Abstract
This systematic review and meta-analysis aimed to evaluate the accuracy of fine-needle aspiration (FNA) and core-needle biopsy (CNB) in diagnosing thyroid cancer. The PubMed, Embase, and Cochrane Library databases were retrieved up to May 2019, and the overall accuracy of FNA and CNB in diagnosing thyroid cancer was evaluated by meta-analysis. The sensitivity, specificity, positive likelihood ratio (PLR), and negative likelihood ratio (NLR) were calculated. The summary receiver operating characteristic (ROC) curve was estimated, and the area under the ROC curve (AUC) was calculated. Ten eligible studies, involving 10,078 patients with 10,842 thyroid nodules, were included. The overall sensitivity and specificity of FNA and CNB for thyroid cancer were 0.72 [95 % confidence interval (CI): 0.69–0.74], 0.99 (95% CI: 0.98–0.99), and 0.83 (95% CI: 0.81–0.85), 0.99 (95% CI: 0.98–0.99), respectively. Other parameters used to assess efficacy included PLR 41.71 (2.15–808.27) and 51.56 (3.20–841.47), NLR 0.31 (0.22–0.42) and 0.22 (0.15–0.32), for FNA and CNB, respectively. Overall, the pooled summary ROC (AUC) value of FNA and CNB was 0.9025 and 0.7926, respectively. No significant difference was observed between the two AUCs of FNA and CNB (P = 0.164). FNA and CNB are still similar as first-line diagnostic tools. FNA remains a good first-line method for detecting thyroid malignancies.
Keywords: biopsy, fine-needle aspiration, meta-analysis, thyroid cancer, systematic review
Introduction
Thyroid nodules are common, and more than 95% are benign. However, the National Cancer Institute indicates that thyroid cancer remains the most common endocrine-related cancer. In 2016, 64,330 new cases were reported in the United States. Thyroid cancer accounts for about 3.8% of all new cancer cases (1). Different types of thyroid malignancies have different survival rates, but most thyroid cancers are highly treatable and can be cured on time. This makes an early and accurate diagnosis of malignant nodules critical, as they require immediate surgical resection and adjuvant radioactive iodine treatment (2).
Fine-needle aspiration (FNA) cytology is the primary tool for evaluating thyroid nodules (3). However, the non-negligible part of the thyroid lesion that receives FNA is interpreted as an indeterminate thyroid nodule, representing the gray area of cytology (4). These thyroid cytology samples show that a single cell population with or without colloids does not allow for the identification of malignant and benign lesions (4). In these cases, the International Guidelines (5) recommend the evaluation of almost all patients by diagnostic surgery. In the histological analysis of surgical specimens, a vast majority (70–80%) of thyroid nodules with indeterminate FNA results are benign (6). Therefore, identifying parameters that may serve as potential markers of malignancy has a significant clinical value. Some of the molecular, cytological, ultrasound, and clinical studies investigated these parameters but yielded controversial results (7–9). Some recent studies reported that the microscopic histological examination of the core-needle biopsy (CNB) could diagnose a large number of uncertain thyroid nodules. This technique has been widely used worldwide (10). It is particularly important in clinical practice that diagnostic surgery can avoid thyroid nodules that are considered benign CNB. Also, the incidence of minor complications of CNB is very low, which is beneficial for patients (11).
Several recent studies showed that CNB could effectively reduce the non-diagnostic rate of thyroid nodules (12–14), and unnecessary and/or diagnostic surgery (15), initially showing non-diagnostic or uncertain results through FNA (16). A recent small-group pilot study (31 patients) reported that the first-line use of CNB was more effective in suspected thyroid nodules compared with FNA (17). Although CNB has an advantage over thyroid nodules with previously non-diagnostic or indeterminate results, the small sample size may lead to inconclusive results on using CNB in evaluating thyroid nodules. This systematic review and meta-analysis compared the accuracy of CNB and FNA in diagnosing thyroid cancer.
Materials and Methods
This meta-analysis was based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines (18).
Search Strategy
As of May 1, 2019, a comprehensive literature search was conducted on electronic databases PubMed (www.pubmed.gov), Embase (www.embase.com), and Cochrane Library (www.cochranelibrary.com) using the following search terms and combinations: “fine needle aspiration or FNA,” “core needle biopsy or CNB,” “thyroid nodules,” and “malignancy.” Also, published research and review studies were manually searched. No language restrictions are applied.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) prospective or retrospective studies involving patients with thyroid nodules using FNA and CNB evaluations; (2) surgical histology as the gold standard; (3) data extracted to construct at least a 2 × 2 test performance table. The exclusion criteria were as follows: investigations of cell lines or animals; reviews, case reports, letters, or meeting records; and insufficient data. Additionally, if more than one study was published using the same case series, the study with the largest sample size was selected.
