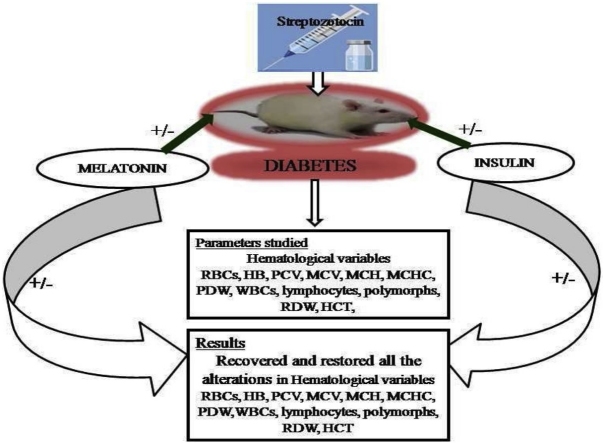
Graphical abstract
Keywords: Melatonin, Insulin, Diabetes, Streptozotocin, WBCs, RBCs glibenclamide
Highlights
-
•
Diabetes is both clinically as well as genetically multifaceted group of disorders characterized by high blood glucose level.
-
•
Hyperglycemic condition arises due to shortage of insulin secretion or resistance developed by the cells of body against the insulin binding on its membrane receptors, or it may be the combination of both.
-
•
Present study was designed to explore therapeutic efficacy of exogenous supplementation of melatonin and insulin against the diabetes induced alterations in hematological variables.
-
•
Combined administration of melatonin and insulin significantly restored the altered hematological variables towards the normal range.
Abstract
The aim of the present was to ameliorate the protective effect of exogenous melatonin and insulin against the diabetes induced alterations in the different hematological variables. Albino rats were administrated streptozotocin at the dose of 15 mg/kg for 6 days. Total 54 rats were randomly selected for the experimental purpose and were divided into two major groups. Group-1 consisting twenty four (24) and were further sub-divided into four (4) different groups viz. group-I served as normal control, group-II served as melatonin treated, group-III served as insulin treated and group-IV served as glibenclamide treated. Group-2 consisting thirty (30) rats were given streptozotocin (STZ) injection (15 mg/kg) for 6 days. After confirmation of diabetes by measuring blood glucose level, animals having blood glucose level above 250 mg/dl) confirmed as diabetic. Thirty (30) Diabetic rats were further subdivided into following sub-groups and were given different therapeutic treatments, Viz group-I served as Diabetic control, group-II treated with melatonin, group-III treated with insulin, group-IV given treatment of melatonin and insulin and group-V were given treatment of glibenclamide respectively. Diabetic rats showed modulation in all the studied hematological variables. Diabetic rats displayed significant decline in RBCs count, HB level and its associated indices (HCT, RDW, MCV, MCH, MCHC), WBCs and its related indices (polymorphs and lymphocytes) and platelet distribution width (PDW %) whereas platelet count showed significant increase. Nonetheless alone as well as combined treatment of exogenous melatonin and insulin restored all altered hematological parameters. However, significant recovery was found in the group in which combined dose of melatonin and insulin was administrated. Therefore, it might be concluded that combined administration of melatonin and insulin will be better remedy to normalize the altered blood profile during the diabetic condition.
1. Introduction
Diabetes is both clinically as well as genetically multifaceted group of disorders characterized by high blood glucose level [1]. Hyperglycemic condition arises due to shortage of insulin secretion or resistance developed by the cells of body against the insulin binding on its membrane receptors, or it may be the combination of both [1]. Epidemiological studies reported that 150 million people are affected by diabetes in the world and it may reach upto 300 million or even more by 2025 [2]. Diabetes is a global public health crisis that threatens the all nations; particularly Asian and European countries are highly influenced [3]. The epidemiological parameters such as sedentary lifestyle, intake of energy rich diet, change in food habits which results in are considered as major factors causing this disorder. Diabetes affects all the physiological functions of an organism by altering the normal utilization and metabolism of glucose the excess glucose self oxidizes. The self oxidation of glucose generates a chain of free radicals which causes membrane damages. Therefore, self oxidation of glucose causes various complications including hepatorenal abnormalities [4,5], neuronal damages [6] and also reproductive complications [7].
Melatonin (N-Acetyl-5-methoxytryptamine) is a chronobiotic hormone predominantly secreted from vertebrate pineal gland and other selected organs called as extra pineal melatonin. It acts directly to detoxify reactive oxygen and reactive nitrogen species and indirectly by stimulating antioxidant enzymes while suppressing the activity of pro-oxidant enzymes. Earlier both in-vivo and in vitro studies reported that melatonin plays a key role in regulation glucose metabolism during pathogenesis of diabetes [8]. With increase in age synthesis of melatonin declines, this in turn may impacts the insulin signaling in aged rats. Melatonin level was found decreased in Goto kakizaki (GK) rats and human with T2D [9]. Melatonin is a well known synchronizing agent (zeitgeber), contributing in the control of circadian rhythms in different parts of the body and hence controls various metabolic and reproductive functions including sleep-wake cycle, food intake, energy metabolism, and gastrointestinal tract action [10,11]. Further, it has been reported from previous studies that melatonin effects sleep, mediators of ingestion like ghrelin and leptin, adiposity and body weight regulation, etc. might be controlling the regulation of glucose homeostasis, metabolic syndrome and risk of diabetes [12,13].
Blood is the principal transporting medium, which carries different fundamental physiological functions such as gaseous transportation (oxygen and carbon dioxide transporter) and nutrient supplementation. It also contains various immune cells which are defensive against the various pathogenic conditions. Blood works as transporter of metabolic products from and to the different areas of the cardiovascular system. Influx of altered biochemical and tissue products in the blood interacts with the components of blood and alters their functional ability. Change in the shape of red blood cells affects their flow rate (hemorheological), other cellular changes includes aggregation and deformability of erythrocytes, nonenzymatic glycosylation [14]. Diabetes leads to decrease in hemoglobin, HCT, MCV, MCH and MCHC level [15]. Diabetes also causes decrement in total white blood cell count (WBCC) and lymphocytes [[16], [17], [18]], while as platelet count increases [19]
Since decades insulin is a well known key regulator of metabolism. In the earlier studies it was reported that melatonin has inhibitory effect on insulin secretion. However, Ramracheya et al., 2008 reported that melatonin has stimulatory action on pancreatic β-cells, therefore stimulates the secretion of insulin via MT1 receptors present on β-cells [20]. Melatonin modulates the insulin secretion by MT1 and MT2 receptors and Gi-protein signaling cascade, it suppresses the insulin secretion by AC/cAMP (MT1 and MT2) and the GC/cGMP system (MT2) hence decreases the insulin release. Nonetheless melatonin couples with Gq, melatonin receptors activates phospholipase C, and hence melatonin induces insulin secretion by IP3-signaling pathway [21].
