Abstract
The sense of vision is the primary means by which we gather information from our surroundings, and vision loss, therefore, severely compromises the life of the affected individuals, their families, and society. Loss of vision becomes more frequent with age, and diabetic retinopathy, age-related macular degeneration, cataracts, and glaucoma are the major causes of vision impairment. To find active pharmacological compounds that might prevent or ameliorate the vision-threatening eye diseases, numerous studies have been performed, and some botanical compounds, including those extracted from ginseng, have been shown to possess beneficial effects in the treatment or prevention of common ocular diseases. In this review, we summarize the recent reports investigating the therapeutic effects of ginseng and ginsenosides on diverse ocular diseases and discuss their therapeutic potential.
Keywords: Age-related macular degeneration, Cataract, Diabetic retinopathy, Ginseng, Glaucoma
1. Introduction
Vision is the most used sense among the five senses and is one of the primary means by which we collect information from our surroundings. Loss of vision, thus, severely compromises the quality of life and has a significant impact on the lives of families and society, as well as the affected individuals [1], [2]. Impaired vision becomes more frequent with age, and the common causes of age-associated vision loss include diabetic retinopathy (DR), cataracts, glaucoma, and age-related macular degeneration (AMD) [3].
Many botanical compounds contain active pharmacological ingredients that have long been used to treat diverse diseases, and such natural compounds have added considerable value to the development of modern pharmaceutics [4]. Natural products have a distinctive diversity of multidimensional chemical structures, and currently, more than 60% of modern drugs in use have their origin in natural compounds [5]. For instance, terpene paclitaxel, an anticancer drug is originally isolated from Taxus baccata, and artemisinin, an antimalarial drug, is derived from Artemisia annua L [6], [7].
Ginseng, the roots of Panax ginseng, P. notoginseng, and P. quinquefolius, has been widely used as a remedy for a number of diseases and is currently being studied for its efficacy in a scientific way. Ginseng contains ginsenosides, gintonin, polysaccharides, peptides, phytosterols, polyacetylenes, polyacetylenic alcohols, fatty acids, etc., and among the active ingredients of ginseng, ginsenoside, which constitutes 2%–3% of ginseng, is the major pharmacological component [8]. About 40 ginsenosides have been identified, and they are generally divided into protopanaxadiols and protopanaxatriols based on their chemical structures (Fig. 1) [8]. Each ginsenoside exerts unique pharmacological activity in various diseases, and some ginsenosides such as ginsenoside Rb1, Rb2, Rc, and Rg3 have gone through clinical trials with human participants to test their antidiabetic, anticancer, and sperm-promoting activities [9], [10], [11], [12], [13], [14].
Fig. 1.
Structure of ginsenosides. (A) Protopanaxadiol- and protopanaxatriol-type saponins, (B) ginsenoside Rg5, (C) ginsenoside Rk1, and (D) compound K. Glc, β-D-glucose; Ara(p), arabinose (pyranose); Ara(f), arabinose (furanose); Rha, α-L-rhamnose; NR1, notoginsenoside R1; Xyl, β-D-xylose.
In an attempt to find plant-derived active ingredients to prevent or ameliorate the vision-threatening eye diseases, ample studies have been performed. As a result, some botanical compounds, such as curcumin, saffron, catechin, lutein, Ginkgo biloba extract, ginseng, resveratrol, and quercetin, have been shown to possess beneficial effects in the treatment or prevention of common ocular diseases [15]. In particular, numerous studies have shown the effects of ginseng extract or ginsenoside on AMD, DR, cataracts, and glaucoma. In this review, we summarize the recent reports investigating the effects of ginseng and ginsenosides on diverse ocular diseases. The studies on the extracts from P. ginseng and P. quinquefolius, saponin fraction of P. notoginseng, ginsenosides Rb1, Rg1, Rg3, and Rk1, notoginsenoside R1 (NR1), compound K, dammarendiol II, and some Chinese herbal medicine containing P. notoginseng will be reviewed, and their therapeutic potential will be discussed.
2. Basic anatomy of the eye
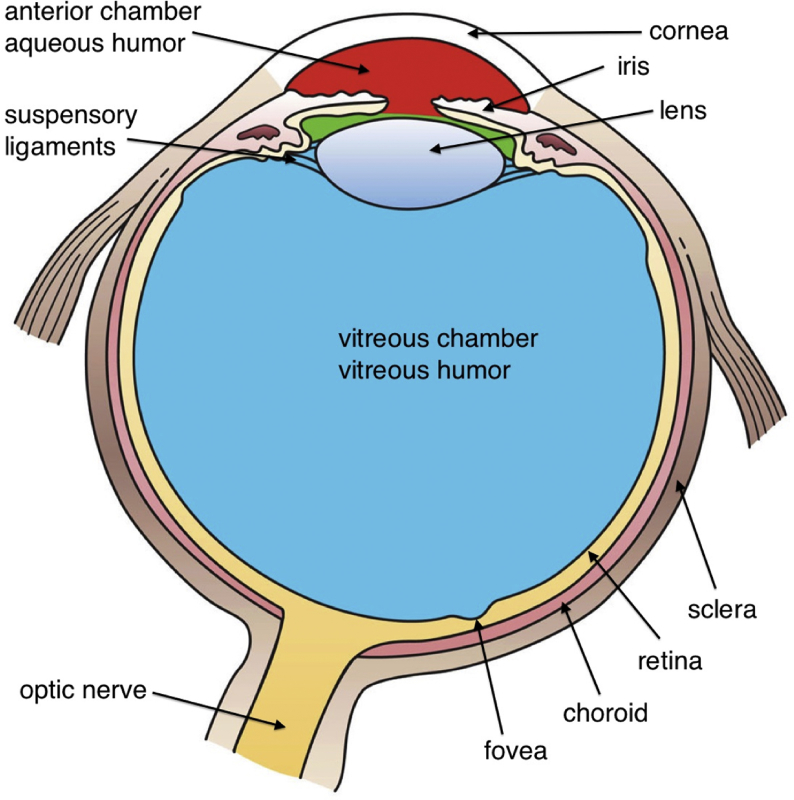
The human eye is a fluid-filled sphere consisting of three layers of tissue (Fig. 2). The outermost layer is composed of the sclera, a tough white fibrous tissue, and the cornea, the transparent front part of the eye that covers the iris, pupil, and the anterior chamber [16]. The cornea controls and focuses the entry of light into the eye and is responsible for 65%–75% of the eye's focusing power [17].
