Summary
Conserved translocator proteins (TSPOs) mediate cell stress responses possibly in a cell-type-specific manner. This work reports on the molecular function of plant TSPO and their possible evolutionary divergence. Arabidopsis thaliana TSPO (AtTSPO) is stress induced and has a conserved polybasic, plant-specific N-terminal extension. AtTSPO reduces water loss by depleting aquaporin PIP2;7 in the plasma membrane. Herein, AtTSPO was found to bind phosphoinositides in vitro, but only full-length AtTSPO or chimeric mouse TSPO with an AtTSPO N-terminus bound PI(4,5)P2in vitro and modified PIP2;7 levels in vivo. Expression of AtTSPO but not its N-terminally truncated variant enhanced phospholipase C activity and depleted PI(4,5)P2 from the plasma membrane and its enrichment in Golgi membranes. Deletion or point mutations within the AtTSPO N-terminus affected PI(4,5)P2 binding and almost prevented AtTSPO-PIP2;7 interaction in vivo. The findings imply functional divergence of plant TSPOs from bacterial and animal counterparts via evolutionary acquisition of the phospholipid-interacting N-terminus.
Subject Areas: Cell Biology, Plant Biology, Plant Physiology
Graphical Abstract
Highlights
-
•
Plant TSPOs possess a specific and structurally conserved polybasic N-terminal extension
-
•
Plant TSPOs bind PI(4,5)P2 in vitro and at the Golgi membrane in vivo
-
•
The polybasic N-terminal extension is required for PI(4,5)P2 binding
-
•
Plant TSPO depletes the plasma membrane of both an aquaporin and a PI(4,5)P2
Cell Biology; Plant Biology; Plant Physiology
Introduction
Translocator proteins (TSPOs) are polytopic membrane proteins present in organisms from prokaryotes to humans (Kreps et al., 2002, Seki et al., 2002, Zimmermann et al., 2004, Papadopoulos et al., 2006, Guillaumot et al., 2009, Fan et al., 2012, Guo et al., 2015, Li et al., 2015). TSPOs share an evolutionarily conserved structure consisting of five α-helical transmembrane segments forming a tryptophan-rich sensory protein/mitochondrial benzodiazepine receptor (TspO/MBR) domain (Fan et al., 2012, Jaremko et al., 2014, Guo et al., 2015, Li et al., 2015). TSPOs are attracting clinical and pre-clinical research attention due to links to human diseases such as metastatic cancer, central nervous system (CNS) ailments including Alzheimer and Parkinson, and neuroinflammations (Papadopoulos et al., 2006, Rupprecht et al., 2010, Gatliff et al., 2017). Natural and synthetic TSPO ligands are widely used as diagnostic tools and treatments for the aforementioned conditions despite the molecular functions remaining unknown (Rupprecht et al., 2010, Morin et al., 2016). Although TSPOs have been extensively studied since their discovery in the 1970s (Braestrup and Squires, 1977), varying findings across species and experimental models have failed to clarify evolutionarily conserved molecular functions. Functional studies of bacterial, mammalian, and plant TSPOs indicate roles in sensing and stress responses (Yeliseev and Kaplan, 1999, Davey and de Bruijn, 2000, Guillaumot et al., 2009, Jaremko et al., 2014, Guo et al., 2015, Li et al., 2015).
In bacteria, TSPOs are involved in responses to nutrient deficiency, stress-related and light-dependent processes such as the synthesis of photosynthetic pigments, and oxygen-dependent respiration (Yeliseev and Kaplan, 1999, Davey and de Bruijn, 2000). Interestingly, a phylogenetically distant rat TSPO rescued the phenotype of a knockout mutant of the photosynthetic bacteria Rhodobacter sphaeroides, indicating functional as well as structural conservation (Yeliseev et al., 1997). In eukaryotes, TSPOs are localized in different intracellular membrane compartments, and deletion of mitochondria-localized Schizosaccharomyces pombe TSPO (SpTSPO) enhanced sensitivity to mineral toxicity and to the drug rapamycin (Doi et al., 2015). Rapamycin and its derivatives are therapeutically attractive immunosuppressants and antitumor agents (Augustine et al., 2007) that function by inhibiting the mechanistic target of rapamycin (mTOR), a key kinase regulating starvation-induced autophagy in eukaryotic cells (Weisman and Choder, 2001, Thoreen et al., 2009, Sancak et al., 2010, Nakashima et al., 2010). In addition, rapamycin may serve as a calorie restriction mimetic to extend lifespan (Takahara and Maeda, 2013). Overexpression of SpTSPO increases cell viability at stationary phase, and deletion of SpTSPO decreases cell population growth on glucose (Doi et al., 2015). Interestingly, inhibition or knockdown of Drosophila TSPO (dTSPO) inhibits wing disk apoptosis in response to γ-irradiation or H2O2 exposure, extends fly lifespan, and reduces neurodegeneration (Lin et al., 2014). In multiple cross-species cell types, TSPO overexpression stimulates an oxidative cellular environment, which is reversed upon knockdown (Vanhee et al., 2011a, Doi et al., 2015, Batoko et al., 2015, Gatliff et al., 2017). TSPO expression is transiently increased during inflammation of the CNS, facilitating imaging using functionalized TSPO-specific ligands (Braestrup and Squires, 1977, Rupprecht et al., 2010). For example, animal TSPO is abundantly expressed in glial cells recruited and activated during neuroinflammation, where it may modulate redox homeostasis (Hong et al., 2006, Lavisse et al., 2012, Banati et al., 2014, Bae et al., 2014, Liu et al., 2015). Involvement of TSPO in reactive oxygen species (ROS) signaling may be linked to porphyrin binding (Batoko et al., 2015, Guo et al., 2015, Marginedas-Freixa et al., 2016, Ozaki et al., 2010, Vanhee et al., 2011a, Verma et al., 1987, Guilarte et al., 2016), because porphyrins are the main endogenous ligands of TSPO in all cell types, and free protoporphyrins are powerful light-dependent ROS generators.
Although TSPO ligands are applied in clinical imaging and therapeutics, TSPO functions remain poorly understood (Li et al., 2016). Mammalian mitochondrial TSPO and the mitochondrial outer membrane partner voltage-dependent anion channel (VDAC1) contribute to establishing a molecular platform for tuning autophagy-mediated removal of ROS-damaged mitochondria (Gatliff et al., 2014). Arabidopsis thaliana TSPO (AtTSPO) is transiently induced by abiotic (osmotic) stress and the stress phytohormone abscisic acid (ABA) (Kreps et al., 2002, Seki et al., 2002, Guillaumot et al., 2009, Vanhee et al., 2011a). The time-limited presence of AtTSPO in plant cells may contribute to osmotic stress responses. Indeed, the mostly Golgi-localized AtTSPO physically interacts with the highly expressed plasma membrane (PM) aquaporin PIP2;7 in both Golgi and ER membranes (Hachez et al., 2014). Under osmotic stress, AtTSPO interacts with PIP2;7 en route to the PM, thereby contributing to reducing water loss (Hachez et al., 2014). The resulting protein complex is subsequently targeted to the vacuole via the autophagic pathway. Plant TSPO may act as a selective autophagy receptor targeting haem and aquaporin to the vacuole for degradation (Vanhee et al., 2011b, Hachez et al., 2014). The underlying molecular mechanisms of these interactions are not clear yet, but TSPO involvement in stress homeostasis could be a conserved ancestral function, albeit with species dependent mechanistic variation (Batoko et al., 2015, Li et al., 2016). TSPOs may be ancient bacterial receptor/stress sensors that have evolved additional interactions, partners, and roles in eukaryotes (Li et al., 2016).
