Abstract
Background
The ascomycete fungi Cylindrocarpon destructans (Cd) and Fusarium solani (Fs) cause ginseng root rot and significantly reduce the quality and yield of ginseng. Cd produces the secondary metabolite radicicol, which targets the molecular chaperone Hsp90. Fs is resistant to radicicol, whereas other fungal genera associated with ginseng disease are sensitive to it. Radicicol resistance mechanisms have not yet been elucidated.
Methods
Transcriptome analyses of Fs and Cd mycelia treated with or without radicicol were conducted using RNA-seq. All of the differentially expressed genes (DEGs) were functionally annotated using the Fusarium graminearum transcript database. In addition, deletions of two transporter genes identified by RNA-seq were created to confirm their contributions to radicicol resistance.
Results
Treatment with radicicol resulted in upregulation of chitin synthase and cell wall integrity genes in Fs and upregulation of nicotinamide adenine dinucleotide dehydrogenase and sugar transporter genes in Cd. Genes encoding an ATP-binding cassette transporter, an aflatoxin efflux pump, ammonium permease 1 (mep1), and nitrilase were differentially expressed in both Fs and Cd. Among these four genes, only the ABC transporter was upregulated in both Fs and Cd. The aflatoxin efflux pump and mep1 were upregulated in Cd, but downregulated in Fs, whereas nitrilase was downregulated in both Fs and Cd.
Conclusion
The transcriptome analyses suggested radicicol resistance pathways, and deletions of the transporter genes indicated that they contribute to radicicol resistance.
Keywords: Cylindrocarpon destructans, Fusarium solani, Ginseng root rot, Radicicol, Transcriptomes
1. Introduction
Panax ginseng, commonly known as ginseng, is an important medicinal plant that is distributed widely, especially in East Asia, including Korea, China, and Japan. In Korea, ginseng is typically cultivated in the same field for 4–6 years and is often threatened by many soil-borne fungal pathogens, including Fusarium solani (Fs), Rhizoctonia solani, Pythium ultimum, and Cylindrocarpon destructans (Cd) [1], [2], [3], [4]. Of these pathogens, Cd is the major cause of ginseng root rot disease, which leads to the most severe losses during ginseng cultivation [5].
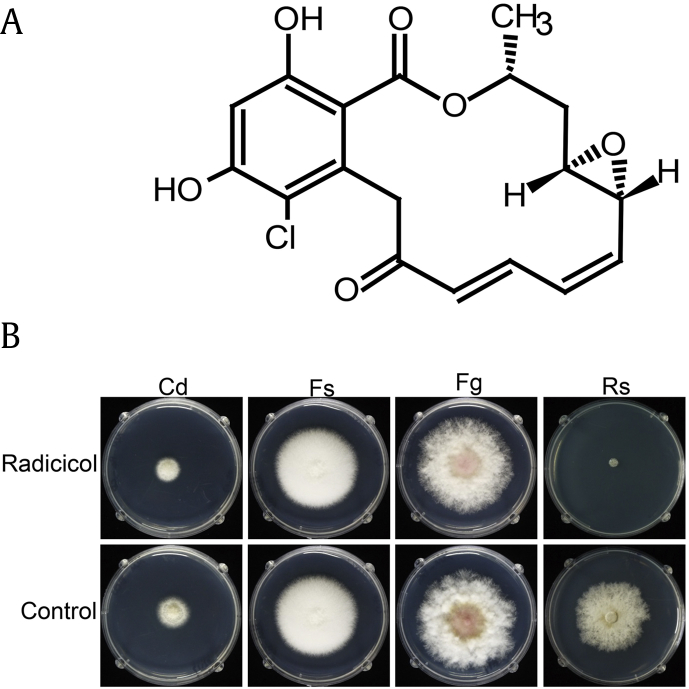
In addition to causing root rot in growing ginseng, Cd (teleomorph Nectria/Neonectria radicicola) leads to replant failure because the fungus can survive in the soil for more than a decade after ginseng harvest. Cd can also cause root rot in other plants, including conifers and fruit trees [6], [7]. Although Cd grows slower than other soil-borne fungi, it is often dominant in ginseng fields, perhaps due to its production of the secondary metabolite radicicol (Fig. 1A) [8], [9].
Fig. 1.
Molecular structure of radicicol (A) and resistance of fungal species to radicicol (B). Each fungal strain was cultivated on MM supplemented with or without 50 mg/L of radicicol for 7 days at 20°C. The radicicol structure was drawn using ACD/ChemSketch. Cd, C. destructans; Fs, F. solani; Fg, F. graminearum; Rs, Rhizoctonia solani.
Radicicol is an antifungal and antibiotic that inhibits signal transduction proteins, including the ATPase activity of Hsp90, which is necessary for proteins to fold and function properly [10], [11]. By interacting with Hsp90, radicicol accelerates the dissociation of the Raf/Hsp90 complex, leading to inhibition of the Ras/MAP kinase signal transduction pathway [12]. Moreover, radicicol suppresses inducible nitric oxide synthase (iNOS) gene expression by blocking p38 kinase signaling [13]. p38 kinase induces iNOS activity via the lipopolysaccharide-induced signal transduction pathway [14], [15]. Suppression of iNOS leads to defects in the regulation of germination, the response to heat stress, and nitrogen uptake [16], [17], [18], [19].
Radicicol contributes to the survival of Cd and allows it to outcompete other microbes in the soil. Previously, we showed that radicicol inhibits the vegetative growth and spore germination of many fungal pathogens and soil-dwelling saprophytic fungi [3]. Interestingly, Fs is not affected by radicicol, indicating that Fs carries the mechanism for radicicol resistance and coexists with Cd in nature.