In the present study melatonin and insulin were administered simultaneously to the rats, in order to check whether exogenous melatonin has same stimulatory effect on insulin secretion. This was analyzed by investigating the various alterations in the hematological parameters caused by insulin deficiency during the diabetic condition.
Previous studies reported that diabetic patients suffering from ulcers are having lower telomerase activity in their leukocytes in comparison to non-diabetic people [18]. Previous studies reported that telomere shorting might be causing decrease in insulin secretion and glucose tolerance in mouse pancreatic β-cells that indicates a bilateral telomere-diabetes connection [19]. Chronic hyperglycemic condition causes elevated oxidative stress which in turn attenuates activity of telomerase of human leukocytes, ultimately causing decreased telomere length [[22], [23], [24]]. Endocrine disruptors (EDs) decreases the function of pancreatic β-cells, growth, peripheral insulin resistance, insulin production, β-cells mass (compensatory, hyperplasia/hypertrophy of β-cells) and reduces insulin output, insulin signaling and elevation apoptosis in β-cells [25]. The present study has been designed to explore the promising and valuable therapeutic potentials of alone and combined treatment of exogenous melatonin (as antioxidant, antigeing and cellular protective) and insulin (metabolic hormone) to compensate the deficiencies of these two hormones by comparing their efficacy with one of the clinically recommended antidiabetic molecule glibenclamide. Simultaneous administration of melatonin and insulin could prevent the glucose induced toxic manifestation on different hematological variables. It may therefore results in glucose homeostatic by regulating internal clock and rhythmic secretion of other metabolic hormones and hence governing the natural physiological rhythms of body function during the diabetic condition.
2. Materials and methods
2.1. Chemicals and reagents
Streptozotocin (STZ), citrate monohydrate, sodium citrate, were purchased from Himedia limited, India and Sisco Reseach limited (SRL), India. Melatonin (MEL) and insulin (INS) were procured from Sigma Aldrich, USA and Actrapid Novo Nordisk, A/S-Denmark, Egyptian Trading Company respectively. Glibenclamide (GN), from Ahmadabad Gujrat, India. Blood was analysed by using automated haematology analyser following the instructions given in the machine operating manual (Analytical, India).
2.2. Animal maintenance
All the experiment on the animals were conducted in accordance with institutional practices of Institutional Animal Ethics Committee (IAEC) of Guru Ghasidas Vishwavidayalaya, (Registration Number: 994/Go/ERe/S/06/CPCSEA) Bilaspur, CG India in its meeting held on 17.10.2015 and within the framework of revised Animals (Specific Procedure) Act of 2002 of Govt. of India on animal welfare.
Male wistar strain albino rats of same age weighing approximately 190 ± 10gm were purchased from Defence Research and Development Establishment (DRDE) Gwalior, M.P. India. Rats were acclimatized for two weeks before the initiation of the experiment under standard temperature, humidity and light with the supplementation of food and water ad libitum. Following acclimatization rats were randomly divided into nine groups containing six rats each.
All animal experimental procedures were approved by the Animal Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) under the Institutional Animal Ethics Committee at SLT institute of Pharmaceutical Sciences, Guru Ghasidas Vishwavidayalaya, Bilaspur, Chhattisgarh, India. All experimental procedures were performed in accordance with the national and international guidelines and regulations and were approved by SLT institute of Pharmaceutical Sciences, Guru Ghasidas Vishwavidayalaya, Bilaspur Institutional Animal Ethics Committee (Reference No. 157/IAEC/Pharmacy/2016).
2.3. Induction and confirmation of diabetes
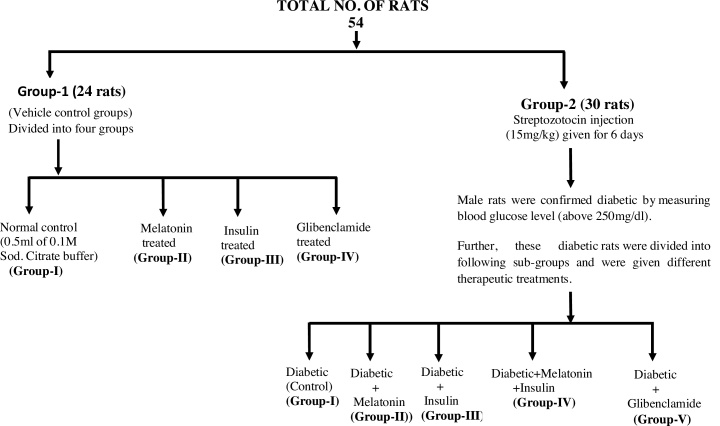
Streptozotocin (STZ) was prepared in 0.1 M citrate buffer (pH-7.4) (15 mg/kg) and was injected intraperitoneally for six consecutive days. Blood glucose levels of animals were monitored using glucometer (ACCUCHECK) after 72 h of streptozotocin treatment. Diabetes was developed and stabilized over a period of 7 days. Total 54 rats were randomly selected for the experimental purpose and were divided into two major groups. Group-1 consisting twenty four (24) and were further sub-divided into four (4) different groups viz. group-I served as normal control, group-II served as melatonin treated, group-III served as insulin treated and group-IV served as glibenclamide treated. Group-2 consisting thirty (30) rats were given streptozotocin (STZ) injection (15 mg/kg) for 6 days. After confirmation of diabetes by measuring blood glucose level, animals having blood glucose level above 250 mg/dl) confirmed as diabetic. Thirty (30) Diabetic rats were further subdivided into following sub-groups and were given different therapeutic treatments, Viz group-I served as Diabetic control, group-II treated with melatonin, group-III treated with insulin, group-IV given treatment of melatonin and insulin and group-V were given treatment of glibenclamide respectively. All the experimental groups named and numbered as documented under experimental design (Table 1), also whole experimental design has been explained in the Flow chart diagram. Doses of melatonin and insulin have been prepared according to the previous method of sugden 1983 and Luippold et al., 2016 respectively with some modifications [26,27].