Terrestrial plants lose water primarily through pores in their aerial parts known as stomata. Turgor and non-turgidity of stomatal guard cells respectively determine pore opening and closing (Mishra et al., 2006). ABA-dependent regulation of stomata involves changes in ROS, calcium, the cytoskeleton, and signaling phosphoinositides (Schroeder et al., 2001, Hetherington and Brownlee, 2004, Lee et al., 2007, Cutler et al., 2010). Dynamic pools of phosphoinositides (PIs), a family of phospholipids located on the cytoplasmic leaflet of cellular membranes, mediate key cellular processes such as signal transduction, structural maintenance, motility, endo-exocytosis, autophagy, and regulation of transporter and ion channel function (Hammond et al., 2012, Holthuis and Menon, 2014, Heilmann, 2016). Spatiotemporal remodeling of PI pools within distinct organelles is an intrinsic feature facilitating orchestration of PI-mediated cellular functions (Hammond et al., 2012, Holthuis and Menon, 2014, Heilmann, 2016). Indeed, PIs are regulated by PI-metabolizing enzymes and must be accessible to effectors. Various regulatory proteins including PI effectors bind these negatively charged lipids through specific binding domains or electrostatic interactions (Kooijman et al., 2007, Hammond et al., 2012, Holthuis and Menon, 2014, Munnik and Nielsen, 2011). Subcellular localization of PIs is tightly governed by the concurrent presence of cognate lipid kinases and phosphatases, giving each cellular membrane a unique and dynamic PI signature and contributing to lipid signaling events (Hammond et al., 2012). For instance, the activity of phospholipase Dα1 (PLDα1) and phospholipase C (PLC) generates the messenger lipids phosphatidic acid (PA) and diacylglycerol (DAG), respectively, and both mediate the effects of ABA on stomata opening and closure (Mishra et al., 2006). In particular, PA binds to the negative ABA signaling regulator ABA insensitive 1 (ABI1), a protein phosphatase 2C, to promote stomatal closure, or to the Gα subunit of heterotrimeric G protein to mediate ABA inhibition of stomatal opening (Testerink and Munnik, 2005, Lee et al., 2007).
In the present work, we provide evidence that an evolutionarily conserved plant-specific polybasic N-terminus confers PI(4,5)P2 binding in plant TSPO, and expression of plant TSPO results in PI(4,5)P2 remodeling through its depletion from the PM and enrichment in the Golgi membrane. Physiologically, PI(4,5)P2 binding to plant TSPO is required for physical interaction with PIP2;7 in vitro and in vivo and subsequent degradation of the protein-lipid complex. Interestingly, addition of the plant-specific polybasic N-terminus to mouse TSPO conferred binding properties to PI(4,5)P2 and, when expressed in plant cells, regulation of PIP2;7 trafficking and abundance in the PM. These findings provide insight into the physiological role of the evolutionary conserved plant-specific polybasic N-terminal extension.
Results
The N-terminal Extension of Arabidopsis TSPO Is Necessary for Protecting Plants against Tissue Dehydration
AtTSPO expression is higher during osmotic stress, but only transiently. We previously showed that AtTSPO physically interacts with defined aquaporin molecules intracellularly, en route to the PM, causing a reduction in water transport across the PM during osmotic stress (Hachez et al., 2014). Consistently, we also showed that constitutive expression in plant cells of AtTSPO or the more stable point mutant AtTSPO (H91A) yielded transgenic plants exhibiting reduced water loss compared with wild-type (WT) plants under dehydration. This role in osmotic stress and the underlying molecular mechanism appear to be unique to plant TSPO. Thus, we wondered whether this activity might be associated with the plant-specific N-terminal extension.
To investigate this, we generated transgenic plants overexpressing a truncated version of AtTSPO lacking the first 41 amino acids (p35S::Venus-AtTSPOΔN) and compared water loss with WT, AtTSPO knockout (KO), and transgenic plants constitutively expressing either AtTSPO (p35S::AtTSPO) or the stable point mutant (p35S::AtTSPOH91A) (Vanhee et al., 2011b). We verified transgene expression in all lines used (Figures 1B and 1D). Under dehydration, KO seedlings had the highest water loss, as expected, whereas p35S::AtTSPO and p35S::AtTSPOH91A plants had the lowest (Figure 1A). Notably, we found no significant difference in water loss between WT and p35S::Venus-AtTSPOΔN plants, suggesting deletion of the plant-specific N-terminus abolished the impact of constitutive AtTSPO expression on the regulation of water loss in transgenic plants. Consistent with prior findings (Hachez et al., 2014), tagging the protein at the N-terminus had no effect on regulation of water loss by AtTSPO (also see below).
Figure 1.
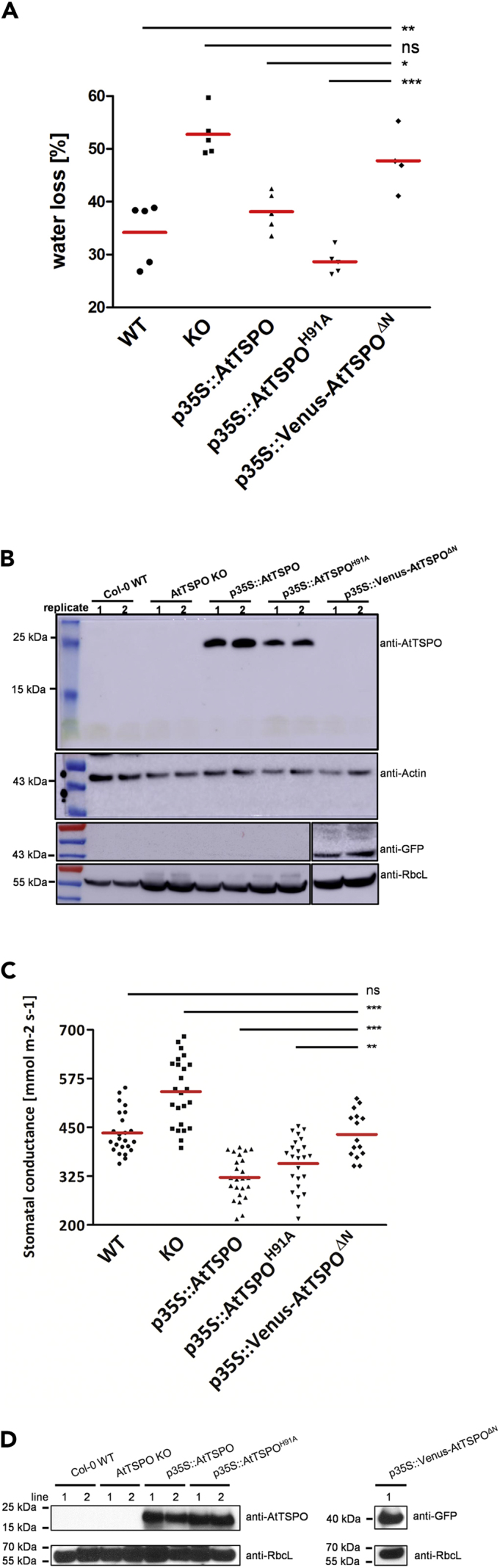
Arabidopsis Plants Expressing N-terminally Truncated AtTSPO Are More Affected by Water Loss than Plants Overexpressing Full-Length AtTSPO
(A) Percentage water loss after 120 min from soil-grown 17-day-old Arabidopsis (whole aerial parts) grown in a phytotron (average temperature 20°C, approx. 65% humidity, 16 h photoperiod, ∼120 μmol.m−2.s−1) and dehydrated under light in the same conditions. WT, wild-type; KO, transfer DNA insertional AtTSPO knockout line; p35S::AtTSPO, AtTSPO-overexpressing line; p35S::AtTSPOH91A, transgenic line stably overexpressing AtTSPO harboring point mutation H91A; p35S::Venus-AtTSPOΔN, line overexpressing Venus-tagged N-terminally truncated AtTSPO. Pooled measurements from five rosettes coming from at least two pots (distributed randomly in the phytotron) are shown, and red horizontal lines define the means for each dataset. Statistical significance was assessed by one-way ANOVA followed by Tukey's tests (*p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant).