Fig. 2.
Basic anatomy of the eye. Cross section of the eye showing major structures (“Three internal chambers of the eye” by Holly Fischer is licensed under CC BY 3.0).
The middle layer consists of the choroid, ciliary body, and iris. The ciliary body is a circular band of muscles that control lens shape and adjust the refractive power of the lens [18]. It also produces aqueous humor and provides nutrients for avascular tissues, such as the cornea [19]. The iris is the colored part of the eye. It adjusts the size of the pupil and modulates the amount of light passing through the pupil. The choroid is a layer of blood vessels that provides oxygen and nourishment to the photoreceptors of the retina [20].
Light focused by the cornea and crystalline lens reaches the retina, the innermost layer of the eye. The retina contains light-sensitive neurons and translates an optical image into neural impulses to transmit to the brain creating visual perception. The vertebrate retina has 10 distinct layers, including retinal pigment epithelium (RPE), photoreceptors, bipolar cells, and ganglion cells, among others [21]. RPE is a single layer of cells above the photoreceptors, and it supports the health and integrity of photoreceptors by supplying essential nutrition and removing waste [22]. Photoreceptors are the main cells that are sensitive to light, and the adult human retina has approximately 63 million photoreceptors [23]. There are two types of photoreceptors, rods and cones [24]. Rods mainly function in dim light and provide peripheral and black-and-white vision, whereas cones provide central and color vision. Macula is located in the central part of the retina, providing sharp central vision, and contains a very high concentration of cones. The fovea is a small dent located in the center of the macula and is responsible for the sharpest vision (Fig. 2).
The lens is suspended from the ciliary body by the suspensory ligaments or zonules that change lens shape with the aid of the ciliary muscle. The space between the lens and the retina is filled with vitreous humor, a thick, gelatinous substance. The vitreous humor accounts for approximately 80% of the eye volume, maintaining the eye shape, and contains phagocytic cells to remove blood and debris that might impede the smooth transmission of light [25].
Bruch's membrane (BM) is the innermost layer of the choroid (Fig. 3). BM forms a pentalaminar structure, which consists of an RPE basement membrane, inner collagenous zone, central elastic fiber bands, outer collagenous zone, and choroid basement membrane [26]. The RPE discards waste products from photoreceptors to the choroid through the BM. In fact, the cause of age-related degeneration seems to be related to a malfunction of BM [27].
Fig. 3.
Bruch's membrane (BM). BM is placed between the retinal pigment epithelium (RPE) and choroidal capillaries and consists of an RPE basement membrane, inner collagenous zone, central elastic fiber bands, outer collagenous zone, and choroid basement membrane (“Illustration of Bruch's membrane” by Evan Mason is licensed under CC BY-SA 4.0).
3. Age-related macular degeneration
AMD is one of the leading causes of visual impairment in people older than 50 years in developed countries [28]. AMD progressively destroys the macula, a pigmented area near the center of the retina, and impairs central vision. In addition to age, risk factors for AMD include smoking, hypercholesterolemia, hypertension, obesity, arteriosclerosis, and family history. AMD can be classified into early-stage AMD with no vision loss and late-stage AMD accompanied by a visual impairment. The late-stage AMD can be further subdivided into wet or neovascular AMD and dry or atrophic AMD [29]. Choroidal neovascularization caused mainly by angiogenic cytokines, such as vascular endothelial growth factor (VEGF) A, is a major contributing factor for neovascular AMD, but AMD is also associated with an increase in oxidative stress, inflammation, and impaired proteasomal or lysosomal function, causing the formation of intracellular and extracellular deposits. Currently, intravitreous anti-VEGF therapy using monoclonal antibodies or aptamers is a major treatment option for neovascular AMD [30].
BM is a five-layered extracellular matrix (ECM) located between the retina and choroid [26]. BM facilitates the exchange of nutrients and metabolic waste between the choriocapillaris and the RPE and supports the differentiation and adhesion of RPE cells. Studies have shown that age-associated thickening of BM, accumulation of lipid and protein deposits, and reduced transport capacity are related to the pathogenesis of AMD, and accordingly, therapeutic approaches to improve transport across BM have been made for AMD prevention or treatment.
By using Bruch's choroid obtained from human donor eyes, Lee et al [31] carried out interesting experiments to evaluate the beneficial effects of ginseng on BM. They mounted human Bruch's choroid preparations in modified Ussing chambers and measured the changes in hydraulic conductivity and diffusional transport of fluorescein isothiocyanate (FITC)-labeled albumin after 24-hr treatment with Korean Red Ginseng extract (KRGE, P. ginseng); ginsenoside Rb1; compound K; or ginsenoside mix containing ginsenosides Rb1, Rb2, Rc, Rd, Rg1, Rg2, Re, and Rf. They observed that the transport capacity of human BM was improved by KRGE, ginsenoside Rb1, and compound K treatment. Furthermore, the perfusion of BM with KRGE or ginsenoside Rb1 leads to the increased removal of protein and lipid deposits, such as cholesterol and phosphatidylcholine, from the membrane.
