Summary
The production of coenzyme B12 using well-characterized microorganisms, such as Escherichia coli, has recently attracted considerable attention to meet growing demands of coenzyme B12 in various applications. In the present study, we designed an auxotrophic selection strategy and demonstrated the enhanced production of coenzyme B12 using a previously engineered coenzyme B12-producing E. coli strain. To select a high producer, the coenzyme B12-independent methionine synthase (metE) gene was deleted in E. coli, thus limiting its methionine synthesis to only that via coenzyme B12-dependent synthase (encoded by metH). Following the deletion of metE, significantly enhanced production of the specific coenzyme B12 validated the coenzyme B12-dependent auxotrophic growth. Further precise tuning of the auxotrophic system by varying the expression of metH substantially increased the cell biomass and coenzyme B12 production, suggesting that our strategy could be effectively applied to E. coli and other coenzyme B12-producing strains.
Subject Areas: Biological Sciences, Bioengineering, Metabolic Engineering
Graphical Abstract
Highlights
-
•
The auxotrophic selection strategy was applied to coenzyme B12 production
-
•
Coenzyme B12-independent methionine synthase was deleted for auxotroph system
-
•
The auxotrophic strategy could significantly enhance the coenzyme B12 production
-
•
Optimization of the auxotroph system further enhanced the coenzyme B12 production
Biological Sciences; Bioengineering; Metabolic Engineering
Introduction
Coenzyme B12, also known as adenosylcobalamin, plays an important role in several metabolic reactions occurring in different organ systems of the body (Guo and Chen, 2018). For example, it is required for proper functioning of the nervous system and synthesis of red blood cells, fatty acids, and amino acids (Ko et al., 2014, Martens et al., 2002). The demand for large-scale production of coenzyme B12 has steadily increased owing to its applications in food, feed additive, and pharmaceutical industries (Fang et al., 2018, Fang et al., 2017). However, chemical synthesis of coenzyme B12 is highly complicated because of its complex structure. To overcome this shortcoming, industrial production of coenzyme B12 through microbial fermentation has been regarded as an efficient method (Biedendieck et al., 2010).
Currently, microorganisms with the inherent ability to synthesize coenzyme B12, including Pseudomonas denitrificans and Propionibacterium freudenreichii (the highest production was 214.3 and 206.0 mg/L, respectively), are widely employed for its industrial production (Fang et al., 2018, Lee et al., 2018, Martens et al., 2002). However, these strains are not well characterized; thus, only limited engineering tools, such as random mutagenesis and plasmid-based gene expression, have been utilized (Fang et al., 2018, Fang et al., 2017, Yin et al., 2019). In addition, these strains are known to have long fermentation cycles and expensive and complex medium requirements (Fang et al., 2017).
The use of genetically well-characterized bacteria can be a compelling strategy for the production of coenzyme B12. In this regard, recent studies have reported the production of coenzyme B12 by exploiting the representative microbial workhorse, Escherichia coli (Fang et al., 2018, Fowler et al., 2010, Ko et al., 2014). It has been demonstrated that E. coli could be used to synthesize coenzyme B12 upon the addition of ado-cobinamide (AdoCbi) and dimethylbenzimidazole (DMBI) (Fowler et al., 2010, Jang et al., 2018) via the native coenzyme B12 salvage pathway (Lawrence and Roth, 1996). Moreover, another group reconstructed the AdoCbi synthetic pathway from P. denitrificans and heterologously introduced it into E. coli BL21(DE3) (Ko et al., 2014). In their study, Ko et al. overexpressed 22 genes using three plasmids; the production of coenzyme B12 (0.65 μg/g dry cell weight [DCW]) was confirmed even without the addition of AdoCbi. More recently, Fang et al. reported the production of unexpectedly high levels of coenzyme B12 (307.00 μg/g DCW) through step-by-step optimization of the synthetic pathway (Fang et al., 2018). The entire synthetic pathway included heterologous expression of 28 genes, and the related genes from different microorganisms were screened for efficient production of coenzyme B12.
In addition to rational strain engineering strategies, selection-based engineering strategies have been valuable for improving the production capabilities of microorganisms. Indeed, recent studies have shown tremendous improvement in the biochemical production by devising genetic circuits with biosensors to detect metabolite levels of interest. Moreover, coupling the production capability with cell survival has led to a remarkable improvement in the titers (Gao et al., 2019, Jang et al., 2019). For example, Xiao et al. have developed a circuit to control the expression of the genes responsible for antibiotic resistance or essential amino acid synthesis under the control of free fatty acid (FFA)-responsive promoter (PAR), only allowing the strains with active production of FFA to grow (Xiao et al., 2016). Consequently, it was highly effective to control the population quality by minimizing the heterogeneity in biological systems, thereby significantly enhancing the production of FFA. Similarly, Rugbjerg et al. have introduced a genetic circuit to couple the gene expression of cell wall synthesis with that of mevalonate production (Rugbjerg et al., 2018); the introduction of the circuit enabled stable plasmid maintenance and consistent mevalonate production during long-term cultivation.