(B) Western blot of total protein extracts from rosettes assayed in (A). Per genotype, probed were two replicates (independent pots).
(C) Stomatal conductance of intact leaves from soil-grown mature plants under phytotron conditions. Genotypes are as in (A). Four plants (2–3 rosette leaves per plant) were tested for each line. Individual measurements are shown and red horizontal lines define the means for each dataset. Statistical analysis was conducted as in (A), with **p < 0.01.
(D) Western blot of total protein extracts from leaves assayed in stomatal conductance experiments.
Water loss is associated with evapotranspiration through stomatal opening, hence we examined stomatal conductance in mature plants also grown on soil. Stomatal conductance measurements on rosette leaves after bolting (Figure 1C) revealed similar trends, indicating that the N-terminal extension is required for regulating water loss mediated by constitutively expressed AtTSPO. Thus, constitutive expression of AtTSPO had a physiological impact on stomatal opening.
The N-terminal Extension of AtTSPO Is Required for Interaction with PIP2;7 in Planta
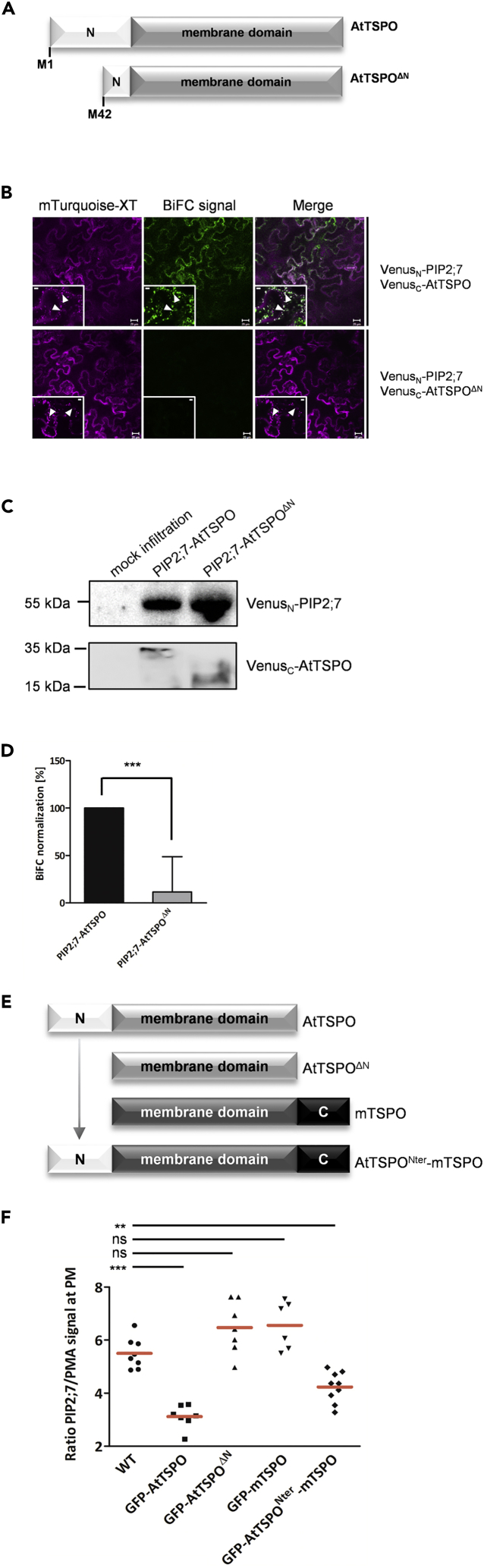
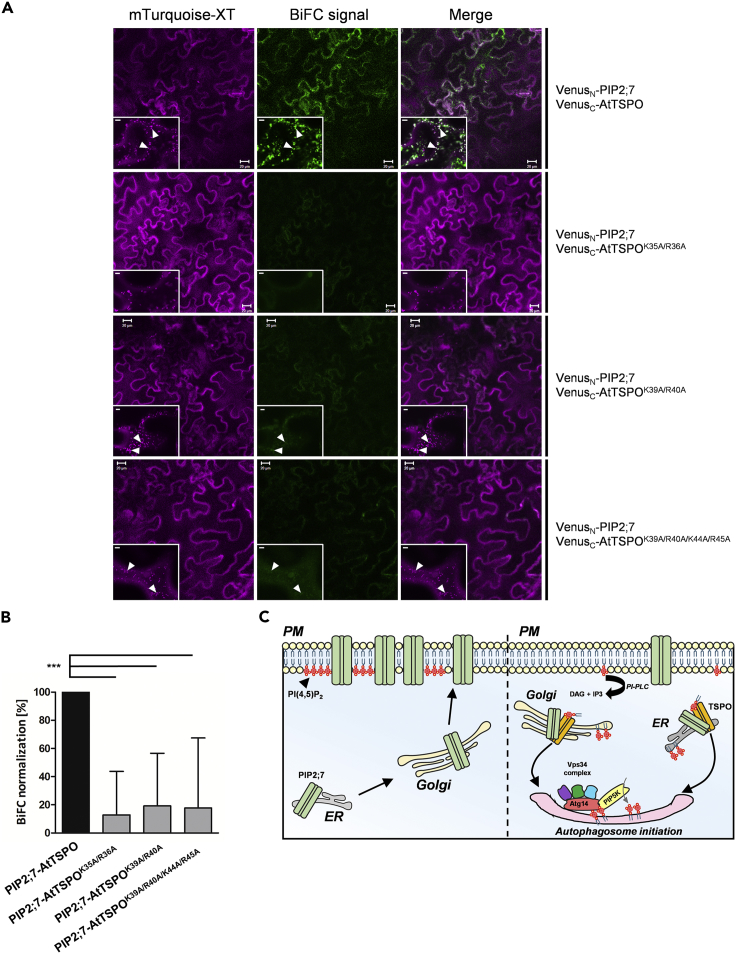
Because AtTSPO-mediated reduction of water loss required the N-terminal extension of AtTSPO and because we previously showed that AtTSPO interacts with PIP2;7 in vivo (Hachez et al., 2014), we examined whether the N-terminal extension is required for this interaction in vivo. We conducted a bimolecular fluorescence complementation (BiFC) experiment using constructs depicted in Figure 2A. Interaction between the full-length AtTSPO and PIP2;7 containing constructs was readily detectable, but there was no BiFC signal using the truncated AtTSPO construct lacking the first 41 residues at the N-terminus (Figure 2B). As shown previously (Hachez et al., 2014), interaction between AtTSPO and PIP2;7 occurs in Golgi membranes (Figure 2B), and truncated AtTSPO was also localized to Golgi membranes (Figures S1 and S2). Expression of all tested constructs was verified by western blot (Figure 2C), and quantification of the BiFC signal (Figure 2D) indicated that Venus fluorescence detectable with the truncated construct was reduced to that of background levels (see Figure S2 for smaller deletions and Figure S3 for signal quantification methodology). We concluded that truncation of the N-terminus abolished the AtTSPO-PIP2;7 interaction in vivo.
Figure 2.
The Plant-specific N-terminal Extension of AtTSPO Is Required for Interaction and Depletion of PIP2;7 In Vivo
(A) Schematic representation of AtTSPO genetic constructs prepared for BiFC analysis. Full-length PIP2;7 and AtTSPO served as positive controls. The N-terminally truncated variant starts with methionine at position 42.
(B) Representative confocal images of tobacco epidermal cells transiently coexpressing VenusN-PIP2;7 and VenusC-AtTSPO or VenusN-PIP2;7 and VenusC-AtTSPOΔN. Xylosyltransferase-mTurquoise fluorescent chimera (magenta) was imaged as a cell transfection control (Golgi marker) and for signal quantification. Low-magnification images qualitatively demonstrate the occurrence or lack of BiFC (green), and insets of high magnification images show Golgi stacks (arrowheads). Bars = 20 μm and 5 μm for low and high magnification, respectively. Experiments were repeated three times.
