Abstract
Endoscopic ear surgery (EES) is an exciting, rapidly developing and innovative field of otologic surgery. Technically and conceptually, EES is a significant departure from traditional microscopic transcanal approaches to the middle ear and canal that has shown very positive results with respect to patient outcomes. This review serves as a primer for the otologist and otology resident embarking on EES and discusses the theory surrounding the learning process, the optical chain for endoscopic surgery as well as other important underlying principles.
Keywords: Endoscopic, Endoscopic Ear Surgery, Optical Chain
1. Introduction
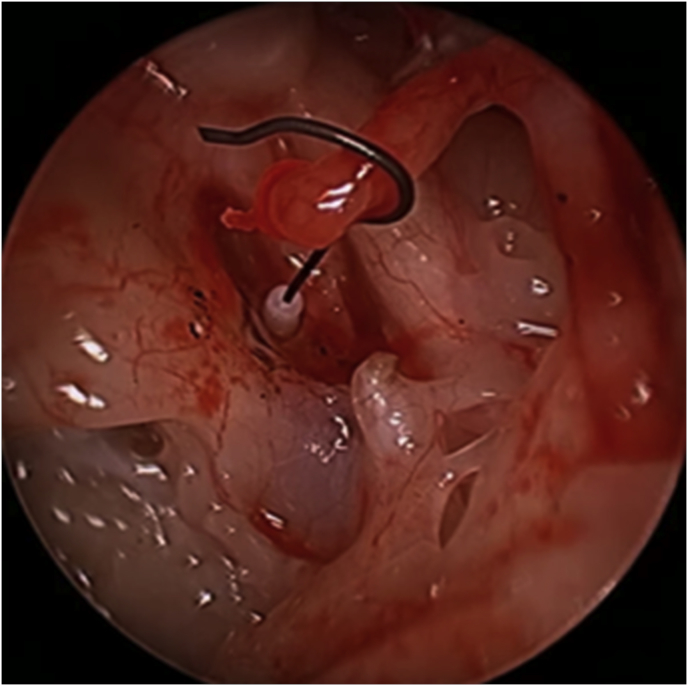
Endoscopic ear surgery (EES) with the wide viewing angle of modern endoscopes (Fig. 1), overcomes many of the limitations of the traditional microscopic approaches to middle ear and canal disease, some of which mandated postauricular approaches in the past. A very broad range of otologic disease can now be successfully managed with the endoscope (Box 1). This review serves as a primer for the otologist embarking on EES and discusses many concepts essential to safe and successful adoption of EES such as learning theory, the optical chain and instrumentation. A broad understanding of endoscopic, fiberoptic, camera and screen technology is essential to optimizing the endoscopic view as well as ensuring patient safety.
Fig. 1.
Angled scope view of attic cholesteatoma sac exemplifying the wide viewing angle of modern endoscopes.
Box 1. Indications for endoscopic ear surgery.
External Ear.
-
•
External canal cholesteatoma
-
•
Biopsies of external canal, toilet of ear
-
•
Canalplasty for anterior overhang or small exostoses
Middle Ear & Tympanic Membrane.
-
•
Perforations – Especially useful in anterior, subtotal and total perforations
-
•
Cholesteatoma – acquired, congenital in the middle ear, attic and antrum
-
•
Ossicular reconstruction
-
•
Neoplasms of the middle ear – paraganglioma, adenoma, hamartoma
-
•
Otosclerosis
Inner Ear.
-
•
Symptomatic cochleovestibular tumour removal
-
•
Small symptomatic internal auditory canal tumours
-
•
Perilymph Fistula
Posterior Petrous Face.
-
•
Sac Surgery for Meniere's disease
-
•
Assistance in Endolymphatic Sac Tumour resection
-
•
CSF Leak identification and closure (Arachnoid granulations)
Middle Cranial Fossa.
-
•
Superior Semicircular Canal Dehiscence: Assistance in closure
-
•
Identification of CSF Leaks
-
•
Meningoencephalocele Identification and closure
Anterior Petrous Apex.
-
•
Extensive cholesteatoma dissection
-
•
Cholesterol granuloma and other petrous apex cyst drainage
Cerebellopontine Angle.
-
•
0° for inspection VII, V, and VI
-
•
30–45° for dissection lateral IAC including endoscopic ear instruments as well as identification of exposed air cells to close and reduce CSF leak
Service delivery.
-
•
Lower equipment cost and more portable than microscope in areas of need
-
•
Teleheath
Alt-text: Box 1
2. Modern learning theory and its application to EES
Certain concepts from modern learning theory are relevant to any surgeon, from junior resident to experienced consultant, looking to introduce a new technique into their practice. The surgeon's emotional state and cognitive bias impact on learning at the time new information is received. With respect to EES, cognitive bias is best exemplified by the way traditional microscopic methods have been used in surgical scenarios in the past. Bjork (1994) describes several “desirable difficulties” that enhance the long-term uptake of a new operative technique:
-
1.
Varied conditions of learning – in this instance moving from standing to sitting and interspersing different first cases, enhances long term hand eye co-ordination.
-
2.
Distributed sessions – an inter-training interval of approximately 7 days is ideal, not block learning (such as repeated 2-day intensive courses).
-
3.
Self-testing – regular review of the relevant anatomy is important to establishing the long-term memory.
2.1. Learning EES in residency
With less experience, residents generally bring low cognitive bias (as to the benefits of microscopic ear surgery) and a more erratic emotional state to the learning environment of EES. Ideally, residents will have been through a period of autonomous scope-holding prior to progressing onto live surgery. Most often this can be achieved through cadaver or 3D printed temporal bone courses. Today's residents are frequently adept with scope handling through their experience with Functional Endoscopic Sinus Surgery.
Knowledge of anatomy is critical, and review of anatomy in standard texts, as well as through focussed endoscopic cadaveric dissections online (http://www.sydneyearendoscopy.com) is of paramount importance.