In the present study, an auxotrophic selection strategy was designed to increase the coenzyme B12 production in E. coli. We leveraged the characteristics of methionine biosynthesis in E. coli; E. coli was engineered to synthesize methionine only if coenzyme B12 existed with cobalamin-dependent homocysteine transmethylase (metH) by deleting the gene encoding for cobalamin-independent homocysteine transmethylase (metE). The growth rate of the E. coli strain lacking the gene metE was highly dependent on the concentration of exogenously added coenzyme B12. When the strategy was applied to the EpACRcob strain, a previously reported coenzyme B12-producing strain (Ko et al., 2014), the specific coenzyme B12 production was substantially improved by autonomous modulation of copy numbers of plasmids. The expression of metH was further varied to optimize the cell biomass and coenzyme B12 production. The engineered strain exhibited significantly enhanced coenzyme B12 production. We believe that this novel strategy would be useful not only for E. coli, but also for several other microorganisms for the production of coenzyme B12.
Results
Construction of Coenzyme B12 Auxotroph System in E. coli
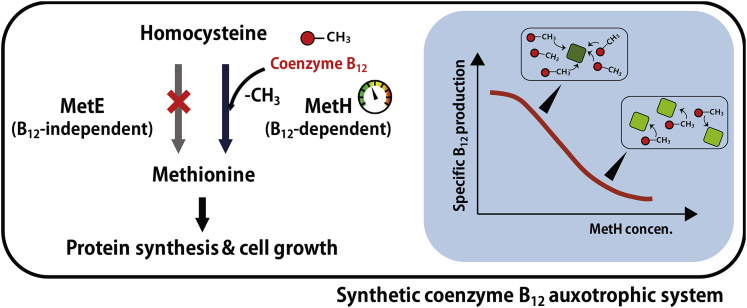
To develop the auxotrophic selection strategy for the production of coenzyme B12, we initially sought an essential enzymatic reaction dependent on coenzyme B12 in E. coli. The most well-known enzyme is cobalamin-dependent homocysteine transmethylase (encoded by metH, Figure 1), which catalyzes the transfer of a methyl group from 5-methyltetrahydrofolate to L-homocysteine to produce methionine and tetrahydrofolate (Lago and Demain, 1969, Neil and Marsh, 1999, Raux et al., 1996). Coenzyme B12 is used as a direct mediator of the methyl group during the transfer process. In fact, E. coli possesses another cobalamin-independent enzyme, encoded by metE, to synthesize methionine (Davis and Mingioli, 1950, Mordukhova and Pan, 2013). Therefore, we decided to delete the metE gene in E. coli for auxotrophic selection. Additionally, we found an early stop codon at the 58th codon of btuB gene (KEGG number; ECD_03851, pseudogene encoding cobalamin outer membrane transporter) in the wild-type E. coli BL21(DE3) strain (Studier et al., 2009), a host used in the previous coenzyme B12 production study (Ko et al., 2014). Because it plays an essential role in coenzyme B12 import (Fowler et al., 2010), the early stop codon was replaced with “CAG” to incorporate glutamine, an identical codon used by other E. coli species (see the Transparent Methods section); thus, the disrupt btuB gene was functionally expressed and the B12 auxotrophic growth was achieved following the addition of coenzyme B12.
Figure 1.
Schematic Diagram of the Overall Strategies Used in the Study
The synthetic coenzyme B12 auxotroph could be constructed by the deletion of chromosomal metE (encoding cobalamin-independent homocysteine transmethylase). The expression of metH (encoding cobalamin-dependent homocysteine transmethylase) was varied using different synthetic promoters to obtain optimal production of coenzyme B12. See also Figure S1.
Upon deleting metE, methionine is produced only by coenzyme B12-dependent MetH and the cells are able to grow in the presence of coenzyme B12. To validate the coenzyme B12-dependent cell growth, the resulting EDMB strain (E. coli BL21(DE3) with metE deletion and functional btuB, Table S1) was cultured in the B12 auxotrophic medium containing all amino acids except methionine (see the Transparent Methods section). As expected, the strain exhibited negligible growth (growth rate <0.01 h−1) because of its inability to synthesize methionine. When the medium was supplemented with varying concentrations of coenzyme B12 ranging from 0 M to 1 μM (∼1.6 mg/L), the specific growth rate gradually increased as the concentration of coenzyme B12 was increased to 500 pM (Figure 2), reaching that of the wild-type (0.51 h−1) when a sufficient amount of coenzyme B12 was present. Although the range of concentrations where the auxotrophic selection functions are at the picomolar level, given that the production of coenzyme B12 is not high in E. coli (Ko et al., 2014), it is expected to be applicable to producing strains. Collectively, these results suggest that the coenzyme B12 auxotrophic system was successfully constructed in E. coli.
Figure 2.