(C) Western blot of total extracts from infiltrated leaf areas imaged by BiFC. PIP2;7 expression was detected by anti-GFP. Full-length and truncated AtTSPO were detected by anti-FLAG (tag originally cloned between VenusC and AtTSPO). Non-infiltrated leaf area served as a negative control.
(D) Signal quantification shows a drastic reduction in BiFC, and hence interaction between PIP2;7 and truncated AtTSPO mutant (gray histogram) compared with full-length AtTSPO (black histogram). Bars represent means +/− SD. Statistical significance was assessed by an independent samples t test using Graphpad Prism (***p < 0.001).
(E) Schematic representation of genetic constructs stably expressed in Arabidopsis suspension cells. The function of the AtTSPO N-terminus was assessed using the mouse homolog (mTSPO) as a negative control and carrier protein for the plant N-terminus. Each construct was N-terminally tagged with GFP.
(F) PM fractions were extracted from Arabidopsis suspension cells and PIP2;7 was quantified by western blot. PM proton ATPase (PMA) served as a reference protein for signal quantification. Individual measurements (PIP2;7 signal normalized against PMA signal from 6 to 9 replicates from two independent PM preparations) are shown, and the red horizontal lines define the means. Statistical significance was analyzed by t-tests (D) or one-way ANOVA followed by Tukey's tests (**p < 0.01; ***p < 0.001; ns, not significant) using Graphpad Prism.
Modification of PIP2;7 Abundance at the PM by AtTSPO Requires the Plant-Specific N-Terminal Extension
Because overexpression of AtTSPO drastically reduces the abundance of PIP2;7 at the PM (Hachez et al., 2014), we tested whether the N-terminal extension is required for depletion. We prepared PM-enriched fractions (Figure S4) from Arabidopsis suspension cell lines stably expressing green fluorescent protein (GFP)-tagged full-length AtTSPO and AtTSPOΔN, and assessed the relative abundance of PIP2;7 by immunoblotting. Consistent with prior findings, the PM fraction from positive control cells expressing full-length AtTSPO exhibited significantly lower amounts of PIP2;7 than did WT cells (Figure 2F). However, no reduction in PIP2;7 levels was seen in AtTSPOΔN-expressing cells compared with WT cells, suggesting the N-terminal domain of AtTSPO is required for PIP2;7 depletion.
Because the N-terminal extension is unique to plant TSPO, we investigated species specificity by introducing the plant-specific N-terminal extension to the mouse TSPO homolog (mTSPO) by generating a chimeric AtTSPONter-mTSPO construct (Figure 2E). As AtTSPO constructs, the chimeric construct also localized within Golgi membranes in plant cells, but unlike the unaltered mTSPO construct that did not decrease PIP2;7 levels, expression of the chimeric construct did diminish PIP2;7 in the PM. However, the efficiency of the reduction (∼30%) was lower than that seen with full-length AtTSPO (∼60%; Figure 2F), indicating some functional specificity of the N-terminal extension in plants, but also some specificity of the transmembrane domain of AtTSPO vs. mTSPO. We concluded that TSPO-dependent PIP2;7 depletion from the PM requires the plant-specific polybasic N-terminal extension.
AtTSPO Binds Defined Signaling Lipids In Vitro via Its Positively Charged N-terminus
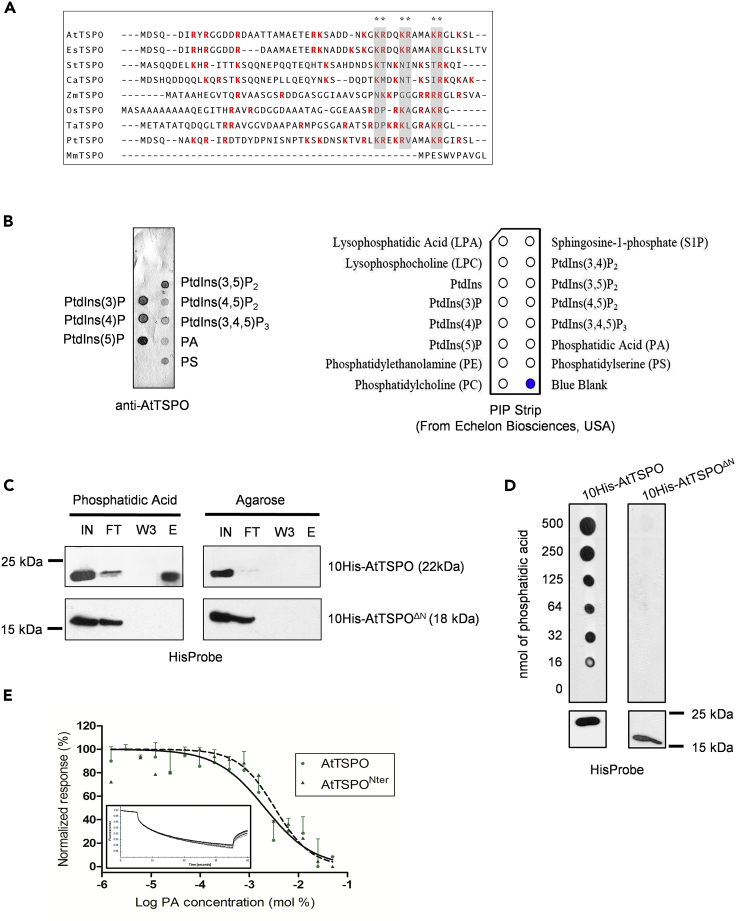
The N-terminal extension in higher plant TSPO is rich in arginines and lysines, despite low overall amino acid conservation (Figure 3A). Because positively charged regions may facilitate electrostatic interactions with negatively charged molecules such as anionic lipids (Zheng et al., 2002, Kooijman et al., 2007, Hammond et al., 2012, Holthuis and Menon, 2014, Heilmann, 2016), we hypothesized that AtTSPO-mediated regulation of PIP2;7 levels in the PM may involve interaction between the N-terminal extension of AtTSPO and membrane lipids. We therefore performed lipid overlays using purified full-length AtTSPO and a commercially available membrane spotted with anionic lipids (Figure 3B). Purified AtTSPOs bound all tested PIs except PI(3,4)P2, as well as phosphatidic acid (PA) and phosphatidylserine (PS; Figure 3B). To confirm the possible interactions with anionic lipids and the putative involvement of the positively charged N-terminal extension, we used PA as a representative anionic lipid for additional analyses in vitro. We used pull-down assays with immobilized PA (Figure 3C) and a membrane-spotted gradient of PA (Figure 3D) to show that purified AtTSPO binds PA in vitro. This interaction required the N-terminal extension, because AtTSPOΔN could not bind PA (Figure 3D). We then expressed and purified the N-terminal peptide (first 49 residues) of AtTSPO almost to homogeneity (Figure S5) and compared its capacity to bind PA with that of full-length AtTSPO using thermophoresis (Figure 3E). The N-terminal peptide (AtTSPONter) did bind PA, with a similar affinity to full-length AtTSPO (Kd = 2.1 mM for purified AtTSPO and 3.3 mM for the peptide; no statistical difference). These results suggest that the plant-specific N-terminal extension of AtTSPO is responsible for binding to defined anionic lipids in vitro, probably via electrostatic/hydrogen bond interactions (Kooijman et al., 2007).
Figure 3.
