Abstract
Endolymphatic sac tumors (ELSTs) are rare, papillary adenomatous tumors that arise from the endothelium of the endolymphatic sac. We demonstrate a difficult case of endolymphatic sac tumor and how it is managed via transcanal endoscopic assisted technique, with discussion of feasibility of transcanal approach to lateral skull base tumor.
Keywords: Endolymphatic sac, Lateral skull base, Transcanal endoscopic assisted technique, Transcanal combined endoscopic microscopic approach
1. Introduction
Endolymphatic sac tumors (ELSTs) are rare, papillary adenomatous tumors that arise from the endothelium of the endolymphatic sac (Kumar et al., 2011; Künzel et al., 2014). These tumors were first recognized as a distinct pathologic entity by Heffner in 1989 (Heffner, 1989). Rare but aggressive, the local destruction of temporal bone by these tumors can result in significant morbidities, including facial nerve weakness, tinnitus, hearing loss (HL), vestibular dysfunction, lower cranial nerve palsies, otalgia and ear discharge (Kumar et al., 2011; Arava et al., 2012; Stanley and Pickett, 2000). They are known to be associated with Von-Hippel Lindau syndrome, despite majority occur sporadically (Arava et al., 2012; Jagannathan et al., 2007). Diagnosis and surgical treatment has been challenging due to its anatomical position in lateral skull base. Classical surgical approaches include middle cranial fossa approach (MCF), translabyrinthine approach (TLA), retrolabrynthine approach (RLA) and transcochlear approach (TCA). These approaches require big incision and lots of bone work before reaching the tumor. With recent increasing popularity of application of endoscope in ear surgeries, the use of endoscope in lateral skull base surgeries has been explored in managing difficult cerebellopontine angle tumors, with advantages of providing wider visualization of previously inaccessible anatomical sites (Presutti et al., 2013; Magnan et al., 1994). The potential of endoscope has in lateral skull base surgery should not be underestimated. In this case report, we demonstrate a difficult case of ELST and how it was managed via transcanal endoscopic assisted technique, with discussion of the feasibility of transcanal approach to lateral skull base tumor.
2. Case
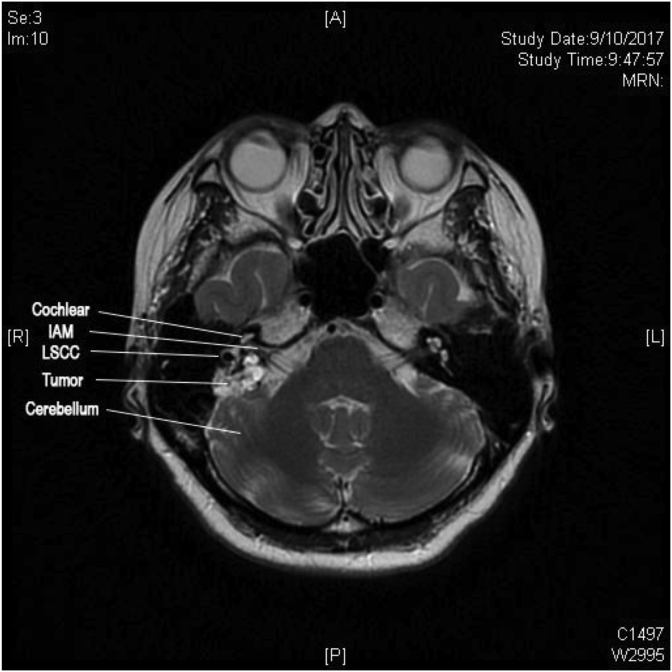
52 year-old woman presented with 7 years of non-positional vertigo, right-sided on-and-off hearing loss, non-pulsatile tinnitus and aural fullness in 2014. There were no cerebellar signs. Otoscopy revealed bilateral normal tympanic membranes and clear external auditory canals. Pure tone audiometry showed right side mild sensorineural hearing loss. She was initially managed as Meniere’s disease. Contrast magnetic resonance image (MRI) reveals a 1.5× 1 × 1.2 cm lesion deep to posteromedial cortex of right temporal bone, but completely separate from vestibulocochlear nerves. Differential diagnosis of cholesteatoma and cartilage bone tumor without intracranial component was made. Serial MRIs showed interval enlargement of the lesion up to 2.1 × 1.3 × 2.2 cm abutting posterior semicircular canal and sigmoid sinus (Fig. 1). It was also accompanied by worsening vertigo and deterioration of hearing to moderate hearing loss. The facial nerve function, however was House-Brackman grade 1 all along.
