini and a new dimerization mode. Acta Crystallogr D Biol Crystallogr. 2006;62(Pt 6):671–677. doi: 10.1107/S0907444906013813. [DOI] [PubMed] [Google Scholar]
- 10.Tejero J., et al. Peroxidase activation of cytoglobin by anionic phospholipids: mechanisms and consequences. Biochim. Biophys. Acta. 2016;1861(5):391–401. doi: 10.1016/j.bbalip.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lechauve C., et al. Cytoglobin conformations and disulfide bond formation. FEBS J. 2010;277(12):2696–2704. doi: 10.1111/j.1742-464X.2010.07686.x. [DOI] [PubMed] [Google Scholar]
- 12.Astudillo L., et al. Reduction of the internal disulfide bond between Cys 38 and 83 switches the ligand migration pathway in cytoglobin. J. Inorg. Biochem. 2013;129:23–29. doi: 10.1016/j.jinorgbio.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Tsujino H., et al. Disulfide bonds regulate binding of exogenous ligand to human cytoglobin. J. Inorg. Biochem. 2014;135:20–27. doi: 10.1016/j.jinorgbio.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Beckerson P., Reeder B.J., Wilson M.T. Coupling of disulfide bond and distal histidine dissociation in human ferrous cytoglobin regulates ligand binding. FEBS Lett. 2015;589(4):507–512. doi: 10.1016/j.febslet.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Beckerson P., et al. Cytoglobin ligand binding regulated by changing haem-co-ordination in response to intramolecular disulfide bond formation and lipid interaction. Biochem. J. 2015;465(1):127–137. doi: 10.1042/BJ20140827. [DOI] [PubMed] [Google Scholar]
- 16.Hankeln T., et al. Neuroglobin and cytoglobin in search of their role in the vertebrate globin family. J. Inorg. Biochem. 2005;99(1):110–119. doi: 10.1016/j.jinorgbio.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Liu X., et al. Cytoglobin regulates blood pressure and vascular tone through nitric oxide metabolism in the vascular wall. Nat. Commun. 2017;8:14807. doi: 10.1038/ncomms14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner A.M., Cook M.R., Gardner P.R. Nitric-oxide dioxygenase function of human cytoglobin with cellular reductants and in rat hepatocytes. J. Biol. Chem. 2010;285(31):23850–23857. doi: 10.1074/jbc.M110.132340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H., et al. Characterization of the mechanism and magnitude of cytoglobin-mediated nitrite reduction and nitric oxide generation under anaerobic conditions. J. Biol. Chem. 2012;287(43):36623–36633. doi: 10.1074/jbc.M112.342378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen M.G., Dewilde S., Fago A. Reactions of ferrous neuroglobin and cytoglobin with nitrite under anaerobic conditions. J. Inorg. Biochem. 2008;102(9):1777–1782. doi: 10.1016/j.jinorgbio.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Amdahl M.B., et al. Efficient reduction of vertebrate cytoglobins by the cytochrome b5/cytochrome b5 reductase/NADH system. Biochemistry. 2017;56(30):3993–4004. doi: 10.1021/acs.biochem.7b00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou D., et al. Oxygen binding and nitric oxide dioxygenase activity of cytoglobin are altered to different extents by cysteine modification. FEBS Open Bio. 2017;7(6):845–853. doi: 10.1002/2211-5463.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeder B.J., Ukeri J. Strong modulation of nitrite reductase activity of cytoglobin by disulfide bond oxidation: implications for nitric oxide homeostasis. Nitric Oxide. 2018;72:16–23. doi: 10.1016/j.niox.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Corti P., Ieraci M., Tejero J. Characterization of zebrafish neuroglobin and cytoglobins 1 and 2: zebrafish cytoglobins provide insights into the transition from six-coordinate to five-coordinate globins. Nitric Oxide. 2016;53:22–34. doi: 10.1016/j.niox.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Keilin D., Hartree E.F. Reaction of methaemoglobin with hydrogen peroxide. Nature. 1950;166(4221):513–514. doi: 10.1038/166513a0. [DOI] [PubMed] [Google Scholar]