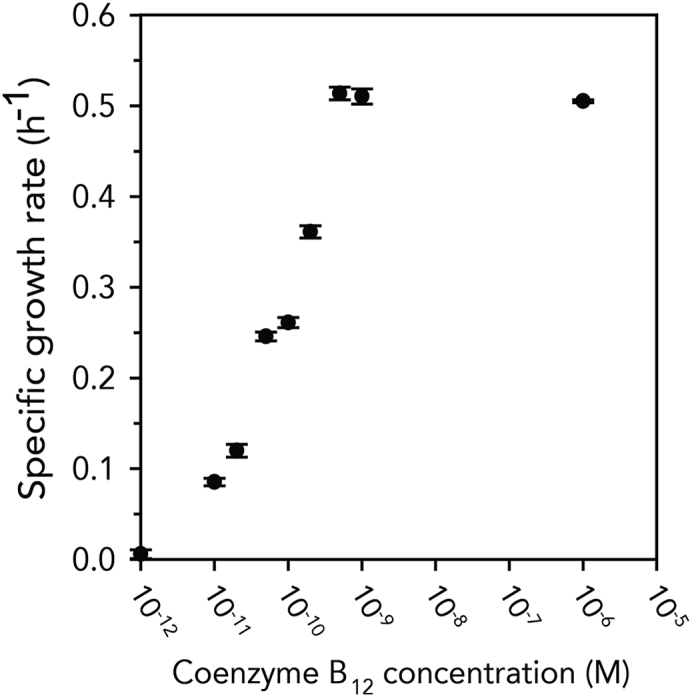
The Validation of Coenzyme B12 Auxotrophic Cell Growth in the EDMB Strain
The specific cell growth rate (h−1) was calculated and plotted on the y axis according to the coenzyme B12 concentration (x axis). Error bars indicate the standard deviations from three independent cultures.
Application of Auxotrophic System for Coenzyme B12 Production
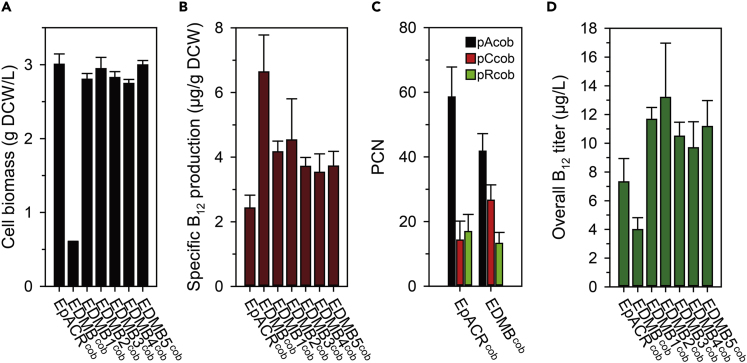
The auxotrophic system was applied to the previously reported coenzyme B12-producing strain, EpACRcob (Tables S1 and S2). The three plasmids (Table S1) harboring AdoCbi synthetic pathway genes from the EpACRcob strain (Ko et al., 2014) were introduced into the EDMB strain. The resulting EDMBcob and EpACRcob strains were initially cultured in the B12 auxotrophic medium; however, a severely reduced cell growth was observed for the EDMBcob strain (the growth rate was 0.03 h−1). Therefore, we decided to use the RB12 medium containing 10 g/L of tryptone instead of the individual amino acids to supplement the low amount of methionine and to enhance the protein synthesis at the early phase of culture. This supplementation was helpful to obtain higher biomass (0.60 g DCW/L, Figure 3A). However, the deletion of metE still resulted in the formation of significantly reduced biomass of the EDMBcob strain (Figure 3A, a 5.01-fold decrease). These results indicated that intracellular methionine synthesis was critical for cell growth and the EDMBcob strain was still affected by the auxotrophic system.
Figure 3.
The Effect of Synthetic Auxotrophic System on Coenzyme B12-Producing Strains
Comparison of cell biomass (A) (See also Figure S2), specific coenzyme B12 production (B), and plasmid copy number (PCN) after 12-h cultivation (C) (see also Figure S3) and overall coenzyme B12 titer (D) after 24-h cultivation. Error bars indicate the standard deviations from experiments conducted in triplicate.
We further quantified the production of coenzyme B12 in both strains to investigate the effect of the selection strategy. The EpACRcob strain exhibited higher production of coenzyme B12 (2.43 μg/g DCW) when compared with the previously reported value (0.65 μg/g DCW) (Ko et al., 2014). Given that most of the culture conditions are the same, this difference in productivity is probably owing to the use of RB12 medium supplemented with glucose as a carbon source, unlike the previously used LB medium (see the Transparent Methods section). Surprisingly, the EDMBcob strain with the metE deletion showed a 2.73-fold increase in specific coenzyme B12 production compared with the EpACRcob strain (Figure 3B, 6.64 μg/g DCW). The result indicates that the auxotrophic selection system was effective in significantly improving the production of coenzyme B12.