Fig. 1.
MRI showing pre-operative tumor involvement (IAM: Internal Acoustic meatus; LSCC: Lateral semicircular canal).
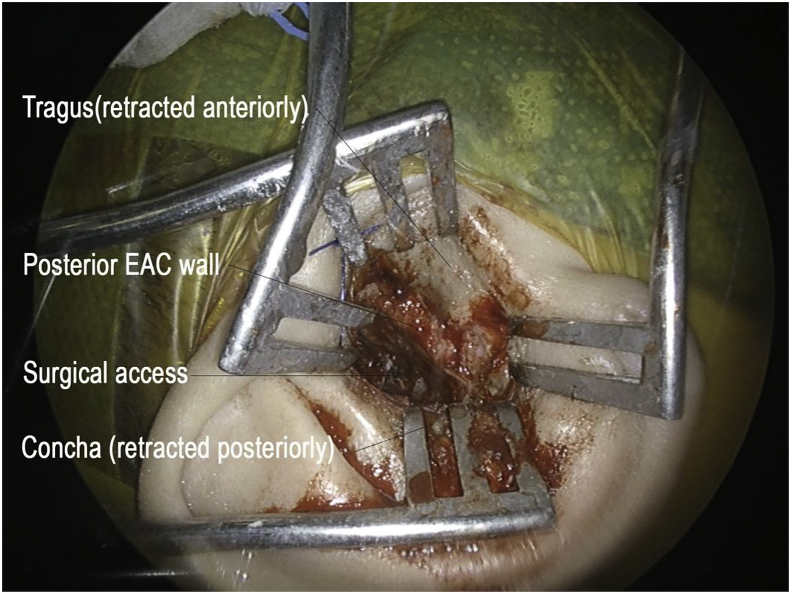
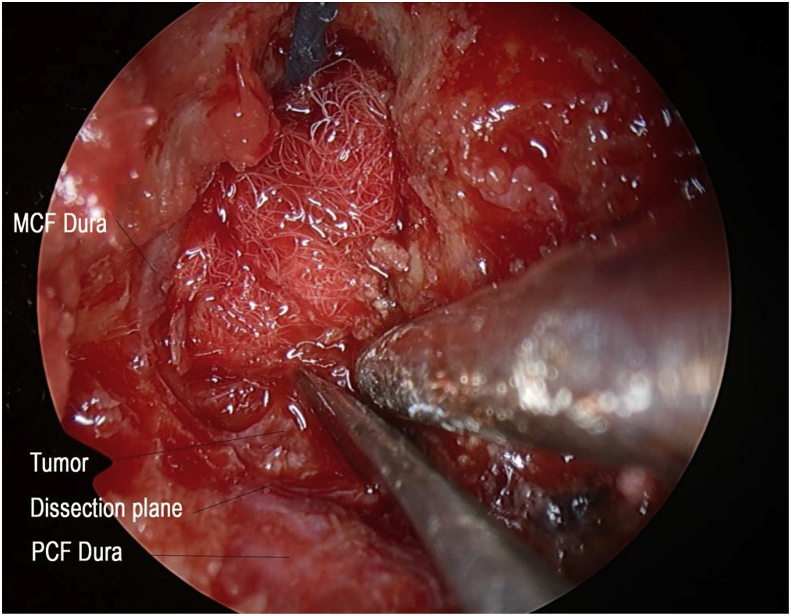
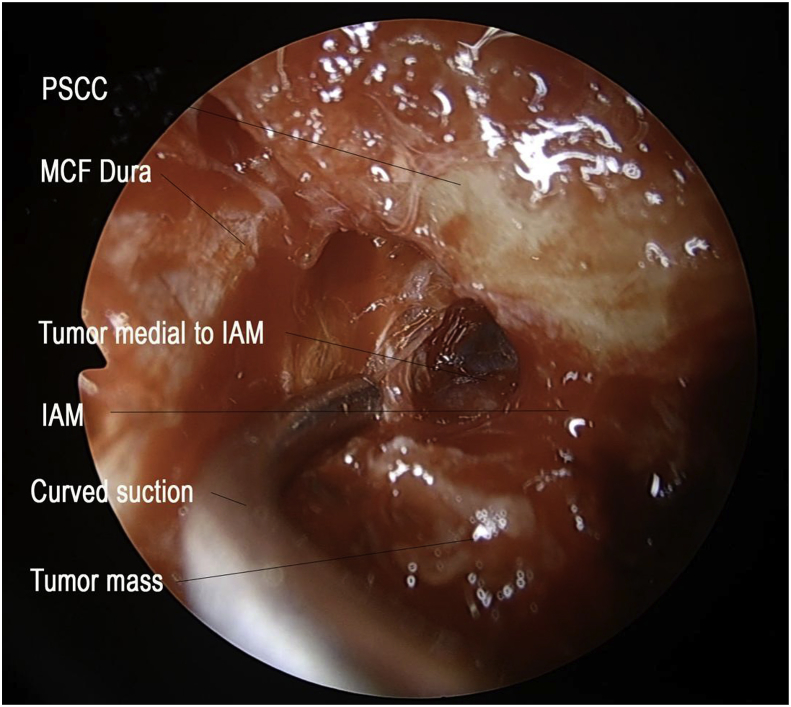
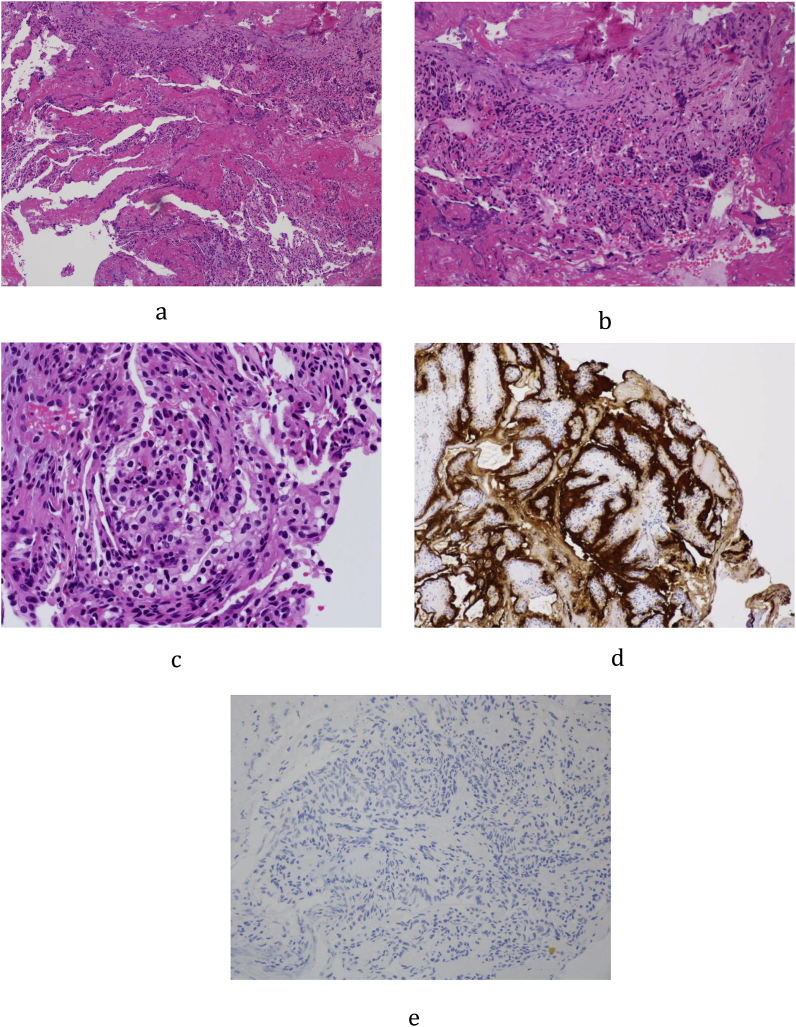
The patient was offered translabyrinthine craniotomy or retrosigmoid approach by the neurosurgeons. However, she preferred a minimally invasive approach with minimal external wound, hence a transcanal combined endoscopic microscopic retrolabyrinth approach was performed for tumor removal. A transcanal meatal incision was made, with superior extension at the root of helix and inferior extension to conchal root (Fig. 2). A meatochonchal flap was raised with traction posteriorly. Microscopic mastoidectomy was performed. Intraoperatively a vascular tumor was encountered with bone infiltration involving mastoid cavity and petrous apex, superiorly adhered to middle cranial fossa dura, posteriorly to posterior cranial fossa dura, medially to dura of internal acoustic meatus (IAM), inferiorly adhered to jugular bulb. With the help of 4 K endoscope with digital enhancement, the tumor plane with the dura was clearly appreciated (Fig. 3). In addition, the view of tumor around and medial to the IAM, originally blocked by the posterior semicircular canal, was clearly viewed with the endoscope and resected with angled dissection instruments (Fig. 4). The tumor was completely excised via transcanal approach. Facial nerve was intact, and valsalva maneuver confirmed no leak of perilymph and cerebrospinal fluid. The pathological diagnosis of endolymphatic sac tumor was challenging. No definitive diagnosis was made in frozen section. In formal pathology, low power magnification shows tumor fragments admixed both blood clot and fibrins (Fig. 5a). In high power magnification, papillary and glandular structures lined by a single layer of epithelium were seen, with low grade nuclear features of tumor cells (Fig. 5b and c). On immunnohistochemical study, the tumor cells were positive for AE1/AE3 (Fig. 5d), with focal positive for synaptophysin and scanty cells show weak positive for PAX8, VHL stain were negative (Fig. 5e). The case was discussed in the pathology intradepartmental meeting and an eventual diagnosis of ELST was made by a consultant pathologist.