Fs, also known as Nectria haematococca, is a widespread soil-borne fungus, and nearly a century ago, it was shown that Fs associates with ginseng root rot [20]. Fs is very close phylogenetically to Cd, and it is not easy to distinguish the two species due to their morphological similarity [21], [22]. The morphological and phylogenetic similarity of these two pathogens led us to hypothesize that they share similar mechanisms of radicicol resistance. In this study, we performed transcriptome analyses of Fs and Cd to identify differentially expressed genes (DEGs) after radicicol treatment. Our transcriptome analyses identified putative pathways of radicicol resistance, and our genetic analyses confirmed that two transporter genes are involved in radicicol resistance.
2. Materials and methods
2.1. Fungal strains and media
The fungal strains F. solani 13chu01-05 (Fs) and C. destructans KACC41077 (Cd) were used for all of the experiments and were stored in 20% glycerol at −80°C. Media, including minimal medium (MM), complete medium (CM), potato dextrose agar, and carboxyl methyl cellulose, were prepared as described in The Fusarium laboratory manual [23].
2.2. Targeted gene deletion and quantitative real-time PCR
The mutants ΔFgAfla and ΔFgAbc containing deletions of the FGSG_09595 and FGSG_04580 genes, respectively, were generated from F. graminearum GZ3639 (Fg) as previously described [24]. In brief, the 5′ and 3′ flanking regions of each target gene were amplified from the wild-type strain using the primer pairs Del-5′F/Del-5′R and Del-3′F/Del-3′R. A hygromycin-resistance cassette (HYG) was amplified from pIGPAPA using the primer pairs HYG-F and HYG-R. The three amplicons were then mixed and fused by polymerase chain reaction (PCR). The final structures for transformation were amplified during a third PCR step using the nested primers nestedF, nestedR, HYG nestedF, and HYG nestedR. Quantitative real-time PCR (qRT-PCR) was performed to validate the expression levels of the target gene in the wild-type strain and deletion mutants. The synthesized cDNA samples from each fungal strain were diluted to 10 ng/μL using distilled water, and 2 μL of cDNA was used for qRT-PCR. The relative transcription levels were normalized by reference gene, cyclophilin (CYP). All of the PCR primers used in this study are listed in Table S1.
2.3. Radicicol resistance test
The radicicol resistance test was performed as previously described [25] with a slight modification. In brief, all of the fungal strains except Cd were cultivated on CM at 25°C for 5 days, whereas Cd was cultivated on CM at 20°C for 5 days. CM agar blocks with freshly grown mycelia were transferred to MM supplemented with 50 mg/L of radicicol. Growth of the mycelia was measured every 2 days. MM containing 5% (v/v) methanol was used as the control, and the experiment was performed three independent times. Differences between mean values for vegetative growth were determined using the post hoc Tukey test in the statistical software R version 3.5.1.
2.4. RNA extraction and sequencing
Fs and Cd were cultivated in potato dextrose agar for 5 days at 25°C and 20°C, respectively. Then, each strain was inoculated into MM liquid with constant shaking at 200 rpm at 25°C or 20°C. After 7 days, mycelia were harvested by centrifugation and transferred to fresh MM supplemented with or without 50 mg/L of radicicol. After an additional 24 hour of incubation, total RNA was extracted using the easy-spin Total RNA Extraction kit (iNtRON Biotechnology, Gyeonggi-do, Korea) following the manufacturer's protocol. Whole transcriptomes of Fs were sequenced using Illumina Hiseq4000 at Macrogen (Seoul, Korea), and whole transcriptomes of Cd were sequenced using Illumina Hiseq2500 at the National Instrumentation Center for Environmental Management (Seoul, Korea).
2.5. Transcriptome and DEG analyses
The Fs reference genome sequence was downloaded from MycoCosm (https://genome.jgi.doe.gov/fungi), and the Cd reference genome sequence was provided by Dr S.-H. Lee (Natural Institute of Horticultural and Herbal Science). RNA-seq reads from the two species were mapped to their respective reference genomes using STAR with the parameters —alignIntronMin 20 —alignIntronMax 10000 because the default intron length setting for this program is too large. Each aligned read was made into a transcriptome assembly using Cufflinks with the options —min-intron-length = 20 and —max-intron-length = 10000, and assemblies were merged together using Cuffmerge. Cuffdiff was used to quantify transcript abundance in terms of fragments per kilobase of transcript per million fragments mapped (FPKM) and to test the statistical significance of observed changes. DEGs were defined as genes with at least a twofold change in FPKM between the high-yield and low-yield groups, with a statistical cutoff of p ≤ 0.05 and a false discovery rate of q < 0.001 [26]. Heat map was generated using heatmap.2 function of the gplots packages in R. Hierarchical clustering was performed using Euclidean measure to obtain distance matrix and complete linkage method for clustering.
2.6. Functional annotation and metabolic pathway mapping
All the assembled transcripts were subjected to the basic local alignment search tool (BLASTX) against the proteins of Fg (ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/240/135/GCF_000240135.3_ASM24013v3/GCF_000240135.3_ASM24013v3_protein.faa.gz) with an E-value < 10−5, and functional annotations were made based on the best hit of the BLASTX results. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway mapping was performed based on the metabolic pathways of Fg due to the lack of metabolic pathway data for Fs and Cd. We manually integrated Fs and Cd metabolic pathway data obtained from the literature into the KEGG pathway to draw the metabolic pathways represented by the gene expression data.
3. Results
3.1. Resistance of Fg to radicicol
The structure of radicicol (Fig. 1A) is similar to the structure of zearalenone, which is produced by Fg. Both Fg and Fs were equally resistant to radicicol, whereas R. solani (Rs) was sensitive to radicicol (Fig. 1B). There was no significant difference in the vegetative growth of Fg, Fs, and Cd on radicicol-containing medium and the control medium.