A stepwise training schedule using operant conditioning in the form of relatively neutral click prompts has been demonstrated to show uniform improvement in cohorts of orthopaedic surgical residents and medical students (Levy et al., 2016). This method could be applied to EES resident training (Box 2).
Box 2. Sydney Endoscopic Ear Surgery Task Specific Checklist. Modified from Lin et al. (2009).
Assessment Criteria.
-
•Basic inventory, setup of equipment
-
•Knowledge of endoscopes, equipment, instruments
-
•Appropriate draping
-
•Instrument/tissue handling
-
•
-
•Assessment of candidacy, pathology and canal size
-
•Endoscopic assessment of canal
-
•Endoscopic assessment of disease location
-
•Assessment of disease radiologically
-
•Determination of canal widening and or mastoidectomy
-
•
-
•Ear preparation, Injection and Hair trimming
-
•Injection
-
•Application of otowick or neuropatty with adrenaline
-
•Hair removal
-
•
-
•Flap elevation
-
•Incision location
-
•Management of bleeding
-
•Demonstration of Prussak's space
-
•Elevation off handle of malleus; observe the anterior malleolar ligament
-
•
-
•Middle ear exploration with 3 mm scopes 0, 30 and 45
-
•Demonstrate safe insertion of angled scopes
-
•Naming structures of retrotympanum
-
•Observing ventilation routes
-
•Naming structures of the hypotympanum and protympanum
-
•Curetting to show limits of Prussak's space and lateral epitympanum; name the regions of the epitympanum
-
•
-
•Ossicle manipulation
-
•Division of incudostapedial joint
-
•Removal of incus and identification of the facial nerve and relationships
-
•Division of the neck of the malleus and head removal
-
•Name the regions of the epitympanum
-
•
-
•
Bone removal methods, drill, curette, sonopet, piezoelectric
-
•
Extended middle ear - dissection with angled scopes and instruments; extended protympanum, antrum, hypotympanum, retrotympanum
-
•
Beyond ME – infracochlear, perigeniculate, transpromontorial
Alt-text: Box 2
2.2. Learning as an established surgeon
In contrast to residents, established surgeons generally bring a high cognitive bias (regarding the benefits of traditional microscopic methods) and a superior ability to control their emotional state than residents. Learning for experienced surgeons commences with prereading, watching anatomy and surgical videos online (http://www.sydneyearendoscopy.com). The established surgeon should then attend at least one course where the endoscopic approach is taught and begin soon after with a step wise progression of cases (see below). Prior to progression toward more advanced techniques, the established surgeon should consider visiting another surgeon familiar with EES methods or asking such a surgeon to attend their operating sessions as a mentor. Fig. 6, Fig. 7 compare the captured images of a left stapedectomy with microscope and endoscope.
Fig. 6.
Microscopic view of left stapedectomy.
Fig. 7.
Endoscopic view of left stapedectomy.
2.3. Learning curve
There is, as with all procedural skills, a learning curve that applies to the attainment of EES competency. It is widely accepted that the learning curve for surgical skills rises in a continuous or step-wise manner, plateauing once competency is attained (Hopper et al., 2007). At present, there is no universally accepted definition of competency with most studies of procedural learning curves using operative time and postoperative complications as a proxy for competent performance (Khan et al., 2014). These measures are prone to bias and do not encompass every domain of competency but they are, however, the most universally comparable measures. In most centers, timetabled operating lists naturally space surgeons’ exposure to techniques and therefore their development of skills. Fortunately, such temporal spacing of learning has been shown to improve skill retention in laparoscopic surgery (Spruit et al., 2015) and the same effect may apply to EES.
Given its relatively recent adoption, published data relating to the learning curve in EES is not currently available. Several otologic procedures congenital aural atresia surgery (Patel and Shelton, 2007), stapedotomy (Yung et al., 2006) and translabyrinthine removal of vestibular schwannomas (Moffat et al., 1996)) have been demonstrated to require performance of 50–60 procedures before competency is attained. A similar learning curve of 40–60 procedures has been observed in endoscopic nasal procedures such as septoplasty (Champagne et al., 2016), transsphenoidal pituitary surgery (Leach et al., 2010) and functional endoscopic sinus surgery (Laeeq et al., 2013). It seems reasonable to assume, given the similarities in techniques, equipment and anatomical regions, that a similar learning curve applies to EES.
2.4. Learning and instruction in endoscopic vs microscopic ear surgery
One of the primary challenges in teaching otologic procedures using the operating microscope is in the different views offered by the primary binocular lenses, the microscope side-port and any digital images displayed on screens. Typically, only the operating surgeon has a binocular view of the operative field that affords depth perception. Supervisors and observers have a view without depth perception and an image that is often of different clarity and brightness.
In contrast, in EES, the learning otologist, supervisor, observers and other operating theatre staff share the one, identical image. This shared image has obvious benefits for the teacher and learner of EES with supervisors able to instruct on the operating screen in real time and appreciate the same view as the operating surgeon during all phases of a procedure. Anecdotally, the EES method also significantly engages the operating room staff in the procedure, more than viewing the microscopic method.