Data Extraction and Quality Assessment
Based on the aforementioned criteria, the two authors carefully extracted information from all eligible studies. Data of general research characteristics were extracted from each study: first author's surname, year of publication, country, gender, mean age, number of patients, number of nodules, period of enrollment, study type, diagnostic methods, and number of true positives, true negatives, false positives, and false negatives. The differences were resolved through discussion and consensus. Two reviewers independently assessed the eligibility of each study based on the aforementioned inclusion criteria and assessed the quality of the methodology based on the Diagnostic Accuracy Research Quality Assessment-2 (QUADAS-2) tool (19). The risk of bias was rated as “low,” “high,” or “unclear,” corresponding to a score of “1,” “0,” and “0,” respectively. The study awarded a cumulative score higher than or equal to 6 was considered as high quality.
Statistic Analysis
Meta DISC1.4 (XI Cochrane Symposium, Barcelona, Spain) was used for data analysis. The pooled sensitivity, specificity, and area under the curve (AUC) were calculated to assess the diagnostic value of FNA and CNB. A heterogeneity analysis was performed using the Cochran Q test and the Higgins I square test. If P < 0.1 or I2 > 50%, a random-effects model was applied. If P > 0.1 or I2 <50%, a fixed-effects model was used. Finally, a summary receiver operating characteristic (SROC) curve was drawn based on the literature, and the area under the receiver operating characteristic curve (SAUC) was calculated. The relative impact of each study on the overall evaluation was assessed by deleting a study for sensitivity analysis. In addition, subgroup analysis and meta-regression were employed to trace the potential sources of study heterogeneity. Publication bias was evaluated by the visual inspection of the symmetry of the funnel plot and the assessment of Begg's and Egger's tests. The trim-and fill-analysis was applied in the case of publication bias. All results showed a 95% confidence interval (CI), and two-sided P < 0.05 was considered statistically significant.
Results
Characteristics of Including Studies
Figure 1 shows the study selection procedure. A comprehensive search yielded 266 studies. The manual review of the references of retrieved studies on the use of FNA-CNB in thyroid nodules yielded three additional studies that met the inclusion criteria for this analysis. After removing duplicate studies and those containing unspecific data that did not meet the inclusion criteria, 10 studies (12, 14, 20–27) were finally included in the present meta-analysis. Table 1 illustrates the characteristics of all the studies included in this meta-analysis. These studies were published between 2001 and 2018 and conducted in four countries (the USA, Denmark, Korea, and Finland). The total number of enrolled patients was 10,078, with individual samples ranging from 52 to 4,553. The reported mean age of the patients ranged from 4.5 to 95.3 years across the eligible studies. The quality of the included studies was assessed using QUADAS-2, as shown in Figure 2. All included studies were of high quality.
Figure 1.
Flow diagram for identification of studies.
Table 1.
Characteristics of the studies included in this meta-analysis.
| References | Country | Male (%) | Mean age | Patients(N) | No. of nodules | Period of enrollment | Study type | Diagnostic methods |
|---|---|---|---|---|---|---|---|---|
| Karstrup et al. (20) | Denmark | 17 | 51 (33–81) | 77 | 77 | 1997–1999 | Retrospective | FNA, CNB |
| Renshaw et al. (21) | USA | 20 | 52 (14–86) | 377 | 377 | 2000–2006 | Retrospective | FNA, CNB |
| Na et al. (12) | Korea | 13 | 46 ± 11.7 | 220 | 225 | 2009–2010 | Prospective | FNA, CNB |
| Sung et al. (14) | Korea | 16 | 44.32 ± 11.86 | 538 | 555 | 2008–2009 | Retrospective | FNA, CNB |
| Hakala et al. (23) | Finland | 23 | 53 ± 17 | 52 | 52 | 2010–2011 | Prospective | FNA, CNB |
| Chen et al. (21) | USA | 21 | 58 (14–88) | 350 | 461 | 2007–2011 | Retrospective | FNA, CNB |
| Choi et al. (22) | Korea | 21 | 55.5 (25–81) | 505 | 505 | 2008–2013 | Retrospective | FNA, CNB |
| Ahn et al. (20) | Korea | 22 | 50.7 (4.5–95.3) | 2,187 | 2,406 | 2004–2014 | Retrospective | FNA, CNB |
| Suh et al. (27) | Korea | 21 | 53.4 ± 12.6 | 4,553 | 4,822 | 2013 | Retrospective | FNA, CNB |
| Hong et al. (24) | Korea | 18 | 46.9 ± 12.9 | 1,219 | 1,362 | 2010–2014 | Retrospective | FNA, CNB |
FNA, fine-needle aspiration; CNB, core needle biopsy.
Figure 2.
Risk-of-bias and applicability concerns summary for each domain of the QUADAS-2 for each included study. (A) Risk-of-bias summary. (B) Risk-of-bias graph. Symbols. (+), low risk of bias; (?), unclear risk of bias; (–), high risk of bias.