Table 1.
Experimental table.
| Group No. | Groups | Treatment | No. of Rats |
|---|---|---|---|
| Major Group-1 (24 Rats) | |||
| I | Control (CON) | 0.1 M Sodium citrate buffer | 6 |
| II | Melatonin (MEL) | 1 mg/kg B. W. (4 weeks) | 6 |
| III | Insulin (INS) | 0.5 ml (20units)/kg for 4weeks | 6 |
| IV | Glibenclamide (GB) | 0.5 mg/kg B. W. (4 weeks). | 6 |
| Major Group-2 (30 Rats) | |||
| I | Diabetic | 15 mg/kg body weight, o. p. | 6 |
| II | Diabetic + Melatonin (MEL) | STZ 15 mg/kg (6days)+1 mg/kg B.W. (4weeks) |
6 |
| III | Diabetic + Insulin (INS) | 15 mg/kg (6days)+0.5 ml/kg for 4weeks | 6 |
| IV | Diabetic + MEL + INS | 15 mg/kg (6days)+1 mg/kg+0.5 ml (20units)/kg (4 weeks) | 6 |
| V | Diabetic + Glibenclamide (GB) | STZ 15 mg/kg+0.5 mg/kg B.W.(4weeks)o.p. | 6 |
Flow chart.
Flow chart representation of experimental design and allocation of rats into different experimental groups.
At the end of experiment (4weeks), animals of each group were sacrificed following complete anesthesia (anesthetic ether). Blood was collected in EDTA coated vial for assessment of hematological variables.
3. Parameters studied
3.1. Assessment of blood glucose level
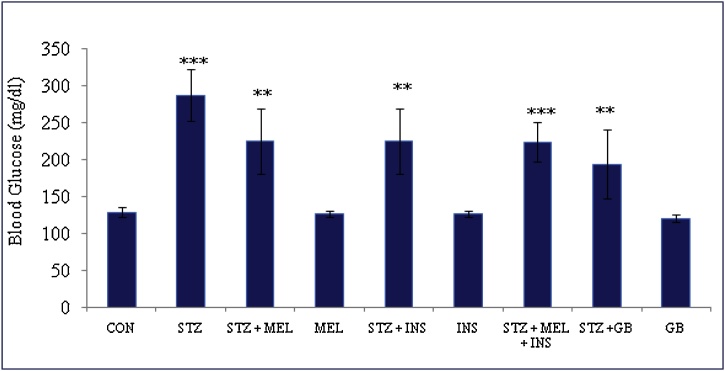
Blood glucose level in all the experimental groups was assessed and recorded during the experimental period (Fig. 1; Table 2)
Fig. 1.
Effect of exogenous melatonin and insulin on blood glucose level of streptozotocin (STZ) induced male diabetic rats. Histogram represents Mean + SE; N = 6. CON = Control, STZ = Streptozotocin, MEL = Melatonin, INS = Insulin, GB = Glibenclamide. **p < 0.01; ***p < 0.001, @F Value 14.8 @Significant at 5% for ANOVA. CON vs STZ; STZ vs STZ + MEL; STZ vs STZ + INS; STZ vs STZ + MEL + INS; STZ vs STZ + GB.
Table 2.
Effect of exogenous melatonin and insulin alone and in combination on STZ induced diabetic rat model with respect to the weekly blood glucose level and serum random sugar level in different experimental groups.
| Groups | Weekly changes in blood glucose level (mg/dl) Before Induction Week-I Week-II Week-III Week-IV | Random sugar level in serum (mg/dl) | ||||
|---|---|---|---|---|---|---|
| CON | 125 ± 10 | 128 ± 8 | 116 ± 3 | 120 ± 5 | 135 ± 4 | 66.34 ± 3.18 |
| Diabetic | 127 ± 15 | 301 ± 11** | 312 ± 10** | 320.33 ± 14*** | 328 ± 16*** | 94 ± 4.45* |
| Diabetic + MEL | 117.25 ± 9 | 394.75 ± 14** | 280.25 ± 15* | 243.25 ± 15** | 221 ± 18** | 69 ± 6.90* |
| MEL | 110 ± 7 | 120 ± 12 | 131 ± 10 | 130.5 ± 8 | 125 ± 14 | 65.17 ± 3.22* |
| Diabetic + INS | 118.25 ± 13 | 394.75 ± 13** | 283.25 ± 6* | 219.25 ± 16* | 167.2 ± 10** | 68.67 ± 7.28 |
| INS | 110 ± 10 | 123 ± 15 | 135 ± 12 | 132.5 ± 12 | 130.5 ± 7 | 65.67 ± 4.36 |
| Diabetic + MEL + INS | 115 ± 7 | 298 ± 9*** | 248 ± 20** | 232 ± 20*** | 224 ± 10*** | 67.84 ± 3.71** |
| Diabetic + GB | 114.25 ± 5 | 384.25 ± 11** | 274 ± 17** | 186.75 ± 13* | 180.5 ± 12** | 66.5 ± 4.51* |
| GB | 117.75 ± 7 | 128 ± 16 | 140.75 ± 11 | 125.5 ± 17 | 144.25 ± 5 | 63.17 ± 4.34 |
@F Value 5.61 (Random sugar level). Data are Mean ± SE;N = 6. Abbreviations: CON = Control; STZ = Streptozotocin; MEL = Melatonin; INS = Insulin GB = Glibenclamide; @ Significant at 5 % for ANOVA. STZ vs CON STZ vs STZ + MEL.STZ vs STZ + MEL + INS.STZ vs STZ + GB Superscripts denotes; *p < 0.05; **p < 0.01; ***p < 0.001.
