Abstract
Nutcracker syndrome is a rare vascular disorder that involves compression of the left renal vein (LRV), most often at the level of the aortomesenteric angle. In some cases, this compression syndrome arises in the setting of unusual vascular anatomic variants. We describe the case of a 43-year-old woman with a duplicated inferior vena cava who was found to have LRV compression on magnetic resonance angiography and venography with intravascular ultrasound. The patient was successfully treated with concurrent transposition of the LRV and left-sided inferior vena cava, with complete resolution of symptoms.
Keywords: Nutcracker syndrome, Duplicated IVC, Renal vein transposition, IVUS
Nutcracker syndrome (NCS) is a rare clinical condition that occurs when the left renal vein (LRV) experiences venous outflow obstruction secondary to external vascular compression, most often between the aorta and superior mesenteric artery (SMA).1 The resultant LRV hypertension potentiates a constellation of symptoms including flank pain, pelvic congestion, and proteinuria or hematuria. Initial diagnosis of NCS has typically relied on the use of duplex ultrasound (DUS), magnetic resonance angiography (MRA), computed tomography angiography (CTA), and venography with venous pressure measurements.2 More recently, intravascular ultrasound (IVUS) has emerged as an important adjunct for diagnosis of venous outflow obstruction, particularly in cases in which renocaval pressure gradients are equivocal.3,4
Duplicated inferior vena cava (IVC) is a congenital venous abnormality that occurs in 0.2% to 3.0% of the population and arises from persistence of the left and right supracardinal veins.5 This anomaly not only can place individuals at increased risk for vascular complications but also can have an impact on surgical and technical decision-making. Whereas nutcracker physiology has been reported in individuals with both normal vascular anatomy and aberrancies including celiacomesenteric trunks6,7 and left-sided IVCs,8, 9, 10, 11, 12, 13 there has been only one report of NCS in a patient with a duplicated IVC, with no discussion of the technical nuances of open surgical treatment.14 We report a unique case of NCS in a patient with a duplicated IVC who was found to have significant LRV compression on MRA and IVUS. She experienced symptom resolution after concurrent transposition of the LRV and left-sided IVC. Consent was obtained for case publication.
Case report
A 43-year-old white woman presented with 1 year of left flank and pelvic pain, gross hematuria, and anemia requiring regular transfusions. The initial workup consisting of urine cytology, cystoscopy, bladder biopsy, ureteroscopy, retrograde pyelography, and autoimmune serologic tests failed to elucidate the cause of her presentation.