3.2. Transcriptome analyses of Fs and Cd upon radicicol treatment
Transcriptome data were deposited in the Sequence Read Archive of the National Center for Biotechnology Information (NCBI) under accession numbers PRJNA473368 for Fs and PRJNA473390 for Cd. We mapped approximately 99.9% of the reads to the assembled transcript sequences using the STAR program. Some reads were mapped in pairs with libraries of three replicates of Fs treated without radicicol (FS), Fs treated with radicicol (RFS), Cd treated without radicicol (CD), and Cd treated with radicicol (RCD). The mapping ratio ranged from 60.8 to 61.2% with an average of 60.9% for FS and ranged from 63.2 to 64.0% with an average of 63.7% for RFS. The mapping ratio ranged from 83.1 to 83.8% with an average of 83.4% for CD and ranged from 78.1 to 82.6% with an average value of 80.0% for RCD (Table S2).
3.3. Functional annotation and DEG identification
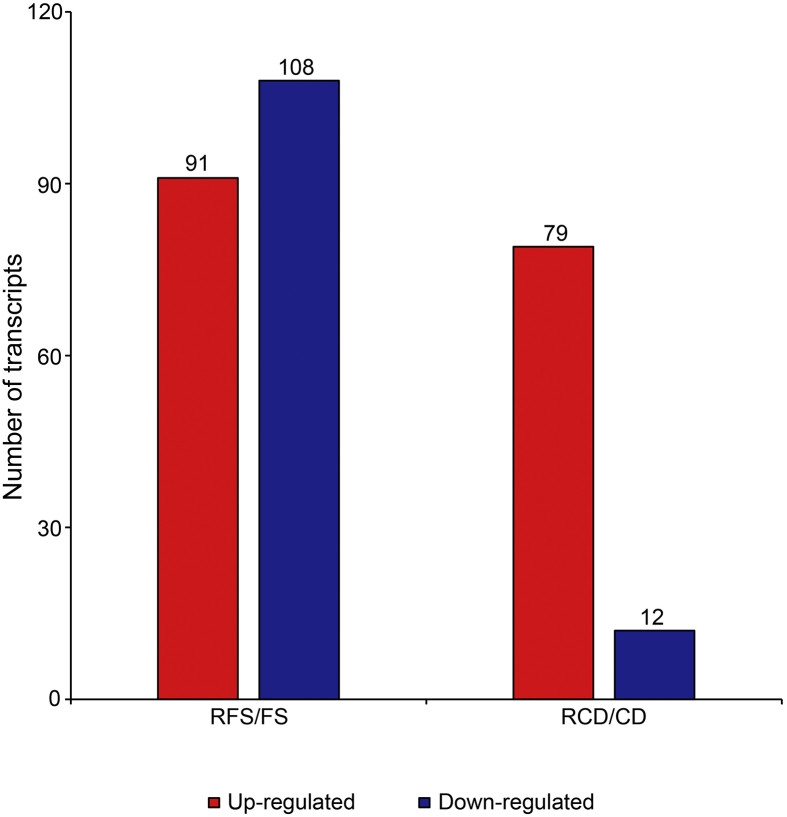
To provide insight into the Fs and Cd transcriptome profiles with and without radicicol treatment, we analyzed the transcript levels of 3801 functionally annotated genes in Fs and 2710 functionally annotated genes in Cd using |log2-fold-change| ≥ 2.0 and p-value ≤ 0.05 as the cutoff criteria for DEGs (Fig. 2). Based on these criteria, we found 199 genes that were expressed differentially in Fs, including 91 upregulated DEGs and 108 downregulated DEGs. We found 91 genes that were expressed differentially in Cd, including 79 upregulated DEGs and 12 downregulated DEGs. Functional annotation using the Fg transcript database showed that genes encoding chitin synthases and genes related to cell wall integrity were upregulated in Fs, whereas genes encoding NADH dehydrogenase and sugar transporters were upregulated in Cd after radicicol treatment (Table S3).
Fig. 2.
The number of differentially regulated transcripts in response to radicicol. The transcripts were functionally annotated using the Fg transcript database. Cd, C. destructans; Fs, F. solani; RCD, Cylindrocarpon destructans treated with radicicol; RFS, Fusarium solani treated with radicicol. All of the transcripts with differential expression met the |log2-fold-change| ≥ 2 and p-value ≤ 0.05 criteria.
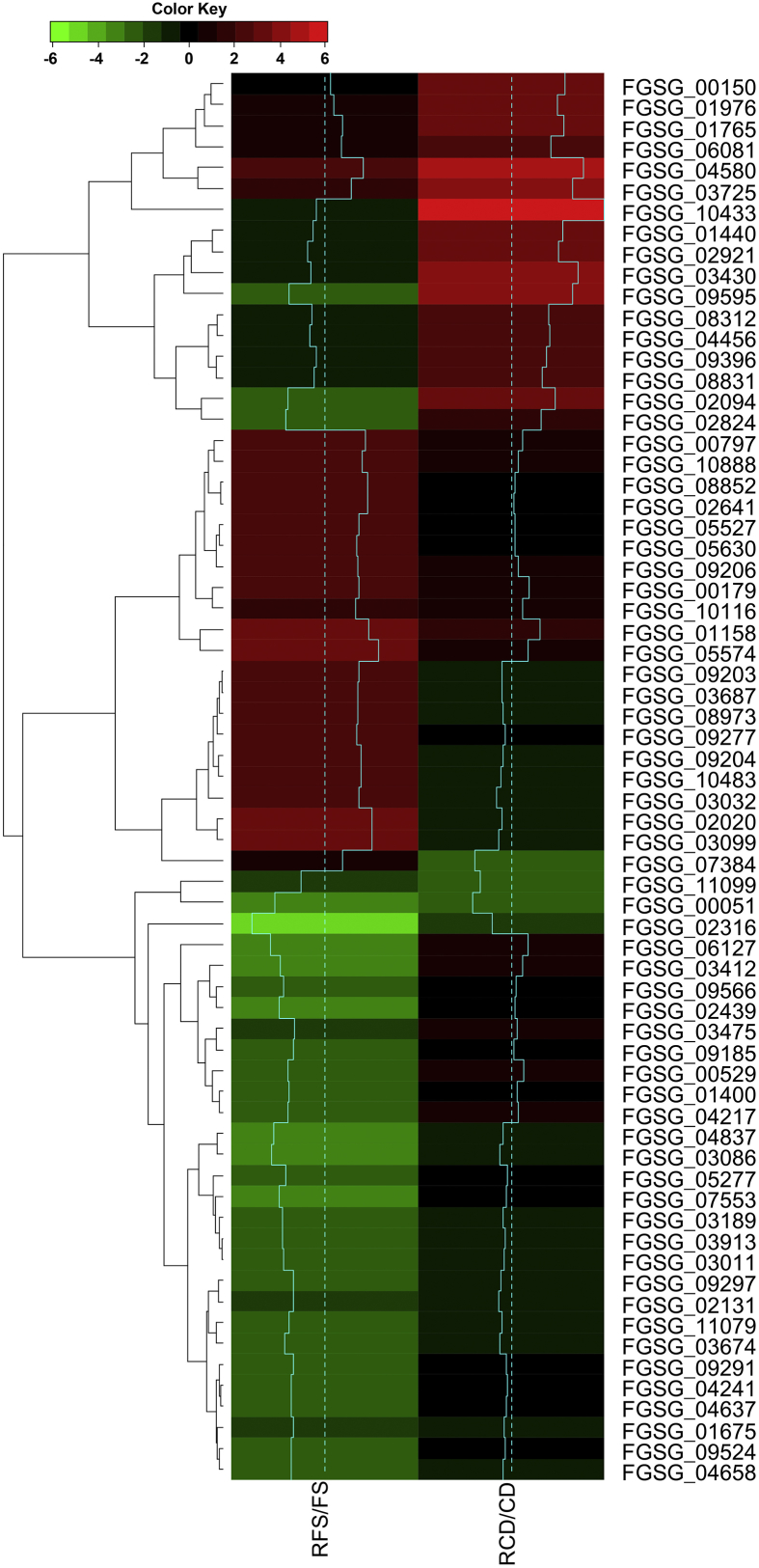
Based on the complete functional annotation using the Fg transcript database, the Fs and Cd DEGs were analyzed as a Venn diagram using Venny 2.1.0 (http://bioinfogp.cnb.csic.es/tools/venny/). The Venn diagram divided the genes into four regions containing 66 upregulated genes in Fs, 82 downregulated genes in Fs, 38 upregulated genes in Cd, and 11 downregulated genes in Cd. Of these genes, only one was upregulated in both Fs and Cd after radicicol treatment. Two genes were downregulated in Fs but upregulated in Cd after radicicol treatment (Fig. 3; Fig. S1). Functional annotation using the Fg transcript database showed that the gene upregulated in both Fs and Cd after radicicol treatment encodes a putative ATP-binding cassette (ABC) transporter, and the two genes that were expressed differently in Fs and Cd encode aflatoxin efflux pump and ammonium permease (mep1) homologs. In addition, we found that the nitrilase gene was downregulated in both Fs and Cd (Table 1).