3. Instrumentation in EES and suggested surgical progression
3.1. Beginning with EES
Once the surgeon has familiarised themselves with the anatomy, indications and procedures by attending an appropriate course then it suggested that the method is commenced immediately in a step-wise manner to reinforce previous learning. Currently available equipment in most hospitals can be used before any specialised endoscopic ear equipment is required. Suggested available equipment to start with includes:
-
•
4 mm sinuscopes 0 and 30°
-
•
HD Camera
-
•
LED or Xenon Light source and shielded fiberoptic light lead – set at 50%
-
•
Basic otology tray
Initially, simple procedures will serve as ideal training for the hand-eye coordination required to progress to more advanced endoscopic procedures. Suggestions for initial EES experience includes:
-
•
Middle ear ventilation tube (grommet) insertion
-
•
Raising a tympanomeatal flap
-
•
Myringoplasty for small central or posterior perforations
-
•
Using the scope for inspection during conventional microscopic surgery
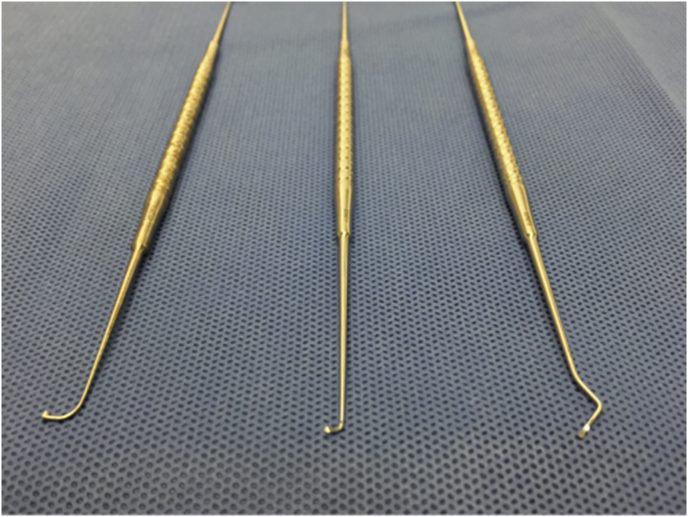
Once the surgeon has gained some familiarity with the principles of EES, specialised EES instruments are useful for more advanced indications such as extensive tympanoplasty, cholesteatoma surgery and surgery beyond the middle ear (Fig. 2). These instruments include:
-
•
Ultra-High definition cameras and high definition/4K screens
-
•
3 mm rigid 0, 30, 45° endoscopes
-
•
Short and long angled Thomassin dissectors
-
•
Angled EES curettes
-
•
Angled suckers
-
•
Angled microscissors
-
•
Angled cup forceps
-
•
Bone removal tools such as guarded drill burrs, osteotomes, piezoelectric device or ultrasonic aspirator
Fig. 2.
Basic EES instruments: Curette (left) and short and long Thomassin dissectors.
Once the surgeon gains confidence with basic as well as more advanced equipment, then progression can occur toward:
-
•
Subtotal or total perforations using angled scopes
-
•
Cholesteatoma using angled scopes
-
•
Ossicular reconstruction
-
•
Stapes surgery
-
•
Beyond the middle ear: Eustachian Tube & Lateral Skull Base Surgery
3.2. Dedicated instruments
There are two primary manufacturers of dedicated and specific endoscopic ear instruments: Karl Storz GmbH (Fig. 3) and Spiggle & Theis Medizintechnik GmbH. The instruments they make are varied, unique and some aspects complement each other well. Karl Storz have a number of tapered curved suction instruments of varying lengths and diameters, and specific dissection instruments. Spiggle & Theis, on the other hand, have suction capability built into all of their instruments which have varying dissection tips (Fig. 4). Newer and improved instruments will no doubt be made in coming years.
Fig. 3.
Karl Storz GmbH specialised EES instrument tray. © Karl Storz GmbH, 2018.
Fig. 4.
Panetti EES instrument tray. © Spiggle & Theis Medizintechnik GmbH, 2018.
3.3. Endoscopes
There are a number of manufacturers of rigid Hopkins rod-lens endoscopes. They come in a variety of diameters, but for EES, the most useful of these are 2.7, 3 and 4 mm. Better picture quality and size is obtained with a larger diameter scope, so the best one to use is the largest one that can fit into an ear canal. The 3 mm diameter appears to offer the best balance between image quality and ability to fit in most ear canals.
Endoscopes also come in a variety of lengths, with 11–14 cm being the standard for most EES scopes. The size range was chosen as it is a good compromise between a longer scope which is too unwieldy and a shorter scope which may clash with the operating instrument in the dominant hand. For more lateral work, such as simple tympanoplasty and ossiculoplasty, a shorter scope may prove feasible.
3.4. Cameras
There are two main types of camera sensors: charged-coupled device (CCD) and complementary metal-oxide semiconductor (CMOS). Traditionally, CCD was the better of these technologies in terms of picture quality, color reproduction and performance in low light. The technology behind CMOS is now dramatically and quickly improving, with many professional digital still and movie cameras now utilising this technology.
Nevertheless, medical technology still predominantly employs CCD, which can either be 1-chip or 3-chip. 1-chip uses a Bayer filter, which means that a dominant color in the red-green-blue spectrum can wash-out the others – leading to a phenomenon called “red-out” if bleeding is present. 3-chip devices have prisms which split the red-green-blue inputs into 3 separate chips, preventing this phenomenon from occurring – and so is preferred in EES where small amounts of bleeding in a confined space can make a big difference.
Weight is also a factor to consider, with some camera manufacturers targeting the endoscopic markets of other surgical disciplines where weight of the camera is of less importance than in EES. The smaller the camera, the less fatigue the surgeon is likely to experience.
3.5. Endoscopic light source
Although there are no reported cases, consideration should be given to the potential for thermal injury to canal skin and inner ear and medial wall structures from endoscopic light sources. In a recent non-sytematic review, Mitchell and Coulson (2017) reported significant variability in the temperatures recorded at the endoscope tip during endoscopic ear surgery. In a human temporal bone model, Kozin et al. (2014) demonstrated a rise in temperature at the endoscope tip from 36 °C to 46 °C within 30–124 s of turning on a Xenon light source at 100% power. The rise in temperature was similar for an LED light source. The authors demonstrated a rapid drop in temperature to within 25% of baseline temperature within 20–88 s of removal of the light source. Of relevance to EES, Kozin et al. (2014) demonstrated a precipitous drop in temperature to below baseline within 20 s of applying suction at the level of the endoscope tip.