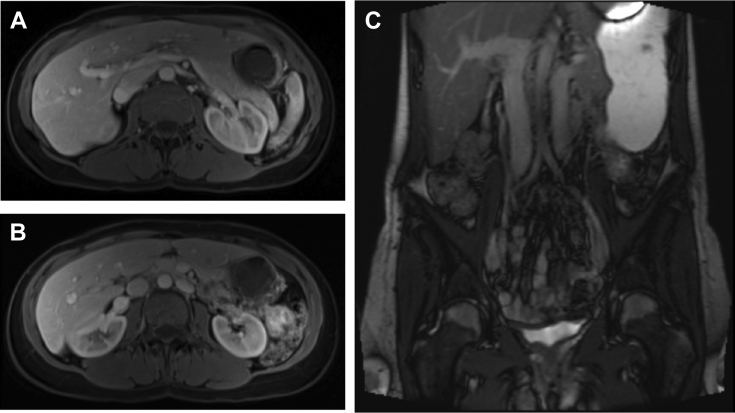
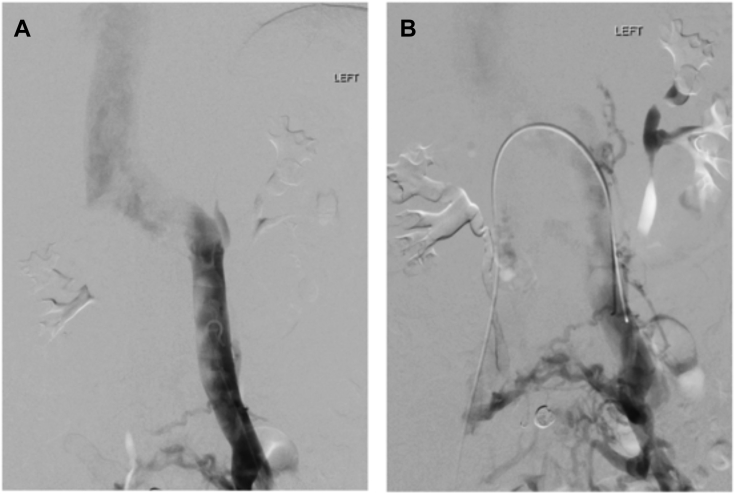
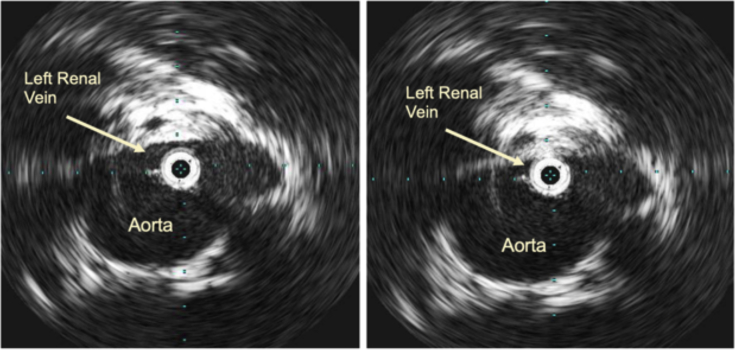
MRA was obtained for further evaluation of hematuria. This demonstrated compression of the LRV as it traversed the aortomesenteric angle (Fig 1, A), resulting in a 3.5-mm-diameter reduction between the hilum and aortomesenteric angle as well as a duplicated left-sided IVC with anomalous drainage into the LRV (Fig 1, B). Initial pelvic and renal venography revealed no visual evidence of LRV compression and unobstructed free filling of contrast material into the right suprarenal IVC (Fig 2, A). In addition, the renocaval pressure gradient was found to be 1 mm Hg. However, there was some suggestion of LRV outflow obstruction, as evidenced by numerous well-developed left-to-right collaterals decompressing venous drainage from the left pelvis (Fig 2, B). Subsequent IVUS imaging demonstrated focal LRV compression as it crossed the aorta, with little residual lumen surrounding the catheter at its most stenotic region (Fig 3).
Fig 1.
Axial magnetic resonance image demonstrating (A) compression of the left renal vein (LRV) as it traverses the aorta-superior mesenteric artery (SMA) angle and (B and C) presence of a duplicated inferior vena cava (IVC).
Fig 2.
Left renal and pelvic venography demonstrating (A) continuous, free filling of contrast material from the left-sided inferior vena cava (IVC) into the left renal vein (LRV) and right suprarenal IVC and (B) presence of well-developed left-to-right venous collaterals draining the left-sided IVC.
Fig 3.
Intravascular ultrasound (IVUS) showing left renal vein (LRV) compression as it crosses the aorta.
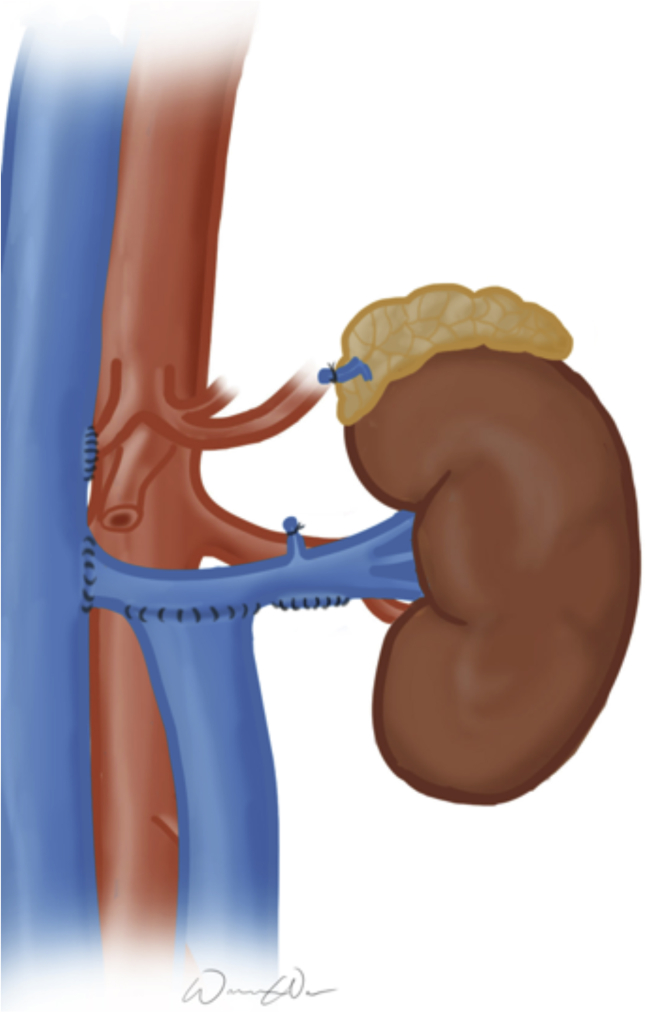
Surgical correction of LRV compression through a transperitoneal approach was offered. After an upper midline laparotomy, the bowel was packed in the right abdomen, the retroperitoneum was entered, and the right- and left-sided IVCs were exposed. The LRV was fully mobilized, requiring division of its branches. The LRV-right IVC junction was sclerotic, suggestive of chronic inflammation and scarification. After transection of the LRV and oversewing of the junction, the LRV was transposed 7 cm inferiorly onto the right-sided IVC. After LRV transposition, considerable kinking of the left-sided IVC was observed; thus, the left-sided IVC was transposed more medially onto the LRV to improve this angulation. All anastomoses were performed in an end-to-side four-quadrant fashion using 3-0 Prolene sutures. Fig 4 illustrates this transposition.