Quantitative Synthesis
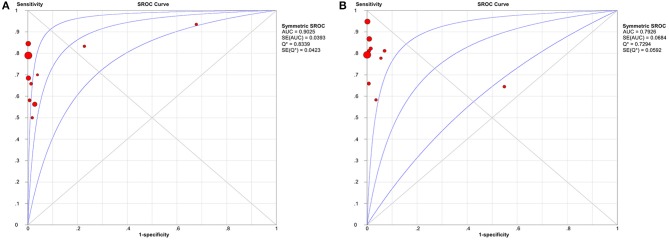
Study data and individual diagnostic estimates are summarized in Table 2. Compared with the gold standard, the random-effects model meta-analysis of the 11 studies showed a pooled sensitivity of 0.72 (95% CI: 0.69–0.74) (Figure 3A) and a pooled specificity of 0.99 (95% CI: 0.98–0.99) (Figure 4A) for diagnosing benign and malignant lesions of thyroid nodules with FNA. For CNB, the pooled sensitivity and specificity were 0.83 (95% CI: 0.81–0.85) (Figure 3B) and 0.99 (95% CI: 0.98–0.99) (Figure 4B), respectively. The pooled positive likelihood ratio of FNA and CNB was 41.71 (2.15–808.27) and 51.56 (3.20–841.47), respectively; the pooled negative likelihood ratio for FNA and CNB was 0.31 (0.22–0.42) and 0.22 (0.15–0.32), respectively, (Table 2). The pooled SROC (AUC) value of FNA and CNB was 0.9025 and 0.7926, respectively; the pooled SE (AUC) value of FNA and CNB was 0.0393 and 0.0684, respectively (Figure 5). No significant difference was observed between the two AUCs of FNA and CNB (P = 0.164). The sensitivity analysis evaluated the impact of a single data set on the summary results by deleting each eligible study in turn. The overall results remained the same after removing any single study (Table 3).
Table 2.
Summary of results of the studies included in this meta-analysis.
| References | Diagnostic methods | TP | FP | FN | TN | Sensitivity (%) | Specificity (%) | PLR | NLR |
|---|---|---|---|---|---|---|---|---|---|
| Karstrup et al. (25) | FNA | 15 | 5 | 3 | 17 | 0.83 (0.59, 0.96) | 0.77 (0.55, 0.92) | 3.67 (1.65, 8.14) | 0.22 (0.07, 0.62) |
| CNB | 14 | 1 | 4 | 17 | 0.78 (0.52, 0.94) | 0.94 (0.73, 1.00) | 14.00 (2.05, 95.56) | 0.24 (0.10, 0.56) | |
| Renshaw and Pinnar (26) | FNA | 29 | 21 | 2 | 10 | 0.94 (0.79, 0.99) | 0.32 (0.17, 0.51) | 1.38 (1.06, 1.79) | 0.20 (0.05, 0.84) |
| CNB | 20 | 17 | 11 | 14 | 0.65 (0.45, 0.81) | 0.45 (0.27, 0.64) | 1.18 (0.78, 1.78) | 0.79 (0.43, 1.45) | |
| Na et al. (12) | FNA | 46 | 0 | 33 | 70 | 0.58 (0.47, 0.69) | 1.00 (0.95, 1.00) | 82.54 (5.18, 1314.94) | 0.42 (0.33, 0.55) |
| CNB | 65 | 1 | 14 | 69 | 0.82 (0.72, 0.90) | 0.99 (0.92, 1.00) | 57.59 (8.21, 404.25) | 0.18 (0.11, 0.29) | |
| Sung et al. (14) | FNA | 218 | 0 | 100 | 237 | 0.69 (0.63, 0.74) | 1.00 (0.98, 1.00) | 326.04 (20.43, 5202.61) | 0.32 (0.27, 0.37) |
| CNB | 276 | 2 | 42 | 235 | 0.87 (0.83, 0.90) | 0.99 (0.97, 1.00) | 102.85 (25.86, 409.11) | 0.13 (0.10, 0.18) | |
| Hakala et al. (23) | FNA | 12 | 0 | 12 | 28 | 0.50 (0.29, 0.71) | 1.00 (0.88, 1.00) | 29 (1.81, 465.42) | 0.51 (0.34, 0.76) |
| CNB | 14 | 1 | 10 | 27 | 0.58 (0.37, 0.78) | 0.96 (0.82, 1.00) | 16.33 (2.31, 115.28) | 0.43 (0.27, 0.70) | |
| Chen et al. (21) | FNA | 3 | 0 | 1 | 12 | 0.75 (0.19, 0.99) | 1.00 (0.74, 1.00) | 18.2 (1.13, 292.75) | 0.31 (0.08, 1.20) |
| CNB | 26 | 4 | 6 | 53 | 0.81 (0.64, 0.93) | 0.93 (0.83, 0.98) | 11.58 (4.44, 30.22) | 0.20 (0.10, 0.42) | |
| Choi et al. (22) | FNA | 27 | 1 | 14 | 75 | 0.66 (0.49, 0.80) | 0.99 (0.93, 1.00) | 50.05 (7.05, 355.12) | 0.35 (0.23, 0.53) |
| CNB | 49 | 0 | 11 | 69 | 0.82 (0.70, 0.90) | 1.00 (0.95, 1.00) | 113.61 (7.16, 1803.15) | 0.19 (0.11, 0.32) | |