3.2. Estimation hematological variables
Assessment of hematological variables is a very common and primary diagnostic important routine for the clinical evaluation of the state of health [28]. Examination of hematological variables helps to explain the blood related functions of consumed substances [29]. It also helps to evaluate that the hematological parameters to assess the level of deadly effects of diabetes on the blood of animals. 5 ml of venous blood was collected aseptically from retro orbital sinus by the method of Riley (1960) [30] by using EDTA tubes for each of the selected study subjects. Red blood cells (RBCs), hemoglobin (HB), hematocrit (HCT), leukocytes, platelets, lymphocytes, polymorphs, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), red blood cell distribution (RDW), platelet distribution width (PDW) were determined by using automated hematology following the manufactures instruction (Analytical, India).
3.3. Statistical analysis
Statistical analysis of data was carried using the Statistical Package for Social Sciences (SPSS) for Microsoft Windows release 8.0 statistical software package. Dunken t-tests were used for multiple comparisons at a significance level of p ≤ 0.05, p ≤ 0.01, p ≤ 0.001. All values are expressed as Mean ± Standard Error of the Mean (SEM) [31].
4. Results
4.1. Combined effect of exogenous melatonin and insulin on blood glucose level
Diabetic rats showed significant increase in blood glucose level. However, combined treatment of exogenous melatonin and insulin given to the diabetic rats revealed significant restoration in the blood glucose near to control range (Fig. 1). Glucose lowering potential of melatonin might be due to its regulatory role (biological clock). Further, melatonin couples with Gq, melatonin receptors activates phospholipase-C, and hence melatonin inducing the insulin secretion by IP3-signaling pathway.
4.2. Combined effect of exogenous melatonin and insulin on weekly changes on blood glucose level
Diabetic control rats revealed significant incremental in blood glucose level. However, combined treatment of exogenous melatonin and insulin given to the diabetic rats revealed significant reversal in blood glucose level from week 1 to week 4 (Table 2).
4.3. Combined effect of exogenous melatonin and insulin on hematological variables
Red blood cells (RBCs), hemoglobin (HB), hematocrit (HCT), leukocytes, lymphocytes, polymorphs, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), red blood cell distribution (RDW), platelet distribution width (PDW) showed remarkable decrement in streptozotocin induced diabetic control rats while combined administration of exogenous melatonin and insulin significantly restored the alterations in all these variables, whereas as platelet count exhibited significant increase in diabetic control rats, however exogenous melatonin and insulin treatment prevented further increase in platelet near to the control range (Table 3, Table 4, Table 5, Table 6).
Table 3.
Combined effect of exogenous melatonin and insulin in an diabetic rat model with respect to the different red hematological parameters viz blood cells (RBCs), red blood cell distribution width (RDW), hemoglobin and hematocrit (HCT).
| Groups | (RBC x10/μl) | RDW (%) | Hemoglobin (g/dl) | HCT (%) |
|---|---|---|---|---|
| CON | 9.03 ± 0.58 | 22.13 ± 1.78 | 14.98 ± 0.99 | 42.97 ± 1.39 |
| Diabetic | 4.93 ± 0.45** | 11.30 ± 1.25** | 10.37 ± 0.68*** | 27.55 ± 2.01*** |
| Diabetic + MEL | 6.01 ± 0.47** | 17.92 ± 0.1.26* | 11.89 ± 0.72** | 35.35 ± 2.86** |
| %protection | 26.35 | 61.12 | 32.97 | 50.59 |
| MEL | 8.84 ± 0.71 | 22.01 ± 2.96 | 14.85 ± 0.45 | 39 ± 3.96 |
| Diabetic + INS | 6.71 ± 0.48* | 20.98 ± 1.19* | 13.1 ± 0.93** | 37.43 ± 3.40* |
| %protection | 43.42 | 89.39 | 59.22 | 64.07 |
| INS | 8.67 ± 0.37 | 22.82 ± 1.46 | 14.31 ± 0.99 | 39.96 ± 2.65 |
| Diabetic + MEL + INS | 9 ± 0.40** | 22.1 ± 1.98 | 15.03 ± 0.47*** | 40.68 ± 2.89** |
| %protection | 99.26 | 99.72 | 101.08 | 85.14 |
| Diabetic + GB | 6.28 ± 0.33** | 19.71 ± 2.18 | 13.8 ± 0.79*** | 33.86 ± 2.66 |
| %protection | 32.92 | 77.65 | 74.40 | 40.92 |
| GB | 8.65 ± 0.88 | 22 ± 1.81 | 14.78 ± 0.37 | 39.98.34 ± 3.89 |
| @F Value | 3.38 | 8.476 | 5.016 | 5.443 |
Data are Mean ± SE; N = 6. Abbreviations: CON = Control; MEL = Melatonin and INS = Insulin; GB = Glibenclamide; RBCs = Red Blood Cells; HCT = Hematocrit; RDW = Red Blood Cell Distribution Width; @ Significant at 5 % for ANOVA. Diabetic vs CON; Diabetic vs Diabetic + MEL; Diabetic vs Diabetic + INS; Diabetic vs Diabetic + MEL + INS; Diabetic vs Diabetic + GB. Superscripts denotes; *p < 0.05; **p < 0.01 and ***p < 0.001.
Table 4.
Combined effect of exogenous melatonin and insulin in diabetic rat model with respect to the main corpuscular Hemoglobin (MCH), main corpuscular Hemoglobin concentration (MCHC) and main corpuscular volume (MCV).
| Groups | MCV (fl) | MCH (pg) | MCHC (%) |
|---|---|---|---|
| CON | 58.22 ± 3.19 | 19.8 ± 1.66 | 42.28 ± 2.09 |
| Diabetic | 44.92 ± 2.14* | 15.05 ± 1.47** | 31.38 ± 1.50** |
| Diabetic + MEL | 53.52 ± 3.09* | 17.81 ± 1.51** | 36.4 ± 2.18* |
| %protection | 64.66 | 58.10 | 46.05 |
| MEL | 57.6 ± 2.28 | 19.14 ± 1.43 | 41.13 ± 2.06 |
| Diabetic + INS | 55.33 ± 2.47* | 19.34 ± 1.64* | 38.26 ± 2.93* |
| %protection | 78.27 | 90.31 | 63.11 |
| INS | 57.93 ± 3.77 | 19.7 ± 0.85 | 40.14 ± 1.80 |
| Diabetic + MEL + INS | 56.85 ± 2.88*** | 19.78 ± 0.89*** | 41.63 ± 2.08** |
| %protection | 89.69 | 101.72 | 94.03 |
| Diabetic + GB | 53.3 ± 2.92* | 17.73 ± 1.42* | 39.01 ± 1.45* |
| %protection | 63.00 | 56.42 | 70.00 |
| GB | 57.41 ± 2.37 | 19.98 ± 1.08 | 41.6 ± 1.92 |
| @ F Value | 4.655 | 1.209 | 11.871 |
Data are Mean ± SE; N = 6. Abbreviations: CON = Control; MEL = Melatonin and INS = Insulin; GB = Glibenclamide; MCV = Mean corpuscular volume, MCH = Mean Corpuscular Hemoglobin; MCHC = Mean Corpuscular Hemoglobin Concentration; Significant at 5 % for ANOVA. Diabetic vs CON; Diabetic vs Diabetic + MEL; Diabetic vs Diabetic+.