Purified AtTSPO Binds Defined Anionic Lipids In Vitro and Binding Requires the N-terminus
(A) The N-terminal extension of plant TSPO is positively charged, as shown by ClustalW alignment of the first 50 residues in higher plant (monocot and dicot) TSPO sequences. At, Arabidopsis thaliana; Es, Eutrema salsugineum; St, Solanum tuberosum; Ca, Capsicum annuum; Zm, Zea mays; Os, Oryza sativa; Ta; Triticum aestivum; Pt; Populus trichocarpa. Positively charged amino acids are red (neutral pH) and conserved lysine/arginine residues near the transmembrane domain are indicated by gray rectangles. The mouse (Mus musculus, Mm) TSPO lacks the plant conserved N-terminal extension.
(B) Initial screening of AtTSPO ligands yielded several candidate anionic lipids. AtTSPO purified from yeast was incubated with spotted lipids in PIP-strip overlay assays (right panel) and detected with anti-AtTSPO antibodies (left panel).
(C) The plant-specific N-terminal extension is involved in AtTSPO-anionic lipid interactions in vitro, as shown by lipid-dependent pull-down of AtTSPO. Purified AtTSPO or AtTSPOΔN[IN] were incubated with PA or agarose resin (negative control). Flow-through [FT], wash (last wash W3), and eluted [E] fractions were probed with HisProbe.
(D) Ten histidine-tagged and N-terminally truncated AtTSPOs expressed and solubilized from yeast microsomes were incubated with a gradient of phosphatidic acid concentrations and detected with HisProbe. The bottom panel shows control detection of both proteins.
(E) Thermophoresis analyses of full-length AtTSPO and its N-terminal peptide titrated against different phosphatidic acid concentrations. Protein samples were labeled with the red fluorescent dye NT-647-NHS, and non-linear fitting of labeled full-length protein (continuous line) yielded a Kd of 2.1 ± 0.4 mM, compared with a Kd of 3.3 ± 0.9 mM for the N-terminal peptide (dotted line). The rectangular insert is a representative fluorescence trace. For AtTSPO shown is the mean ± SD of three experiments and for AtTSPOΔN shown is a recorded value from one experiment.
Among PIs that bind to AtTSPO (Figure 3B), we were particularly interested in PI(4,5)P2, because its roles in ABA signaling, plant osmotic stress responses (Mishra et al., 2006, Lee et al., 2007), and autophagosome initiation and fusion with the lytic compartment (Tan et al., 2016, Baba et al., 2019) indicate possible mechanistic links to autophagic regulation of the stress-induced AtTSPO-PIP2;7 interaction (Hachez et al., 2014). In addition, enzymatic hydrolysis of PI(4,5)P2 at the plasma membrane can generate secondary signaling lipids such as DAG and PA, which can affect ABA responses (Mishra et al., 2006). Using thermophoresis, we checked whether the N-terminal peptide is required for AtTSPO PI interactions (Figure 4A). Titration of histidine-tagged full-length AtTSPO and truncated AtTSPOΔN against a range of PI(4,5)P2 concentrations revealed a decrease in fluorescence for full-length AtTSPO, indicating binding to PI(4,5)P2 (Figure 4A). By contrast, no binding was observed with AtTSPOΔN (Figure 4A).
Figure 4.
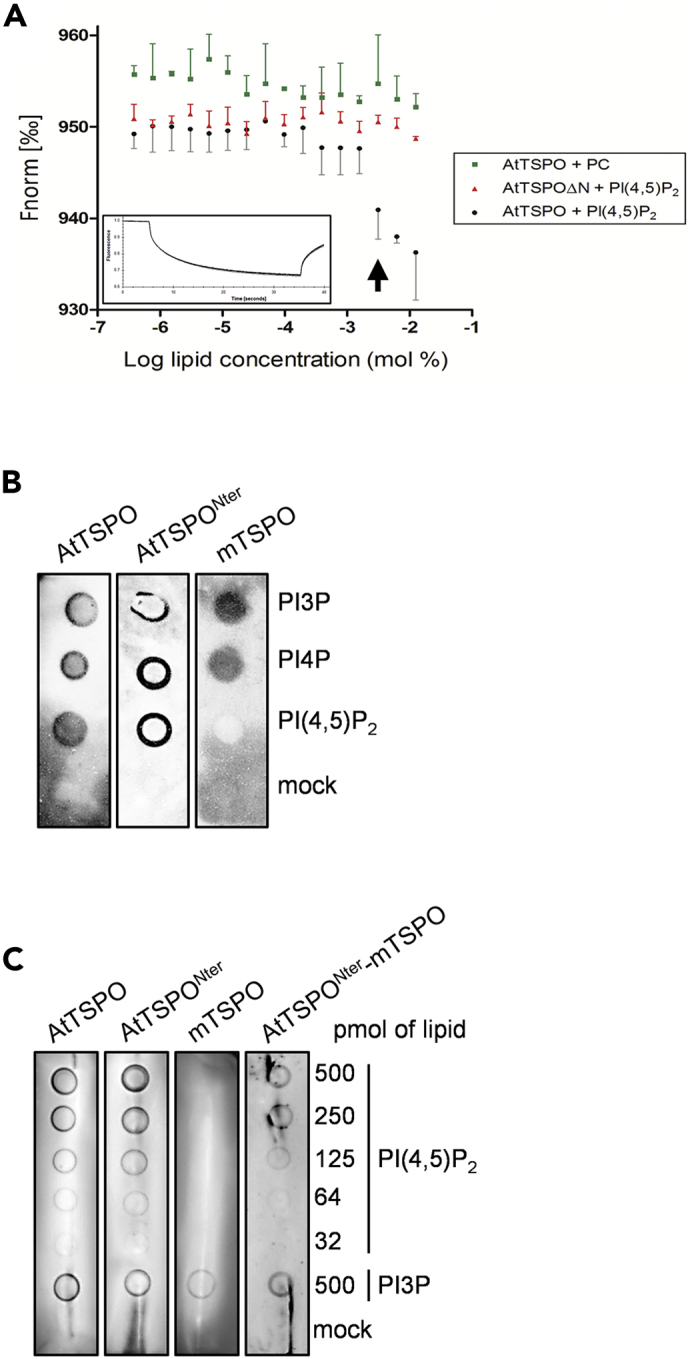
The AtTSPO N-terminal Peptide Binds PI(4,5)P2
(A) N-terminally truncated AtTSPO cannot bind PI(4,5)P2in vitro. Thermophoresis was performed by titrating full-length and N-terminally deleted TSPO against different PI(4,5)P2 concentrations. Protein samples were labeled at polyhistidine tags by RED-tris-NTA. Thermophoretic movement of labeled TSPO increases (normalized fluorescence decreases) upon binding PI(4,5)P2. Phosphatidylcholine served as a negative control lipid. Both datasets (AtTSPO + PI(4,5)P2 and AtTSPOΔN + PI(4,5)P2) were compared using two-way ANOVA followed by Bonferroni multiple comparison post-hoc tests. The threshold lipid concentration yielding statistically significant data (p <0.01) is 1.56 mM (arrow). The non-linear regression-based estimate of the Kd for the AtTSPO-PI(4,5)P2 interaction is 2.3 ± 0.7 mM. The rectangular insert is a representative fluorescence trace. For each sample shown is the mean ± SD of three experiments.
(B) Purified mouse TSPO homolog lacking the plant-specific N-terminus does not bind PI(4,5)P2in vitro, unlike the full-length plant protein and isolated N-terminal peptide. Five hundred pmol of phosphoinositide was spotted on a nitrocellulose membrane, incubated with purified proteins and detected with anti-AtTSPO (for isolated N-terminus) or HisProbe (for AtTSPO and mTSPO). A mock control of 2% n-dodecyl-β-D-maltoside (DDM) was included.
(C) Chimeric mouse TSPO fused to the AtTSPO N-terminus binds PI(4,5)P2in vitro. Purified proteins were incubated with different PI(4,5)P2 concentrations and detected using anti-AtTSPO antibodies (for isolated N-terminal peptide and N-terminus-fused mTSPO) and HisProbe (for AtTSPO and mTSPO). PI3P was spotted as a positive control for mTSPO binding. DDM (2%) served as a mock control. Experiments in (B) and (C) were performed at least twice.