Fig. 2.
External incision of transcanal approach (EAC: External acoustic canal).
Fig. 3.
Tissue plane between tumor and dura with 4 K system (MCF: middle cranial fossa; PCF: posterior cranial fossa).
Fig. 4.
Endoscopic approach to Internal Acustic Meatus with curved instruments (PSCC: posterior semicircular canal; MCF: middle cranial fossa; IAM: internal acoustic meatus).
Fig. 5.
a: Low power magnification showing tumor fragments admixed both blood clot and fibrins.
b: Higher power magnification showing papillary and glandular structures lined by a single layer of epithelium.
c: High power magnification showing low grade nuclear features of tumor cells.
d: Cytoderatin stain (AE1/AE3) highlights the lining cells.
e: VHL stain were negative.
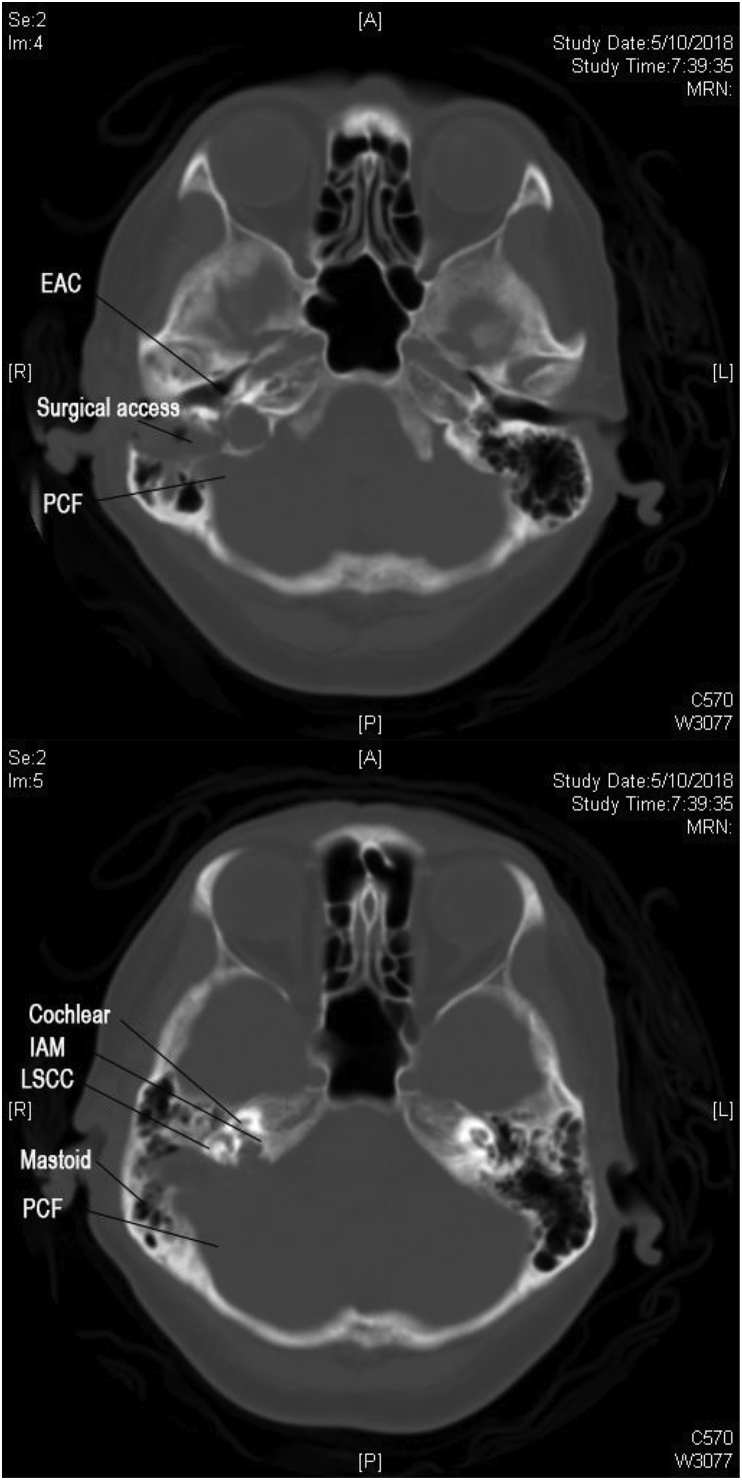
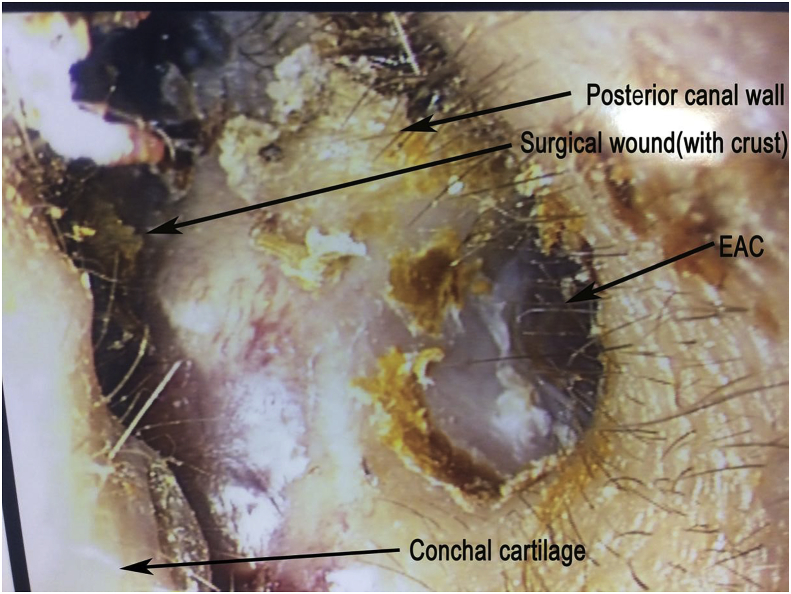
Post-operatively, the patient retained normal facial nerve function with static pre-operative moderate to severe hearing loss. She was discharged from hospital day-1 post-operation. Post-operative Computed Tomography (CT) scan showing the transcanal endoscopic assisted approach with minimal bone destruction and reaching the IAM perfectly (Fig. 6a–b). 2-weekpost-operative follow-up reveals intact tympanic membrane and intra-auricular wound (Fig. 7) with good cosmetic outcome (Fig. 8). The patient would be continuously follow-up by multidisciplinary team comprising of neurosurgeons, ENT surgeons and Oncology team, with further adjuvant treatments to be decided in multidisciplinary meeting. Follow-up investigations include pure-tone audiogram 1-year post-operative for hearing monitoring, and imaging (MRI brain 1–2year post-operatively, CT abdomen and pelvis 2 year post-operative) for surveillance of recurrence and other associated tumors of ELST.
Fig. 6.
a,b: CT scan showing transcanal approach to Internal Acoustic Meatus and Jugular Bulb.
Fig. 7.
Intra-auricular wound, 2 weeks post op (EAC: External auditory canal).
Fig. 8.