Because the only genomic differences are the deletion of metE and functional expression of btuB, it was hypothesized that the increase in the specific production could be attributed to the altered expression levels of AdoCbi synthetic genes. Plasmids typically exhibit a huge heterogeneity in their plasmid copy numbers (PCNs), which has often affected the production performance of microorganisms (Jahn et al., 2016, Kang et al., 2018). To test this hypothesis, the copy number of each plasmid in both strains was measured by quantitative PCR (qPCR). As mentioned, the copy numbers of the pCcob (ColA origin) and pRcob (RSF1030 origin) plasmids in the EpACRcob strain (Figure 3C) were observed to be relatively lower (14.2 and 16.9 copies per cell, respectively) than that known (20–40 copies and 100 copies, respectively), which could be due to different culture conditions, use of multiple plasmids, and metabolic burden from several heterologous gene expression (Jahn et al., 2016, Zhong et al., 2011). Meanwhile, the pACob plasmid (p15A origin) exhibited a significantly higher PCN (58.5 copies/cell) than the known PCN (10–12 copies/cell). Moreover, the high PCN of pAcob is consistent with the previously measured high mRNA levels of the genes in the pAcob plasmid (Ko et al., 2014). The measurement revealed that the EDMBcob strain showed dramatic changes in the copy number compared with the EpACRcob strain. The copy number of the pCcob plasmid (26.6 copies/cell) was almost 2-fold higher than that in the EpACRcob strain. The increased copy number of the pCcob plasmid led to enhanced expression of the genes responsible for converting hydrogenobyrinic acid a,c-diamide to AdoCbi (Figure S1). On the contrary, PCN of both pAcob and pRcob plasmids (41.8 and 13.2 copies/cell, respectively) was 1.40-fold and 1.28-fold lower than that of the EpACRcob strain. The decreased copy number might be beneficial to minimize the wasteful usage of resources in gene expression. There might be other potential factors; nevertheless, the introduction of the auxotrophic selection system affected PCNs, improving the production of coenzyme B12.
Optimization of metH Expression for Enhancing Coenzyme B12 Production
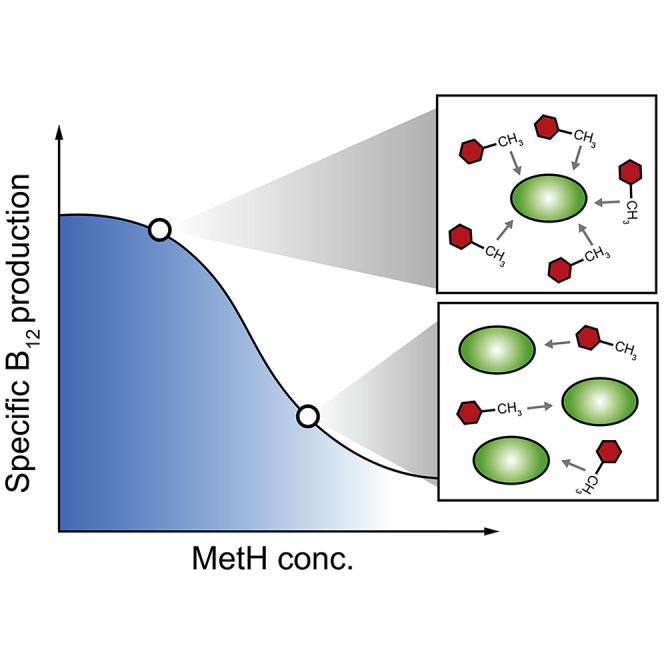
Despite the enhanced specific production, the volumetric titer of the EDMBcob strain was lower than that of the EpACRcob strain by 1.83-fold (3.98 μg/L, Figure 3D) because of the decreased cell biomass of the EDMBcob strain. This result suggested that an additional tuning of the auxotrophic system was required to restore the cell biomass and thereby increase the overall titer.
It has been known that MetH has a higher catalytic efficiency than MetE (Gonzalez et al., 1992). However, methionine is mostly synthesized by MetE and not MetH because the expression of metH is induced only when cobalamin is present in the media (Helliwell et al., 2011, Roth et al., 1996). Therefore, it was believed that reduced cell biomass of the EDMBcob strain presumably resulted from insufficient methionine synthesis with low metH expression at an early stage of cultivation. To validate our assumption, the expression of metH was measured by qPCR (Figure 4). Indeed, the metH expression was very low at the beginning of the cultivation (4 h), reinforcing our assumption that this interfered with the early biomass accumulation. The expression of metH was subsequently induced after synthesis of coenzyme B12 (10 h); however, the cell biomass could not be recovered (Figure S2). This is probably due to the metabolic imbalance that the proteins essential for cell growth could not be sufficiently synthesized along with the synthesis of numerous coenzyme B12 (Darlington et al., 2018, Segall-Shapiro et al., 2014). Collectively, this result suggested that increasing the metH expression would improve the initial cell growth as well as the overall titer.
Figure 4.