The RPE is a monolayer of pigmented cells between BM and retinal photoreceptors [32]. RPE cells support the integrity and survival of retinal photoreceptors, and RPE cell death is one of the hallmarks of AMD. Betts et al [33] reported that ginsenoside Rb1 induced a proliferation of adult RPE cells (ARPE-19) in vitro, whereas it inhibited the release of VEGF from ARPE-19 cells.
Taken together, KRGE and ginsenoside could be valued to slow down the onset and progression of AMD by improving transport capacity across BM or inhibiting VEGF production in ARPE-19 cells.
4. Cataracts
The role of the ocular lens is to bend light rays to form a clear image on the retina, and its transparency is critical for providing visual acuity [34]. The lens is composed of layers of elongated fiber cells that are filled with water-soluble α-, β-, and γ-crystallin proteins [35]. The cyrstallin proteins form soluble oligomers and function in maintaining high refractive power and transparency of the lens.
Cataract is an age-associated loss of transparency of the ocular lens caused by an accumulation of protein aggregates in the lens [34], [36]. During aging, crystalline proteins are damaged by oxidative stress resulting from reactive oxygen species (ROS) and UV irradiation, and the ensuing insoluble protein aggregates lead to the opacity of the lens and interference with vision. Although aging is a leading risk factor for developing cataracts, a cataract could also be associated with a genetic mutation of crystallin, prolonged use of steroids, diabetes, inflammation, and infection in the eye. The treatment for cataracts is surgery involving the replacement of the dysfunctional and cloudy lens with artificial ones. In addition, the use of phytochemical antioxidants to inhibit protein damage and aggregate formation has been suggested [15].
Sun ginseng is a type of heat-processed ginseng obtained by steaming a raw ginseng at a higher temperature (above 100°) than that used for red ginseng [37]. Sun ginseng contained an increased amount of ginsenosides Rg3, Rg5, and Rk1 and had improved biological activities compared with white ginseng and red ginseng [38], [39]. Lee et al [40] compared the protective effects of saponin and nonsaponin fractions of sun ginseng extract on cataract in a selenite-induced rat cataract model. They found that the saponin fraction had no effects on cataract, whereas the nonsaponin fraction exerted protective effects on the incidence and progression of cataracts by decreasing the size of the cataractous lens area and by lowering cataract grade representing cataract severity. Future studies need to be conducted to identify a single compound with anticataract activity from the nonsaponin fraction of sun ginseng extract and to develop prophylactic and therapeutic agents for cataract.
5. Glaucoma
Glaucoma is the second principal cause of blindness after cataracts and is characterized by high intraocular pressure (IOP), irreversible optic neuropathy, and visual field loss [41]. Because optic nerve impairment through apoptosis of retinal ganglion cell is mainly caused by high IOP, the major treatment for glaucoma is to reduce IOP with eye drops, oral drugs, laser treatment, or surgery [42]. In addition to lowering IOP, neuroprotectants, which prevent the death of retinal ganglion cells, might be effective in the prevention and treatment of glaucoma, and botanical compounds such as Ginkgo bibloba extract and danshen (Salvia miltiorrhiza), glutamate antagonists, calcium channel blockers, and nerve growth factor have been proposed as possible neuroprotectants [15], [43].
Glaucoma patients using antiglaucoma eye drops often suffer from dry eye syndrome, such as ocular discomfort, visual disturbance, tear film instability, and hyperosmolarity that may lead to ocular surface damage [44]. Although ginseng has not shown direct effects on glaucoma, daily supplementation of 3 g of Korean Red Ginseng (KRG) for 8 weeks showed beneficial effects on dry eye syndrome in glaucoma patients by improving tear film stability and the total ocular surface disease index score, suggesting the use of KRG as a nutritional supplement for antiglaucoma eye drops.
The glaucomatous optic nerve damage model can be established by injecting 0.3% carbomer solution into the anterior chamber of rabbits. Wang et al [45] reported that intravitreous injection of ginsenoside Rg1 with or without ultrasound-targeted microbubble destruction was effective in the treatment of glaucomatous optic nerve damage in rabbits. They observed that ginsenoside Rg1 reduced IOP, increased the thickness of the retina, and lowered the oxidative stress by reducing malondialdehyde and nitric oxide and by increasing superoxide dismutase. Because a well-controlled regulation of IOP is critical for normal eye function, it would be worth investigating other types of ginsenosides and ginsenoside Rg1 for their IOP-lowering activities for glaucoma.
6. Diabetic retinopathy
Diabetes mellitus (DM) is one of the core public health challenges worldwide which can affect many organs, including eyes. Diabetic eye diseases involve diverse pathological eye conditions, such as DR, diabetic macular edema, cataracts, and glaucoma, all of which have the potential to progress to vision loss. DR is the most common cause of vision loss associated with DM [46]. High blood glucose damages the wall of tiny blood vessels in the retina, resulting in leakage of fluid and blood and distortion of vision. In advanced-stage DR, new but fragile blood vessels are formed on the retina, leading to scars and retinal detachment, followed by permanent vision loss [47]. Studies have shown that chronic inflammation is one of the prominent features of DR, and many physiological abnormalities, such as increases in tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, VEGF, IL-8, and IL-6, have been found in the retina or vitreous humor of diabetic animals and patients. Accordingly, antiinflammatory therapy along with common DR treatment, including glycemic control, laser surgery, vitrectomy, and anti-VEGF therapy, would be beneficial for the prevention or delay of disease progression [48].