Most current cameras with automatically adapt gain to lower light settings, so it is prudent to use the lowest light source power setting that gives adequate illumination of the operative field. Lower settings have been shown not to affect static image interpretation (McCallum et al., 2018). In most cases, a power of 50% will achieve these goals. Where the endoscope must remain in the canal for periods exceeding 2 min, applying suction or irrigation will help minimize the risk of thermal injury.
3.6. Zoom
Some cameras have a manual focusing ring, whilst others rely on digital zoom. As with all digital photography, manual zoom provides a crisper/clearer image.
3.7. Post processing
Some manufacturers are now starting to offer post-processing options which make darker areas stand out more, or blood vessels more vibrantly displayed. These are certainly helpful options to have available but not essential.
3.8. 3D systems
3D image enhancement: Another development in the pursuit of enhancing reality in surgical vision is the development of 3D vision and flexible endoscope tips. 3D vision is possible by incorporating video feeds from 2 different cameras. Some systems incorporate two chip-on-tip HD sensors to obtain 3D vision (Karl Storz GmbH, 2018). Others use multiple lenses to map the surgical field through a single optical channel, likened to the 3D vision attained by an insect's eye (VisionSense, 2018). Recent studies have compared a task-specific use of 2D and 3D endoscopy for the purposes of endonasal skull base and neurosurgery in both experienced and novice surgeons. Overall, the enhancement of depth perception in a 3D system was perceived as superior and demonstrated an advantage in specific tasks such as vascular and neural dissection. The 3D system was disliked by more senior surgeons, but preferred by less experienced surgeons, with authors suggesting that it could shorten the learning curve (Inoue et al., 2013; Marcus et al., 2014). At present 3D systems are only available in 4 mm or greater sizes, which limits their application in the ear canal.
3.9. High & Ultra-High definition
Standard definition in medical equipment is being phased out and replaced with high definition. High definition means at least 720 pixels in height per frame; 720p, 1080p and 1440p are each considered high definition. Ultra-high definition or 4k technology displays at 2160p, while 8k technology displays a 4320p mode. The video mode relates to the amount of pixels that fit in each image frame because it is linked to the size of the screen's frame (width x height). A 720p screen will have a frame size of 1280 × 720pixels, a 1080i or p will have a 1920 × 1080pixels frame size. Ultra HD will have a 3840 × 2160 size.
Another characteristic of a monitor is the aspect ratio. It refers to the ratio between width and height, or the dimensions of video screens and video picture elements. The screen aspect ratio of a traditional television screen is 4:3. High definition monitors use an aspect ratio of 16:9. Pixel shapes correspond to the shape of the aspect ratio. Simplistically, pixels for the 4:3 format are thin and for the 16:9 format are wide.
3.10. The monitor
The monitor is composed of the display device, the circuitry, the casing, the backlight and power supply. The modality of display device is the usual way in which we differentiate and name each type of monitor. Modern monitors typically have a thin film transistor liquid crystal display (TFT-LCD or LCD) with a LED (Light Emitting Diode) backlight, while older monitors used a cathode ray tube (CRT) display. The latter are bulkier, and do not have the low heat-emitting LED lighting system. Because LCD monitors are slimmer and lighter, they can be easily suspended from the ceiling, which has improved the ergonomic features and space utilization in operating rooms in recent time.
LCD is perceived as superior in terms of overall image quality. Although CRT was preferred at off-axis viewing angles (Brown et al., 2004), the introduction of in-place switching (IPS) to LCD has improved the strong viewing angle dependence and low quality color reproduction that had plagued early iterations. Monitor adjustments in brightness, contrast and sharpness, may improve visualization and reduce visual strain when the option for an increment in resolution is not available. The enhancement of these features will translate into more detail (Berber and Siperstein, 2001; Berci et al., 1995; Seagull et al., 2011).
3.11. Video interface and connectors
The video source must be connected by an interface to a video display device in order for the signal to be transmitted. In the case of analog signals, the method of transmission includes Y/C (Y=Luminance, C=Chrominance) or S-Video (Super-Video). RGB (Red, Green and Blue) is another method of analog transmission that may be superior to the previously mentioned in that it transmits colors via separate channels, thus providing a more vivid image. Another analog interface connection worth mentioning is the VGA or Video Graphics Array. Developed by IBM® in the late 80's, it was the standard interface between computers and for example, projectors.
Newer LCD monitors, the DVI or Digital Visual Interface is now standard. It is used in many operating room monitors as well as in laptops and projectors since it is compatible with both digital and analog signals. High-Definition Multimedia Interface (HDMI) is a new audio/video digital interface. It is not compatible with analog signals therefore it will only work with newer video system models, and some argue that its audio component serves little purpose in endoscopic surgery (Milad et al., 2014). The caveat is that all components on the optical chain must be compatible with an HDMI cable or interface, and this may not be available at all times.
4. The optical chain
The optical chain consists of all the elements of the endoscopic system that create and deliver the image to the surgeon's eye. This consists of the light source, light lead, endoscope, camera, camera processing system and video display. Optimizing the optical chain is essential for the best surgical image. It is clearly important for the surgeon to understand how to trouble shoot the system. Defects in image quality can be attributed to “downstream” equipment issues (Fig. 5), occurring from the lens at its interface with the camera unit or to “upstream” issues from the camera unit to the monitor the images are ultimately displayed on. Simple first steps to improve the optical chain include:
-
•
Lowering the ambient operating room light to improve contrast.
-
•
Check the glass – look at your light lead and scope to see they are not damaged.
-
•
Tweak the digital – experiment with settings in the camera and screen to refine the picture.
Fig. 5.