INS; Diabetic vs Diabetic + MEL + INS and Diabetic vs Diabetic + GB.
Superscripts denotes; *p < 0.05; **p < 0.01.
Table 5.
Combined effect of exogenous melatonin and insulin in diabetic rat model with respect to the leukocytes, platelets and platelet distribution width (PDW).
| Groups | PDW (%) | Platelets (x103/μl) |
|---|---|---|
| CON | 27.36 ± 0.92 | 90.5 ± 2.20 |
| Diabetic | 20.07 ± 1.19** | 97 ± 32.01* |
| Diabetic + MEL | 24.2 ± 1.93* | 95.5 ± 3.08* |
| %protection | 56.65 | 23.07 |
| MEL | 27.43 ± 1.86 | 89.67 ± 2.61 |
| Diabetic + INS | 24.61 ± 1.83* | 90.33 ± 2.39 * |
| %protection | 62.27 | 102.61 |
| INS | 27.16 ± 1.73 | 89.67 ± 2.05 |
| Diabetic + MEL + INS | 27.21 ± 2.25 ** | 90.33 ± 2.71 ** |
| %protection | 97.94 | 104.15 |
| Diabetic + GB | 22.86 ± 1.38* | 94.16 ± 42.36* |
| %protection | 38.27 | 43.69 |
| GB | 27.75 ± 2.87 | 89.5 ± 3.36 |
| @ F Value | 1.152 | 0.233 |
Data are Mean ± SE; N = 6. Abbreviations: CON = Control; MEL = Melatonin and INS = Insulin,GB = Glibenclamide; PDW = Platelet distribution width @Significant at 5 % for ANOVA. Diabetic vs CON. Diabetic vs Diabetic + MEL, Diabetic vs Diabetic + INS; Diabetic vs Diabetic + MEL + INS. Diabetic vs Diabetic + GB. Superscripts denotes; *p < 0.05 ; **p < 0.01.
Table 6.
Combined effect of exogenous melatonin and insulin in diabetic rat model with respect to the polymorphs, leukocytes and lymphocytes.
| Groups | Leukocytes (/Cmm) | Polymorphs (%) | Lymphocytes (%) |
|---|---|---|---|
| CON | 20470 ± 1388.60** | 74.2 ± 2.15 | 22.95 ± 1.44 |
| Diabetic | 12399 ± 772.24** | 45.43 ± 12.23** | 11.03 ± 1.95*** |
| Diabetic + MEL | 15561.83 ± 1017.28* | 60 ± 4.31** | 16.15 ± 1.36** |
| %protection | 39.18 | 50.64 | 54.11 |
| MEL | 20147.33 ± 702.14 | 72.34 ± 1.91 | 20.2 ± 0.72 |
| diabetic + INS | 14938.67 ± 104.35* | 64.83 ± 2.93* | 14.21 ± 1.76* |
| %protection | 31.46 | 67.43 | 64.93 |
| INS | 20063.83 ± 429.99*** | 73.26 ± 2.29** | 10 ± 1.18 |
| Diabetic + MEL + INS | 17361.17 ± 830.26 *** | 62.88 ± 2.52*** | 21.86 ± 1.21** |
| %protection | 61.48 | 60.65 | 93.03 |
| Diabetic + GB | 19340.83 ± 712.11** | 63.33 ± 2.13** | 13.1 ± 1.47* |
| %protection | 86.00 | 62.21 | 57.46 |
| GB | 20328.33 ± 1253.61 | 73.4 ± 1.94 | 19.29 ± 0.97 |
| @ F Value | 14.184 | 38.480 | 24.54 |
Data are Mean ± SE; N = 6. Abbreviations: CON = Control; INS = Insulin; GB = Glibenclamide; @ Significant at 5 % for ANOVA. Diabetic vs CON; Diabetic vs Diabetic + MEL; Diabetic vs Diabetic + INS; Diabetic vs Diabetic + MEL + INS; Diabetic vs Diabetic + GB.
Superscripts denotes; *p < 0.05; **p < 0.01; ***p < 0.001.
5. Discussion
Present study focused on coadminstrtaion of melatonin and insulin alone as well as in combination. Diabetic rats showed significant increase in the blood glucose level. However melatonin and insulin alone and in combination significantly lowered the blood mean as well as weekly blood glucose level. The results of the present study were strongly supported by previous finding which suggested that melatonin positively impacts on the β-cell proliferation/regeneration as well as inhibit apoptosis [32,33]. The known basic mechanism for the streptozotocin leads to the production of antibodies. These auto-antibodies in turn act on the β-cell of the pancreas. Therefore, causes apoptosis of pancreatic β-cell, which finally leads to the impaired insulin synthesis and secretion [34,35]. Pancreas damage is connected with the development of the diabetes. Destruction of pancreatic islet cells lead to decrease in insulin secretion and glucose increases in blood [36,37]. This increased blood glucose causes oxidative stress and might be altering other physiological processes such reduced RBC count, hemoglobin glycosylation and other hematological impairments. Therefore, it has confirmed that melatonin protects the β-cell as well as accelerates their function. In case of the diabetes level of melatonin and insulin decreases which can be compensated by exogenous supplementation of melatonin along with insulin. This might signifies that protective action by counteracting diabetes induced stress, hence attenuates the oxidative stress induced β-cell damages and also protects them against the functional overstrain [36,38,39].