- 26.Lardinois O.M., et al. Identification of protein radicals formed in the human neuroglobin-H2O2 reaction using immuno-spin trapping and mass spectrometry. Biochemistry. 2008;47(39):10440–10448. doi: 10.1021/bi800771k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicolis S., et al. Reactivity and endogenous modification by nitrite and hydrogen peroxide: does human neuroglobin act only as a scavenger? Biochem. J. 2007;407(1):89–99. doi: 10.1042/BJ20070372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beckerson P., Svistunenko D., Reeder B. Effect of the distal histidine on the peroxidatic activity of monomeric cytoglobin. F1000Res. 2015;4:87. doi: 10.12688/f1000research.5971.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeder B.J., Svistunenko D.A., Wilson M.T. Lipid binding to cytoglobin leads to a change in haem co-ordination: a role for cytoglobin in lipid signalling of oxidative stress. Biochem. J. 2011;434(3):483–492. doi: 10.1042/BJ20101136. [DOI] [PubMed] [Google Scholar]
- 30.Trandafir F., et al. Neuroglobin and cytoglobin as potential enzyme or substrate. Gene. 2007;398(1–2):103–113. doi: 10.1016/j.gene.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 31.Mammen P.P., et al. Cytoglobin is a stress-responsive hemoprotein expressed in the developing and adult brain. J. Histochem. Cytochem. 2006;54(12):1349–1361. doi: 10.1369/jhc.6A7008.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabula Muris C., et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature. 2018;562(7727):367–372. doi: 10.1038/s41586-018-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalluri A.S., et al. Single-cell analysis of the normal mouse aorta reveals functionally distinct endothelial cell populations. Circulation. 2019;140(2):147–163. doi: 10.1161/CIRCULATIONAHA.118.038362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wirka R.C., et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat. Med. 2019;25(8):1280–1289. doi: 10.1038/s41591-019-0512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakatani K., et al. Cytoglobin/STAP, its unique localization in splanchnic fibroblast-like cells and function in organ fibrogenesis. Lab. Invest. 2004;84(1):91–101. doi: 10.1038/labinvest.3700013. [DOI] [PubMed] [Google Scholar]
- 36.Kwon Y.S., et al. Cytoglobin deficiency potentiates Crb1-mediated retinal degeneration in rd8 mice. Dev. Biol. 2019 doi: 10.1016/j.ydbio.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt M., et al. Divergent distribution of cytoglobin and neuroglobin in the murine eye. Neurosci. Lett. 2005;374(3):207–211. doi: 10.1016/j.neulet.2004.10.071. [DOI] [PubMed] [Google Scholar]
- 38.Ostojic J., et al. Neuroglobin and cytoglobin: oxygen-binding proteins in retinal neurons. Invest. Ophthalmol. Vis. Sci. 2006;47(3):1016–1023. doi: 10.1167/iovs.05-0465. [DOI] [PubMed] [Google Scholar]
- 39.Mimura I., et al. Cytoglobin, a novel globin, plays an antifibrotic role in the kidney. Am. J. Physiol. Ren. Physiol. 2010;299(5):F1120–F1133. doi: 10.1152/ajprenal.00145.2010. [DOI] [PubMed] [Google Scholar]
- 40.Nishi H., et al. Cytoglobin, a novel member of the globin family, protects kidney fibroblasts against oxidative stress under ischemic conditions. Am. J. Pathol. 2011;178(1):128–139. doi: 10.1016/j.ajpath.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Randi E.B., et al. Antioxid Redox Signal; 2020. The Anti-oxidative Role of Cytoglobin in Podocytes: Implications for a Role in Chronic Kidney Disease. [DOI] [PubMed] [Google Scholar]
- 42.Singh S., et al. Calcineurin activates cytoglobin transcription in hypoxic myocytes. J. Biol. Chem. 2009;284(16):10409–10421. doi: 10.1074/jbc.M809572200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang S., et al. Cytoglobin promotes cardiac progenitor cell survival against oxidative stress via the upregulation of the NFkappaB/iNOS signal pathway and nitric oxide production. Sci. Rep. 2017;7(1):10754. doi: 10.1038/s41598-017-11342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jourd'heuil F.L., et al. The hemoglobin homolog cytoglobin in smooth muscle inhibits apoptosis and regulates vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2017 doi: 10.1161/ATVBAHA.117.309410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Straub A.C., et al. Endothelial cell expression of haemoglobin alpha regulates nitric oxide signalling. Nature. 2012;491(7424):473–477. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lilly B., et al. Endothelial cell-induced cytoglobin expression in vascular smooth muscle cells contributes to modulation of nitric oxide. Vasc. Pharmacol. 2018;110:7–15. doi: 10.1016/j.vph.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrow D., et al. Notch and vascular smooth muscle cell phenotype. Circ. Res. 2008;103(12):1370–1382. doi: 10.1161/CIRCRESAHA.108.187534. [DOI] [PubMed] [Google Scholar]