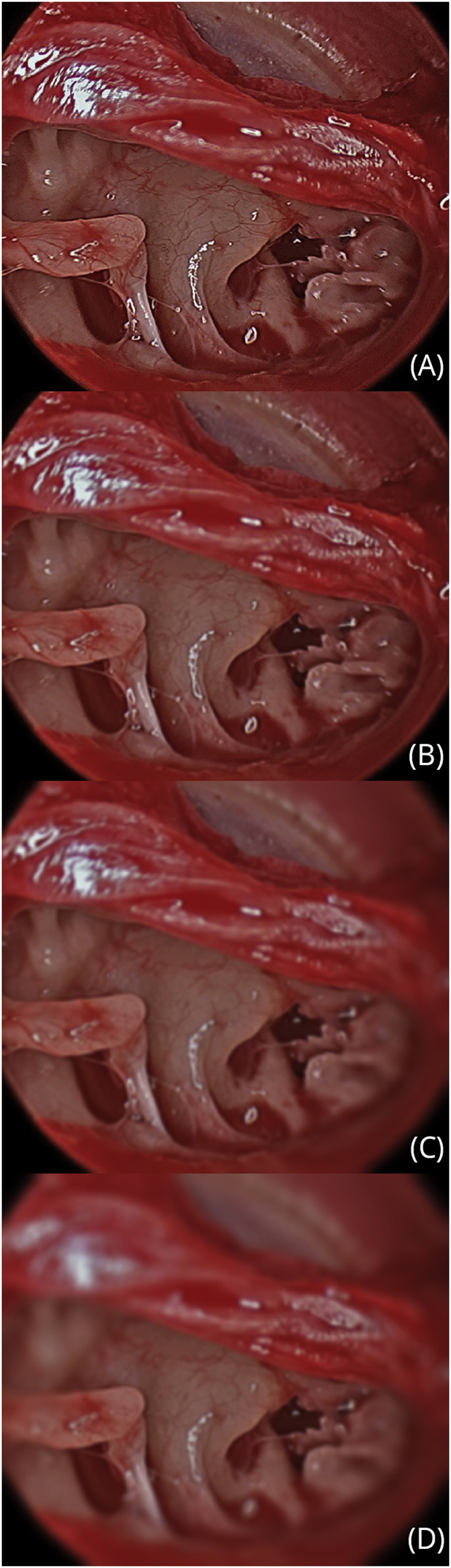
Downstream equipment issues causing poor image quality. A demonstration of the effect progressive scope damage on the endoscopic image. (Credit Dr Nicholas Jufas).
5. Anaesthetic considerations
A relatively bloodless operating field is desirable for EES. Excessive bleeding requires the otologist to more frequently switch between microsuction and dissection instruments, frustrating attempts at adopting the method. As discussed above, excessive bleeding can cause “red out” in 1-chip CCD video systems, reducing the quality of images.
Wormald et al. (2005) demonstrated that the use of TIVA (remifentanil and propofol) compared with volatile inhalational anaesthetics (sevoflurane) and fentanyl was associated with lower surgical grade (less bleeding requiring less frequent suctioning) in endoscopic sinus surgery. TIVA gives the anesthesiologist tighter control over hemodynamic variables (pulse rate and mean arterial pressure), aiming for a MAP of 50 mmHg and pulse rate of 50.
Infiltration of local anaesthetic should be performed, either preoperatively or after sterile preparation of the skin, with 1% Ropivicaine and 1:50000 adrenaline. Caution is required in children under 3 years as the mastoid tip is not formed yet. When injecting near the mastoid tip a finger is placed in the tympanomastoid groove to disperse local anaesthesia away from the facial nerve.
A single canal injection (25G – 30G) very slowly in the vascular strip is often all that is required, as well as tragal and conchal injections. Overinjection should be avoided as this may greatly reduce vision in the canal. Neuro Patties or an Otowick (Medtronic, 2018) with 1:1000 adrenaline should be placed in the bony medial canal whilst hair is then cut from the lateral meatus. Great care (and time) should be taken to cut the hair from the lateral meatus to avoid remaining hairs dirtying the endoscope on entry to the canal each time.
6. Operating room configuration
There are several specific considerations that must be made in configuring the operating room for EES. Some of the changes discussed below may represent a significant change to the current practice of the otologist, anesthesiologist and/or nursing staff in the early stages of EES adoption.
6.1. Microscope
The microscope remains in the operating room for all EES cases. In the early adoption phase the microscope is draped for all cases to allow the surgeon quick access for confirming EES views with the old microscopic view. With experience the microscope remains in the room but can be undraped.
6.2. Ergonomics
Freedom of movement and posture is greatly reduced in minimally invasive surgical procedures, increasing the risk of musculoskeletal injuries (Janki et al., 2017). Complex endoscopic ear procedures can exceed 3 h in duration, requiring otologists to maintain fixed position of the neck, back and non-dominant hand for extended periods.
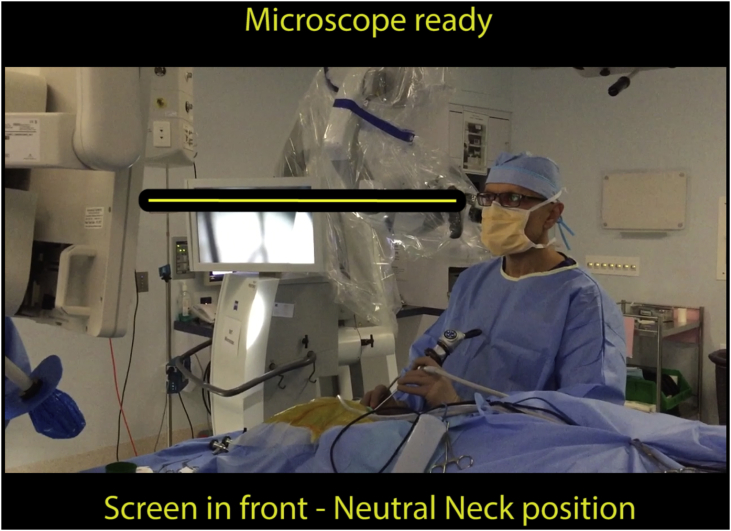
Many of the principles that apply to other forms of endoscopic surgery are applicable to EES. The most important ergonomic principle, and the simplest to adhere to is the maintenance of a neutral neck, shoulder and upper back position (Fig. 8). This is achieved with a screen that is adjustable in height, positioned after the otologist has assumed the position that will be maintained for the duration of the procedure. Positioning the centre of the screen at eye height avoids the temptation to extend the neck during the procedure.