Fig. 3.
Log2-fold-change heat map. Heat map showing upregulated and downregulated transcript (|log2-fold-change| ≥ 2, p-value ≤ 0.05) common in two treatments. Each gene was functionally annotated using Fg transcript database. The color gradation from green to red represents log2 (radicicol treatment/control) values from a minimum of −6 to a maximum of 6. The 0 point (black) indicates similar expression levels in the radicicol-treated and control samples. FS, F. solani without radicicol; RFS, F. solani treated with radicicol; CD, C. destructans without radicicol; RCD, C. destructans treated with radicicol.
Table 1.
Commonly expressed genes in Cylindrocarpon destructans (Cd) and Fusarium solani (Fs) in response to radicicol.
| F. graminearum gene ID | Putative function | Transcript level |
|||
|---|---|---|---|---|---|
| RCD/CD |
RFS/FS |
||||
| log2FC | p-value | log2FC | p-value | ||
| FGSG_04580 | ABC transporter (ABCG, PDR) | 4.7341 | 5 × 10−5 | 2.5126 | 5 × 10−5 |
| FGSG_09595 | Aflatoxin efflux pump | 4.0339 | 5 × 10−5 | −2.3219 | 5 × 10−5 |
| FGSG_02094 | Ammonium permease 1 | 2.8911 | 5 × 10−5 | −2.4211 | 5 × 10−5 |
| FGSG_00051 | Nitrilase | −2.5658 | 5 × 10−5 | −3.2662 | 0.0053 |
CD, Cylindrocarpon destructans treated without radicicol; FS, Fusarium solani treated without radicicol; RCD, Cylindrocarpon destructans treated with radicicol; RFS, Fusarium solani treated with radicicol
3.4. Functional classification of genes
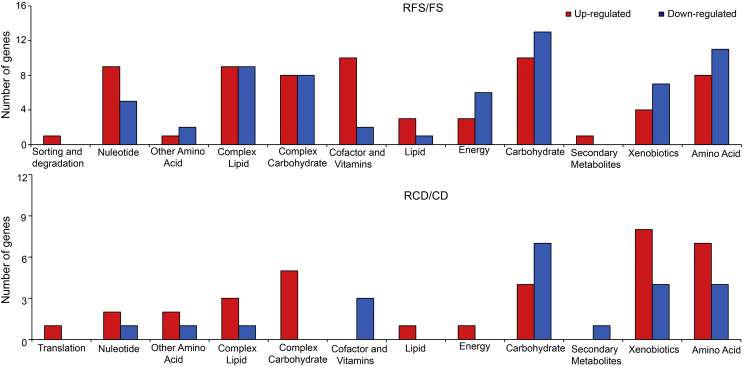
For a more detailed analysis, we applied |log2-fold-change| ≥ 1.0 and p-value ≤ 0.05 cutoff criteria to the DEGs in Fs and Cd. This analysis divided the genes into 13 functional groups (Fig. 4). Genes involved in nucleotide metabolism, lipid metabolism, and carbohydrate metabolism showed similar expression patterns in Fs and Cd, but genes involved in translation, sorting and degradation, metabolism of complex lipids, metabolism of complex carbohydrates, metabolism of cofactors and vitamins, energy metabolism, biosynthesis of secondary metabolites, biodegradation of xenobiotics, and amino acid metabolism showed different expression patterns in Fs and Cd. Expression of biodegradation of xenobiotics and amino acid metabolism genes differed the most between Fs and Cd as their expression patterns were the opposite of one another. These results suggest that Fs and Cd have both shared and unique pathways for radicicol resistance.