Compared to other types of ocular diseases, numerous studies have investigated the therapeutic activity of ginseng and ginsenosides on DR in vitro and in vivo. TNF-α is suggested as an indirect inducer of angiogenesis by increasing the expression of VEGF. Intragastric administration of ginsenoside Rg3 lowered the expression of TNF-α and VEGF in the ganglion cell layer and inner nuclear layer in the retina of diabetic rats induced by streptozotocin [49]. Ginsenoside Rb1 also inhibited the release of VEGF from adult RPE cells, as previously mentioned, suggesting that ginsenoside Rb1 might have beneficial effects on DR, as well as AMD, by attenuating retinal neovascularization [33]. Furthermore, the treatment of human retina microvascular endothelial cells with ginsenoside Rk1 showed a significant decrease in VEGF-induced and advanced glycation end products–induced retinal endothelial permeability, thereby reducing retinal edema [50].
In addition to the inhibition of VEGF expression, impeding the apoptosis of retinal ganglion cells could also be helpful for the treatment of early-stage DR because apoptosis of retinal ganglion cells leads to the gradual loss of retinal neurons in the early phase of DR. Ginsenosides Rg1, Rh1, Rd, and Re are four major components in the crude saponin fraction of P. notoginseng (CSPN), and CSPN has been shown to deter palmitate-induced apoptosis and loss of postsynaptic density protein-95, which plays a critical role in synaptic development and neural plasticity in retinal ganglion cells (RGC-5 cells) [51]. In addition, CSPN prevented RGC-5 cells from apoptosis by reducing the generation of ROS and endoplasmic reticulum stress. Similarly, Fan et al [52], [53] reported that P. notoginseng saponins (PNS) and NR1, a unique ingredient of P. notoginseng, increased cell viability in high glucose–treated rat retinal capillary endothelial cells (RCECs) accompanied by a decrease in glucose-induced generation of ROS. PNS also induced the activities of total superoxide dismutase, Mn-SOD, catalase, and glutathione peroxidase, whereas NR1 maintained a balanced cellular redox homeostasis by increasing the ratios of oxidized nicotinamide adenine dinucleotide (NAD+)/reduced NAD+ (NADH), reduced nicotinamide adenine dinucleotide phosphate (NADPH)/oxidized nicotinamide adenine dinucleotide phosphate (NADP+), and glutatione (GSH)/glutatione disulfide (GSSG) in RCECs. The results suggest a protective role of PNS and NR1 against oxidative injury in DR, in part through their antioxidative function.
Chinese herbal medicine, such as Fufang Xueshuantong and the compound danshen dripping pill (CDDP), contains P. notoginseng and has been shown to possess protective effects against DR [54], [55]. Jian et al [54] combined the main constituents of Fufang Xueshuantong (cFXT), which included saponins of P. notoginseng (NR1, ginsenoside Rg1, ginsenoside Rb1, and other saponins), harpagoside, cryptotanshinone, tanshinone I, and astragaloside A, and found that cFXT treatment relieved retinal lesions. In more detail, cFXT diminished the proliferation of acellular capillaries and abnormal expression of VEGF and pigment epithelium-derived factor, intercellular adhesion molecule 1, endothelin-1 (ET-1), and occludin in the retinas of diabetic rats. In a randomized, double-blind, placebo-controlled clinical trial with 223 patients with nonproliferative DR, CDDP containing P. notoginseng, as well as Salvia miltiorrhiza and borneol, reduced the symptoms of DR [55]. In fundoscopic examination, CDDP improved capillary hemangioma, hard exudates, and retinal hemorrhage, although the clinical observation time was relatively short (24 weeks) for a chronic disease. In addition, an aqueous extract of Radix Astragali, Radix Angelica sinensis, and P. notoginseng also prevented the pathogenesis and/or progression of DR when it was administered to Goto-Kakizaki rats, a nonobese model of spontaneous type 2 diabetes, and streptozotocin-induced Sprague-Dawley rats [56]. Radix Astragali, Radix Angelica sinensis, and P. notoginseng decreased the expression of inflammatory factors, such as IL-1β, IL-6, TNF-α, nuclear factor-kappa B, monocyte chemoattractant protein 1, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1, in the retinas of Goto-Kakizaki rats, and inhibited leukostasis, acellular capillaries, and vascular leakage in diabetic rats.
Along with P. notoginseng, North American ginseng (P. quinquefolius) also showed preventive effects on DR [57]. A 75% ethanol extract of the North American ginseng root contained ginsenosides Rg1, Re, Rb1, Rc, Rb2, and Re and reduced oxidative stress and the levels of retinal ECM, VEGF, and ET-1 in type I and type 2 diabetic mice.
During the ginsenoside biosynthesis process, dammarenediol-II is a precursor of protopanaxadiols and protopanaxatriols, which turn into panaxadiol-type ginsenoside and panaxatriol-type ginsenoside, respectively. Ha et al [58] demonstrated that dammarenediol-II inhibited VEGF-induced generation of intracellular ROS, stress fiber formation, and vascular endothelial (VE)-cadherin disruption in human umbilical vein endothelial cells. Furthermore, dammarenediol-II prevented VEGF-induced vascular leakage in the retina of diabetic mice, confirming the protective role of dammarenediol-II against DR in vivo.
