Abstract
Objective
The aim of this study was to improve muscle flaps and to evaluate surgical outcomes with the use of a novel specialized retractor, which is a surgical instrument used to locate and shape a bony seat for minimally invasive cochlear implantation.
Methods
50 patients aged 1–75 years with sensorineural hearing loss who required cochlear implantation were recruited. A small incision (<3 cm) was made, and the novel specialized retractor was used in the study group during cochlear implantation. The incision length, surgical outcomes and operative time were recorded and analyzed.
Results
The incision length, total operative time and drilling bony time were shorter in the study group than in the control group (P < 0.05, respectively). All patients recovered well after the surgery without any severe complications.
Conclusion
The use of a novel specialized retractor standardized the surgical processes of cochlear implantation. The retractor helped locate and control the size of the bony well during bone drilling. The tool reduced the technical difficulty and improved the efficacy of this minimally invasive operation.
Keywords: Minimally invasive surgery, Cochlear implantation, Retractor, Muscle flap
1. Introduction
Cochlear implantation is an effective treatment for severe to profound sensorineural hearing loss. However, post-surgical complications can still occur, such as skin incision infection, musculoperiosteal flap infection, hematoma, implant displacement, and implant damage.
Surgical complications can occur during implant fixation, such as drilling of the bony well. A larger incision provides a better operative field for bone drilling but increases the risk of skin infection, musculoperiosteal flap necrosis and subcutaneous hematoma formation (Mangus et al., 2012). A small incision is more conducive to reducing postoperative scarring and recovery time (Bajaj et al., 2005) but provides a narrow field of vision, which increases the surgical difficulty and may result in damage to the musculoperiosteal flap. A small incision also requires the surgeon to adjust the size of the bony seat repeatedly, which prolongs the operative time. Furthermore, the risk of postoperative implant displacement will increase due to non-optimal fixation (Davids et al., 2009).
Feasible methods or techniques in cochlear implantation have been reported to solve the above problems. For example, Riskalla designed an illuminated retractor for drilling of the well (Riskalla et al., 2010). The suprameatal approach and Veria operation were introduced to protect the facial nerve without mastoidectomy (Yin et al., 2008; Balachandran et al., 2014). Matsumoto and Balachandran developed methods for image-guided otologic surgery via a linear path from the lateral skull to the cochlea (Balachandran et al., 2014; Matsumoto et al., 2012). Robotic cochlear implantation has been used to increase the consistency and accuracy of surgical outcomes (Weber et al., 2017; Caversaccio et al., 2017).
We try to solve problems in an economical manner. Therefore, in this study, we aimed to improve the outcomes and efficiency of small-incision cochlear implantation with modified retractors and surgical methods.
2. Materials and methods
2.1. Subjects
This prospective study was carried out at the Department of Otolaryngology - Head and Neck Surgery at the General Hospital of the People’s Liberation Army from June, 2012 to June, 2018. A total of 50 patients underwent Nurotron cochlear implantation and were randomly divided into the study group (n = 25) and the control group (n = 25).
2.2. Cochlear implantation protocol
The experimental group was treated with the improved small-incision method described in this study, which includes the use of a new retractor and a modified muscle flap. The control group underwent surgery involving a routine small “C”-shaped incision, and the muscle flap was cut below the skin incision. Before surgery, each patient’s medical history was recorded, and the patient underwent a physical examination, a complete set of hearing tests, CT scan of the temporal bone, and brain MRI. The position and size of the incision on the surgical side, the operative time, the implant model, and the occurrence of related complications were recorded after surgery. A ruler was used to measure the incision.
The patients were placed in the supine position under general anesthesia with the head turned toward the contralateral side. A small “S”-shaped incision was made behind the ear on the surgical side. The subcutaneous tissue was separated. A bow-shaped anterior incision was made to divide the periosteum on the mastoid surface, and the incision was extended downward to the attachment of the sternocleidomastoid muscle on the mastoid, which formed the front edge of the musculoperiosteal flap. Then, the incision was directed upward to form the posterior edge, and the base of the muscle flap was located at the posterosuperior part (Fig. 1). The musculoperiosteal flap was separated along the surface of the skull to form a bag-like structure for the implant, and a retractor with drilling and positioning functions was placed posterosuperiorly. The skin and musculoperiosteal flap were lifted to reveal the mastoid bone. The outer wall of the posterior tympanum was drilled to expose the round window. The incision was extended from the upper end in an “S” shape. The overall length of the incision was limited to approximately 3.0 cm.