Fig. 4.
Functional classification of DEGs. RFS/FS and RCD/CD genes were classified into 13 groups, and all of the genes were functionally annotated using the Fg transcript database. All of the transcripts with differential expression met the |log2-fold-change| ≥ 1 and p-value ≤ 0.05 criteria. CD, C. destructans without radicicol; DEGs, differentially expressed genes; FS, F. solani without radicicol; RCD, C. destructans treated with radicicol; RFS, F. solani treated with radicicol.
3.5. Influence of FgAbc and FgAfla on radicicol resistance
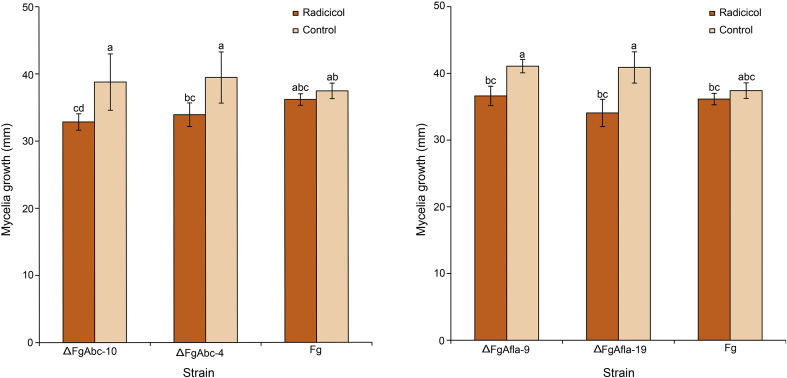
Transcriptome analyses showed that genes encoding a putative ABC transporter and an aflatoxin efflux pump were upregulated in Cd upon radicicol treatment. We identified the homologs of both genes using the Fg database, and we deleted each gene individually from wild-type Fg strain GZ3639. In the meantime, we observed the gene expression level of each target gene using qRT-PCR to confirm knock out of each gene (Fig. S2). The FgAbc and FgAfla mutants showed slightly reduced vegetative growth on radicicol-containing medium than on radicicol-free medium (Fig. 5), indicating that the ABC transporter and the aflatoxin efflux pump contribute to radicicol resistance although they are not major contributors to radicicol resistance.
Fig. 5.
Resistance of Fg mutant strains to radicicol. Strains were cultivated on MM with or without 50 mg/L of radicicol for 7 days at 20°C. ΔFgAbc (left), the ABC transporter gene deletion mutant; ΔFgAfla (right), the aflatoxin efflux pump gene deletion mutant; Fg, F. graminearum. Bars not sharing a letter are significantly different according to Tukey's test, and data are presented as mean ± standard deviation.
4. Discussion
Transcriptome analyses showed that only one gene, a putative ABC transporter, was upregulated in both Fs and Cd in response to exogenous radicicol treatment, suggesting that one mechanism of radicicol resistance in Fs and Cd involves pumping radicicol out of the cytosol. Interestingly, the Cd ABC transporter is most similar to ZRA1, which is an ABC transporter involved in zearalenone production in Fg [27], and radicicol and zearalenone are structurally similar. In this study, we deleted the putative ABC transporter and aflatoxin efflux pump genes in Fg to determine whether they contributed to radicicol resistance because we cannot yet create targeted gene deletions in Fs and Cd. Vegetative growth of the FgAbc and FgAfla mutants was lower on radicicol-containing medium than on radicicol-free medium, suggesting that these genes contribute to radicicol resistance, although they are not major factors (Fig. S3). In addition, a higher level of the ABC transporter transcript was found in Fg after radicicol treatment, which supports our FgAbc growth data (Fig. S4).
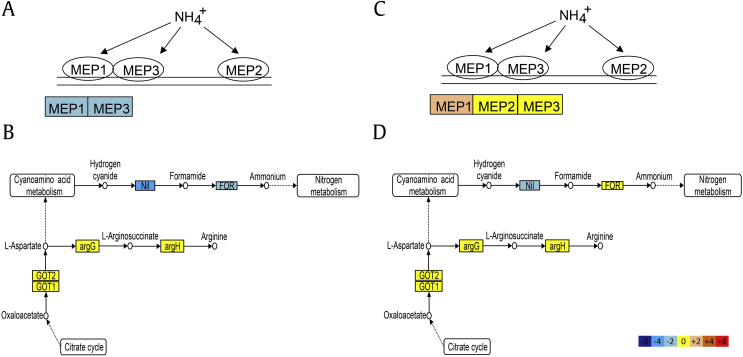
In addition, RNA-seq analyses showed downregulation of a putative nitrilase gene in both Fs and Cd after radicicol treatment (Fig. S1). Nitrilase catalyzes the hydrolysis of nitriles to carboxylic acids, including the conversion of a cyano group to a carboxylic group in cyanoamino acid metabolism [28]. The KEGG pathway of cyanoamino acid metabolism shows that all amino acids go through a series of steps to produce hydrogen cyanide, which then converts to formamide and ammonium, which are involved in nitrogen metabolism (Fig. 6). Functionally, nitrilase is similar to cyanide hydratase, which converts hydrogen cyanide to formamide. Therefore, downregulation of nitrilase in Fs and Cd may influence the production of formamide, which forms the structural base of nitrogenous bases, carboxylic acids, amino acids, acyclic nucleosides, sugars, and amino sugars [29], [30]. Moreover, RNA-seq analyses showed that expression of formamidase, which converts formamide to ammonium, is altered in Fs upon radicicol treatment. These data suggest that radicicol has an inhibitory effect on cyanoamino acid metabolism, especially in the conversion of hydrogen cyanide and formamide to ammonium, and thus may affect uptake of nitrogen by Fs as occurring in a pseudomonad [31].