| Ahn et al. (20) | FNA | 174 | 5 | 135 | 176 | 0.56 (0.51, 0.62) | 0.97 (0.94, 0.99) | 20.38 (8.54, 48.65) | 0.45 (0.39, 0.51) |
| CNB | 45 | 0 | 23 | 71 | 0.66 (0.54, 0.77) | 1.00 (0.95, 1.00) | 94.96 (5.97, 1511.4) | 0.34 (0.25, 0.48) | |
| Suh et al. (27) | FNA | 340 | 3 | 90 | 1,757 | 0.79 (0.75, 0.83) | 1.00 (1.00, 1.00) | 463.88 (149.6, 1438.4) | 0.21 (0.17, 0.25) |
| CNB | 524 | 0 | 136 | 1,043 | 0.79 (0.76, 0.82) | 1.00 (1.00, 1.00) | 1656.82 (103.67, 26478.25) | 0.21 (0.18, 0.24) | |
| Hong et al. (24) | FNA | 238 | 0 | 43 | 405 | 0.85 (0.80, 0.89) | 1.00 (0.99, 1.00) | 686.74 (43.01, 10965.62) | 0.15 (0.12, 0.20) |
| CNB | 267 | 0 | 14 | 405 | 0.95 (0.92, 0.97) | 1.00 (0.99, 1.00) | 770.25 (48.25, 12295.09) | 0.05 (0.03, 0.08) | |
| Pooled value | FNA | 1,409 | 36 | 451 | 3,031 | 0.72 (0.69, 0.74) | 0.99 (0.98, 0.99) | 41.71 (2.15, 808.27) | 0.31 (0.22, 0.42) |
| CNB | 1,476 | 26 | 293 | 2,180 | 0.83 (0.81, 0.85) | 0.99 (0.98, 0.99) | 51.56 (3.20, 841.47) | 0.22 (0.15, 0.32) |
FNA, fine-needle aspiration; CNB, core needle biopsy; FN, False-negative; FP, False-positive; TN, True-negative; TP, True-positive; PLR, positive likelihood ratio; NLR, negative likelihood ratio.
Figure 3.
Pooled sensitivity forest plot of two methods in diagnosing thyroid cancer. (A) FNA; (B) CNB.
Figure 4.
Pooled specificity forest plot of two methods in diagnosing thyroid cancer. (A) FNA; (B) CNB.
Figure 5.
Summary receiver operator characteristic curve (SROC) with the area under the ROC curve (AUC) of the two methods in diagnosing thyroid cancer. (A) FNA; (B) CNB. AUC, area under the ROC curve; SROC, summary receiver operator characteristic curve.
Table 3.
The influence of individual studies using the leave-one-out approach.
| Study excluded | Diagnostic methods | Sensitivity (95% CI) | Specificity (95% CI) | AUC |
|---|---|---|---|---|
| Karstrup et al. (25) | FNA | 0.72 (0.69, 0.74) | 0.99 (0.98, 0.99) | 0.91 |
| CNB | 0.83 (0.81, 0.85) | 0.99 (0.98, 0.99) | 0.79 | |
| Renshaw and Pinnar (26) | FNA | 0.71 (0.69, 0.74) | 0.99 (0.99, 1.00) | 0.83 |
| CNB | 0.83 (0.81, 0.85) | 1.00 (0.99, 1.00) | 0.88 | |
| Na et al. (12) | FNA | 0.73 (0.70, 0.75) | 0.99 (0.98, 0.99) | 0.90 |
| CNB | 0.83 (0.81, 0.85) | 0.99 (0.98, 0.99) | 0.79 | |
| Sung et al. (14) | FNA | 0.73 (0.70, 0.75) | 0.99 (0.98, 0.99) | 0.90 |
| CNB | 0.82 (0.79, 0.84) | 0.99 (0.98, 0.99) | 0.78 | |
| Hakala et al. (23) | FNA | 0.72 (0.70, 0.74) | 0.99 (0.98, 0.99) | 0.91 |
| CNB | 0.83 (0.81, 0.85) | 0.99 (0.98, 0.99) | 0.79 | |
| Chen et al. (21) | FNA | 0.72(0.69, 0.74) | 0.99 (0.98, 0.99) | 0.90 |
| CNB | 0.83 (0.81, 0.85) | 0.99 (0.98, 0.99) | 0.77 | |
| Choi et al. (22) | FNA | 0.72 (0.70, 0.74) | 0.99 (0.98, 0.99) | 0.90 |
| CNB | 0.83 (0.81, 0.85) | 0.99 (0.98, 0.99) | 0.79 | |
| Ahn et al. (20) | FNA | 0.76 (0.73, 0.78) | 0.99 (0.98, 0.99) | 0.92 |
| CNB | 0.83 (0.82, 0.85) | 0.99 (0.98, 0.99) | 0.79 | |
| Suh et al. (27) | FNA | 0.69 (0.66, 0.72) | 0.97 (0.96, 0.98) | 0.89 |
| CNB | 0.85 (0.83, 0.87) | 0.97 (0.96, 0.98) | 0.78 | |
| Hong et al. (24) | FNA | 0.69 (0.66, 0.71) | 0.99 (0.98, 0.99) | 0.90 |
| CNB | 0.80 (0.78, 0.82) | 0.98 (0.98, 0.99) | 0.79 |
FNA, fine-needle aspiration; CNB, core needle biopsy; AUC, the area under the curve.