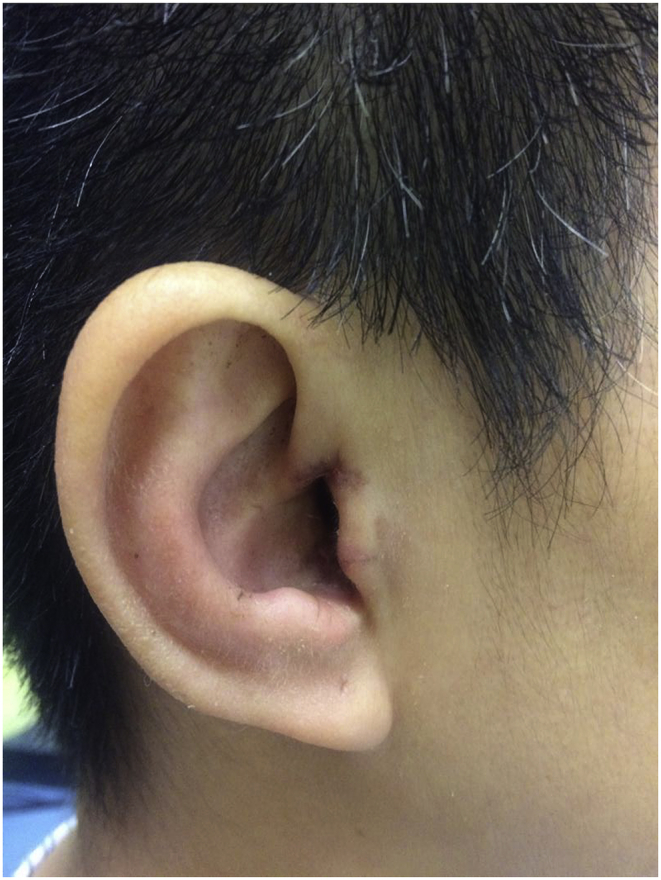
Post operative 2 weeks external cosmetic appearance.
3. Discussion
This case demonstrates a difficult diagnosis of endolymphatic sac tumor, due to its rarity, non-specific presentations, atypical radiological findings and inconclusive intra-operative frozen section. Classically in CT scans, ELST is a heterogenous mass located in retrocochlear petrous temporal bone, with surrounding destructive bone changes of ‘moth-eaten’ pattern. The jugular foramen is usually spared, which is useful for differentiating ELSTs from jugulare tumor (Kumar et al., 2011; Künzel et al., 2014). In this case however, the jugular bulb was dehisced and was partially involved. ELST is heterogeneous hyperintense in T2 MRI images, while areas of altered gradient susceptibility would be seen on gradient weight images due to its hypervascularity proning to multiple foci of haemorrhage (Kumar et al., 2011; Künzel et al., 2014). The differential diagnosis of ELST includes middle ear tumor, choroid plexus papilloma, paraganglioma, haemangioma, meningioma and bony lesions like chondrosarcoma (Kumar et al., 2011; Künzel et al., 2014; Arava et al., 2012).
Open surgery is the traditional primary treatment for ELST. The choice of open surgical approach depends on the presence of functional hearing, tumor size and localization. In Bambakidis Stage I & II ELSTs (Bambakidis et al., 2004), transmastoid, translabyrinthine, retrolabyrinthine, retrosigmoid approaches could be adopted. Transmastoid retrolabyrinthine approach allows visualization of posterior SCC and preservation of cranial nerve VII & VIII during dissection of endolymphatic sac. It also provides access to resect dural leaflet of ELS and transform to retrolabyrinthine-transdural approach if there are relevant involvement (Wick et al., 2018). Retrosigmoid approach provides better visualization and control of cerebellopontine angle tumor extension and facilitate dural resection, but there is no visualization of posterior SCC during endolymphatic sac dissection and the tumor can be spread intracranially if there was no dural involvement (Wick et al., 2018). Both approaches however, would require a large skin excision and bone drilling, compromising the recovery and cosmetic outcome. As for Bambakidis Stage III & IV ELSTs (Bambakidis et al., 2004), more complex approaches such as staged anterior and posterior fossa approach, subtemporal craniotomy with petrosectomy, modified transcochclear approach are required depending on the structures involved. Our patient has a Bambakidis stage I ELST without dural involvement, and the sparing of dural dissection has made the transcanal endoscopic-microscopic approach possible and superior to conventional open retrosigmoid, transmastoid translabyrinthine approaches. Transmastoid translabyrinthine approach would sacrifice the hearing. Retrosigmoid approach bears a risk of intradural seeding of an extra-dural lesion, especially when the pathology was initially uncertain. These two approaches would also raise a concern of large external wound hence poor cosmetic outcome for a relatively young lady. The main advantage of transcanal approach is its small external wound and minimal bone drilling, requiring only a small meatal incision for surgical access and offering a better cosmetic outcome and hastening the post-operative recovery. Transcanal endoscopic-microscopic approach extracts and combines the unique advantages of microscope & endoscope respectively, preserving the use of two-hands operation in microscope should tumor bleeding is significant, while overcoming the limited view of deep structures by the use of endoscope. In fact, endoscope has been widely used as adjuncts in microscopic middle ear surgeries, with degreed endoscopes providing a wider surgical field visualizing anatomical sites previously not seen, and curved instruments for angled dissections (Pollak, 2017; Sajjadi, 2013; Kanona et al., 2015).