Fig. 1.
A, The red line illustrates the shape of the musculoperiosteal flap, and the green line shows the “S”-shaped incision used in the experimental group.
B, The borders of the musculoperiosteal flap (inferior and posterior).
C, The musculoperiosteal flap held up by a tweezer.
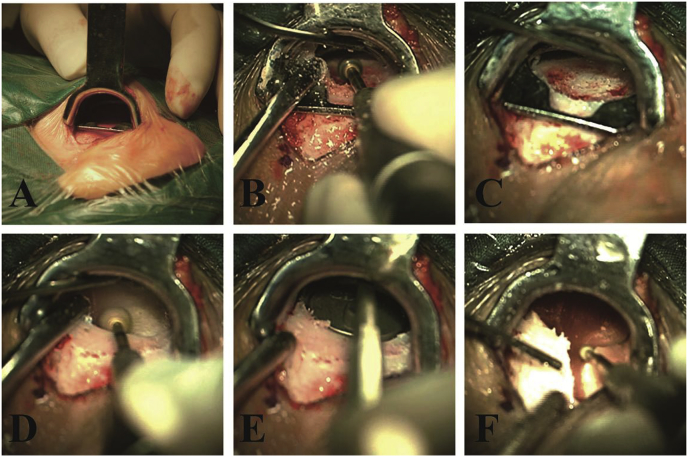
The novel retractor with drilling and positioning functions was used in the study group. The device consisted of 3 parts, including a handle, skin retractor plate and positioning plate (Fig. 2). The surgeon held the handle and inserted the skin retractor and positioning plate into the surgical region through the skin incision; the skin retractor was oriented toward the outer side (skin), and the positioning plate was close to the inner side (bone surface). The skin retractor plate provided sufficient space and good visualization for surgery. Additionally, the positioning plate enabled more accurate bone drilling. Then, the bony well was considered suitable for the receiver/stimulator after slight modification of the edges (Fig. 3).
Fig. 2.
The novel retractor consists of 3 parts, including the operating handle (A), skin retractor plate (B) and positioning plate (C).
Fig. 3.
A, The retractor with drilling and positioning functions was placed between the musculoperiosteal flap and the skull surface.
B, Drilling of the bony well.
C, Shape of the bony well.
D, Modification of the edges.
E, Mold of the receiver-stimulator.
F, Drilling of the bony well for wire.
The receiver/stimulator was placed, and the electrode was inserted into the scala tympani through the round window. The round window was closed, and the musculoperiosteal flap was sutured. The auditory nerve response was then evaluated by neural response telemetry. The surgical area was closed by an intradermal suture (Fig. 4).
Fig. 4.
The surgical area was closed using an intradermal suture.
2.3. Ethics statement
The use of human clinical materials in this study was approved by the Ethics Committee of the General Hospital of the People’s Liberation Army. All patients or their caregivers provided written informed consent.
2.4. Data analysis
Continuous variables were expressed as the mean ± SD unless otherwise specified. Continuous variables were compared by the t-test or the Mann-Whitney U-test where appropriate. Pearson’s X2-test was used to compare categorical variables. All statistical analyses were performed with SPSS 17.0, and a P value < 0.05 was considered statistically significant.
3. Results
The study included a total of 50 patients, with 34 males and 16 females aged from 1 to 75 years. Table 1 presents the baseline characteristics of the study and control group patients.
Table 1.
Baseline characteristics of the subjects.
| Study Group (n = 25) | Control Group (n = 25) | P | |
|---|---|---|---|
| Age | 13.8 ± 14.4 | 14.6 ± 17.3 | 0.464 |
| Gender (Male/Female) | 16/9 | 18/7 | 0.368 |
| Operation side (Right/Left) | 16/9 | 15/10 | 0.085 |
| Complications | 1 | 2 | 0.222 |
P values for comparisons between the patients in the study group and the patients in the control group derived using Student’s t-test, the Mann-Whitney test or Pearson’s X2-test.
Cochlear implant surgery (Nurotron cochlear CS-10A) was successfully completed in 50 patients, with 19 cases in the left ear and 31 cases in the right ear. A subcutaneous hematoma occurred in 1 case in the study group and in 2 cases in the control group. The subcutaneous hematomas were absorbed though a pressure bandage without puncture or reoperation. No other complications such as infection or flap necrosis occurred.