Subgroup Analyses
Due to the existence of significant heterogeneity across the whole analyses, subgroups were analyzed depending on the country, number of patients, period of enrollment, and study type. As exemplified in Table 4, FNA achieved a high AUC value of 0.90 in the diagnosis of thyroid cancer (overall), especially in pre-2010 enrollment (AUC = 0.91) and in a retrospective study (AUC = 0.91). Moreover, stratified analyses in terms of country evidenced that CNB presented an AUC of 0.97 in Asian countries better than in occidental countries (AUC = 0.77).
Table 4.
Subgroup analysis of diagnostic effect.
| Subgroup | Diagnostic methods | Number of studies | Sensitivity (95% CI) | Specificity (95% CI) | AUC |
|---|---|---|---|---|---|
| Overall | FNA | 10 | 0.72 (0.69, 0.74) | 0.99 (0.98, 0.99) | 0.90 |
| CNB | 10 | 0.83 (0.81, 0.85) | 0.99 (0.98, 0.99) | 0.79 | |
| Country | |||||
| Occidental | FNA | 4 | 0.77 (0.66, 0.86) | 0.72 (0.62, 0.81) | 0.89 |
| CNB | 4 | 0.70 (0.61, 0.79) | 0.83 (0.75, 0.89) | 0.77 | |
| Asian | FNA | 6 | 0.72 (0.69, 0.74) | 1.00 (0.98, 1.00) | 0.89 |
| CNB | 6 | 0.84 (0.82, 0.85) | 1.00 (1.00, 1.00) | 0.96 | |
| Number of patients | |||||
| ≤ 500 | FNA | 5 | 0.67 (0.59, 0.75) | 0.84 (0.78, 0.89) | 0.89 |
| CNB | 5 | 0.76 (0.69, 0.82) | 0.88 (0.83, 0.92) | 0.77 | |
| >500 | FNA | 5 | 0.72 (0.70, 0.75) | 1.00 (0.99, 1.00) | 0.90 |
| CNB | 5 | 0.84 (0.82, 0.86) | 1.00 (1.00, 1.00) | 0.97 | |
| Period of enrollment | |||||
| Pre-2010 | FNA | 4 | 0.69 (0.65, 0.73) | 0.93 (0.90, 0.95) | 0.91 |
| CNB | 4 | 0.84 (0.80, 0.87) | 0.94 (0.91, 0.96) | 0.76 | |
| Post-2010 | FNA | 6 | 0.73 (0.70, 0.76) | 1.00 (0.99, 1.00) | 0.88 |
| CNB | 6 | 0.82 (0.80, 0.84) | 1.00 (0.99, 1.00) | 0.86 | |
| Study type | |||||
| Prospective | FNA | 2 | 0.56 (0.46, 0.66) | 1.00 (0.96, 1.00) | —— |
| CNB | 2 | 0.77 (0.67, 0.84) | 0.98 (0.93, 1.00) | —— | |
| Retrospective | FNA | 8 | 0.73 (0.71, 0.75) | 0.99 (0.98, 0.99) | 0.91 |
| CNB | 8 | 0.83 (0.81, 0.85) | 0.99 (0.98, 0.99) | 0.80 | |
FNA, fine-needle aspiration; CNB, core needle biopsy; AUC, the area under the curve.
Heterogeneity Analysis
The source of heterogeneity was examined by a meta-regression analysis. None of the examined factors, including country (FNA: P = 0.657; CNB: P = 0.187), number of patients (FNA: P = 0.523; CNB: P = 0.296), period of enrollment (FNA: P = 0.771; CNB: P = 0.666), and study type (FNA: P = 0.333; CNB: P = 0.346), was responsible for heterogeneity across studies in meta-regression.
Publication Bias
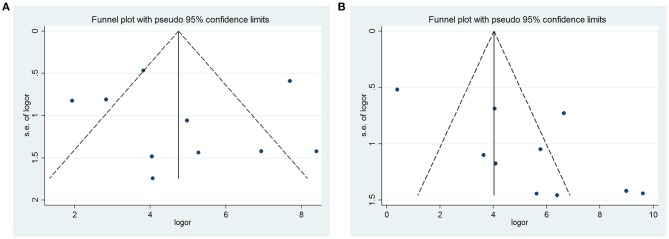
Finally, the Begg's and Egger's regression tests showed no evidence of asymmetrical distribution in the funnel plot in FNA (Begg's test P = 0.858; Egger's test P = 0.766) (Figure 6A). However, the P-value of Begg's test confirmed the existence of publication bias for CNB (Begg's test P = 0.210; Egger's test P = 0.017; Figure 6B). The trim-and-fill method showed no need for additional studies (Figure S1).