Next, we checked whether the purified N-terminus of AtTSPO (AtTSPONter) alone was sufficient for interaction with PI(4,5)P2 (Figure 4B). Lipid overlay experiments showed that both full-length AtTSPO (consistent with previous observations) and its N-terminal peptide could bind PI3P, PI4P, PI5P, and PI(4,5)P2. By contrast, mTSPO did not bind PI(4,5)P2, suggesting that the binding determinant for PI(4,5)P2 is not within the membrane domain of TSPO that is conserved between species. The chimeric mouse TSPO protein (AtTSPONter-mTSPO) harboring the plant-specific N-terminal extension could bind PI(4,5)P2, albeit with reduced affinity compared with full-length AtTSPO or its N-terminal peptide alone (Figure 4C). We used PI3P as a positive control lipid for testing the binding activity of all purified protein variants. Together, these results demonstrate that the plant-specific N-terminus of AtTSPO is a bona fide determinant for binding to PI(4,5)P2 and other PIs.
AtTSPO-mediated Depletion of PI(4,5)P2 at the PM Requires Its N-terminal Extension
The subcellular distribution of plant PIs is membrane-specific (Kooijman et al., 2007, Hammond et al., 2012, Holthuis and Menon, 2014, Heilmann, 2016, Simon et al., 2014). Under normal growth conditions, PI(4,5)P2 is mainly localized at the PM in Arabidopsis (Simon et al., 2014), whereas AtTSPO occurs in the ER and mainly in Golgi apparatus (Guillaumot et al., 2009) (Figure S1). To identify where AtTSPO interacts with PI(4,5)P2 in cells, we used a plant-validated genetically encoded PI(4,5)P2-specific biosensor (Simon et al., 2014). We first generated stable transgenic Arabidopsis suspension cell lines expressing a double mCherry-tagged phospholipase C PI(4,5)P2-binding domain under the control of a mild constitutive promoter to limit possible interference with cell physiology. Using ABA treatment to mimic osmotic stress conducive to AtTSPO expression (Guillaumot et al., 2009, Vanhee et al., 2011a), we observed a partial redistribution of the PI(4,5)P2 biosensor from the PM to the cytosol, indicating depletion of the free lipid pool in the membrane or concomitant catabolism and/or reduced biosynthesis of PI(4,5)P2 after ABA treatment (Figure S8).
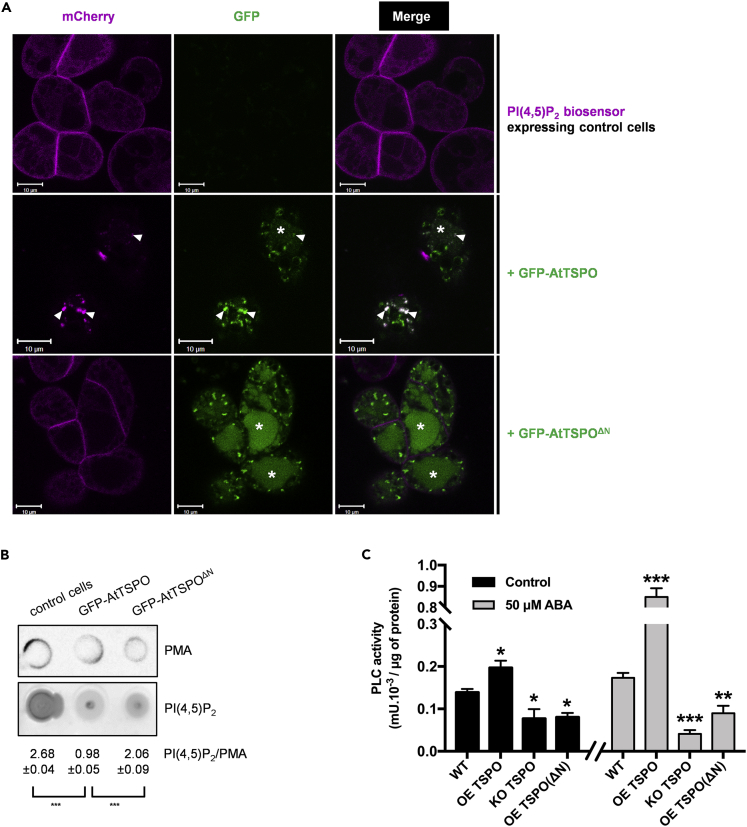
To investigate whether expression of AtTSPO modified the distribution of PI(4,5)P2 in cells, we retransformed PI(4,5)P2 biosensor-expressing cells to constitutively express GFP-tagged AtTSPO or AtTSPOΔN. Confocal imaging revealed localization of the biosensor at the cell periphery in the absence of AtTSPO, consistent with previous studies showing this construct mainly localized at the PM (Simon et al., 2014) (Figure 5A, upper panels, control cells). Notably, in cells coexpressing GFP-AtTSPO (Figure 5A, middle panels), little or no PI(4,5)P2 biosensor fluorescence was detected at the cell periphery, but strong fluorescence was seen in mobile punctate structures within cells, colocalized with GFP-AtTSPO in Golgi stacks, because GFP-AtTSPO is mainly localized in Golgi membranes (Guillaumot et al., 2009). By contrast, coexpressing the truncated form of AtTSPO (GFP-AtTSPOΔN), which also localizes to Golgi membranes, had no distinct effect on biosensor distribution (Figure 5A, bottom panels; see also Figure S2E). GFP fluorescence from both full-length and truncated AtTSPO was detected in cell vacuoles (asterisks), suggesting truncation of the plant-specific N-terminus did not preclude AtTSPO targeting to and degradation in the vacuole.
Figure 5.
PI(4,5)P2 Biosensor Was Depleted from the PM and Partially Colocalized with AtTSPO in Golgi Membranes in the Presence of Full-length AtTSPO but Not AtTSPOΔN
(A) Representative confocal images of Arabidopsis suspension cells stably coexpressing the PI(4,5)P2biosensor (mCherry-tagged double pleckstrin homology domain from phospholipase C; magenta) and full-length AtTSPO or AtTSPOΔN (GFP-tagged; green). Arrowheads indicate mCherry and GFP signals colocalizing at Golgi membranes. * indicates GFP fluorescence in the vacuole. Bars = 10 μm.
(B) Solubilized PM-enriched fractions from cells imaged as in (A) were spotted on a nitrocellulose membrane, and PI(4,5)P2 was detected using monoclonal anti-PI(4,5)P2 antibodies. For signal quantification (ImageJ 1.51 software) we used PM proton ATPase (PMA) as a control.
(C) Ten-day-old Arabidopsis seedlings grown on MS agar plates were incubated in liquid MS medium with or without 50 μM ABA for 24 h, and PLC activity was measured in triplicate using an EnzCheck direct phospholipase C assay kit. Statistical significance was analyzed by one-way ANOVA followed by Tukey's test (***p < 0.001; **p < 0.01; *p < 0.05). Data in (B) and (C) are means ± SD from three technical replicates, and all experiments were performed at least twice.
To ascertain whether expression of AtTSPO decreases PI(4,5)P2 levels at the PM, we prepared PM-enriched fractions from cell lines and performed dot blot experiments to measure relative PI(4,5)P2 content with antibody specific for this lipid (Yakir-Tamang and Gerst, 2009) (Figure 5B) and anti-H+-ATPase (PMA) to normalize the quantified signal. As expected, AtTSPO expression decreased PI(4,5)P2 levels by ∼ two-thirds, whereas PI(4,5)P2 was decreased by ∼23% when N-terminally truncated AtTSPO was expressed (Figure 5B). These results suggest that the plant-specific N-terminus is required for efficient AtTSPO-induced depletion of PI(4,5)P2 from the PM and its concomitant enrichment and colocalization with AtTSPO in Golgi membranes.