In diabetic rats red blood cells (RBC) count was found decreased significantly, due to the non-enzymatic glycosylation of RBC membrane protein which is directly associated with the hyperglycemic condition. Because high glucose level leads to the generation of toxic products alteration which causes many such as reduced production of bone marrow [40], affects the shape of RBCs result in anemia and low hemoglobin production [41], hence impairs their flow properties. Oxidation of these abnormally synthesized glycosylated membrane proteins in association with the hyperglycemic situation leads to the production of lipid peroxides. These peroxides increases membrane stiffness, decreases cellular deformability, decreases life span of RBCs and lipid fluidity and also causes hemolysis of RBCs [42]. Therefore, it might be a strong reason that diabetes patients also suffer from anemia [43,44]. Anemia leads to the impaired healing wound, macrovascular diseases [45]. Administration of exogenous melatonin and insulin significantly normalized the RBC count because melatonin scavenges the peroxides, reduces membrane damages, simultaneously insulin competes for the regulation of glucose metabolism and hence prevents glycosylation of proteins [46,47]. It could be inferred that melatonin and insulin might have stimulated the production or secretion of erythropoietin and stimulated stem cells in the bone marrow to produce red blood cells.
Reduction in the RBC indices such as HB, MCV, HCT, MCH and MCHC specified that inconsistent hemoglobin synthesis, anemia [48], failure of blood osmoregulation and plasma osmolarity [15]. The stimulatory effect of melatonin and insulin boosted the rapid synthesis of RBC which is supported by the increased in the level of HB, HCT, MCV, MCH and MCHC. Improved level of these parameters is correlated with increased level of hemoglobin in melatonin and administrated group of rats. Hence increases and restored the oxygen carrying capacity blood [49].
In diabetic rats total WBC count and its associated indices showed remarkable decrease, which is supported by previous studies that total white blood cell count (WBCC) and lymphocyte count decreases [16]. The decrease in WBC and lymphocytes could be interrelated to inhibition of leukocytosis from the bone marrow which might be due to the poor defensive mechanism against infection [17]. Diabetic rats given the exogenous supplementation of melatonin and insulin for four weeks significantly restored the WBC count and lymphocytes near the control level. Long term poor glycemic control happened because of the insulin absence/ deficiency [18].
Polymorphs include neutrophils, eosinophils and basophils are involved in different immune defense process. Neutrophils are the main leukocytes, functions as first line of defense; their decreased count might be contributing reduction in functional activity, which in turn impairs healing of wounds. Prolonged healing of wounds is the main problem in diabetic which in long term leads to the severe issues such as amputation of the infected organ. Therefore, diabetic patients are susceptible to any kind of infection, results in high morbidity and mortality. Decrease in the neutrophils count is also directly associated with the hyperglycemia induced neutrophils dysfunction via advanced glycosylation, production of oxygen free radicals, protein glycosylation. Eosinophils and basophils are especially involved with allergy and parasitic infections by controlling activation state of macrophages in the adipose tissue. Diabetes rats showed significant reduction in the polymorph count (neutrophils, eosinophils and basophils). However, rats receiving exogenous melatonin and insulin showed significant increment in these immune cells by preventing production of oxygen free radicals, protein glycosylation and advanced glycosylation. Present study is in agreement with the earlier studies reported that melatonin is a well known immune modulator, free radical scavenger. Insulin regulates the glucose metabolism, hence prevents the auto-oxidation of glucose, non-enzymatic protein glycosylation. Therefore, combined therapy of melatonin and insulin might have boosted the immune system by stimulating the immune system to increase the formation of polymorphs.
In the present study diabetes rats showed increment in platelet count. Earlier studies are in support, which reported that diabetes causes multiple abnormalities such as platelet hyper-reactivity with higher adhesiveness, activation and aggregation [16], increases in the rate of insulin resistance. The platelet abnormalities are related with increased clotting, impaired clot breakdown, endothelial dysfunction, and platelet hyper-reactivity. All these factors amplify the threat artherothrombotic incidents in diabetes [16]. The combined administration of melatonin and insulin significantly recovered the platelet count towards the control, might prevented their hyper-reactivity.
6. Conclusion
Findings of the present suggests that diabetes resulted in alterations in hematological parameters viz. decrease red blood cell count, hemoglobin, white blood cell count and associated physiological processes including decrease in blood volume, anemia, oxygen carrying capacity, including compromised immune status and clotting related problems. Since all these impairments were recovered following combined treatment of melatonin and insulin comparable with the glibenclamide treated diabetic rats as well as control healthy rats. It may be concluded that since the integrated and administration of melatonin and insulin might have inhibited the membrane protein peroxidative damages, non-enzymatic glycosylation of protein, stimulating the white blood cell formation and prevented the platelet aggregation, hence recovered and restored the diabetes induced hematological alterations in rats. Therefore, such co-administration of melatonin and insulin can be suggested as a beneficial therapeutic combination therapy for diabetic cases / disorder. However further studies are required to find out the pharmacological doses and molecular target of melatonin and insulin.
Declaration of Competing Interest
Authors declares that there is not conflict of interest.
Acknowledgments
Authors are highly grateful to the Department of Zoology, Guru Ghasidas Vishwavidyalaya, Bilaspur, Chhattisgarh for providing necessary available research facilities. Further, support of DBT Builder Project, Department of Biotechnology, Ministry of Science and Technology (Grant number-BT/PR-7020/INF22/172-2012) New-Delhi India, is highly acknowledged.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.01.020.