Figure 6.
Funnel plot for publication bias test. Each point represents a separate study for the indicated association. (A) FNA; (B) CNB.
Discussion
This systematic review and meta-analysis evaluated the accuracy of FNA and CNB in diagnosing thyroid malignancy. The study found a pooled sensitivity of 0.72 (95% CI: 0.69–0.74) and a pooled specificity of 0.99 (95% CI: 0.98–0.99) for FNA. For CNB, the pooled sensitivity and specificity were 0.83 (95% CI: 0.81–0.85) and 0.99 (95% CI: 0.98–0.99), respectively. The pooled SROC (AUC) value of FNA and CNB was 0.9025 and 0.7926, respectively. No significant difference was observed between the two AUCs of FNA and CNB (P = 0.164).
Besides pooling the diagnostic characteristics of the index test, identification of heterogeneity is also an important goal of a meta-analysis. The present study assessed the between-study heterogeneity using subgroup and meta-regression analyses. The subgroup analysis indicated that FNA achieved a high AUC value of 0.90 in the diagnosis of thyroid cancer (overall), especially in pre-2010 enrollment (AUC = 0.91) and in a retrospective study (AUC = 0.91). Moreover, stratified analyses in terms of country evidenced that CNB presented an AUC of 0.97 in Asian countries better than in occidental countries (AUC = 0.77). However, none of the examined factors, including country, number of patients, period of enrollment, and study type, was responsible for heterogeneity across studies in meta-regression. These heterogeneities might be due to technical differences in institutions or operators, nodule characteristics, number of passages, or lack of standardized pathological criteria for CNB.
Previous meta-analytical studies yielded conflicting results. Li et al. found no significant differences in the values of the preoperative diagnosis of thyroid nodules between FNA and CNB (28). The two meta-analyses by Tandon et al. (29) and Novoa et al. (30) evaluated the role of FNA and CNB in the diagnosis of head and neck malignancies. Both studies found that CNB was more accurate and specific than FNA, and its negative predictive value was better when applied to all head and neck tumors. However, neither of these studies compared the diagnostic value of FNA and CNB for thyroid malignancies. The present meta-analysis, comprising 10,078 patients with 10,842 thyroid nodules from 10 studies, was the largest study investigating the accuracy of FNA and CNB in diagnosing thyroid malignancy.
In clinical follow-up and surgery for thyroid nodules, FNA is a classic, cost-effective, minimally invasive, easy-to-apply standard of choice. However, the rate of dissatisfaction/non-diagnosis results varies (2–40%) among centers (2, 5, 31, 32). These results are directly related to the technical capabilities of the personnel performing the procedures and evaluating the materials. Besides, among observers, the incidence of AUS/FLUS (a heterogeneous diagnostic group that may lead to uncertainty in patient treatment) varies (3–32.2%) (2, 5, 31, 32). Recent studies explored the potential role of CNB as a first-line tool for diagnosing thyroid nodules. A study showed that CNB had low rates of non-diagnostic results (1.3%), inconclusive results (5.9%), complications (0.2%), high diagnostic accuracy (97.6%), and unnecessary surgery (0.5%) (33). In another study, the diagnostic accuracy of CNB was significantly higher than that of FNA (96.8 vs. 78%, P < 0.001), and the incidence of false-negative and uncertain results in suspected US characteristic nodules reduced (17). Hong et al. (24) suggested that CNB could prevent unnecessary repetitive biopsy procedures or diagnostic surgery because 13.5% of nodules had no conclusive results. Also, CNB could achieve additional malignant diagnosis in 27% of malignant tumors compared with FNA. When executed by experienced operators, CNB has been reported as safe (34) and tolerable (11). Therefore, although FNA has been widely used as a first-line diagnostic tool, CNB can be used by experienced operators as an alternative first-line diagnostic tool for thyroid nodules.
This meta-analysis had several limitations. First, it was a retrospective study, and the number of patients in some studies was relatively small. Second, the meta-analysis showed considerable heterogeneity in the pooled proportions. Third, differences existed in the sample collection technology. For example, FNA could be accomplished by capillary or aspiration techniques, and some retrospective studies did not specify which techniques were used.
In summary, FNA and CNB are still similar as first-line diagnostic tools. FNA remains a good first-line method for detecting thyroid malignancies. Additional controlled studies on larger, homogeneous patient groups are needed to validate further the practicability of the aforementioned two methods.