Fig 4.
Concurrent left renal vein (LRV) and left-sided inferior vena cava (IVC) transposition for a patient with nutcracker syndrome (NCS) in the setting of a duplicated IVC.
There were no perioperative complications. The patient reported prompt resolution of hematuria and had no further anemia postoperatively. At 1-month follow-up, she reported significant improvement of left pelvic and flank pain, which resolved during the ensuing 6 months. At 3-year follow-up, the patient denied any symptom recurrence.
Discussion
NCS is a rare vascular condition characterized by LRV compression between the aorta and SMA, resulting in venous hypertension and a spectrum of clinical sequelae. Currently, DUS, MRA, and CTA serve as initial noninvasive imaging modalities in patients with suspected NCS.2,15 For DUS, an LRV diameter to peak velocity ratio >5 is 60% to 90% sensitive and 89% to 100% specific for NCS; in particular, DUS is useful because of its capacity to record velocities and to accentuate NCS findings with positional changes.2 For CTA and MRA, an LRV hilar to aortomesenteric diameter ratio >4.9, a beak sign, and an SMA to aorta angle <41 degrees offer the highest diagnostic accuracy.16 However, hemodynamic assessments of LRV compression and venous outflow obstruction are often best accomplished through venography and venous pressure measurements.17 Conventionally, the diagnosis of NCS has been defined as a renocaval pressure gradient ≥3 mm Hg, which has been evidenced to reflect significant LRV hypertension.16,18,19 However, there is considerable debate about this cutoff. Many asymptomatic individuals have renocaval pressure gradients ≥3 mm Hg; conversely, patients with long-standing NCS may lack high-pressure gradients because of collateralization and vascular decompression.20,21 Thus, it is uncertain whether this gradient can serve as a definitive diagnostic tool for nutcracker physiology.
IVUS has emerged as an important adjunct to standard imaging modalities for diagnosis of NCS. Given that this technique has recently become the “gold standard” for identification of other venous compression syndromes because of its high sensitivity and ability to provide intraluminal measurements and characteristics,22, 23, 24 IVUS has been increasingly employed to help identify LRV compression.2,3,25 Specifically, IVUS may play a critical role in detecting nutcracker physiology in patients such as ours, in whom venography revealed a normal renocaval pressure gradient. Although there are no formal IVUS criteria established for NCS, IVUS has been effectively used to identify planar LRV compression that is unappreciable on two-dimensional imaging and to visualize intraluminal webs and spurs.2,16
Our patient had the added complexity of having a duplicated IVC. Whereas this congenital anomaly has been associated with complications such as deep venous thrombosis,26, 27, 28 its connection with NCS has been observed only once. In a case report by Yoshida et al,14 a female patient with a duplicated IVC was found to have NCS after presenting with left lower extremity deep venous thrombosis; she was successfully treated with LRV pharmacomechanical thrombolysis and stenting.