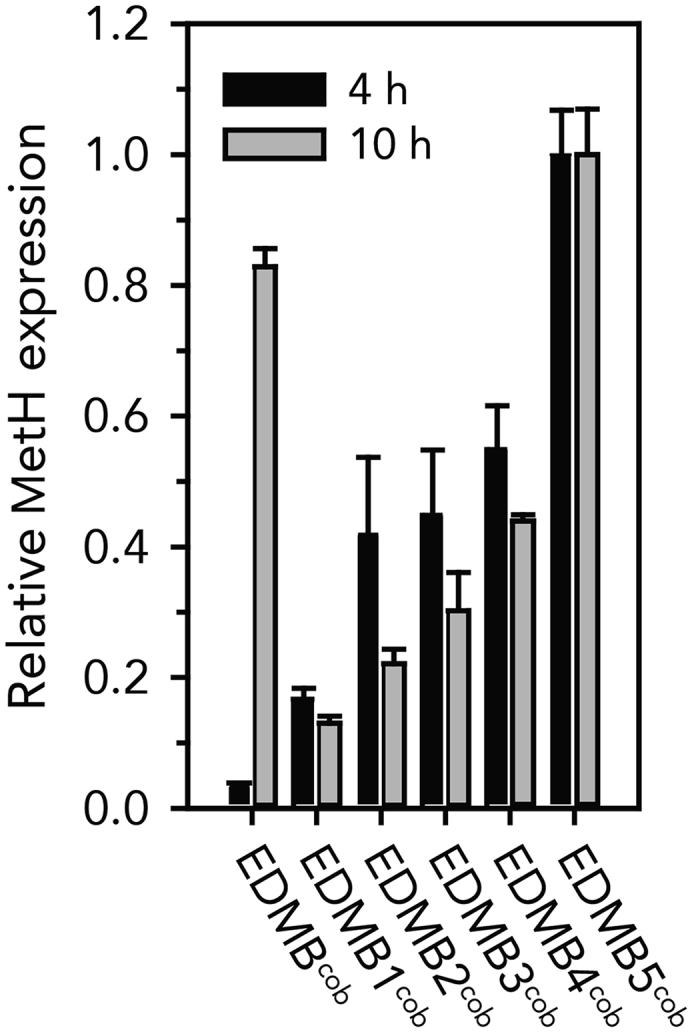
Comparison of Normalized Amounts of metH Transcript in Engineered Strains
Error bars indicate the standard deviations from experiments conducted in triplicate.
Therefore, we tried to deregulate the expression of metH using synthetic constitutive promoters (http://parts.igem.org/Promoters/Catalog/Anderson) (Kang et al., 2018, Noh et al., 2018, Noh et al., 2017). To restore the cell biomass, the metH expression needed to be increased than before as its insufficient expression produced less cell biomass. However, excessive expression could also lower the specific coenzyme B12 production. Consequently, multiple strains with varied expression levels of metH were generated by introducing synthetic metH cassettes with different-strength constitutive promoters (Tables S1 and S2). The resultant strains (EDMB1-5cob) were cultivated, and the expression of metH was measured in the same manner (Figure 4). Although there was a gap between predicted strength and measured metH expression levels (Iverson et al., 2016, Kelwick et al., 2015, Noh et al., 2017), varied metH expression levels (up to 7.84-fold) were successfully identified in these engineered strains as intended. Moreover, these strains showed higher metH expression up to 28.6-fold than the EDMBcob strain at an early stage of cultivation and appeared to maintain a relatively constant level during cultivation.
The synthetic expression of metH caused noticeable changes in both cell biomass and specific coenzyme B12 production. All engineered strains, EDMB1-5cob, displayed notably increased cell biomass compared with the EDMBcob strain (Figures 3A and S2). In particular, the cell biomass was generally enhanced at 12 and 18 h as the expression of metH increased (Figure S2), and almost 5-fold increased cell biomass was observed in all engineered strains at 24 h (Figure 3A). These values obtained at 24 h corresponded to the recovery of cell biomass to more than 90% of EpACRcob strain without metE deletion. This indicated that sufficient methionine could be synthesized by the deregulated metH expression in engineered strains (Figure 4). On the contrary, specific coenzyme B12 production was decreased as a result of the enhanced metH expression (Figure 3B). Especially, specific production generally decreased as the metH expression increased, up to 1.78-fold for the EDMB5cob strain with the highest metH expression as intended. Nevertheless, the significantly enhanced cell biomass increased the overall titer (Figure 3D). Among the strains, the EDMB2cob strain with moderate metH expression level showed the highest coenzyme B12 production (13.2 μg/L), which was 3.31-fold and 1.80-fold higher than that of the EDMBcob and EpACRcob strains, respectively. In addition, the PCNs of EDMB1-5cob strains showed a similar tendency as that of EDMBcob strain (Figure S3), indicating they were still affected by the synthetic auxotroph system. Collectively, these results show that the synthetic auxotroph system with precise controlled metH expression could be successfully applied to coenzyme B12-producing strains.
Discussion
Recent studies have successfully demonstrated the production of coenzyme B12 in a well-known microbial workhorse E. coli (Fang et al., 2018, Ko et al., 2014). However, the heterologous expression of synthetic genes has been an obstacle to further improvement. In the present study, a novel strategy to enhance the coenzyme B12 production was designed and applied to previously constructed coenzyme B12-producing E. coli (Ko et al., 2014). Initially, the coenzyme B12 synthetic auxotrophic system was constructed using the characteristics of methionine synthesis in E. coli. Next, this system was applied to the previously reported producer strain to greatly improve the coenzyme B12 production. We found modulated copy numbers in the coenzyme B12-producing plasmids after the application of the auxotrophic selection system, which could explain the observed improvement in the production.