Author Contributions
LL and YL carried out the studies, participated in collecting data, and drafted the manuscript. LL and MZ performed the statistical analysis and participated in its design. LH, HC, and WD helped to draft the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study has received funding by National Natural Science Foundation of China (81372858).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.00044/full#supplementary-material
Filled funnel plot of any PEP using trim-and-fill method.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. (2016) 66:7–30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Yassa L, Cibas ES, Benson CB, Frates MC, Doubilet PM, Gawande AA, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. (2007) 111:508–16. 10.1002/cncr.23116 [DOI] [PubMed] [Google Scholar]
- 3.Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard BA, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol. (2014) 81:1–122. 10.1111/cen.12515 [DOI] [PubMed] [Google Scholar]
- 4.Baloch ZW, Fleisher S, LiVolsi VA, Gupta PK. Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration cytology. Diagn. Cytopathol. (2002) 26:41–4. 10.1002/dc.10043 [DOI] [PubMed] [Google Scholar]
- 5.Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid. (2009) 19:1159–65. 10.1089/thy.2017.0500 [DOI] [PubMed] [Google Scholar]
- 6.Trimboli P, Treglia G, Guidobaldi L, Saggiorato E, Nigri G, Crescenzi A, et al. Clinical characteristics as predictors of malignancy in patients with indeterminate thyroid cytology: a meta-analysis. Endocrine. (2014) 46:52–9. 10.1007/s12020-013-0057-1 [DOI] [PubMed] [Google Scholar]
- 7.Giovanella L, Ceriani L, Treglia G. Role of isotope scan, including positron emission tomography/computed tomography, in nodular goitre. Best Pract Res Clin Endocrinol Metabol. (2014) 28:507–18. 10.1016/j.beem.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 8.Trimboli P, Fulciniti F, Zilioli V, Ceriani L, Giovanella L. Accuracy of international ultrasound risk stratification systems in thyroid lesions cytologically classified as indeterminate. Diagn Cytopathol. (2017) 45:113–7. 10.1002/dc.23651 [DOI] [PubMed] [Google Scholar]
- 9.Trimboli P, Virili C, Romanelli F, Crescenzi A, Giovanella L. Galectin-3 performance in histologic and cytologic assessment of thyroid nodules: A systematic review and Meta-analysis. Int J Mol Sci. (2017) 18:1756. 10.3390/ijms18081756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trimboli P, Crescenzi A. Thyroid core needle biopsy: taking stock of the situation. Endocrine. (2015) 48:779–85. 10.1007/s12020-014-0382-z [DOI] [PubMed] [Google Scholar]
- 11.Nasrollah N, Trimboli P, Rossi F, Amendola S, Guidobaldi L, Ventura C, et al. Patient's comfort with and tolerability of thyroid core needle biopsy. Endocrine. (2014) 45:79–83. 10.1007/s12020-013-9979-x [DOI] [PubMed] [Google Scholar]
- 12.Na DG, Kim JH, Sung JY, Baek JH, Jung KC, Lee H, et al. Core-needle biopsy is more useful than repeat fine-needle aspiration in thyroid nodules read as nondiagnostic or atypia of undetermined significance by the Bethesda system for reporting thyroid cytopathology. Thyroid. (2012) 22:468–75. 10.1089/thy.2011.0185 [DOI] [PubMed] [Google Scholar]
- 13.Suh CH, Baek JH, Lee JH, Choi YJ, Kim KW, Lee J, et al. The role of core-needle biopsy in the diagnosis of thyroid malignancy in 4580 patients with 4746 thyroid nodules: a systematic review and meta-analysis. Endocrine. (2016) 54:315–28. 10.1007/s12020-016-0991-9 [DOI] [PubMed] [Google Scholar]
- 14.Sung JY, Na DG, Kim KS, Yoo H, Lee H, Kim JH, et al. Diagnostic accuracy of fine-needle aspiration versus core-needle biopsy for the diagnosis of thyroid malignancy in a clinical cohort. Eur Radiol. (2012) 22:1564–72. 10.1007/s00330-012-2405-6. [DOI] [PubMed] [Google Scholar]
- 15.Choi YJ, Baek JH, Ha EJ, Lim HK, Lee JH, Kim JK, et al. Differences in risk of malignancy and management recommendations in subcategories of thyroid nodules with atypia of undetermined significance or follicular lesion of undetermined significance: the role of ultrasound-guided core-needle biopsy. Thyroid. (2014) 24:494–501. 10.1089/thy.2012.0635 [DOI] [PubMed] [Google Scholar]