Fig. 6.
Pathways identified by KEGG enrichment analysis. Nitrogen metabolism and cyanoamino acid metabolism pathways were identified for Fs (A–B) and C. destructans (C–D). The color gradation from blue to red represents log2 (radicicol treatment/control) values from a minimum of −8 to a maximum of +8. The 0 point (yellow) indicates similar expression levels in the radicicol-treated and control samples. The schematic diagram shows ammonia transfer by ammonia permease. MEP, ammonium permease; Nil, nitrilase; FOR, formamidase; GOT, aspartate aminotransferase; argG, argininosuccinate synthase; argH, argininosuccinate lyase.
The mep1 gene, which encodes for a membrane transport protein, was downregulated in Fs, but upregulated in Cd after radicicol treatment (Fig. S1). Among the three types of MEPs, MEP1 and MEP3 are low affinity ammonium permeases that allow cells to grow under nitrogen-limiting conditions with ammonium present at relatively low concentrations. MEP1 expression is highest in cells grown on poor nitrogen sources and in environments with low concentrations of ammonium [32], [33], [34]. MM contains inorganic nitrate as the sole nitrogen source; thus, Fs and Cd cultivated in MM are exposed to a low concentration of ammonium. Although inorganic nitrate is a good nitrogen source for fungi, it is typically not utilized unless cells lack a preferred nitrogen source, such as ammonium [35]. Downregulation of nitrilase by radicicol may result in further ammonium deficiency. Thus, upregulation of mep1 in Cd may be a response to compensate for the low concentration of ammonium present in the environment.
Nitrogen is an essential component of nucleic acids, adenosine triphosphate, amino acids, and proteins [36]. In Cd, the only DEG involved in nitrogen metabolism and cyanoamino acid metabolism was nitrilase; however, in Fs, radicicol treatment resulted in downregulation of nitrilase and formamidase genes (Fig. 6). Moreover, downregulation of mep1 and mep3 could affect the ability of Fs to uptake nitrogen. These results may explain the opposing expression patterns of genes involved in amino acid metabolism in Fs and in Cd (Fig. 4).
To successfully colonize plant hosts, pathogens must prevent the accumulation of intercellular toxins and natural toxic compounds. One strategy is to pump toxic compounds out of the cell through efflux pumps [37]. The aflatoxin efflux pump belongs to the major facilitator superfamily (MFS) of membrane transport proteins and is a more selective transporter than the ABC transporter [27]. In addition, MFS transporters facilitate movement of small solutes in response to chemiosmotic gradients [38], [39], [40], [41]. Thus, we propose two opposing reasons for upregulation of an MFS transporter after radicicol treatment in Cd; first, upregulation of an MFS transporter prevents extracellular radicicol from entering the cells, and second, radicicol can only be introduced into cells by an MFS transporter if the extracellular radicicol concentration is higher than the intracellular radicicol concentration.
Although radicicol treatment in Cd leads to downregulation of nitrilase, which contributes to nitrogen uptake, upregulation of an ABC transporter, an MFS transporter, and mep1 could compensate for this reduction in nitrogen uptake (Fig. 4); thus, Cd can both produce radicicol and protect itself from radicicol threats. Interestingly, although radicicol treatment resulted in the downregulation of an MFS transporter, mep1, mep3, nitrilase, and formamidase in Fs, Fs still survives radicicol treatment, suggesting that Fs uses a different mechanism to resist radicicol.
Our DEG data suggest that in addition to the ABC transporter, genes involved in nucleotide synthesis were upregulated in Fs and allowed Fs to overcome the negative effects of radicicol (Fig. 4). Cells require both pyrimidines and purines for nucleotide biosynthesis [42], and they can be obtained from the preferred nitrogen source glutamine [35], [43]. Glutamine can also be converted to glutamate, which is important in amino acid biosynthesis and glycolysis [44]. Our transcriptome analyses showed that expression of genes related to glutamine and glutamate metabolism were unaffected by radicicol treatment; thus, glutamine and glutamate metabolism may compensate for the negative effect of radicicol on extracellular ammonium and nitrogen uptake in Fs.
Furthermore, radicicol treatment resulted in upregulation of genes related to cell wall integrity and chitin synthases in Fs. Previous studies have shown that radicicol interacts with Hsp90, which leads to inhibition of MAP kinase signal transduction, which then results in defects in cell wall integrity [12], [13], [45], [46], [47], [48]. Because chitin forms the structural base of the cell wall in fungi [49], Fs must upregulate chitin synthesis to preserve the integrity of cell wall from the negative effects of radicicol. Our DEG data suggest that the upregulation of cell wall integrity genes in response to radicicol allows Fs to survive in the presence of radicicol.