Optimizing complex metabolic pathways such as coenzyme B12 to enhance the production has been a labor-intensive work in metabolic engineering (Smanski et al., 2014). Our successful application of the auxotroph system, which could significantly enhance the coenzyme B12 production without optimization of individual gene expressions, suggests enough potential that the auxotroph system can be effectively used for complex pathway optimization. In addition, it was shown that the selection efficiency could be optimized through precisely regulating the expression of a key enzyme, which implies that the system can be optimized for different production levels like other selection-based strategies (Rugbjerg et al., 2018, Xiao et al., 2016).
The strategy would be applied to other coenzyme B12-producing strains such as P. denitrificans and P. freudenreichii. These strains also possess cobalamin-dependent homocysteine methyltransferase and are known to have a similar regulation system (Ainala et al., 2013, Falentin et al., 2010). Given that these strains produce high amounts of coenzyme B12, the expression of cobalamin-dependent methyltransferase may need to be optimized at lower levels. Alternatively, the coenzyme B12-binding residues could be intentionally disrupted to lower the affinity base on the elucidated structure of cobalamin-dependent methyltransferase (Drennan et al., 1994, Seo et al., 2018). In addition, other cobalamin-dependent enzymes, such as methylmalonyl-CoA mutase (essential for odd-chain fatty acid synthesis) and glycerol dehydratase (essential for glycerol utilization), could be utilized using a similar strategy (Banerjee and Ragsdale, 2003, Neil and Marsh, 1999). Moreover, these coenzyme B12 synthetic auxotrophic systems could be applied to evolutionary engineering approaches (Lim et al., 2018, Seok et al., 2018). The short-term change in PCNs was validated to enhance the coenzyme B12 production in the current study; however, it could be used to screen genetically effective mutants as a powerful screening method in long-term evolution. Taken together, we expect our strategy to be widely applied for the efficient production of coenzyme B12.
Limitations of the Study
The system described in the present study may be limited by the binding affinity of MetH to coenzyme B12. When this system is applied to a superior coenzyme B12-producing strain (Fang et al., 2018), its operational range needs to be investigated or modified, if necessary. As discussed, the coenzyme B12-binding residues or expression levels can be altered for effective tuning of the dynamic range. Furthermore, other enzymes involved in different reactions could be considered also. Nonetheless, the improved coenzyme B12 production with our parental strain (EpACRcob) shows great potential. The present study could be valuable for understanding the heterogeneity during biochemical production and its minimization by introducing a selection strategy.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This research was supported by the C1 Gas Refinery Program (NRF-2018M3D3A1A01055754) and the National Research Foundation of Korea grant (NRF-2019R1A2C2084631) funded by the Ministry of Science and ICT. This research was also supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry and Energy (MOTIE) of the Republic of Korea (no. 20194030202330).
Author Contributions
M.H.N., H.G.L., D.M., S.P., and G.Y.J. conceived the project. M.H.N. and H.G.L. designed and conducted the experiments. M.H.N., H.G.L., D.M., S.P., and G.Y.J. conducted data analysis and interpretation and wrote the manuscript. S.P. and G.Y.J. critically revised the manuscript. G.Y.J. supervised the project. All the authors read and approved the final manuscript.
Declaration of Interests
The authors declare that they have no competing interests.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100890.
Supplemental Information
References
- Ainala S.K., Somasundar A., Park S. Complete genome sequence of Pseudomonas denitrificans ATCC 13867. Genome Announc. 2013;1 doi: 10.1128/genomeA.00257-13. e00257–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R., Ragsdale S.W. The many faces of vitamin B12 : catalysis by cobalamin-dependent enzymes. Annu. Rev. Biochem. 2003;72:209. doi: 10.1146/annurev.biochem.72.121801.161828. [DOI] [PubMed] [Google Scholar]