Various pre-operative and post-operative adjuvant treatments have been mentioned in literature review. Pre-operative embolization is believed to be beneficial to minimize intra-operative bleeding and facilitate complete resection (Nevoux et al., 2014; Megerian and Semaan, 2007). In fact, the tumor was found to be very vascular during dissection, which is characteristic of the cystic subtype of ELST (Mendenhall et al., 2018). It however, may not be practical as ELST, as demonstrated in our case, is diagnosed post-operatively in histology and often mistaken as other differential pre-operatively.
Gross total resection was report to have >90% long term cure rate (Wick et al., 2018). However, with restricted access or involvement adjacent structures, subtotal resection is not uncommon and 70% may have progressive disease (Wick et al., 2018). For incomplete resection, radiotherapy has been proposed as adjuvant treatment (Balasubramaniam et al., 2009). A systematic review on outcome of radiation therapy for ELST has reported a higher tumor control rate of stereotactic radiosurgery than external beam radiation (77.8% ⇔ 47.4%), but the finding was not statistically significant (Wick et al., 2018). Nonetheless, the role of radiotherapy is still controversial, with quoted 50% recurrence post-radiation (Husseini et al., 2013; Bell et al., 2011), and currently there is no study comparing post-operative surveillance and adjuvant radiotherapy (Sun et al., 2012). A multidisciplinary decision making with consideration of completeness of resection, patient factors, and treatment risks is currently adopted among different case reports (Künzel et al., 2014; Poletti et al., 2013; Carlson et al., 2013), and until further evidence is available, the choice of post-operative management of ELSTs should be individualised. It should be noted adjuvant treatments for ELST is not the focus of this case report, and the authors call for more structural reviews on the current evidence for pre- and post-operative management of ELST. However, the importance of long-term follow up in ELST should be emphasized, as recurrence of ELST can occur as late as 13 year, with majority of the recurrence between 24 and 48 month post-radiotherapy (Wick et al., 2018). Our patient was considered as gross total resection. Amount discussion on adjuvant radiotherapy versus close monitoring with imaging, patient has a strong desire on the latter. Her follow up contrast MRI was arranged in 6 months after the operation.
This case has demonstrated that transcanal endoscopic-assisted technique provides a new surgical approach with combined advantages of microscopes and endoscopes in accessing petrous apex and lateral skull base lesions. From our experience, we believe that the new approach can be applied to Bambakidis Stage I & II ELSTs, with the potential of further application in managing other lateral skull base lesions. However, traditional open approaches may still be necessary for Stage III & IV ELSTs given the complexity and extend of the dissections required. With more experience, transcanal endoscopic-assisted technique may be part of personalized approach in management of lateral skull base lesion.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.joto.2019.06.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Arava S., Soumya R.M., Chitragar S., Safaya R., Chandrashekhar S.H., Thakar A. Papillary endolymphatic sac tumor: a case report. Case Rep. Otolaryngol. 2012;2012(163851) doi: 10.1155/2012/163851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniam S., Deshpande R.B., Misra B.K. Gamma knife radiosurgery in jugular foramen endolymphatic sac adenocarcinoma. J. Clin. Neurosci. 2009;16(5):710–711. doi: 10.1016/j.jocn.2008.07.086. [DOI] [PubMed] [Google Scholar]
- Bambakidis N.C., Megerian C.A., Ratcheson R.A. Differential grading of endolymphatic sac tumor extension by virtue of von Hippel-Lindau disease status. Otol. Neurotol. 2004;25(5):773–781. doi: 10.1097/00129492-200409000-00021. [DOI] [PubMed] [Google Scholar]