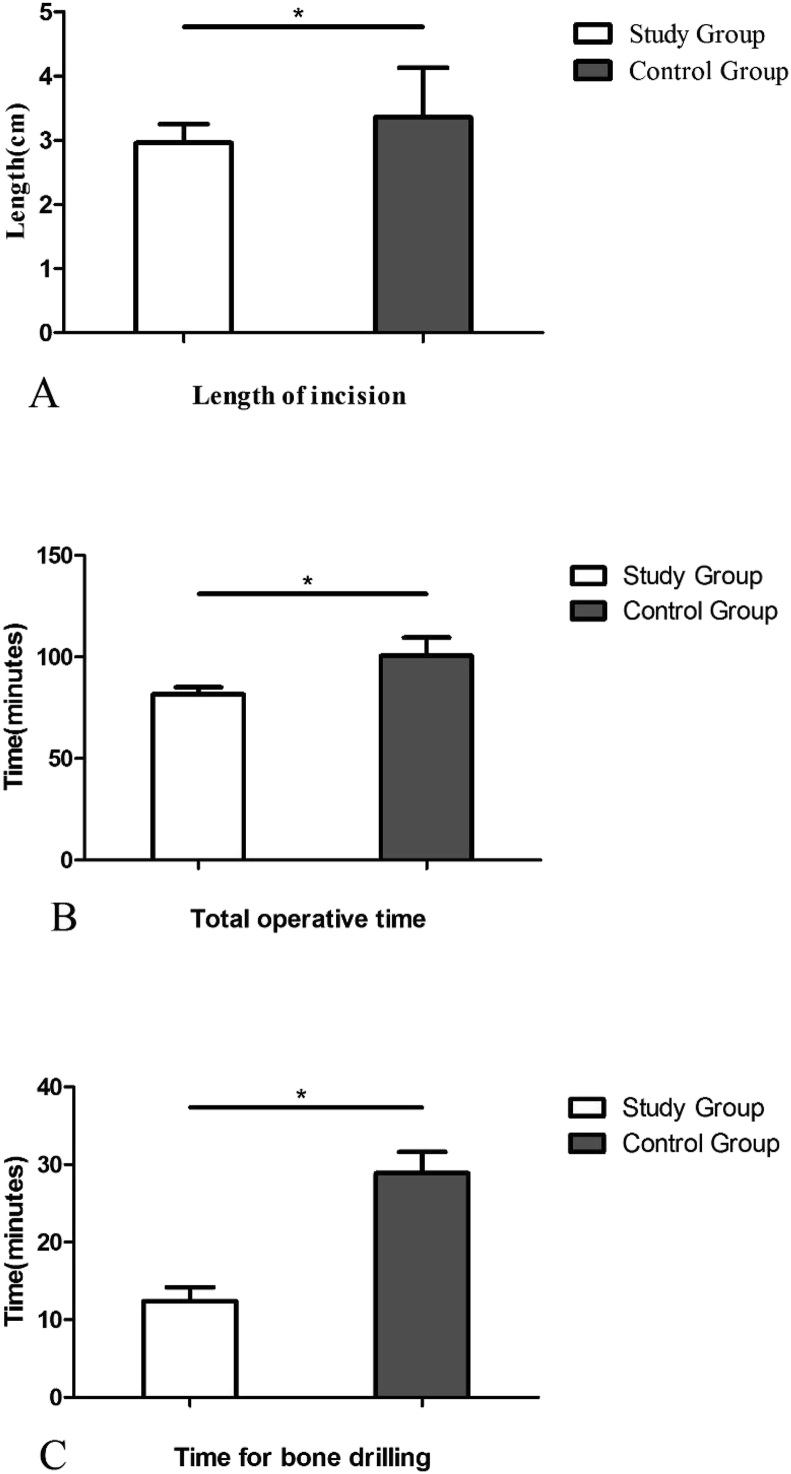
The length of the incision was 2.9 ± 0.3 cm in the study group and 3.3 ± 0.8 cm in the control group (P < 0.01). The total operative time was 81.6 ± 3.4 min in the study group and 100.5 ± 9.1 min in the control group (P < 0.01). The mean time required for bone drilling was 12.4 ± 1.8 min in the study group and 28.9 ± 2.7 min in the control group (P < 0.01) (Fig. 5).
Fig. 5.
The incision length (A), the total operative time (B) and the time for bone drilling (C) were shorter in the study group than in the control group.