Unlike in that case, our patient presented with clinical signs and symptoms that were more typical of nutcracker physiology. In addition, we made the decision to undertake open repair, given the high migration rates associated with LRV stenting29 and the known vascular anomaly. The most commonly reported technique for open repair of NCS is LRV transposition, with reimplantation of the LRV more caudally onto the IVC.30 This procedure demands meticulous surgical planning and execution because rates of postoperative LRV stenosis or occlusion have exceeded 20% in some series.30 In our case, the presence of a duplicated IVC compelled us to be particularly cognizant of the technical challenges that could arise intraoperatively. Although we were able to successfully transpose the LRV beneath the original LRV-caval junction, issues that emerged because of the patient's vascular anomaly necessitated a second transposition of the left-sided IVC medially to prevent further flow obstruction. Therefore, knowledge of these anatomic variants and their impact on surgical methods is important for effective NCS treatment.
Conclusions
NCS in the setting of a duplicated IVC is a rare variant of LRV entrapment that warrants increased consideration during medical and surgical evaluation. Along with conventional imaging methods, IVUS can serve as a useful adjunct in nutcracker physiology identification, particularly when the diagnosis is unclear. Open repair with concurrent transposition of the LRV and left-sided IVC can lead to effective symptom resolution in such cases of NCS.
From the New England Society for Vascular Surgery
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Kurklinsky A.K., Rooke T.W. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc. 2010;85:552–559. doi: 10.4065/mcp.2009.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ananthan K., Onida S., Davies A.H. Nutcracker syndrome: an update on current diagnostic criteria and management guidelines. Eur J Vasc Endovasc Surg. 2017;53:886–894. doi: 10.1016/j.ejvs.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Jayaraj A., Gloviczki P., Peeran S., Canton L. Hybrid intervention for treatment of the nutcracker syndrome. J Vasc Surg Cases. 2015;1:268–271. doi: 10.1016/j.jvsc.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gill A.E., Ciszak T., Braun H., Hawkins C.M. Intravascular ultrasound versus digital subtraction angiography: direct comparison of intraluminal diameter measurements in pediatric and adolescent imaging. Pediatr Radiol. 2017;47:450–457. doi: 10.1007/s00247-016-3771-z. [DOI] [PubMed] [Google Scholar]
- 5.Ang W.C., Doyle T., Stringer M.D. Left-sided and duplicate inferior vena cava: a case series and review. Clin Anat. 2013;26:990–1001. doi: 10.1002/ca.22090. [DOI] [PubMed] [Google Scholar]
- 6.Peterson J., Hage A.N., Diljak S., Long B.D., Marcusa D.P., Stribley J.M. Incidental anatomic finding of celiacomesenteric trunk associated with ‘nutcracker phenomenon,’ or compression of the left renal vein. Am J Case Rep. 2017;18:1334–1342. doi: 10.12659/AJCR.906283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Zoubi N.A., Al-Ghalayini I.F., Al-Okour R. Nutcracker syndrome associated with celiacomesentric trunk anomaly: case report. Int J Nephrol Renovasc Dis. 2017;10:285–288. doi: 10.2147/IJNRD.S146814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markovic J., Shortell C. Right gonadal vein transposition for the treatment of anterior nutcracker syndrome in a patient with left-sided inferior vena cava. J Vasc Surg Venous Lymphat Disord. 2016;4:340–342. doi: 10.1016/j.jvsv.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Yang B.Z., Li Z., Wang Z.G. Nutcracker syndrome due to left-sided inferior vena cava compression and treated with superior mesenteric artery transposition. J Vasc Surg. 2012;56:816–818. doi: 10.1016/j.jvs.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A., Naik N., Gulati G.S. Mesoaortic entrapment of a left inferior vena cava. Indian J Radiol Imaging. 2010;20:63–65. doi: 10.4103/0971-3026.59758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pulgarin Ricardo L.G., Isaza Zapata S., Uribe Gonzalez R. Left inferior vena cava with nutcracker syndrome: a case report. Radiol Case Rep. 2017;13:32–34. doi: 10.1016/j.radcr.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitoz S., Yalcinkaya F. Compression of left inferior vena cava: a form of nutcracker syndrome. J Clin Ultrasound. 2008;36:101–104. doi: 10.1002/jcu.20365. [DOI] [PubMed] [Google Scholar]