- 16.Wolinski K, Stangierski A, Ruchala M. Comparison of diagnostic yield of core-needle and fine-needle aspiration biopsies of thyroid lesions: systematic review and meta-analysis. Eur Radiol. (2017) 27:431–6. 10.1007/s00330-016-4356-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trimboli P, Nasrollah N, Guidobaldi L, Taccogna S, Modica DDC, Amendola S, et al. The use of core needle biopsy as first-line in diagnosis of thyroid nodules reduces false negative and inconclusive data reported by fine-needle aspiration. World J Surg Oncol. (2014) 12:61. 10.1186/1477-7819-12-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. 10.1136/bmj.b2535 [DOI] [PubMed] [Google Scholar]
- 19.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Internal Med. (2011) 155:529–36. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 20.Ahn SH, Park SY, Choi SI. Comparison of consecutive results from fine needle aspiration and core needle biopsy in thyroid nodules. Endocr Pathol. (2017) 28:332–8. 10.1007/s12022-017-9496-1 [DOI] [PubMed] [Google Scholar]
- 21.Chen B, Jain A, Dagis A, Chu P, Vora L, Maghami E, et al. Comparison of the efficacy and safety of ultrasound-guided core needle biopsy versus fine-needle aspiration for evaluating thyroid nodules. Endocr Pract. (2014) 21:128–35. 10.4158/EP14303 [DOI] [PubMed] [Google Scholar]
- 22.Choi YJ, Baek JH, Suh CH, Shim WH, Jeong B, Kim JK, et al. Core-needle biopsy versus repeat fine-needle aspiration for thyroid nodules initially read as atypia/follicular lesion of undetermined significance. Head Neck. (2017) 39:361–9. 10.1002/hed.24597 [DOI] [PubMed] [Google Scholar]
- 23.Hakala T, Kholová I, Sand J, Saaristo R, Kellokumpu-Lehtinen P. A core needle biopsy provides more malignancy-specific results than fine-needle aspiration biopsy in thyroid nodules suspicious for malignancy. J Clin Pathol. (2013) 66:1046–50. 10.1136/jclinpath-2013-201559 [DOI] [PubMed] [Google Scholar]
- 24.Hong MJ, Na DG, Kim SJ, Kim DS. Role of core needle biopsy as a first-line diagnostic tool for thyroid nodules: a retrospective cohort study. Ultrasonography. (2018) 37:244–53. 10.14366/usg.17041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karstrup S, Balslev E, Juul N, Eskildsen PC, Baumbach L. US-guided fine needle aspiration versus coarse needle biopsy of thyroid nodules. Eur J Ultrasound. (2001) 13:1–5. 10.1016/s0929-8266(01)00116-1 [DOI] [PubMed] [Google Scholar]
- 26.Renshaw AA, Pinnar N. Comparison of thyroid fine-needle aspiration and core needle biopsy. Am J Clin Pathol. (2007) 128:370–4. 10.1309/07TL3V58337TXHMC [DOI] [PubMed] [Google Scholar]
- 27.Suh CH, Baek JH, Choi YJ, Kim TY, Sung TY, Song DE, et al. Efficacy and safety of core-needle biopsy in initially detected thyroid nodules via propensity score analysis. Sci Rep. (2017) 7:8242. 10.1038/s41598-017-07924-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L, Chen BD, Zhu HF, Wu S, Wei D, Zhang JQ, et al. Comparison of pre-operation diagnosis of thyroid cancer with fine needle aspiration and core-needle biopsy: a meta-analysis. Asian Pac J Cancer Prev. (2014) 15:7187–93. 10.7314/apjcp.2014.15.17.7187 [DOI] [PubMed] [Google Scholar]
- 29.Tandon S, Shahab R, Benton JI, Ghosh SK, Sheard J, Jones TM. Fine-needle aspiration cytology in a regional head and neck cancer center: comparison with a systematic review and meta-analysis. Head Neck. (2008) 30:1246–52. 10.1002/hed.20849 [DOI] [PubMed] [Google Scholar]
- 30.Novoa E, Gürtler N, Arnoux A, Kraft M. Role of ultrasound-guided core-needle biopsy in the assessment of head and neck lesions: a meta-analysis and systematic review of the literature. Head Neck. (2012) 34:1497–503. 10.1002/hed.21821 [DOI] [PubMed] [Google Scholar]
- 31.Choi SH, Baek JH, Lee JH, Choi YJ, Hong MJ, Song DE, et al. Thyroid nodules with initially non-diagnostic, fine-needle aspiration results: comparison of core-needle biopsy and repeated fine-needle aspiration. Eur Radiol. (2014) 24:2819–26. 10.1007/s00330-014-3325-4. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer Cytopathol. (2007) 111:306–15. 10.1002/cncr.22955 [DOI] [PubMed] [Google Scholar]
- 33.Suh CH, Baek JH, Lee JH, Choi YJ, Kim JK, Sung TY, et al. The role of core-needle biopsy as a first-line diagnostic tool for initially detected thyroid nodules. Thyroid. (2016) 26:395–403. 10.1089/thy.2015.0404 [DOI] [PubMed] [Google Scholar]
- 34.Ha EJ, Baek JH, Lee JH, Kim JK, Choi YJ, Sung TY, et al. Complications following US-guided core-needle biopsy for thyroid lesions: a retrospective study of 6,169 consecutive patients with 6,687 thyroid nodules. Eur Radiol. (2017) 27:1186–94. 10.1007/s00330-016-4461-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Filled funnel plot of any PEP using trim-and-fill method.