4. Discussion
Since cochlear implantation was introduced into clinical practice, considerable efforts have been devoted to improving the surgical methods for cochlear implantation in different aspects. Based on the previous work of others, Brannon Mangus summarized and supplemented the principles for incision: adequate exposure for placement of the receiver-stimulator, an incision position that is not too close to the receiver-stimulator, and maintenance of a good blood supply in the flap. Earlier surgical techniques for cochlear implantation, such as large “C”-shaped incisions and inverted “U”-shaped incisions, frequently caused skin numbness, infection, and necrosis due to large incisions and large potential dead space in the skin and musculoperiosteal flap in the surgical area (Gibson et al., 1995). Gerard M. O’Donoghue (Caversaccio et al., 2017) improved Gibson’s concept of a small-incision technique and promoted minimally invasive surgery in which a postaural incision of the same width as the receiver/stimulator was made (O’Donoghue and Nikolopoulos, 2002). Surgeries involving small incisions have been found to be associated with a low incidence of infection of the skin and musculoperiosteal flap, a high survival rate, fast postoperative recovery, a smaller scar, and a better appearance. Stratigoleas ED reported a total of 22 complications (12.5%), including 1 case of flap infection, 1 case of stitch abscess, 2 cases of delayed mastoiditis and 1 case of reoperation for repositioning of the receiver/stimulator (Obholzer and Craham, 2004). Thus, this surgical technique still requires improvement.
A smaller incision in cochlear implant surgery provides a smaller direct visual field and operating space beneath the skin and the musculoperiosteal flap. Additionally, a greater demand for surgeons to have advanced skills is evident. When promoting the surgical procedure with a small incision, O’Donoghue stated the key points for surgeons when drilling the bony well: stand on the opposite side of the surgical site and wear a headlight while operating (Stratigouleas et al., 2006). Nevertheless, problems such as a small visual field remain even when these techniques are performed with the current “S”-shaped incision, which is difficult for inexperienced surgeons to execute. Moreover, an assistant must keep holding the handle to expose the surgical field when using traditional retractors, which increases the instability of the external force. The assistant has poor operative field visibility and may encounter difficulty with prompt adjustment of the retractor direction toward the drilling position, which prolongs the operative time. To facilitate exposure of the surgical field through small incisions, Obholazer RJ designed a simple skin distraction device, and Dalchow CV added a light supply to a nasal retractor (Obholzer and Craham, 2004; Dalchow and Werner, 2005). Considering the economic effectiveness, we designed a new retractor with positioning and molding functions, although light is still provided by a headlight. This retractor helps accurately locate the drilling position for preparation of the bony well. By lifting the skin and musculoperiosteal flap, the retractor provided a good surgical field and protected the skin and flap. Molds of various sizes and shapes can be designed according to different models of cochlear implants and can guide the surgeon during drilling such that a reasonable amount of bone tissue is drilled in the appropriate area. The mold used in this study was designed according to the Nurotron cochlear implant. Although the positioning plate had a flat surface that did not fit the curved skull surface perfectly, the surgeon could drill the rudimentary bony well and easily modify the well for the receiver/stimulator. The time required to drill the bony well was markedly shortened. Furthermore, the surgeon could stably hold the operating handle using only one hand and save energy for another surgical procedure. Some otologists have used a temporalis pocket technique in which the receiver-stimulator is enveloped by a temporalis pocket structure and the muscular flap is formed by the temporalis muscle and periosteum, with no drilling of a bony well (Balkany et al., 2009). However, this technique cannot prevent receiver-stimulator displacement due to the occurrence of a subcutaneous hematoma or infection. Therefore, we tended to drill the bony well and improve the tongue-shaped muscular flap. The posteriorly and inferiorly-based musculoperiosteal flap was extended anteriorly to the superoposterior ridge of the external auditory canal and downward to the attachment of the sternocleidomastoid muscle on the mastoid, and its posterior end was 2.5 cm from the posterior wall of the external auditory canal. When drilling the bony well, the improved musculoperiosteal flap was fully lifted by the retractor plate and did not affect the exposure of the surgical field. The receiver-stimulator fixed in the bony well was covered by the musculoperiosteal flap after suturing; thus, the flap completely separated the implant from the skin. No overlap was present between the skin incision and the tongue-shaped muscular flap incision. The improved muscle flap may reduce complications such as hematoma and implant displacement.