Fig. 8.
Ideal neck, shoulder and upper back position for EES with the centre of the screen at eye height. Consider standing, especially for right-handed surgeons operating on the left ear.
Both standing and sitting positions can be adopted for EES. If microscope use is likely, a sitting position should be adopted to reduce the likelihood the patient or table will need to be moved intraoperatively. Many otologists comfortable with the operating microscope for transcanal procedures will find a sitting position more natural. Standing, however, allows the surgeon a degree of freedom in positioning the camera and body toward the attic, antrum and retrotympanum which is hard to achieve sitting.
For the right handed surgeon the left ear is generally easier to operate upon for two reasons. Firstly, the scope is held in the left hand in the inferior canal and the pathology is usually directly accessed with the operating right hand without a risk of instrument cross over. Secondly, the surgeon may intermittently and gently rest their wrist on the patient's shoulder to reduce camera shake.
A stable image that is maintained throughout the procedure, is essential in EES. Careful consideration should be given to reducing the risk of muscle strain and fatigue while maintaining the comfort and safety of the patient. Adjustable armrests firmly fixed to the operating table or the surgeon's chair provide steady support for the camera arm while operating. Mayo or hand tables with towels or folded drapes can also be used. Alternatively, where handedness allows, the forearm can be rested intermittently on the patient's shoulder with folded towels used to protect the patient.
Where the surgeon holds the camera in the non-dominant hand is a matter of personal preference, however, in the learning period various options should be tried. Three common options include, holding the body of the camera with the whole hand, holding the camera with index and thumb with the light lead between index and middle and finally holding the body with thumb and index and having the light lead between middle and ring finger.
Endoscope holders are available but their usage is not common and their benefits unclear. Theoretically, their use frees up a hand to participate in dissection, useful when both suction and curette are required for the same step of the procedure for example. Endoscope holders, however, reduce the space available in the canal, risk ossicular or canal injury if the patient were to move during surgery and risk significant heat transfer from a static endoscope tip.
6.3. Equipment configuration
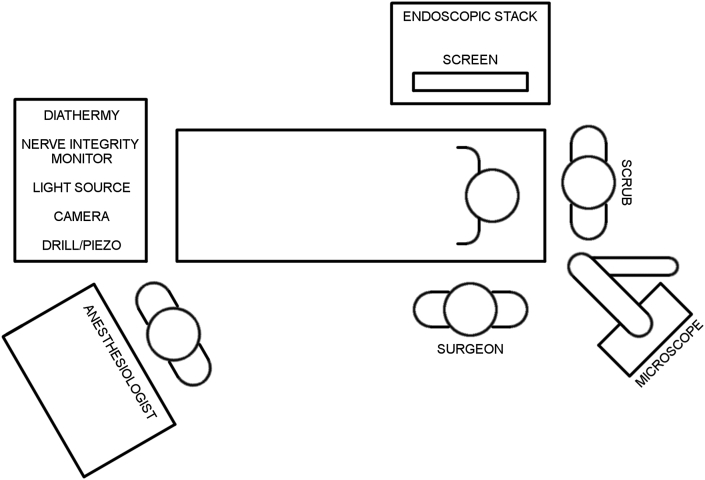
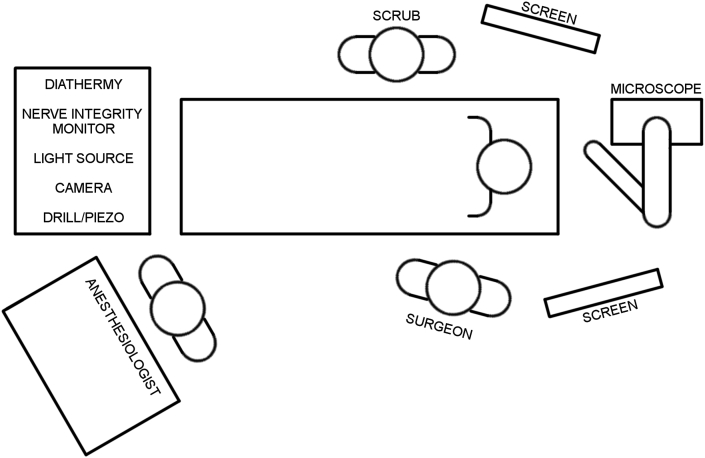
There are many suitable ways to configure operating theatre equipment for EES. Factors influencing configuration include the size of the operating theatre, likelihood of microscope use and any fixed or installed theatre equipment. Configurations may change over time to suit otologist experience, handedness and preferences. Fig. 9, Fig. 10 illustrate a recommended configuration for single and dual-screen endoscopic case respectively. Specific positioning of instrument tables should be determined by ergonomics and surgeon handedness.
Fig. 9.
Recommended operating theatre configuration for single screen endoscopic case.
Fig. 10.
Recommended operating theatre configuration for dual screen endoscopic case.
7. Considerations in endoscopic cholesteatoma management: ideal initial cases
When commencing endoscopic cholesteatoma surgery, patient assessment is critical to identify ideal endoscopic candidates. For example, a patient with limited attic disease, no evidence of infection and a wide canal (Fig. 11) is an ideal initial EES case, as are most cases of congenital cholesteatoma (Fig. 12). Other characteristics ideal for initial EES are included in the list below.
Fig. 11.