Because the biosynthesis of PI(4,5)P2 is catalyzed by PI4P 5-kinase at the PM via phosphorylation of PI4P (Kooijman et al., 2007, Ischebeck et al., 2008, Hammond et al., 2012, Holthuis and Menon, 2014, Heilmann, 2016), AtTSPO-induced accumulation of PI(4,5)P2 in Golgi membranes may be linked to enrichment of PI4P 5-kinase and relatively low levels of PI4P present in the Golgi compartment (Simon et al., 2014). We transiently expressed the mCherry-tagged human PI4P 5-kinase (mCherry-HsPIPK1α) fusion protein known to be active in plant cells (Ischebeck et al., 2008, Ischebeck et al., 2011) and compared its localization in WT tobacco and plants stably expressing YFP-AtTSPO. No difference in kinase subcellular localization was observed, suggesting that the trafficking of expressed mCherry-HsPIPK1α in the plant cell is not detectably affected by AtTSPO expression (Figure S9). Phosphoinositide-specific PLC (PI-PLC) and non-specific PLC (NPC) family lipases have been implicated in ABA signaling and salt and drought stress (Lee and Assmann, 1991, Hirayama et al., 1995, Sanchez and Chua, 2001, Hunt et al., 2003, Im et al., 2007, Peters et al., 2010), conditions conducive to AtTSPO induction. During receptor-regulated PLC signaling in animal cells, PI(4,5)P2 levels can drop rapidly at the PM (Peters et al., 2010.; Yen et al., 2018). We wondered whether expression of AtTSPO in plant seedlings with or without additional ABA treatment could modify PLC activity. As shown in Figure 5C (black histograms), control seedlings (without ABA treatment) overexpressing AtTSPO (OE TSPO) displayed ∼25% more PLC activity than WT seedlings. By comparison, seedlings lacking AtTSPO (KO TSPO) or overexpressing N-terminally truncated AtTSPO (OE TSPO[ΔN]) exhibited similar PLC activities (∼50% that of WT seedlings). Preincubation of seedlings in ABA marginally enhanced PLC activity in WT seedlings (∼20% compared with untreated samples) but dramatically increased activity in OE TSPO seedlings (∼4-fold), suggesting the effect of AtTSPO on PLC activity is enhanced by ABA signaling. Interestingly, ABA treatment had no effect on PLC activity in KO TSPO seedlings or transgenic seedlings overexpressing N-terminally truncated AtTSPO. These results suggest that PLC activity at the PM is modulated by ABA signaling and TSPO expression in plants, and TSPO-dependent enhancement of PLC activity requires the plant-specific N-terminal polybasic extension.
Lysine/Arginine Pairs in the N-terminal Extension of AtTSPO Are Required for Both PI(4,5)P2 Binding and Interaction with Aquaporin PIP2;7 In Vivo
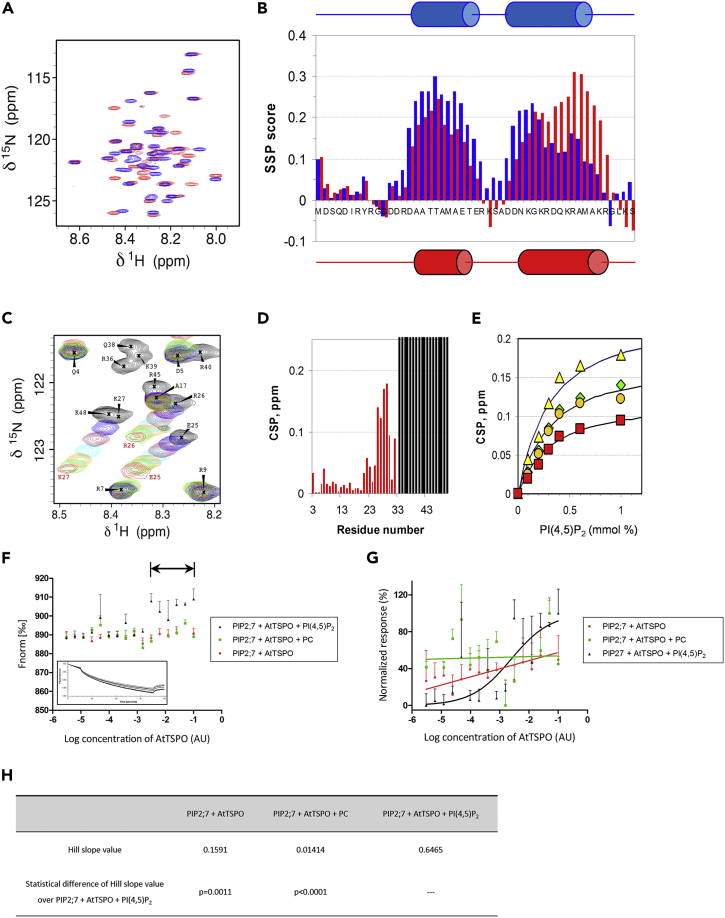
To identify key amino acids in the N-terminus of AtTSPO involved in interactions with PI(4,5)P2, we performed NMR analysis of the purified N-terminus (first 50 amino acids) labeled with 5N and 13C (Figure S5). The very good spectral quality of the purified N-terminal construct (Figure 6A) allowed effective recording of 2D and triple resonance 3D data, facilitating full 1H, 15N, and 13C assignments. Chemical shift analysis suggested the peptide was poorly structured and did not adopt a stable globular fold. Calculation of secondary structure propensity (SSP) (Marsh et al., 2006) revealed that two regions (R14-R26 and D31-K44) had a tendency to form α-helices (Figure 6B). The N-terminal peptide showed no interaction with calcium or zwitterionic lipids such as dimyristoylphosphatidylcholine (DMPC) bicelles, but it interacted with negatively charged dimyristoylphosphatidylglycerol bicelles, according to heteronuclear single quantum coherence spectroscopy (HSQC) spectra (Figure 6A), leading to increased stabilization of the C-terminal R36-R45 helical segment (Figure 6B). Titration of the peptide with PI(4,5)P2 micelles induced larger changes in 1H-15N HSQC spectra than did DMPG bicelles (Figures 6A and 6C), suggesting stronger interactions with PI(4,5)P2. H-15N cross-peaks of residues belonging to the 34–49 segment completely disappeared (Figure 6C), indicating a tight association between the C-terminal region of the peptide and PI(4,5)P2. Other residues along 20–32 segment exhibited a progressive chemical shift variation upon titration corresponding to fast exchange (Figures 6C and 6D). Monitoring these chemical shift perturbations during titration (Figure 6D) allowed estimation of a Kd in the range of 0.15 mM for the interaction between these residues and PI(4,5)P2.
Figure 6.
The Recombinant AtTSPO N-terminal Peptide Interacts with PI(4,5)P2 and This Anionic Lipid Is Required for AtTSPO Interaction with the Aquaporin PIP2;7 In Vitro
(A) 2D 1H-15N HSQC spectrum (500 MHz, 30°C) of 100 μM AtTSPONter in the absence (blue) and in the presence (red) of 25 mM DMPG/50 mM DHPC bicelles.
(B) Secondary Structure Propensity (SSP) score in the absence (blue) and in the presence (red) of DMPG/DHPC bicelles.
(C) Selected region in 2D 1H-15N HSQC showing different behaviors of residues upon titration of AtTSPONter with increasing amounts of PI(4,5)P2 (0 mM, black; 0.1 mM, blue; 0.2 mM, maroon; 0.3 mM, cyan; 0.4 mM, orange; 0.6 mM, green; 1 mM, red). Residues R36, Q38, K39, R40, and K48 disappear at the first addition of PI(4,5)P2, whereas residues E25, R26, and K27 show progressive chemical shift perturbations.