Appendix A. Supplementary data
The following is supplementary data to this article:
References
- 1.Johar D., Maher A., Aboelmagd O., Hammad A., Morsi M., Warda H.F., Awad H.I., Mohamed T.A., Zaky S. Whole-food phytochemicals antioxidative potential in alloxan-diabetic rats. Toxicol. Rep. 2018;5:240–250. doi: 10.1016/j.toxrep.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wild S., Roglic G., Green A., Sicree R., King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(2004):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(2014):81–90. [Google Scholar]
- 4.Rai S., Hajam Y.A., Basheer M., Ghosh H. Biochemical and histopathological inflections in Hepato-renal Tissues of Streptozotocin (STZ) induced diabetic male rats: impact of exogenous melatonin administration. J. Clin. Res. Bioeth. 2016;7:10–4172. [Google Scholar]
- 5.Hajam Y.A., Rai S. Melatonin and insulin modulates the cellular biochemistry, histoarchitecture and receptor expression during hepatic injury in diabetic rats. Life Sci. 2019;239 doi: 10.1016/j.lfs.2019.117046. [DOI] [PubMed] [Google Scholar]
- 6.Hajam Y.A., Rai S., Shree S., Basheer M., Ghosh H. Retrieval of reproductive complications by exogenous melatonin treatment in streptozotocin induced diabetic rat model. Res. Rev: J. Zool. Sci. 2017;5:96–104. [Google Scholar]
- 7.Hajam Y.A., Rai S., Roy A., Basheer M., Ghosh H. Repossession of brain complications in a streptozotocin induced diabetic rat by exogenous melatonin administration. Int. J. Zool. Res. 2017;13:64–73. [Google Scholar]
- 8.Peschke E., Mühlbauer E. New evidence for a role of melatonin in glucose regulation. Best Pract. Res. Clin. Endocrinol. Metab. 2010;24:829–841. doi: 10.1016/j.beem.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Peschke E., Frese T., Chankiewitz E., Peschke D., Preiss U., Schneyer U., Spessert R., Mühlbauer E. Diabetic Goto Kakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin‐receptor status. J. Pine Res. 2006;40:135–143. doi: 10.1111/j.1600-079X.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 10.Mirzaei K., Xu M., Qi Q., De Jonge L., Bray G.A., Sacks F., Qi L. Variants in glucose-and circadian rhythm–related genes affect the response of energy expenditure to weight-loss diets: the pounds lost trial. Am. J. Clin. Nutr. 2013;99:392–399. doi: 10.3945/ajcn.113.072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiter R.J., Tan D.X., Korkmaz A., Ma S. Obesity and metabolic syndrome: association with chronodisruption, sleep deprivation, and melatonin suppression. Ann. Med. 2012;44:564–577. doi: 10.3109/07853890.2011.586365. [DOI] [PubMed] [Google Scholar]
- 12.Konturek P.C., Brzozowski T., Konturek S.J. Gut clock: implication of circadian rhythms in the gastrointestinal tract. J. Physiol. Pharmacol. 2011;62:139–150. [PubMed] [Google Scholar]
- 13.Zanuto R., Siqueira‐Filho M.A., Caperuto L.C., Bacurau R.F., Hirata E., Peliciari‐ Garcia R.A., do Amaral F.G., Marçal A.C., Ribeiro L.M., Camporez J.P., Carpinelli A.R. Melatonin improves insulin sensitivity independently of weight loss in old obese rats. J. Pine. Res. 2013;55:156–165. doi: 10.1111/jpi.12056. [DOI] [PubMed] [Google Scholar]
- 14.Caimi G., Serra A., Lo Presti R., Grifò G., Romano A., Catania A., Francavilla G., D’Asaro S., Montana M., Sarno A. Red cell Ca2+ content (total and cytosolic) and erythrocyte membrane fluidity in several clinical conditions. Clin. Hemorheol. Microcirc. 1993;13(1993):227–235. [Google Scholar]
- 15.Stookey J.D., Burg M., Sellmeyer D.E., Greenleaf J.E., Arieff A., Van H.L., Gardner C., King J.C. A proposed method for assessing plasma hypertonicity in vivo. Eur. J. Clin. Nutr. 2007;61:143. doi: 10.1038/sj.ejcn.1602481. [DOI] [PubMed] [Google Scholar]
- 16.Hillson R. Diabetes and the blood–white cells and platelets. Pract. Diabetes. 2015;32:159–160. [Google Scholar]
- 17.Oyedemi S.O., Yakubu M.T., Afolayan A.J. Antidiabetic activities of aqueous leaves extract of Leonotis leonurus in streptozotocin induced diabetic rats. J. Med. Plants Res. 2011;5:119–125. [Google Scholar]
- 18.Jarald E., Joshi S.B., Jain D.C. Diabetes vs herbal medicines. Iran. J. Pharmacol. Ther. 2008;7:97–100. [Google Scholar]
- 19.Baltzis D., Meimeti E., Grammatikopoulou M.G., Roustit M., Mavrogonatou E., Kletsas D., Efraimidou S., Manes C., Nikolouzakis T.K., Tsiaoussis J., Tsatsakis A.M. Assessment of telomerase activity in leukocytes of type 2 diabetes mellitus patients having or not foot ulcer: possible correlation with other clinical parameters. Exp. Ther. Med. 2018;15:3420–3424. doi: 10.3892/etm.2018.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramracheya R.D., Muller D.S., Squires P.E., Brereton H., Sugden D., Huang G.C., Amiel S.A., Jones P.M., Persaud S.J. Function and expression of melatonin receptors on human pancreatic islets. J. Pine Res. 2008;44:273–279. doi: 10.1111/j.1600-079X.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- 21.She M., Laudon M., Yin W. Melatonin receptors in diabetes: a potential new therapeutical target. Eur. J. Pharmacol. 2014;744:220–223. doi: 10.1016/j.ejphar.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Kuhlow D., Florian S., von-Figura G., Weimer S., Schulz N., Petzke K.J., Zarse K., Pfeiffer A.F., Rudolph K.L., Ristow M. Telomerase deficiency impairs glucose metabolism and insulin secretion. Aging (Albany NY) 2010;2:650–658. doi: 10.18632/aging.100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salpea K.D., Talmud P.J., Cooper J.A., Maubaret C.G., Stephens J.W., Abelak SHu K. E. mphries, Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis. 2010;209:42–50. doi: 10.1016/j.atherosclerosis.2009.09.070. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serra V., Grune T., Sitte N., Saretzki G., von Zglinicki T. Telomere length as a marker of oxidative stress in primary human fibroblast cultures. Ann. N. Y. Acad. Sci. 2000;908:327–330. doi: 10.1111/j.1749-6632.2000.tb06666.x. [DOI] [PubMed] [Google Scholar]