Ideal initial EES case: limited attic disease, uninfected and in a patient with a wide canal.
Fig. 12.
Ideal initial EES case: congenital cholesteatoma.
7.1. Optimal disease & patient characteristics for EES
-
•
Congenital cholesteatoma
-
•
Acquired cholesteatoma in mesotympanum and confluent areas (especially retrotympanum, protympanum and anterior epitympanum), with minimal mastoid extension
-
•
Minimal inflammation in the middle ear
-
•
Sclerotic mastoid
7.2. Preoperative assessment for EES cholesteatoma surgery
-
•
The lateral meatus and bony canal relative to disease location should be assessed to determine the largest endoscope size that can be used and whether a canal widening procedure (meatoplasty or canalplasty) is required.
-
•
The status of middle ear – whether inflamed or not will help assist the surgeon in determining the need for preoperative topical therapy and microscopy during some of the middle ear work.
-
•The status of the mastoid is assessed with imaging including:
-
•Fine cut CT temporal bones is essential in pre-operative evaluation of cholesteatoma to determine the extent of disease. With the limitations of current instrumentation, a mastoidectomy will be required if disease extends into the mastoid beyond the posterior aspect of the lateral semicircular canal or into a deep type C Sinus tympani.
-
•A non-echo planar diffusion weighted magnetic resonance imaging (non-EPI DWI MRI) is useful to determine mastoid, intralabyrinthine and intracranial spread. Caution is required regarding negative prediction rates in discharging ears and with disease less than 4 mm.
-
•
8. Pitfalls and pearls when starting
-
•
Slow down and add time to the operating list when starting EES
-
•
Start in a deliberate manner with wide ear canals and simple uninflamed pathology
-
•
Pick the ideal cases to start endoscopic cholesteatoma upon and appropriately preoperatively assess the patient
-
•
Spend time trimming hair
-
•
Have the microscope in the OR ready for use
-
•
Avoid 45 and 70° scopes when starting
-
•
Don't give up too early. The first few cases of tympanomeatal flap elevation may take time but they are useful for developing hand eye coordination
-
•Manage bleeding by:
-
-Anaesthetic control of mean arterial pressure and pulse
-
-Neuropatties or cottoinoids with 1:1000 adrenaline, ensuring to allow it time to work before suctioning
-
-Haemostatic agents like surgicel or floseal
-
-Warm saline irrigation
-
-Microbipolar forceps
-
-Low power protected time monopolar for the vascular strip incision
-
-
9. Common mistakes in early EES
-
•
Poor bleeding management – in early cases spending time to control the bleeding is one of the most important factors in getting comfortable with the endoscopic methods
-
•
Trauma to the canal with angled scope and instrument movement – early on with angled scope insertion this should be performed in a two handed manner. Slowly progressing to single handed making sure to identify and treat any canal trauma.
-
•
Understanding the limitations of the equipment – the endoscopic otologic equipment is slightly different to the usual otologic tray and takes time to understand its limitations, such the reach and angulation of Thomassin instruments.
-
•
Short tympanomeatal flap – the limited depth perception in EES can result in the inexperienced otologist creating a tympanomeatal flap that is too short for the intended procedure. An adequate flap length is particularly important where, for example, an extensive atticotomy will be performed. To achieve an adequate tympanomeatal flap length, the diameter of the round knife (approximately 3 mm) can be used as a guide in early EES. A tympanomeatal flap 5–6 mm is adequate for most cases, approximately twice the diameter of the round knife.
-
•
Failing to convert earlier to a mastoidectomy – when starting the endoscopic approach particularly with attic and antral disease, the temptation is to spend a lot of time chasing cholesteatoma to try and avoid a mastoidectomy. Consider adopting a “10 min by the clock rule”. Here the surgeon considers converting to mastoidectomy if they are chasing the same antral disease for more than 10 min by the clock measured by the scrub nurse.
-
•
Overdoing the atticotomy – Usually a small atticotomy in conjuction with angled scopes gives the endoscopic surgeon an adequate view of disease. When chasing posterior attic and antral disease, the temptation is to continue extending the atticotomy. The concern is with reconstruction of large defects which are challenging after large atticotomies. Sometimes the patient is better off with a small atticotomy and a canal wall up mastoidectomy.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Berber E., Siperstein A.E. Understanding and optimizing laparoscopic videosystems. Surg. Endosc. 2001;15(8):781–787. doi: 10.1007/s004640000391. [DOI] [PubMed] [Google Scholar]
- Berci G., Wren S.M., Stain S.C., Peters J., Paz-Partlow M. Individual assessment of visual perception by surgeons observing the same laparoscopic organs with various imaging systems. Surg. Endosc. 1995;9(9):967–973. doi: 10.1007/BF00188452. [DOI] [PubMed] [Google Scholar]
- Bjork R.A. Memory and metamemory considerations in the training of human beings. In: Metcalfe J., Shimamura A., editors. Metacognition: Knowing about Knowing. MIT Press; Cambridge, MA: 1994. pp. 185–205. [Google Scholar]
- Brown S.I., White C., Wipat K., Hanna G.B., Frank T.G., Cuschieri A. Characterizing the "gold standard" image for laparoscopic surgery. Surg. Endosc. 2004;18(8):1192–1195. doi: 10.1007/s00464-003-8278-7. [DOI] [PubMed] [Google Scholar]