- Bell D., Gidley P., Levine N., Fuller G.N. Endolymphatic sac tumor (aggressive papillary tumor of middle ear and temporal bone): sine qua non radiology-pathology and the University of Texas MD Anderson Cancer Center experience. Ann. Diagn. Pathol. 2011;15(2):117–123. doi: 10.1016/j.anndiagpath.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Carlson M.L., Thom J.J., Driscoll C.L., Haynes D.S., Neff B.A., Link M.J., Wanna G.B. Management of primary and recurrent endolymphatic sac tumors. Otol. Neurotol. 2013;34(5):939–943. doi: 10.1097/MAO.0b013e31828680da. [DOI] [PubMed] [Google Scholar]
- Heffner D.K. Low-grade adenocarcinoma of probable endolymphatic sac origin A clinicopathologic study of 20 cases. Cancer. 1989;64:2292–2302. doi: 10.1002/1097-0142(19891201)64:11<2292::aid-cncr2820641119>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Husseini S.T., Piccirillo E., Taibah A., Paties C.T., Almutair T., Sanna M. The Gruppo Otologico experience of endolymphatic sac tumor. Auris Nasus Larynx. 2013;40(1):25–31. doi: 10.1016/j.anl.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Jagannathan J., Butman J.A., Lonser R.R., Vortmeyer A.O., Zalewski C.K., Brewer C. 2007. Endolymphatic Sac Tumor Demonstrated by Intralabyrinthine Hemorrhage. [DOI] [PubMed] [Google Scholar]
- Kanona H., Virk J.S., Owa A. Endoscopic ear surgery: a case series and first United Kingdom experience. World J. Clin. Cases: WJCC. 2015;3(3):310. doi: 10.12998/wjcc.v3.i3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Ramakrishnaiah R., Muhhamad Y., Van Hemert R., Angtuaco E. Endolymphatic sac tumor. Radiol. Case Rep. 2011;6(3) doi: 10.2484/rcr.v6i3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzel J., Agaimy A., Hornung J., Lell M., Ganslandt O., Semrau S., Zenk J. Sporadic endolymphatic sac tumor–a diagnostic and therapeutic challenge. Int. J. Clin. Exp. Pathol. 2014;7(5):2641. [PMC free article] [PubMed] [Google Scholar]
- Magnan J., Chays A., Lepetre C., Pencroffi E., Locatelli P. Surgical perspectives of endoscopy of the cerebellopontine angle. Am. J. Otol. 1994;15(3):366–370. [PubMed] [Google Scholar]
- Megerian C.A., Semaan M.T. Evaluation and management of endolymphatic sac and duct tumors. Otolaryngol. Clin. 2007;40(3):463–478. doi: 10.1016/j.otc.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Mendenhall W.M., Suárez C., Skálová A., Strojan P., Triantafyllou A., Devaney K.O. Current treatment of endolymphatic sac tumor of the temporal bone. Adv. Ther. 2018:1–12. doi: 10.1007/s12325-018-0730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevoux J., Nowak C., Vellin J.F., Lepajolec C., Sterkers O., Richard S., Bobin S. Management of endolymphatic sac tumors: sporadic cases and von Hippel-Lindau disease. Otol. Neurotol. 2014;35(5):899–904. doi: 10.1097/MAO.0000000000000299. [DOI] [PubMed] [Google Scholar]
- Poletti A.M., Dubey S.P., Barbò R., Pericotti S., Fiamengo B., Colombo G., Mazzoni A. Sporadic endolymphatic sac tumor: its clinical, radiological, and histological features, management, and follow-up. Head Neck. 2013;35(7):1043–1047. doi: 10.1002/hed.22962. [DOI] [PubMed] [Google Scholar]
- Pollak N. Endoscopic and minimally-invasive ear surgery: a path to better outcomes. World J. Otorhinolaryngol. Head Neck Surg. 2017;3(3):129–135. doi: 10.1016/j.wjorl.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presutti L., Alicandri-Ciufelli M., Cigarini E., Marchioni D. Cochlear schwannoma removed through the external auditory canal by a transcanal exclusive endoscopic technique. The Laryngoscope. 2013;123(11):2862–2867. doi: 10.1002/lary.24072. [DOI] [PubMed] [Google Scholar]
- Sajjadi H. Endoscopic middle ear and mastoid surgery for cholesteatoma. Iran. J. Otorhinolaryngol. 2013;25(71):63. [PMC free article] [PubMed] [Google Scholar]
- Stanley J.J., Pickett B.P. Lippincott Williams and Wilkins; Philadelphia: 2000. Endolymphatic Sac Tumors; pp. 156–171. [Google Scholar]
- Sun Y.H., Wen W., Wu J.H., Song J.M., Guan H., Wang K.X., Xu M.Q. Endolymphatic sac tumor: case report and review of the literature. Diagn. Pathol. 2012;7(1):36. doi: 10.1186/1746-1596-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick C.C., Eivaz N.A., Yeager L.H., Hunter J.B., Isaacson B., Kutz J.W., Jr. Case series and systematic review of radiation outcomes for endolymphatic sac tumors. Otol. Neurotol. 2018;39(5):550–557. doi: 10.1097/MAO.0000000000001804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.