- Biedendieck R., Malten M., Barg H., Bunk B., Martens J.H., Deery E., Leech H., Warren M.J., Jahn D. Metabolic engineering of cobalamin (vitamin B12) production in Bacillus megaterium. Microb. Biotechnol. 2010;3:24–37. doi: 10.1111/j.1751-7915.2009.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington A.P.S., Kim J., Jiménez J.I., Bates D.G. Dynamic allocation of orthogonal ribosomes facilitates uncoupling of co-expressed genes. Nat. Commun. 2018;9:695. doi: 10.1038/s41467-018-02898-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B.D., Mingioli E.S. Mutants of Escherichia coli requiring methionine or vitamin B12. J. Bacteriol. 1950;60:17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan C.L., Huang S., Drummond J.T., Matthews R.G., Ludwig M.L. How a protein binds B12 : a 3.0 Å x-ray structure of B12-binding domains of methionine synthase. Science. 1994;266:1669–1674. doi: 10.1126/science.7992050. [DOI] [PubMed] [Google Scholar]
- Falentin H., Deutsch S.M., Jan G., Loux V., Thierry A., Parayre S., Maillard M.B., Dherbécourt J., Cousin F.J., Jardin J. The complete genome of Propionibacterium freudenreichii CIRM-BIA1T, a hardy Actinobacterium with food and probiotic applications. PLoS One. 2010;5:e11748. doi: 10.1371/journal.pone.0011748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H., Kang J., Zhang D. Microbial production of vitamin B12: a review and future perspectives. Microb. Cell Fact. 2017;16:15. doi: 10.1186/s12934-017-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H., Li D., Kang J., Jiang P., Sun J., Zhang D. Metabolic engineering of Escherichia coli for de novo biosynthesis of vitamin B12. Nat. Commun. 2018;9:4917. doi: 10.1038/s41467-018-07412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler C.C., Brown E.D., Li Y. Using a riboswitch sensor to examine coenzyme B12 metabolism and transport in E. coli. Chem. Biol. 2010;17:756–765. doi: 10.1016/j.chembiol.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Gao C., Xu P., Ye C., Chen X., Liu L. Genetic circuit-assisted smart microbial engineering. Trends Microbiol. 2019;27:1011–1024. doi: 10.1016/j.tim.2019.07.005. [DOI] [PubMed] [Google Scholar]
- Gonzalez J.C., Banerjee R.V., Huang S., Sumner J.S., Matthews R.G. Comparison of cobalamin-independent and cobalamin-dependent methionine synthases from Escherichia coli : two solutions to the same chemical problem. Biochemistry. 1992;31:6045–6056. doi: 10.1021/bi00141a013. [DOI] [PubMed] [Google Scholar]
- Guo M., Chen Y. Coenzyme cobalamin: biosynthesis, overproduction and its application in dehalogenation—a review. Rev. Environ. Sci. Biotechnol. 2018;17:259–284. [Google Scholar]
- Helliwell K.E., Wheeler G.L., Leptos K.C., Goldstein R.E., Smith A.G. Insights into the evolution of vitamin B12 auxotrophy from sequenced algal genomes. Mol. Biol. Evol. 2011;28:2921–2933. doi: 10.1093/molbev/msr124. [DOI] [PubMed] [Google Scholar]
- Iverson S.V., Haddock T.L., Beal J., Densmore D.M. CIDAR MoClo: improved MoClo assembly standard and new E. coli part library enable rapid combinatorial design for synthetic and traditional biology. ACS Synth. Biol. 2016;5:99–103. doi: 10.1021/acssynbio.5b00124. [DOI] [PubMed] [Google Scholar]
- Jahn M., Vorpahl C., Hübschmann T., Harms H., Müller S. Copy number variability of expression plasmids determined by cell sorting and droplet digital PCR. Microb. Cell Fact. 2016;15:211. doi: 10.1186/s12934-016-0610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S., Kim M., Hwang J., Jung G.Y. Tools and systems for evolutionary engineering of biomolecules and microorganisms. J. Ind. Microbiol. Biotechnol. 2019;46:1313–1326. doi: 10.1007/s10295-019-02191-5. [DOI] [PubMed] [Google Scholar]
- Jang S., Jang S., Noh M.H., Lim H.G., Jung G.Y. Novel hybrid input part using riboswitch and transcriptional repressor for signal inverting amplifier. ACS Synth. Biol. 2018;7:2199–2204. doi: 10.1021/acssynbio.8b00213. [DOI] [PubMed] [Google Scholar]
- Kang C.W., Lim H.G., Yang J., Noh M.H., Seo S.W., Jung G.Y. Synthetic auxotrophs for stable and tunable maintenance of plasmid copy number. Metab. Eng. 2018;48:121–128. doi: 10.1016/j.ymben.2018.05.020. [DOI] [PubMed] [Google Scholar]
- Kelwick R., Kopniczky M., Bower I., Chi W., Chin M.H.W., Fan S., Pilcher J., Strutt J., Webb A.J., Jensen K. A forward-design approach to increase the production of poly-3-hydroxybutyrate in genetically engineered Escherichia coli. PLoS One. 2015;10:e0117202. doi: 10.1371/journal.pone.0117202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y., Ashok S., Ainala S.K., Sankaranarayanan M., Chun A.Y., Jung G.Y., Park S. Coenzyme B12 can be produced by engineered Escherichia coli under both anaerobic and aerobic conditions. Biotechnol. J. 2014;9:1526–1535. doi: 10.1002/biot.201400221. [DOI] [PubMed] [Google Scholar]