- 48.Lin C.H., Lilly B. Notch signaling governs phenotypic modulation of smooth muscle cells. Vasc. Pharmacol. 2014;63(2):88–96. doi: 10.1016/j.vph.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Pongsuchart M., et al. Novel lymphoid enhancer-binding factor 1-cytoglobin axis promotes extravasation of osteosarcoma cells into the lungs. Canc. Sci. 2018;109(9):2746–2756. doi: 10.1111/cas.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaw R.J., et al. Promoter methylation of P16, RARbeta, E-cadherin, cyclin A1 and cytoglobin in oral cancer: quantitative evaluation using pyrosequencing. Br. J. Canc. 2006;94(4):561–568. doi: 10.1038/sj.bjc.6602972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shivapurkar N., et al. Cytoglobin, the newest member of the globin family, functions as a tumor suppressor gene. Canc. Res. 2008;68(18):7448–7456. doi: 10.1158/0008-5472.CAN-08-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaw R.J., et al. Cytoglobin is upregulated by tumour hypoxia and silenced by promoter hypermethylation in head and neck cancer. Br. J. Canc. 2009;101(1):139–144. doi: 10.1038/sj.bjc.6605121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fordel E., et al. Cytoglobin expression is upregulated in all tissues upon hypoxia: an in vitro and in vivo study by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004;319(2):342–348. doi: 10.1016/j.bbrc.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 54.Fordel E., et al. Hypoxia/ischemia and the regulation of neuroglobin and cytoglobin expression. IUBMB Life. 2004;56(11–12):681–687. doi: 10.1080/15216540500037406. [DOI] [PubMed] [Google Scholar]
- 55.Tian S.F., et al. Mechanisms of neuroprotection from hypoxia-ischemia (HI) brain injury by up-regulation of cytoglobin (CYGB) in a neonatal rat model. J. Biol. Chem. 2013;288(22):15988–16003. doi: 10.1074/jbc.M112.428789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo X., Philipsen S., Tan-Un K.C. Study of the hypoxia-dependent regulation of human CYGB gene. Biochem. Biophys. Res. Commun. 2007;364(1):145–150. doi: 10.1016/j.bbrc.2007.09.108. [DOI] [PubMed] [Google Scholar]
- 57.Sato-Matsubara M., et al. Fibroblast growth factor 2 (FGF2) regulates cytoglobin expression and activation of human hepatic stellate cells via JNK signaling. J. Biol. Chem. 2017;292(46):18961–18972. doi: 10.1074/jbc.M117.793794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomes B.R.B., et al. Cytoglobin attenuates neuroinflammation in lipopolysaccharide-activated primary preoptic area cells via NF-kappaB pathway inhibition. Front. Mol. Neurosci. 2019;12:307. doi: 10.3389/fnmol.2019.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo X., Philipsen S., Tan-Un K.C. Characterization of human cytoglobin gene promoter region. Biochim. Biophys. Acta. 2006;1759(5):208–215. doi: 10.1016/j.bbaexp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Latina A., et al. DeltaNp63 targets cytoglobin to inhibit oxidative stress-induced apoptosis in keratinocytes and lung cancer. Oncogene. 2016;35(12):1493–1503. doi: 10.1038/onc.2015.222. [DOI] [PubMed] [Google Scholar]
- 61.Arcidiacono P., et al. p63 is a key regulator of iRHOM2 signalling in the keratinocyte stress response. Nat. Commun. 2018;9(1):1021. doi: 10.1038/s41467-018-03470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McRonald F.E., et al. Down-regulation of the cytoglobin gene, located on 17q25, in tylosis with oesophageal cancer (TOC): evidence for trans-allele repression. Hum. Mol. Genet. 2006;15(8):1271–1277. doi: 10.1093/hmg/ddl042. [DOI] [PubMed] [Google Scholar]
- 63.Moncada S., Palmer R.M.J., Higgs E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43(2):109–142. [PubMed] [Google Scholar]
- 64.McDonald L.J., Murad F. Nitric oxide and cyclic GMP signaling. Proc Soc Exp Biol Med. 1996;211(1):6. doi: 10.3181/00379727-211-43950a. [DOI] [PubMed] [Google Scholar]
- 65.Rapoport R.M., Draznin M.B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983;306:174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- 66.Francis S.H., et al. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010;62(3):525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marsden P.A., et al. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J. Biol. Chem. 1993;268(23):17478–17488. [PubMed] [Google Scholar]