Apart from testing the therapeutic activities of ginseng and ginsenosides, the changes in the expression retinal genes in diabetic rat upon KRG treatment have also been analyzed [59]. Although KRG treatment did not improve DM, it reversed the expression of DR-associated genes that were differentially expressed in diabetic rats. For example, Gfap encoding a Glial fibrillary acidic protein, a representative marker associated with the polyol pathway in DR, Crygb encoding Gamma-crystallin B, and Igf1r (insulin-like growth factor 1 receptor) were upregulated in the retinal cells of diabetic rats, whereas they were all downregulated in those of KRG-treated rats, implying that KRG might have therapeutic effects on DR by regulating the expression of relevant genes.
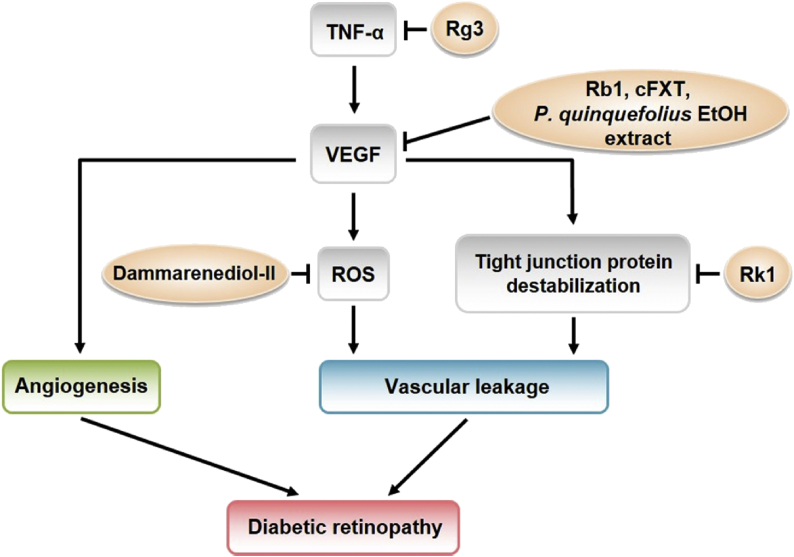
The therapeutic effects of P. quinquefolius, Fufang Xueshuantong containing saponins of P. notoginseng, ginsenosides Rb1, Rg3, and Rk1, and dammarenediol-II on VEGF signaling pathway in DR have been summarized in Fig. 4.
Fig. 4.
Therapeutic effect of ginseng on VEGF signaling pathway in diabetic retinopathy ginsenoside Rg3 inhibits the expression of VEGF by suppressing TNF-α production. Ginsenoside Rb1, cFXT, and P. quinquefolius ethanol extracts repress the expression of VEGF. Dammarenediol-II inhibits the VEGF-induced ROS production. Ginsenoside Rk1 increases the stability of tight junction proteins and reduces retinal endothelial permeability. cFXT, constituents of Fufang Xueshuantong; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-alpha; VEGF, vascular endothelial growth factor.
7. Closing remarks
The contributing factors to diverse ocular diseases are multifarious and are mostly under investigation. Pharmacological treatments to prevent or defer disease progression and subsequent blindness would bring in an increase in the quality of life and a decrease in economic burdens. Although ginseng has been widely used through history for the preventive and therapeutic intervention against diverse diseases, its application in eye diseases has been limited. Nonetheless, attempts have been insistently made to reveal the prophylactic and protective effects of ginseng and ginsenosides on diverse ocular diseases (Table 1). The exact molecular mechanisms have not yet been clearly identified, but antiinflammation, antioxidation, elimination of waste products, and inhibition of VEGF action have been proposed to be involved. Given an increasing interest in ginseng for their roles in ocular diseases, novel therapeutic possibilities of ginseng will be uncovered in the near future.
Table 1.
Bioactivity of Ginseng compounds.
| Disease | Compound | Bioactivity | Reference |
|---|---|---|---|
| AMD | P. ginseng ginsenoside Rb1 | Increased removal of protein and lipid deposit from human BM | [31] |
| P. ginseng ginsenoside Rb1 Compound K | Improved transport capacity of the human BM | [31] | |
| Ginsenoside Rb1 | Induced proliferation of adult RPE cell | [33] | |
| Inhibited release of VEGF from ARPE-19 cell | [33] | ||
| Cataract | Nonsaponin fraction of sun ginseng | Decrease in the incidence and progression of cataract in a rat model | [40] |
| Glaucoma | P. ginseng | Relieving the side effects of antiglaucoma eyedrop in glaucoma patients | [44] |
| Ginsenoside Rg1 | Reduce IOP, increased thickness of retina, and lower oxidative stress in a rabbit model | [45] | |
| DR | Ginsenoside Rb1 | Inhibition of VEGF release from ARPE-19 cell | [33] |
| Ginsenoside Rg3 | Decreased expression of TNF-α and VEGF in ganglion cells | [49] | |
| Ginsenoside Rk1 | Reduction in retinal edema in human retina microvascular endothelial cells | [50] | |
| P. ginseng | Reduced expression of Gfap, Crygb, and Igf1r (DR marker gene) in rat retinal cell | [59] | |
| P. quinquefolius ethanol extract | Reduction in oxidative stress level of retinal ECM, VEGF, and ET-1 in mouse model | [57] | |
| CSPN | Prevention of apoptosis and loss of PSD-95 in ganglion cells | [51] | |
| PNS and NR1 | Increase cell viability in rat RCEC | [52], [53] | |
| cFXT | Decrease in the proliferation of acellular capillaries | [54] | |
| RRP | Prevention of the pathogenesis and/or progression of DR in a rat model | [56] | |
| Dammarenediol-II | Inhibition of VEGF-induced generation of intracellular ROS in HUVEC | [58] | |
| Prevention of VEGF-induced vascular leakage in the retina of a mouse model | [58] | ||
| CDDP | Reduced symptoms of DR in a clinical trial | [55] |
ARPE-19, adult retinal epithelial cell; AMD, age-related macular degeneration; BM, Bruch's membrane; CDDP, compound Danshen Dripping pill; cFXT, constituents of Fufang Xueshuantong; CSPN, crude saponin fraction of P. notoginseng; DR, diabetic retinopathy; ECM, extracellular matrix; ET-1, endothelin-1; HUVEC, human umbilical vein endothelial cell; IOP, intraocular pressure; NR1, notoginsenoside R1; PNS, P. notoginseng saponin; PSD-95, postsynaptic density protein-95; RCEC, retinal capillary endothelial cells; ROS, reactive oxygen species; RPE, retinal pigment epithelial; RRP, Radix Astragali, Radix Angelica sinensis, and P. notoginseng; TNF-α, tumor necrosis factor-alpha; VEGF, vascular endothelial growth factor.