- Champagne C., Regloix S.B., Genestier L., Crambert A., Maurin O., Pons Y. Endoscopic septoplasty: learning curve. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016;133(3):167–170. doi: 10.1016/j.anorl.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Hopper A.N., Jamison M.H., Lewis W.G. Learning curves in surgical practice. Postgrad. Med. 2007;83(986):777–779. doi: 10.1136/pgmj.2007.057190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue D., Yoshimoto K., Uemura M., Yoshida M., Ohuchida K., Kenmotsu H., Hashizume M. Three-dimensional high-definition neuroendoscopic surgery: a controlled comparative laboratory study with two-dimensional endoscopy and clinical application. J. Neurol. Surg. Cent. Eur. Neurosurg. 2013;74(6):357–365. doi: 10.1055/s-0033-1345100. [DOI] [PubMed] [Google Scholar]
- Janki S., Mulder E., JNM I.J., Tran T.C.K. Ergonomics in the operating room. Surg. Endosc. 2017;31(6):2457–2466. doi: 10.1007/s00464-016-5247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl Storz GmbH Human medicine – otorhinolaryngology, 2018. https://www.karlstorz.com/de/en/ear-nose-throat.htm
- Khan N., Abboudi H., Khan M.S., Dasgupta P., Ahmed K. Measuring the surgical 'learning curve': methods, variables and competency. BJU Int. 2014;113(3):504–508. doi: 10.1111/bju.12197. [DOI] [PubMed] [Google Scholar]
- Kozin E.D., Lehmann A., Carter M., Hight E., Cohen M., Nakajima H.H., Lee D.J. Thermal effects of endoscopy in a human temporal bone model: implications for endoscopic ear surgery. Laryngoscope. 2014;124(8):E332–E339. doi: 10.1002/lary.24666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeeq K., Lin S.Y., Varela D.A., Lane A.P., Reh D., Bhatti N.I. Achievement of competency in endoscopic sinus surgery of otolaryngology residents. Laryngoscope. 2013;123(12):2932–2934. doi: 10.1002/lary.23509. [DOI] [PubMed] [Google Scholar]
- Leach P., Abou-Zeid A.H., Kearney T., Davis J., Trainer P.J., Gnanalingham K.K. Endoscopic transsphenoidal pituitary surgery: evidence of an operative learning curve. Neurosurgery. 2010;67(5):1205–1212. doi: 10.1227/NEU.0b013e3181ef25c5. [DOI] [PubMed] [Google Scholar]
- Levy I.M., Pryor K.W., McKeon T.R. Is teaching simple surgical skills using an operant learning program more effective than teaching by demonstration? Clin. Orthop. Relat. Res. 2016;474(4):945–955. doi: 10.1007/s11999-015-4555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.Y., Laeeq K., Ishii M., Kim J., Lane A.P., Reh D., Bhatti N.I. Development and pilot-testing of a feasible, reliable, and valid operative competency assessment tool for endoscopic sinus surgery. Am. J. Rhinol. Allergy. 2009;23(3):354–359. doi: 10.2500/ajra.2009.23.3275. [DOI] [PubMed] [Google Scholar]
- Marcus H.J., Hughes-Hallett A., Cundy T.P., Di Marco A., Pratt P., Nandi D., Yang G.Z. Comparative effectiveness of 3-dimensional vs 2-dimensional and high-definition vs standard-definition neuroendoscopy: a preclinical randomized crossover study. Neurosurgery. 2014;74(4):375–380. doi: 10.1227/NEU.0000000000000249. discussion 380-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum R., McColl J., Iyer A. The effect of light intensity on image quality in endoscopic ear surgery. Clin. Otolaryngol. 2018;43(5):1266–1272. doi: 10.1111/coa.13139. [DOI] [PubMed] [Google Scholar]
- Medtronic Merocel. 2018. http://www.merocel.com/
- Milad M.P., Moy I., Pavone M.E. The chain is only as strong as its weakest link: understanding the endoscopic optical chain. Austin J. Obstet. Gynecol. 2014;1(3) [Google Scholar]
- Mitchell S., Coulson C. Endoscopic ear surgery: a hot topic? J. Laryngol. Otol. 2017;131(2):117–122. doi: 10.1017/S0022215116009828. [DOI] [PubMed] [Google Scholar]
- Moffat D.A., Hardy D.G., Grey P.L., Baguley D.M. The operative learning curve and its effect on facial nerve outcome in vestibular schwannoma surgery. Am. J. Otol. 1996;17(4):643–647. [PubMed] [Google Scholar]
- Patel N., Shelton C. The surgical learning curve in aural atresia surgery. Laryngoscope. 2007;117(1):67–73. doi: 10.1097/01.mlg.0000240163.73601.27. [DOI] [PubMed] [Google Scholar]
- Seagull F.J., Sutton E., Lee T., Godinez C., Lee G., Park A. A validated subjective rating of display quality: the Maryland Visual Comfort Scale. Surg. Endosc. 2011;25(2):567–571. doi: 10.1007/s00464-010-1220-x. [DOI] [PubMed] [Google Scholar]
- Spiggle & Thies Medizintechnik GmbH Endoscopic/microscopic instrument set for middle ear surgery - panetti instrument set. 2018. https://www.spiggle-theis.com/en/products/otology/panetti-instrument-set
- Spruit E.N., Band G.P., Hamming J.F. Increasing efficiency of surgical training: effects of spacing practice on skill acquisition and retention in laparoscopy training. Surg. Endosc. 2015;29(8):2235–2243. doi: 10.1007/s00464-014-3931-x. 2210.1007/s00464-00014-03931-x. Epub 02014 Oct 00416. [DOI] [PubMed] [Google Scholar]
- VisionSense Visionsense VSiii system. 2018. http://www.visionsense.com/vsiii-system/
- Wormald P.J., van Renen G., Perks J., Jones J.A., Langton-Hewer C.D. The effect of the total intravenous anesthesia compared with inhalational anesthesia on the surgical field during endoscopic sinus surgery. Am. J. Rhinol. 2005;19(5):514–520. [PubMed] [Google Scholar]
- Yung M.W., Oates J., Vowler S.L. The learning curve in stapes surgery and its implication to training. Laryngoscope. 2006;116(1):67–71. doi: 10.1097/01.mlg.0000184509.01049.06. [DOI] [PubMed] [Google Scholar]