- 68.Nadaud S., et al. Gene structure, polymorphism and mapping of the human endothelial nitric oxide synthase gene. Biochem. Biophys. Res. Commun. 1994;198(3):1027–1033. doi: 10.1006/bbrc.1994.1146. [DOI] [PubMed] [Google Scholar]
- 69.Gardner P.R. Nitric oxide dioxygenase function and mechanism of flavohemoglobin, hemoglobin, myoglobin and their associated reductases. J. Inorg. Biochem. 2005;99(1):247–266. doi: 10.1016/j.jinorgbio.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 70.Lancaster J.J. Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc.Natl.Acad.Sci.USA. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hall C.N., Garthwaite J. What is the real physiological NO concentration in vivo? Nitric Oxide. 2009;21(2):92–103. doi: 10.1016/j.niox.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu X., et al. Diffusion-limited reaction of free nitric oxide with erythrocytes. J. Biol. Chem. 1998;273:18709–18713. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 73.Halligan K.E., Jourd'heuil F.L., Jourd'heuil D. Cytoglobin is expressed in the vasculature and regulates cell respiration and proliferation via nitric oxide dioxygenation. J. Biol. Chem. 2009;284(13):8539–8547. doi: 10.1074/jbc.M808231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zweier J., Ilangovan G. Antioxid Redox Signal; 2019. Regulation of Nitric Oxide Metabolism and Vascular Tone by Cytoglobin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.MacMicking J., Xie Q.W., Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 76.Van Thuy T.T., et al. Possible involvement of nitric oxide in enhanced liver injury and fibrogenesis during cholestasis in cytoglobin-deficient mice. Sci. Rep. 2017;7:41888. doi: 10.1038/srep41888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thuy le T.T., et al. Absence of cytoglobin promotes multiple organ abnormalities in aged mice. Sci. Rep. 2016;6:24990. doi: 10.1038/srep24990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thuy le T.T., et al. Cytoglobin deficiency promotes liver cancer development from hepatosteatosis through activation of the oxidative stress pathway. Am. J. Pathol. 2015;185(4):1045–1060. doi: 10.1016/j.ajpath.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 79.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 80.Zweier J.L., Samouilov A., Kuppusamy P. Non-enzymatic nitric oxide synthesis in biological systems. Biochem.Biophys.Acta. 1999;1411:250–262. doi: 10.1016/s0005-2728(99)00018-3. [DOI] [PubMed] [Google Scholar]
- 81.Gladwin M.T., Kim-Shapiro D.B. The functional nitrite reductase activity of the heme-globins. Blood. 2008;112(7):2636–2647. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rassaf T., et al. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ. Res. 2014;114(10):1601–1610. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- 83.Hendgen-Cotta U.B., et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. U. S. A. 2008;105(29):10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Totzeck M., et al. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation. 2012;126(3):325–334. doi: 10.1161/CIRCULATIONAHA.111.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Backer J., et al. The effect of reactive oxygen and nitrogen species on the structure of cytoglobin: a potential tumor suppressor. Redox Biol. 2018;19:1–10. doi: 10.1016/j.redox.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McRonald F.E., Risk J.M., Hodges N.J. Protection from intracellular oxidative stress by cytoglobin in normal and cancerous oesophageal cells. PloS One. 2012;7(2) doi: 10.1371/journal.pone.0030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li D., et al. Cytoglobin up-regulated by hydrogen peroxide plays a protective role in oxidative stress. Neurochem. Res. 2007;32(8):1375–1380. doi: 10.1007/s11064-007-9317-x. [DOI] [PubMed] [Google Scholar]
- 88.Singh S., et al. Cytoglobin modulates myogenic progenitor cell viability and muscle regeneration. Proc. Natl. Acad. Sci. U. S. A. 2014;111(1):E129–E138. doi: 10.1073/pnas.1314962111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hodges N.J., et al. Cellular protection from oxidative DNA damage by over-expression of the novel globin cytoglobin in vitro. Mutagenesis. 2008;23(4):293–298. doi: 10.1093/mutage/gen013. [DOI] [PubMed] [Google Scholar]