Conflicts of interest
The author declares no conflicts of interest.
Acknowledgments
This work was supported by the National Research Foundation (NRF) funded by the Ministry of Science, ICT, and Future Planning (2015R1C1A2A01054457 to H.M.), and by the Chung-Ang University Research Scholarship Grants in 2018 (S.H.).
References
- 1.West S.K., Munoz B., Rubin G.S., Schein O.D., Bandeen-Roche K., Zeger S., German S., Fried L.P. Function and visual impairment in a population-based study of older adults. The SEE project. Salisbury Eye Evaluation. Invest Ophthalmol Vis Sci. 1997;38:72–82. [PubMed] [Google Scholar]
- 2.Klein R., Klein B.E., Lee K.E., Cruickshanks K.J., Gangnon R.E. Changes in visual acuity in a population over a 15-year period: the beaver dam eye study. Am J Ophthalmol. 2006;142:539–549. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Lin J.B., Tsubota K., Apte R.S. A glimpse at the aging eye. NPJ Aging Mech Dis. 2016;2:16003. doi: 10.1038/npjamd.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler M.S. The role of natural product chemistry in drug discovery. J Nat Prod. 2004;67:2141–2153. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 5.Mathur S., Hoskins C. Drug development: lessons from nature. Biomed Rep. 2017;6:612–614. doi: 10.3892/br.2017.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wani M.C., Taylor H.L., Wall M.E., Coggon P., McPhail A.T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 7.Chang Z. The discovery of Qinghaosu (artemisinin) as an effective anti-malaria drug: a unique China story. Sci China Life Sci. 2016;59:81–88. doi: 10.1007/s11427-015-4988-z. [DOI] [PubMed] [Google Scholar]
- 8.Lü J.M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang W.H., Tsai Y.L., Huang C.Y., Hsieh C.C., Chaunchaiyakul R., Fang Y., Lee S.D., Kuo C.H. Null effect of ginsenoside Rb1 on improving glycemic status in men during a resistance training recovery. J Int Soc Sports Nutr. 2015;12:34. doi: 10.1186/s12970-015-0095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J.C., Chen L.D., Tsauer W., Tsai C.C., Chen B.C., Chen Y.J. Effects of Ginsenoside Rb2 and Rc on inferior human sperm motility in vitro. Am J Chin Med. 2001;29:155–160. doi: 10.1142/S0192415X01000174. [DOI] [PubMed] [Google Scholar]
- 11.Lu P., Su W., Miao Z.H., Niu H.R., Liu J., Hua Q.L. Effect and mechanism of ginsenoside Rg3 on postoperative life span of patients with non-small cell lung cancer. Chin J Integr Med. 2008;14:33–36. doi: 10.1007/s11655-007-9002-6. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z.J., Cheng J., Huang Y.P., Han S.L., Liu N.X., Zhu G.B., Yao J.G. [Effect of adjuvant chemotherapy of ginsenoside Rg3 combined with mitomycin C and tegafur in advanced gastric cancer] Zhonghua Wei Chang Wai Ke Za Zhi. 2007;10:64–66. [PubMed] [Google Scholar]
- 13.Sun Y., Lin H., Zhu Y., Feng J., Chen Z., Li G., Zhang X., Zhang Z., Tang J., Shi M. A randomized, prospective, multi-centre clinical trial of NP regimen (vinorelbine+cisplatin) plus Gensing Rg3 in the treatment of advanced non-small cell lung cancer patients. Zhongguo Fei Ai Za Zhi. 2006;9:254–258. doi: 10.3779/j.issn.1009-3419.2006.03.09. [DOI] [PubMed] [Google Scholar]
- 14.Li Y., Wang Y., Niu K., Chen X., Xia L., Lu D., Kong R., Chen Z., Duan Y., Sun J. Clinical benefit from EGFR-TKI plus ginsenoside Rg3 in patients with advanced non-small cell lung cancer harboring EGFR active mutation. Oncotarget. 2016;7:70535–70545. doi: 10.18632/oncotarget.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huynh T.P., Mann S.N., Mandal N.A. Botanical compounds: effects on major eye diseases. Evid Based Complement Alternat Med. 2013;2013:549174. doi: 10.1155/2013/549174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCaa C.S. The eye and visual nervous system: anatomy, physiology and toxicology. Environ Health Perspect. 1982;44:1–8. doi: 10.1289/ehp.82441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S.Y., Yu H.C., Wang I.J., Sun C.K. Infrared-based third and second harmonic generation imaging of cornea. J Biomed Opt. 2009;14:044012. doi: 10.1117/1.3183805. [DOI] [PubMed] [Google Scholar]
- 18.Beebe D.C. Development of the ciliary body: a brief review. Trans Ophthalmol Soc U K. 1986;105(Pt 2):123–130. [PubMed] [Google Scholar]
- 19.Malhotra A., Minja F.J., Crum A., Burrowes D. Ocular anatomy and cross-sectional imaging of the eye. Semin Ultrasound CT MR. 2011;32:2–13. doi: 10.1053/j.sult.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Willoughby C.E., Ponzin D., Ferrari S., Lobo A., Landau K., Omidi Y. Anatomy and physiology of the human eye: effects of mucopolysaccharidoses disease on structure and function–a review. Clin Exp Ophthalmol. 2010;38:2–11. [Google Scholar]