The purpose of cochlear implant surgery is to restore hearing and verbal communication skills in patients with severe to extremely severe sensorineural hearing loss. A small incision, a shortened operative time, and reduced surgical complications are important factors in the process of achieving this goal. In this study, a novel retractor with drilling and positioning functions was used to achieve a small incision, good exposure, and a short operative time, and this device is worthy of promotion for broad application in future clinical work.
Acknowledgment
This work was supported by the Special Funds for National Excellent Doctoral Dissertation of Higher Education from the Ministry of Education (Project Number: 2007B67) and the National Major Research Project (2014CB943003). The sketch was done by Wei Huang.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Bajaj Y., Wyatt M., Hartley B. Small postaural incision for paediatric cochlear implantation. Cochlear Implants Int. 2005;6(2):77–84. doi: 10.1179/cim.2005.6.2.77. [DOI] [PubMed] [Google Scholar]
- Balachandran R., Reda F.A., Noble J.H. Minimally invasive image-guided cochlear implantation for pediatric patients: clinical feasibility study. Otolaryngol. Head Neck Surg. 2014;150(4):631–637. doi: 10.1177/0194599813519050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkany T.J., Whitley M., Shapira Y. The temporalis pocket technique for cochlear implantation: an anatomic and clinical study. Otol. Neurotol. 2009;30(7):903–907. doi: 10.1097/MAO.0b013e3181b4e904. [DOI] [PubMed] [Google Scholar]
- Caversaccio M., Gavaghan K., Wimmer W. Robotic cochlear implantation: surgical procedure and first clinical experience. Acta Otolaryngol. 2017;137(4):447–454. doi: 10.1080/00016489.2017.1278573. [DOI] [PubMed] [Google Scholar]
- Dalchow C.V., Werner J.A. A new instrument for minimal access surgery in cochlear implantation. Otol. Neurotol. 2005;26(4):678–679. doi: 10.1097/01.mao.0000178135.74106.39. [DOI] [PubMed] [Google Scholar]
- Davids T., Ramsden J.D., Gordon K.A., James A.L., Papsin B.C. Soft tissue complications after small incision pediatric cochlear implantation. The Laryngoscope. 2009;119(5):980–983. doi: 10.1002/lary.20204. [DOI] [PubMed] [Google Scholar]
- Gibson W.P., Harrison H.C., Prowse C. A new incision for placement of cochlear implants. J. Laryngol. Otol. 1995;109(9):821–825. doi: 10.1017/s0022215100131421. [DOI] [PubMed] [Google Scholar]
- Mangus B., Rivas A., Tsai B.S., Haynes D.S., Roland J.T., Jr. Surgical techniques in cochlear implants. Otolaryngol. Clin. N. Am. 2012;45(1):69–80. doi: 10.1016/j.otc.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Matsumoto N., Oka M., Cho B. Cochlear implantation assisted by noninvasive image guidance. Otol. Neurotol. 2012;33(8):1333–1338. doi: 10.1097/MAO.0b013e318268d1e9. [DOI] [PubMed] [Google Scholar]
- O’Donoghue G.M., Nikolopoulos T.P. Minimal access surgery for pediatric cochlear implantation. Otol. Neurotol. 2002;23(6):891–894. doi: 10.1097/00129492-200211000-00014. [DOI] [PubMed] [Google Scholar]
- Obholzer R.J., Craham J.M. A novel retractor for use in cochlear implantation. Otol. Neurotol. 2004;25(1):83–85. doi: 10.1097/00129492-200401000-00017. [DOI] [PubMed] [Google Scholar]
- Riskalla A., Wall K., O’Connor A.F., Jiang D. An illuminated retractor for minimal access surgery in cochlear implantation: how we do it. Acta Otolaryngol. 2010;130(10):1199–1200. doi: 10.3109/00016481003743068. [DOI] [PubMed] [Google Scholar]
- Stratigouleas E.D., Perry B.P., King S.M., Syms C.A., 3rd Complication rate of minimally invasive cochlear implantation. Otolaryngol. Head Neck Surg. 2006;135(3):383–386. doi: 10.1016/j.otohns.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Weber S., Gavaghan K., Wimmer W. Instrument flight to the inner ear. Sci. Robot. 2017;2(4) doi: 10.1126/scirobotics.aal4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S., Chen Z., Wu Y. Suprameatal approach for cochlear implantation in 45 Chinese children. Int. J. Pediatr. Otorhinolaryngol. 2008;72(3):397–403. doi: 10.1016/j.ijporl.2007.12.001. [DOI] [PubMed] [Google Scholar]