- 13.Yildiz A.E., Cayci F.S., Genc S., Cakar N., Fitoz S. Right nutcracker syndrome associated with left-sided inferior vena cava, hemiazygos continuation and persistant left superior vena cava: a rare combination. Clin Imaging. 2014;38:340–345. doi: 10.1016/j.clinimag.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida R.D., Yoshida W.B., Costa R.F., Nacif M.S., Sobreira M.L., Jaldin R.G. Nutcracker syndrome and deep venous thrombosis in a patient with duplicated inferior vena cava. J Vasc Surg Venous Lymphat Disord. 2016;4:231–235. doi: 10.1016/j.jvsv.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Menard M.T. Nutcracker syndrome: when should it be treated and how? Perspect Vasc Surg Endovasc Ther. 2009;21:117–124. doi: 10.1177/1531003509338402. [DOI] [PubMed] [Google Scholar]
- 16.Butros S.R., Liu R., Oliveira G.R., Ganguli S., Kalva S. Venous compression syndromes: Clinical features, imaging findings and management. Br J Radiol. 2013;86:20130284. doi: 10.1259/bjr.20130284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takebayashi S., Ueki T., Ikeda N., Fujikawa A. Diagnosis of the nutcracker syndrome with color Doppler sonography: correlation with flow patterns on retrograde left renal venography. AJR Am J Roentgenol. 1999;172:39–43. doi: 10.2214/ajr.172.1.9888735. [DOI] [PubMed] [Google Scholar]
- 18.Kim K.W., Cho J.Y., Kim S.H., Yoon J.H., Kim D.S., Chung J.W. Diagnostic value of computed tomographic findings of nutcracker syndrome: correlation with renal venography and renocaval pressure gradients. Eur J Radiol. 2011;80:648–654. doi: 10.1016/j.ejrad.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 19.Zerhouni E., Siegelman S., Walsh P., White R. Elevated pressure in the left renal vein in patients with varicocele: preliminary observations. J Urol. 1980;123:512–513. doi: 10.1016/s0022-5347(17)55996-0. [DOI] [PubMed] [Google Scholar]
- 20.Hohenfellner M., Steinbach F., Schultz-Lampel D., Schantzen W., Walter K., Cramer B.M. The nutcracker syndrome: new aspects of pathophysiology, diagnosis and treatment. J Urol. 1991;146:685–688. doi: 10.1016/s0022-5347(17)37893-x. [DOI] [PubMed] [Google Scholar]
- 21.Ali-El-Dein B., Osman Y., Shehab El-Din A.B., El-Diasty T., Mansour O., Ghoneim M.A. Anterior and posterior nutcracker syndrome: a report on 11 cases. Transplant Proc. 2003;35:851–853. doi: 10.1016/s0041-1345(02)04026-5. [DOI] [PubMed] [Google Scholar]
- 22.Raju S. Iliac vein outflow obstruction in “primary” chronic venous disease. Phlebolymphology. 2008;15:12–16. [Google Scholar]
- 23.Neglén P., Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg. 2002;35:694–700. doi: 10.1067/mva.2002.121127. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed H.K., Hagspiel K.D. Intravascular ultrasonographic findings in May-Thurner syndrome (iliac vein compression syndrome) J Ultrasound Med. 2001;20:251–256. doi: 10.7863/jum.2001.20.3.251. [DOI] [PubMed] [Google Scholar]
- 25.Mahmood S.K., Oliveira G.R., Rosovsky R.P. An easily missed diagnosis: flank pain and nutcracker syndrome. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-009447. bcr2013009447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alkhouli M., Morad M., Narins C.R., Raza F., Bashir R. Inferior vena cava thrombosis. JACC Cardiovasc Interv. 2016;9:629–643. doi: 10.1016/j.jcin.2015.12.268. [DOI] [PubMed] [Google Scholar]
- 27.Sitwala P.S., Ladia V.M., Brahmbhatt P.B., Jain V., Bajaj K. Inferior vena cava anomaly: a risk for deep vein thrombosis. N Am J Med Sci. 2014;6:601–603. doi: 10.4103/1947-2714.145486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel S., Cheema A., Karawadia T., Carson M. Management of acute lower extremity deep venous thrombosis in a patient with duplicated inferior vena cava and contraindication to anticoagulation: case and review of the literature. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-222974. bcr-2017-222974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Z., Zheng X., He Y., Fang X., Li D., Tian L. Stent migration after endovascular stenting in patients with nutcracker syndrome. J Vasc Surg Venous Lymphat Disord. 2016;4:193–199. doi: 10.1016/j.jvsv.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Erben Y., Gloviczki P., Kalra M., Bjarnason H., Reed N.R., Duncan A.A. Treatment of nutcracker syndrome with open and endovascular interventions. J Vasc Surg Venous Lymphat Disord. 2015;3:389–396. doi: 10.1016/j.jvsv.2015.04.003. [DOI] [PubMed] [Google Scholar]