- 21.Smerdon D. Anatomy of the eye and orbit. Trends Anaesthesia Crit Care. 2000;11:286–292. [Google Scholar]
- 22.Fuhrmann S., Zou C., Levine E.M. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp Eye Res. 2014;123:141–150. doi: 10.1016/j.exer.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonas J.B., Schneider U., Naumann G.O. Count and density of human retinal photoreceptors. Graefes Arch Clin Exp Ophthalmol. 1992;230:505–510. doi: 10.1007/BF00181769. [DOI] [PubMed] [Google Scholar]
- 24.Brzezinski J.A., Reh T.A. Photoreceptor cell fate specification in vertebrates. Development. 2015;142:3263–3273. doi: 10.1242/dev.127043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skeie J.M., Roybal C.N., Mahajan V.B. Proteomic insight into the molecular function of the vitreous. PLoS One. 2015;10:e0127567. doi: 10.1371/journal.pone.0127567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Booij J.C., Baas D.C., Beisekeeva J., Gorgels T.G., Bergen A.A. The dynamic nature of Bruch's membrane. Prog Retin Eye Res. 2010;29:1–18. doi: 10.1016/j.preteyeres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Lee C.J., Vroom J.A., Fishman H.A., Bent S.F. Determination of human lens capsule permeability and its feasibility as a replacement for Bruch's membrane. Biomaterials. 2006;27:1670–1678. doi: 10.1016/j.biomaterials.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Mathenge W. Age-related macular degeneration. Commun Eye Health. 2014;27:49–50. [PMC free article] [PubMed] [Google Scholar]
- 29.Kaarniranta K., Machalińska A., Veréb Z., Salminen A., Petrovski G., Kauppinen A. Estrogen signalling in the pathogenesis of age-related macular degeneration. Curr Eye Res. 2015;40:226–233. doi: 10.3109/02713683.2014.925933. [DOI] [PubMed] [Google Scholar]
- 30.Salminen A., Kauppinen A., Hyttinen J.M., Toropainen E., Kaarniranta K. Endoplasmic reticulum stress in age-related macular degeneration: trigger for neovascularization. Mol Med. 2010;16:535–542. doi: 10.2119/molmed.2010.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee Y., Hussain A.A., Seok J.H., Kim S.H., Marshall J. Modulating the transport characteristics of Bruch's membrane with steroidal glycosides and its relevance to Age-Related Macular Degeneration (AMD) Invest Ophthalmol Vis Sci. 2015;56:8403–8418. doi: 10.1167/iovs.15-16936. [DOI] [PubMed] [Google Scholar]
- 32.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 33.Betts B.S., Parvathaneni K., Yendluri B.B., Grigsby J., Tsin A.T. Ginsenoside-Rb1 induces ARPE-19 proliferation and reduces VEGF release. ISRN Ophthalmol. 2011;2011:184295. doi: 10.5402/2011/184295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreau K.L., King J.A. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol Med. 2012;18:273–282. doi: 10.1016/j.molmed.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bloemendal H., de Jong W., Jaenicke R., Lubsen N.H., Slingsby C., Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Truscott R.J., Friedrich M.G. The etiology of human age-related cataract. Proteins don't last forever. Biochim Biophys Acta. 2016;1860:192–198. doi: 10.1016/j.bbagen.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim W.Y., Kim J.M., Han S.B., Lee S.K., Kim N.D., Park M.K., Kim C.K., Park J.H. Steaming of ginseng at high temperature enhances biological activity. J Nat Prod. 2000;63:1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- 38.Lee H., Kim J., Lee S.Y., Park J.H., Hwang G.S. Processed Panax ginseng, Sun ginseng, decreases oxidative damage induced by tert-butyl hydroperoxide via regulation of antioxidant enzyme and anti-apoptotic molecules in HepG2 cells. J Ginseng Res. 2012;36:248–255. doi: 10.5142/jgr.2012.36.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song K.C., Chang T.S., Lee H., Kim J., Park J.H., Hwang G.S. Processed Panax ginseng, Sun ginseng increases type I collagen by regulating MMP-1 and TIMP-1 expression in human dermal fibroblasts. J Ginseng Res. 2012;36:61–67. doi: 10.5142/jgr.2012.36.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S.M., Sun J.M., Jeong J.H., Kim M.K., Wee W.R., Park J.H., Lee J.H. Analysis of the effective fraction of Sun ginseng extract in selenite induced cataract rat model. J Korean Ophthalmol Soc. 2010;51:733–739. [Google Scholar]
- 41.Quigley H.A., Broman A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prum B.E., Lim M.C., Mansberger S.L., Stein J.D., Moroi S.E., Gedde S.J., Herndon L.W., Rosenberg L.F., Williams R.D. Primary open-angle glaucoma suspect preferred practice pattern(®) guidelines. Ophthalmology. 2016;123:P112–P151. doi: 10.1016/j.ophtha.2015.10.055. [DOI] [PubMed] [Google Scholar]