Overall, our study demonstrates that Cd and Fs respond to radicicol using both shared and unique mechanisms. The putative ABC transporter was highly upregulated in both Fs and Cd, but the MFS membrane transporter, which has high similarity to the aflatoxin efflux pump, was upregulated only in Cd. Although the expression patterns of the ABC and MFS transporters were not identical in Fs and Cd, growth of our FgAbc and FgAfla deletion mutants showed that both transporters contribute to radicicol resistance. Our RNA-seq analyses showed that radicicol affects nitrogen metabolism and uptake. The Cd transcriptome data suggest that enhancement of ammonium uptake under nitrogen-limiting conditions can be triggered by radicicol. Furthermore, the Fs transcriptome data suggest that normal glutamine and glutamate metabolism and enhancement of cell wall integrity and chitin synthase genes contribute to the survival of Fs in radicicol-containing environments. Further studies on the transmission of the radicicol signal, which induces expression of radicicol resistance genes, will facilitate elucidation of the radicicol resistance mechanisms in Fs and Cd and will provide information for ginseng disease control in the field.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This work was supported by Rural Development Administration, Republic of Korea (PJ010119).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2018.11.005.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Liu C.X., Xiao P.G. Recent advances on ginseng research in China. J Ethnopharmacol. 1992;36:27–38. doi: 10.1016/0378-8741(92)90057-x. [DOI] [PubMed] [Google Scholar]
- 2.Chung H.S. Studies on Cylindrocarpon destructans (Zins.) Scholten causing root rot of ginseng. Rep Tottori Mycol Inst. 1975;12:127–138. [Google Scholar]
- 3.Kang Y., Lee S.H., Lee J. Development of a selective medium for the fungal pathogen Cylindrocarpon destructans using radicicol. Plant Pathol J. 2014;30:432–436. doi: 10.5423/PPJ.NT.08.2014.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yun T.K. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16:S3–S5. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park J.W., Lee S.W., Simon J.E., Häggblom M. A novel molecular diagnostic tool for detection of Ilyonectria radicicola, causal agent of root rot disease of ginseng. J Med Act Plants. 2017;5:20–27. [Google Scholar]
- 6.Yeon B.Y., Hyeon G.S., Bae Y.S., Lee S.W., Seong N.S., Kang S.W. Changes of soil chemical properties and root injury ratio by progress years of post-harvest in continuous cropping soils of ginseng. Kor J Med Crop Sci. 2007;15:157–161. [Google Scholar]
- 7.Seifert K.A., McMullen C.R., Yee D., Reeleder R.D., Dobinson K.F. Molecular differentiation and detection of ginseng-adapted isolates of the root rot fungus Cylindrocarpon destructans. Phytopathology. 2003;93:1533–1542. doi: 10.1094/PHYTO.2003.93.12.1533. [DOI] [PubMed] [Google Scholar]
- 8.Nozawa K., Nakajima S. Isolation of radicicol from Penicillium luteo-aurantium, and meleagrin, a new metabolite from Penicillium meleagrinum. J Nat Prod. 1979;42:374–377. [Google Scholar]
- 9.Delmotte P., Delmotte-Plaquee J. A new antifungal substance of fungal origin. Nature. 1953;171:344. doi: 10.1038/171344a0. [DOI] [PubMed] [Google Scholar]
- 10.Schulte T.W., Akinaga S., Murakata T., Agatsuma T., Sugimoto S., Nakano H., Lee Y.S., Simen B.B., Argon Y., Felts S. Interaction of radicicol with members of the heat shock protein 90 family of molecular chaperones. Mol Endocrinol. 1999;13:1435–1448. doi: 10.1210/mend.13.9.0339. [DOI] [PubMed] [Google Scholar]
- 11.Roe S.M., Prodromou C., O'Brien R., Ladbury J.E., Piper P.W., Pearl L.H. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42:260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S.V., Agatsuma T., Nakano H. Targeting of the protein chaperone, HSP90, by the transformation suppressing agent, radicicol. Oncogene. 1998;16:2639–2645. doi: 10.1038/sj.onc.1201790. [DOI] [PubMed] [Google Scholar]
- 13.Jeon Y.J., Kim Y.K., Lee M., Park S.M., Han S.B., Kim H.M. Radicicol suppresses expression of inducible nitric-oxide synthase by blocking p38 kinase and nuclear factor-κB/Rel in lipopolysaccharide-stimulated macrophages. J Pharmacol Exp Ther. 2000;294:548–554. [PubMed] [Google Scholar]
- 14.Lowenstein C.J., Glatt C.S., Bredt D.S., Snyder S.H. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci U S A. 1992;89:6711–6715. doi: 10.1073/pnas.89.15.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J.C., Young P.R. Role of CSBP/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leukoc Biol. 1996;59:152–157. doi: 10.1002/jlb.59.2.152. [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Higgins V.J. Nitric oxide has a regulatory effect in the germination of conidia of Colletotrichum coccodes. Fungal Genet Biol. 2005;42:284–292. doi: 10.1016/j.fgb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Gong X., Fu Y., Jiang D., Li G., Yi X., Peng Y. L-arginine is essential for conidiation in the filamentous fungus Coniothyrium minitans. Fungal Genet Biol. 2007;44:1368–1379. doi: 10.1016/j.fgb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Kong W., Huang C., Chen Q., Zou Y., Zhang J. Nitric oxide alleviates heat stress-induced oxidative damage in Pleurotus eryngii var. tuoliensis. Fungal Genet Biol. 2012;49:15–20. doi: 10.1016/j.fgb.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Kunert J. Effect of nitric oxide donors on survival of conidia, germination and growth of Aspergillus fumigatus in vitro. Folia Microbiol (Praha) 1995;40:238–244. doi: 10.1007/BF02814199. [DOI] [PubMed] [Google Scholar]
- 20.Hara K. Yokendo; Tokyo: 1930. Pathologia agriculturalis plantarum; pp. 481–483. [Google Scholar]
- 21.Chaverri P., Salgado C., Hirooka Y., Rossman A.Y., Samuels G.J. Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon-like anamorphs. Stud Mycol. 2011;68:57–78. doi: 10.3114/sim.2011.68.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabral A., Groenewald J.Z., Rego C., Oliveira H., Crous P.W. Cylindrocarpon root rot: multi-gene analysis reveals novel species within the Ilyonectria radicicola species complex. Mycological Progress. 2012;11:655–688. [Google Scholar]