- 25.Petrakis D., Vassilopoulou L., Mamoulakis C., Psycharakis C., Anifantaki A., Sifakis S., Docea A., Tsiaoussis J., Makrigiannakis A., Tsatsakis A. Endocrine disruptors leading to obesity and related diseases. Int. J. Environ. Res. Public Health. 2017;14(2017):1282. doi: 10.3390/ijerph14101282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luippold G., Bedenik J., Voigt A., Grempler R. Short-and longterm glycemic control of streptozotocin-induced diabetic rats using different insulin preparations. PLoS One. 2016;11 doi: 10.1371/journal.pone.0156346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugden D. Psychopharmacological effects of melatonin in mouse and rat. J. Pharmacol. Exp. Ther. 1983;227:587–591. [PubMed] [Google Scholar]
- 28.Yakubu M.T., Akanji M.A., Oladiji A.T. Hematological evaluation in male albino rats following chronic administration of aqueous extract of Fadogia agrestis stem. Pharmacogn. Mag. 2007;3:34. [Google Scholar]
- 29.Hoff brand A.V., Pettit J.E. 4th edition. Blackwell Science; 2000. Hematological Parameters in Essentials of Haematology; pp. 21–25. 2000. [Google Scholar]
- 30.Riley V. Adaptation of orbital bleeding technic to rapid serial blood studies. Proc. Soc. Exp. Biol. Med. 1960;104:751–754. doi: 10.3181/00379727-104-25975. [DOI] [PubMed] [Google Scholar]
- 31.Snedecor G.W., Cochran W.G. 8thedn. Iowa State Univ. Press Iowa; Ames: 1989. 1989. Statistical Methods. [Google Scholar]
- 32.Ferreiro J.L., Gómez-Hospital J.A., Angiolillo D.J. Platelet abnormalities in diabetes mellitus. Diab. Vasc. Dis. Res. 2010;7(2010):251–259. doi: 10.1177/1479164110383994. [DOI] [PubMed] [Google Scholar]
- 33.Kanter M., Uysal H., Karaca T., Sagmanligil H.O. Depression of glucose levels and partial restoration of pancreatic β-cell damage by melatonin in streptozotocin-induced diabetic rats. Arch. Toxicol. 2006;80:362–369. doi: 10.1007/s00204-005-0055-z. [DOI] [PubMed] [Google Scholar]
- 34.Simsek N., Kaya M., Kara A., Can I., Karadeniz A., Kalkan Y. Effects of melatonin on islet neogenesis and beta cell apoptosis in streptozotocin-induced diabetic rats: an immunohistochemical study. Domestic Anim. Endocrinol. 2012;43:47–57. doi: 10.1016/j.domaniend.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Boslem E., Meikle P.J., Biden T.J. Roles of ceramide and sphingolipids in pancreatic β-cell function and dysfunction. Islets. 2012;4:177–187. doi: 10.4161/isl.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hajam Y.A., Rai S., Basheer M., Ghosh H., Singh S. Protective role of melatonin in streptozotocin induced pancreatic damages in diabetic Wistar Rat. Pak. J. Biol. Sci. 2018;21(2018):423–431. doi: 10.3923/pjbs.2018.423.431. [DOI] [PubMed] [Google Scholar]
- 37.Szkudelski T. Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp. Biol. Med. 2012;237:481–490. doi: 10.1258/ebm.2012.011372. [DOI] [PubMed] [Google Scholar]
- 38.Karamitri A., Renault N., Clement N., Guillaume J.L., Jockers R. Minireview: toward the establishment of a link between melatonin and glucose homeostasis: association of melatonin MT2 receptor variants with type 2 diabetes. Mol. Endocrinol. 2013;27(2013):1217–1233. doi: 10.1210/me.2013-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiter R.J., Paredes S.D., Manchester L.C., Tan D.X. Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol. 2009;44:175–200. doi: 10.1080/10409230903044914. [DOI] [PubMed] [Google Scholar]
- 40.Abbas M., Siddiqi M.H., Khan K., Zahra K. Haematological evaluation of sodium fluoride toxicity in oryctolagus cunniculus. Toxicol. Rep. 2017;4(2017):450–454. doi: 10.1016/j.toxrep.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh M., Shin S. Changes in erythrocyte aggregation and deformability in diabetes mellitus: a brief review. Indian J. Exper. Biol. 2009;47(2009):7–15. [PubMed] [Google Scholar]
- 42.Kolanjiappan K., Manoharan S., Kayalvizhi M. Measurement of erythrocyte lipids, lipid peroxidation, antioxidants and osmotic fragility in cervical cancer patients. Clin. Chim. Acta. 2002;326:143–149. doi: 10.1016/s0009-8981(02)00300-5. [DOI] [PubMed] [Google Scholar]
- 43.Jaman S., Sawgat R. Association of mean platelet volume and platelet distribution width with Hba1c. J. Endocrinol. Diab. 2017;4(2017):1–6. [Google Scholar]
- 44.Jaman M.S., Rahman M.S., Swarna R.R., Mahato J., Ibrahim M.A., Siddique M., Miah M., Ayshasiddeka M. Diabetes and red blood parameters. Ann. Clin. Endocrinol. Metabol. 2018;2:1–9. [Google Scholar]
- 45.Abate A., Birhan W., Alemu A. Association of anemia and renal function test among diabetes mellitus patients attending Fenote Selam Hospital, West Gojam, Northwest Ethiopia: a cross sectional study. BMC Blood Disord. 2013;13:6. doi: 10.1186/2052-1839-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiter R.J., Mayo J.C., Tan D.X., Sainz R.M., Alatorre‐Jimenez M., Qin L. Melatonin as an antioxidant: under promises but over delivers. J. Pine. Res. 2016;61(2016):253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 47.Montilla P.L., Vargas J.F., Túnez I.F., Carmen M., de Agueda M., Valdelvira M.E.D., Cabrera E.S. Oxidative stress in diabetic rats induced by streptozotocin: protective effects of melatonin. J. Pine Res. 1998;25:94–100. doi: 10.1111/j.1600-079x.1998.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 48.Saheed S., Oladipipo A.E., Abdulazeez A.A., Olarewaju S.A., Ismaila N.O., Emmanuel I.A., Fatimah Q.D., Aisha A.Y. Toxicological evaluations of Stigma maydis (corn silk) aqueous extract on hematological and lipid parameters in Wistar rats. Toxicol. Rep. 2015;2:638–644. doi: 10.1016/j.toxrep.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahmoud A.M. Hematological alterations in diabetic rats-role of adipocytokines and effect of citrus flavonoids. EXCLI J. 2013;12(2013):647. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.