- Lago B.D., Demain A.L. Alternate requirement for vitamin B12 or methionine in mutants of Pseudomonas denitrificans, a vitamin B12-producing bacterium. J. Bacteriol. 1969;99:347–349. doi: 10.1128/jb.99.1.347-349.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J.G., Roth J.R. Evolution of coenzyme B12 synthesis among enteric bacteria: evidence for loss and reacquisition of a multigene complex. Genetics. 1996;142:11–24. doi: 10.1093/genetics/142.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Lama S., Kim J.R., Park S.H. Production of 1,3-propanediol from glucose by recombinant Escherichia coli BL21(DE3) Biotechnol. Bioproc. Eng. 2018;23:250–258. [Google Scholar]
- Lim H.G., Jang S., Jang S., Seo S.W., Jung G.Y. Design and optimization of genetically encoded biosensors for high-throughput screening of chemicals. Curr. Opin. Biotechnol. 2018;54:18–25. doi: 10.1016/j.copbio.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Martens J.H., Barg H., Warren M., Jahn D. Microbial production of vitamin B12. Appl. Microbiol. Biotechnol. 2002;58:275–285. doi: 10.1007/s00253-001-0902-7. [DOI] [PubMed] [Google Scholar]
- Mordukhova E.A., Pan J.G. Evolved cobalamin-independent methionine synthase (MetE) improves the acetate and thermal tolerance of Escherichia coli. Appl. Environ. Microbiol. 2013;79:7905–7915. doi: 10.1128/AEM.01952-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil E., Marsh G. Coenzyme B12 (cobalamin)-dependent enzymes. Essays Biochem. 1999;34:139–154. doi: 10.1042/bse0340139. [DOI] [PubMed] [Google Scholar]
- Noh M.H., Lim H.G., Park S., Seo S.W., Jung G.Y. Precise flux redistribution to glyoxylate cycle for 5-aminolevulinic acid production in Escherichia coli. Metab. Eng. 2017;43:1–8. doi: 10.1016/j.ymben.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Noh M.H., Lim H.G., Woo S.H., Song J., Jung G.Y. Production of itaconic acid from acetate by engineering acid-tolerant Escherichia coli W. Biotechnol. Bioeng. 2018;115:729–738. doi: 10.1002/bit.26508. [DOI] [PubMed] [Google Scholar]
- Raux E., Lanois A., Levillayer F., Warren M.J., Brody E., Rambach A., Thermes C. Salmonella typhimurium cobalamin (vitamin B12) biosynthetic genes: functional studies in S. typhimurium and Escherichia coli. J. Bacteriol. 1996;178:753–767. doi: 10.1128/jb.178.3.753-767.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Lawrence J., Bobik T. Cobalamin (coenzyme B12 ): Synthesis and biological significance. Annu. Rev. Microbiol. 1996;50:137–181. doi: 10.1146/annurev.micro.50.1.137. [DOI] [PubMed] [Google Scholar]
- Rugbjerg P., Sarup-Lytzen K., Nagy M., Sommer M.O.A. Synthetic addiction extends the productive life time of engineered Escherichia coli populations. Proc. Natl. Acad. Sci. U S A. 2018;115:2347–2352. doi: 10.1073/pnas.1718622115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall-Shapiro T.H., Meyer A.J., Ellington A.D., Sontag E.D., Voigt C.A. A ‘resource allocator’ for transcription based on a highly fragmented T7 RNA polymerase. Mol. Syst. Biol. 2014;10:742. doi: 10.15252/msb.20145299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J.H., Min W.K., Lee S.G., Yun H., Kim B.G. To the final goal: can we predict and suggest mutations for protein to develop desired phenotype? Biotechnol. Biopro. Eng. 2018;23:134–143. [Google Scholar]
- Seok J.Y., Yang J., Choi S.J., Lim H.G., Choi U.J., Kim K.J., Park S., Yoo T.H., Jung G.Y. Directed evolution of the 3-hydroxypropionic acid production pathway by engineering aldehyde dehydrogenase using a synthetic selection device. Metab. Eng. 2018;47:113–120. doi: 10.1016/j.ymben.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Smanski M.J., Bhatia S., Zhao D., Park Y.J., Woodruff L.B.A., Giannoukos G., Ciulla D., Busby M., Calderon J., Nicol R. Functional optimization of gene clusters by combinatorial design and assembly. Nat. Biotechnol. 2014;32:1241–1249. doi: 10.1038/nbt.3063. [DOI] [PubMed] [Google Scholar]
- Studier F.W., Daegelen P., Lenski R.E., Maslov S., Kim J.F. Understanding the differences between genome sequences of Escherichia coli B strains REL606 and BL21(DE3) and comparison of the E. coli B and K-12 genomes. J. Mol. Biol. 2009;394:653–680. doi: 10.1016/j.jmb.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Bowen C.H., Liu D., Zhang F. Exploiting nongenetic cell-to-cell variation for enhanced biosynthesis. Nat. Chem. Biol. 2016;12:339–344. doi: 10.1038/nchembio.2046. [DOI] [PubMed] [Google Scholar]
- Yin J., Zheng W., Gao Y., Jiang C., Shi H., Diao X., Li S., Chen H., Wang H., Li R. Single-stranded DNA-binding protein and exogenous RecBCD inhibitors enhance phage-derived homologous recombination in Pseudomonas. iScience. 2019;14:1–14. doi: 10.1016/j.isci.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C., Peng D., Ye W., Chai L., Qi J., Yu Z., Ruan L., Sun M. Determination of plasmid copy number reveals the total plasmid DNA amount is greater than the chromosomal DNA amount in Bacillus thuringiensis YBT-1520. PLoS One. 2011;6:e16024. doi: 10.1371/journal.pone.0016025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.