- 23.Leslie J.F., Summerell B.A. Blackwell Publishing; Iowa: 2006. The Fusarium laboratory manual. [Google Scholar]
- 24.Yu J.H., Hamari Z., Han K.H., Seo J.A., Reyes-Dominguez Y., Scazzocchio C. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet Biol. 2004;41:973–981. doi: 10.1016/j.fgb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Park J., Lee H.H., Youn K., Kim S., Jung B., Lee J., Seo Y.S. Transcriptome analyses to understand effects of the Fusarium deoxynivalenol and nivalenol mycotoxins on Escherichia coli. J Biotechnol. 2014;192:231–239. doi: 10.1016/j.jbiotec.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Xie Y., Wang X. Comparative transcriptomic analysis identifies genes responsible for fruit count and oil yield in the oil tea plant Camellia chekiangoleosa. Sci Rep. 2018;8:6637. doi: 10.1038/s41598-018-24073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S., Son H., Lee J., Lee Y.R., Lee Y.W. A putative ABC transporter gene, ZRA1, is required for zearalenone production in Gibberella zeae. Curr Genet. 2011;57:343–351. doi: 10.1007/s00294-011-0352-4. [DOI] [PubMed] [Google Scholar]
- 28.Raybuck S.A. Microbes and microbial enzymes for cyanide degradation. Biodegradation. 1992;3:3–18. doi: 10.1007/BF00189632. [DOI] [PubMed] [Google Scholar]
- 29.Nolan L.M., Harnedy P.A., Turner P., Hearne A.B., O'Reilly C. The cyanide hydratase enzyme of Fusarium lateritium also has nitrilase activity. FEMS Microbiol Lett. 2003;221:161–165. doi: 10.1016/S0378-1097(03)00170-8. [DOI] [PubMed] [Google Scholar]
- 30.Saladino R., Botta G., Pino S., Costanzo G., Di Mauro E. Genetics first or metabolism first? The formamide clue. Chem Soc Rev. 2012;41:5526–5565. doi: 10.1039/c2cs35066a. [DOI] [PubMed] [Google Scholar]
- 31.White J.M., Jones D.D., Huang D., Gauthier J.J. Conversion of cyanide to formate and ammonia by a pseudomonad obtained from industrial wastewater. J Ind Microbiol. 1988;3:263–272. [Google Scholar]
- 32.Marini A.M., Vissers S., Urrestarazu A., André B. Cloning and expression of the MEP1 gene encoding an ammonium transporter in Saccharomyces cerevisiae. EMBO J. 1994;13:3456–3463. doi: 10.1002/j.1460-2075.1994.tb06651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou R., Jiang C., Zheng Q., Wang C., Xu J.R. The AreA transcription factor mediates the regulation of deoxynivalenol (DON) synthesis by ammonium and cyclic adenosine monophosphate (cAMP) signalling in Fusarium graminearum. Mol Plant Pathol. 2015;16:987–999. doi: 10.1111/mpp.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorenz M.C., Heitman J. The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae. EMBO J. 1998;17:1236–1247. doi: 10.1093/emboj/17.5.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marzluf G.A. Genetic regulation of nitrogen metabolism in the fungi. Microbiol Mol Biol Rev. 1997;61:17–32. doi: 10.1128/mmbr.61.1.17-32.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.March J. John Wiley & Sons; 1992. Advanced organic chemistry: reactions, mechanisms, and structure. [Google Scholar]
- 37.Coleman J.J., Mylonakis E. Efflux in fungi: la pièce de résistance. PLoS Pathog. 2009;5:e1000486. doi: 10.1371/journal.ppat.1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pao S.S., Paulsen I.T., Saier M.H., Jr. Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang P.K., Yu J., Yu J.H. aflT, a MFS transporter-encoding gene located in the aflatoxin gene cluster, does not have a significant role in aflatoxin secretion. Fungal Genet Biol. 2004;41:911–920. doi: 10.1016/j.fgb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Gao S.G., Chen J. Maize for Food, Feed, Nutrition and Environmental Security; Bangkok: 2014. Understanding of curvularia lunata pathogenicity differentiation induced by resistance varieties. [Google Scholar]
- 41.Walmsley A.R., Barrett M.P., Bringaud F., Gould G.W. Sugar transporters from bacteria, parasites and mammals: structure–activity relationships. Trends Biochem Sci. 1998;23:476–481. doi: 10.1016/s0968-0004(98)01326-7. [DOI] [PubMed] [Google Scholar]
- 42.Momin K.I., Bondge A.S., Surawanshi V.B., Dawale J.K. Synthesis and characterization of vital 6-aryl purines and their biological evaluation. Asian J Research Chem. 2017;10:845–851. [Google Scholar]
- 43.Garrett R.H., Grisham C.M. 5th ed. Brooks/Cole Cengage Learning; Pacific Grove: 2012. Biochemistry. [Google Scholar]
- 44.Watford M. Glutamine and glutamate: nonessential or essential amino acids? Anim Nutr. 2015;1:119–122. doi: 10.1016/j.aninu.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Truman A.W., Millson S.H., Nuttall J.M., Mollapour M., Prodromou C., Piper P.W. In the yeast heat shock response, Hsf1-directed induction of Hsp90 facilitates the activation of the Slt2 (Mpk1) mitogen-activated protein kinase required for cell integrity. Eukaryot Cell. 2007;6:744–752. doi: 10.1128/EC.00009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qi M., Elion E.A. MAP kinase pathways. J Cell Sci. 2005;118:3569–3572. doi: 10.1242/jcs.02470. [DOI] [PubMed] [Google Scholar]
- 47.Piper P.W., Truman A.W., Millson S.H., Nuttall J. Hsp90 chaperone control over transcriptional regulation by the yeast Slt2 (Mpk1) p and human ERK5 mitogen-activated protein kinases (MAPKs) Biochem Soc Trans. 2006;34:783–785. doi: 10.1042/BST0340783. [DOI] [PubMed] [Google Scholar]
- 48.Jain R., Valiante V., Remme N., Docimo T., Heinekamp T., Hertweck C., Gershenzon J., Haas H., Brakhage A.A. The MAP kinase MpkA controls cell wall integrity, oxidative stress response, gliotoxin production and iron adaptation in Aspergillus fumigatus. Mol Microbiol. 2011;82:39–53. doi: 10.1111/j.1365-2958.2011.07778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowman S.M., Free S.J. The structure and synthesis of the fungal cell wall. Bioessays. 2006;28:799–808. doi: 10.1002/bies.20441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.