- 90.Thi Thanh Hai N., et al. Selective overexpression of cytoglobin in stellate cells attenuates thioacetamide-induced liver fibrosis in mice. Sci. Rep. 2018;8(1):17860. doi: 10.1038/s41598-018-36215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geuens E., et al. A globin in the nucleus! J. Biol. Chem. 2003;278(33):30417–30420. doi: 10.1074/jbc.C300203200. [DOI] [PubMed] [Google Scholar]
- 92.Flores E.R., et al. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature. 2002;416(6880):560–564. doi: 10.1038/416560a. [DOI] [PubMed] [Google Scholar]
- 93.Pan S., Berk B.C. Glutathiolation regulates tumor necrosis factor-alpha-induced caspase-3 cleavage and apoptosis: key role for glutaredoxin in the death pathway. Circ. Res. 2007;100(2):213–219. doi: 10.1161/01.RES.0000256089.30318.20. [DOI] [PubMed] [Google Scholar]
- 94.Sykes M.C., Mowbray A.L., Jo H. Reversible glutathiolation of caspase-3 by glutaredoxin as a novel redox signaling mechanism in tumor necrosis factor-alpha-induced cell death. Circ. Res. 2007;100(2):152–154. doi: 10.1161/01.RES.0000258171.08020.72. [DOI] [PubMed] [Google Scholar]
- 95.Schmidt M., et al. Cytoglobin is a respiratory protein in connective tissue and neurons, which is up-regulated by hypoxia. J. Biol. Chem. 2004;279(9):8063–8069. doi: 10.1074/jbc.M310540200. [DOI] [PubMed] [Google Scholar]
- 96.Xinarianos G., et al. Frequent genetic and epigenetic abnormalities contribute to the deregulation of cytoglobin in non-small cell lung cancer. Hum. Mol. Genet. 2006;15(13):2038–2044. doi: 10.1093/hmg/ddl128. [DOI] [PubMed] [Google Scholar]
- 97.Fujita Y., et al. Melanoma transition is frequently accompanied by a loss of cytoglobin expression in melanocytes: a novel expression site of cytoglobin. PloS One. 2014;9(4) doi: 10.1371/journal.pone.0094772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thuy le T.T., et al. Promotion of liver and lung tumorigenesis in DEN-treated cytoglobin-deficient mice. Am. J. Pathol. 2011;179(2):1050–1060. doi: 10.1016/j.ajpath.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rowland L.K., et al. Putative tumor suppressor cytoglobin promotes aryl hydrocarbon receptor ligand-mediated triple negative breast cancer cell death. J. Cell. Biochem. 2019;120(4):6004–6014. doi: 10.1002/jcb.27887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang J., et al. Cytoglobin ameliorates the stemness of hepatocellular carcinoma via coupling oxidative-nitrosative stress signals. Mol. Carcinog. 2019;58(3):334–343. doi: 10.1002/mc.22931. [DOI] [PubMed] [Google Scholar]
- 101.Chen H., Zhao X., Meng T. Expression and biological role of cytoglobin in human ovarian cancer. Tumour Biol. 2014 doi: 10.1007/s13277-014-1941-x. [DOI] [PubMed] [Google Scholar]
- 102.Oleksiewicz U., et al. Cytoglobin has bimodal: tumour suppressor and oncogene functions in lung cancer cell lines. Hum. Mol. Genet. 2013;22(16):3207–3217. doi: 10.1093/hmg/ddt174. [DOI] [PubMed] [Google Scholar]
- 103.Emara M., Turner A.R., Allalunis-Turner J. Hypoxic regulation of cytoglobin and neuroglobin expression in human normal and tumor tissues. Canc. Cell Int. 2010;10:33. doi: 10.1186/1475-2867-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Teranishi Y., et al. Involvement of hepatic stellate cell cytoglobin in acute hepatocyte damage through the regulation of CYP2E1-mediated xenobiotic metabolism. Lab. Invest. 2015;95(5):515–524. doi: 10.1038/labinvest.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peskin A.V., et al. The high reactivity of peroxiredoxin 2 with H(2)O(2) is not reflected in its reaction with other oxidants and thiol reagents. J. Biol. Chem. 2007;282(16):11885–11892. doi: 10.1074/jbc.M700339200. [DOI] [PubMed] [Google Scholar]
- 106.Flohe L., et al. Glutathione peroxidase, V. The kinetic mechanism. Hoppe Seylers Z Physiol Chem. 1972;353(6):987–999. doi: 10.1515/bchm2.1972.353.1.987. [DOI] [PubMed] [Google Scholar]
- 107.Marquez L.A., Huang J.T., Dunford H.B. Spectral and kinetic studies on the formation of myeloperoxidase compounds I and II: roles of hydrogen peroxide and superoxide. Biochemistry. 1994;33(6):1447–1454. doi: 10.1021/bi00172a022. [DOI] [PubMed] [Google Scholar]
- 108.Khan K.K., et al. The role of distal histidine in peroxidase activity of myoglobin--transient-kinetics study of the reaction of H2O2 with wild-type and distal-histidine-mutanted recombinant human myoglobin. Eur. J. Biochem. 1998;257(3):547–555. doi: 10.1046/j.1432-1327.1998.2570547.x. [DOI] [PubMed] [Google Scholar]