- 43.Doozandeh A., Yazdani S. Neuroprotection in glaucoma. J Ophthalmic Vis Res. 2016;11:209–220. doi: 10.4103/2008-322X.183923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bae H.W., Kim J.H., Kim S., Kim M., Lee N., Hong S., Seong G.J., Kim C.Y. Effect of Korean Red Ginseng supplementation on dry eye syndrome in glaucoma patients – a randomized, double-blind, placebo-controlled study. J Ginseng Res. 2015;39:7–13. doi: 10.1016/j.jgr.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L., Cao T., Chen H. Treatment of glaucomatous optic nerve damage using ginsenoside Rg1 mediated by ultrasound targeted microbubble destruction. Exp Ther Med. 2018;15:300–304. doi: 10.3892/etm.2017.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engelgau M.M., Geiss L.S., Saaddine J.B., Boyle J.P., Benjamin S.M., Gregg E.W., Tierney E.F., Rios-Burrows N., Mokdad A.H., Ford E.S. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 47.Tarr J.M., Kaul K., Chopra M., Kohner E.M., Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560. doi: 10.1155/2013/343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das A. Diabetic retinopathy: battling the global epidemic. Invest Ophthalmol Vis Sci. 2016;57:6669–6682. doi: 10.1167/iovs.16-21031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun H.Q., Zhou Z.Y. Effect of ginsenoside-Rg3 on the expression of VEGF and TNF-α in retina with diabetic rats. Int J Ophthalmol. 2010;3:220–223. doi: 10.3980/j.issn.2222-3959.2010.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maeng Y.S., Maharjan S., Kim J.H., Park J.H., Suk Yu Y., Kim Y.M., Kwon Y.G. Rk1, a ginsenoside, is a new blocker of vascular leakage acting through actin structure remodeling. PLoS One. 2013;8:e68659. doi: 10.1371/journal.pone.0068659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang D.D., Zhu H.Z., Li S.W., Yang J.M., Xiao Y., Kang Q.R., Li C.Y., Zhao Y.S., Zeng Y., Li Y. Crude saponins of Panax notoginseng have neuroprotective effects to inhibit palmitate-triggered endoplasmic reticulum stress-associated apoptosis and loss of postsynaptic proteins in staurosporine differentiated RGC-5 retinal ganglion cells. J Agric Food Chem. 2016;64:1528–1539. doi: 10.1021/acs.jafc.5b05864. [DOI] [PubMed] [Google Scholar]
- 52.Fan Y., Qiao Y., Huang J., Tang M. Protective effects of Panax notoginseng saponins against high glucose-induced oxidative injury in rat retinal capillary endothelial cells. Evid Based Complement Alternat Med. 2016;2016:5326382. doi: 10.1155/2016/5326382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan C., Qiao Y., Tang M. Notoginsenoside R1 attenuates high glucose-induced endothelial damage in rat retinal capillary endothelial cells by modulating the intracellular redox state. Drug Des Devel Ther. 2017;11:3343–3354. doi: 10.2147/DDDT.S149700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jian W., Yu S., Tang M., Duan H., Huang J. A combination of the main constituents of Fufang Xueshuantong Capsules shows protective effects against streptozotocin-induced retinal lesions in rats. J Ethnopharmacol. 2016;182:50–56. doi: 10.1016/j.jep.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 55.Lian F., Wu L., Tian J., Jin M., Zhou S., Zhao M., Wei L., Zheng Y., Wang Y., Zhang M. The effectiveness and safety of a danshen-containing Chinese herbal medicine for diabetic retinopathy: a randomized, double-blind, placebo-controlled multicenter clinical trial. J Ethnopharmacol. 2015;164:71–77. doi: 10.1016/j.jep.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 56.Gao D., Guo Y., Li X., Li Z., Xue M., Ou Z., Liu M., Yang M., Liu S., Yang S. An aqueous extract of Radix Astragali, Angelica sinensis, and Panax notoginseng is effective in preventing diabetic retinopathy. Evid Based Compl Alternat Med. 2013;2013:578165. doi: 10.1155/2013/578165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sen S., Chen S., Wu Y., Feng B., Lui E.K., Chakrabarti S. Preventive effects of North American ginseng (Panax quinquefolius) on diabetic retinopathy and cardiomyopathy. Phytother Res. 2013;27:290–298. doi: 10.1002/ptr.4719. [DOI] [PubMed] [Google Scholar]
- 58.Kim S.H., Jung S.H., Lee Y.J., Han J.Y., Choi Y.E., Hong H.D., Jeon H.Y., Hwang J., Na S., Kim Y.M. Dammarenediol-ii prevents VEGF-mediated microvascular permeability in diabetic mice. Phytother Res. 2015;29:1910–1916. doi: 10.1002/ptr.5480. [DOI] [PubMed] [Google Scholar]
- 59.Yang H., Son G.W., Park H.R., Lee S.E., Park Y.S. Effect of Korean Red Ginseng treatment on the gene expression profile of diabetic rat retina. J Ginseng Res. 2016;40:1–8. doi: 10.1016/j.jgr.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]