(D) 1H,15N Chemical Shift Perturbations (CSP) after addition of 1 mM PI(4,5)P2. Residues 34–49 that disappear at the first point of the titration are indicated by gray shading. CSPs were calculated as |Δδ(1H)| + |Δδ(15N)|/10.
(E) CSP of E26 (green diamonds), R27 (orange circles), K28 (yellow triangles), and A30 (red squares) as a function of PI(4,5)P2 concentration added.
(F) Thermophoresis analyses of YFP-PIP2;7 titrated against different AtTSPO concentrations. PIP2;7 movement was followed by YFP fluorescence. Either 50 μM PI(4,5)P2, phosphatidylcholine (PC, negative control), or no lipid was added. Thermophoretic motion of Venus-PIP2;7 decreases (normalized fluorescence increases) upon binding of AtTSPO in the presence of 50 μM PI(4,5)P2 but not PC, nor in the absence of lipids. The horizontal arrow indicates the bound state plateau region. The rectangular insert is a representative fluorescence trace. For each sample shown is the mean ± SD of three experiments.
(G) Data from (F) were normalized and fitted using non-linear regression, yielding a typical sigmoidal binding curve only in the presence of PI(4,5)P2.
(H) Statistical comparison of Hill slope values from (G). Low pvalues indicate no similarity in steepness between fitted curves.
To confirm that PI(4,5)P2 is required for AtTSPO-PIP2;7 interactions, we used thermophoresis to monitor the contribution of PI(4,5)P2 to the interaction in vitro (Figures 6F and 6G). We used a transgenic line expressing Venus-PIP2;7 (Hachez et al., 2014) to solubilize the protein from the microsomal fraction and titrated it against different AtTSPO concentrations in the presence and absence of PI(4,5)P2 in trans. As shown in Figures 6F and 6G, there was no interaction between AtTSPO and Venus-PIP2;7 in the absence of PI(4,5)P2 or in the presence of 50 μM phosphatidylcholine. By contrast, in the presence of 50 μM PI(4,5)P2, Venus-PIP2;7 fluorescence increased, generating a double plateau pattern, clearly indicating a protein-protein interaction. Indeed, a non-linear regression model for binding could only be fitted in the presence of PI(4,5)P2 (Figures 6G and 6H). Thus, AtTSPO-PIP2;7 interactions are modulated by PI(4,5)P2.
PI(4,5)P2 Mediates AtTSPO-PIP2;7 Interactions In Vivo
Analysis of the N-terminal extension of AtTSPO revealed several key amino acids potentially interacting with PI(4,5)P2, and the C-terminal region of the peptide was most affected upon interaction. Interestingly, this segment contains three positively charged lysine/arginine pairs—K35-R36, K39-R40, and K44-R45—that may contribute to the interaction (Figures 3A and 6C). To test whether interaction with PI(4,5)P2 is required for AtTSPO to interact with PIP2;7, we generated point substitutions of these residue pairs and tested binding strength to PIP2;7 by BiFC. Double substitution mutants AtTSPOK35A/R36A and AtTSPOK39A/R40A and quadruple mutant AtTSPOK39A/R40A/K44A/R45A (Figure 7) were generated. A Golgi-localized xylosyltransferase tagged with the monomeric variant of a cyan fluorescent protein (mTurquoise) was used as a transfection control (Gookin and Assmann, 2014) and for both colocalization and normalization of the BiFC signal (Figure S3). As expected, coexpression of WT AtTSPO and PIP2;7 generated a mainly Golgi-localized BiFC signal (Figure 7A, upper panels, arrowheads). Notably, all tested mutant combinations yielded substantially lower BiFC signals (Figure 7A), less than 20% that of the WT protein (Figure 7B). These results suggest that mutations affecting PI(4,5)P2 binding to the N-terminus of AtTSPO also reduced the efficiency of its interaction with aquaporin PIP2;7 in vivo.
Figure 7.
Specific Positively Charged Lysine-Arginine Pairs in the N-terminal Region of AtTSPO Mediate Interaction with PIP2;7 In Vivo
(A) Representative confocal images of tobacco epidermal cells transiently coexpressing VenusN-PIP2;7 and VenusC-AtTSPO or VenusN-PIP2;7 and VenusC-AtTSPO point mutants. Xylosyltransferase-mTurquoise fluorescent chimera (magenta) served as a cell transfection control (Golgi marker) and for signal quantification. Full-length PIP2;7 and AtTSPO served as a positive control for the BiFC signal. Low-magnification images qualitatively demonstrate the occurrence and distribution of BiFC (green) in the transfected area, and insets of high-magnification images show Golgi stacks (arrowheads). For PIP2;7-AtTSPOK35A/R36A, colocalization of the BiFC signal and Golgi marker was not detected. Bars = 20 μm and 5 μm for low and high magnification, respectively. Experiments were repeated three times.
(B) Signal quantification showing a drastic reduction in BIFC between PIP2;7 and AtTSPO mutants compared with full-length AtTSPO. Statistical analysis was based on one-way ANOVA followed by Tukey's tests (***p < 0.001). Bars represent means +/− SD. See transparent methods section for quantification procedure.
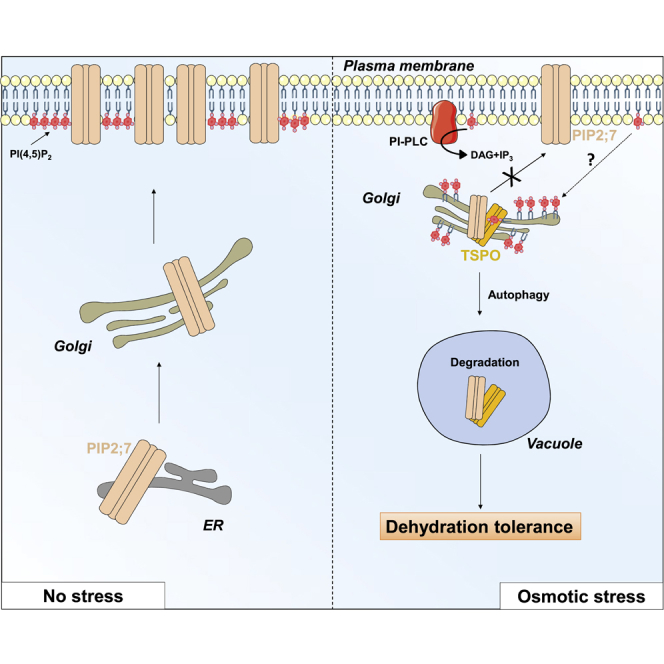
(C) Hypothetical model of PI(4,5)P2-dependent PIP2;7 downregulation by AtTSPO during stress. Under normal conditions (left), synthesized PiP2;7 is targeted to the PM through the biosynthetic secretory pathway. Under osmotic stress conditions (right), expressed AtTSPO is targeted to the ER and Golgi membranes.AtTSPO stimulates PLC activity, depleting PI(4,5)P2 at the PM and generating DAG and PA that modulate the activity of PP2C phosphatases involved in ABA-dependent reduction of water loss through stomata. Decreased PI(4,5)P2 at the PM prevents recruitment of PIP2;7-containing vesicles. Golgi/ER-localized AtTSPO binds PI(4,5)P2 synthesized de novo in these compartments, inducing structural changes needed for recognition and interaction with PIP2;7 en route to the PM, causing redirection to nascent autophagosomes. AtTSPO cannot directly deplete PI(4,5)P2 from the PM but may form a protein complex that recruits PIP5K to organelle/autophagosome initiation sites. AtTSPO-mediated enrichment of ER/Golgi membranes with PI(4,5)P2 may initiate autophagy. Autophagosomal membranes also recruit PIP5K that generates PI(4,5)P2 and the autophagy regulator Atg14/Barkor that interacts with both the enzyme and PI(4,5)P2. PI(4,5)P2 binding to Atg14 regulates its interaction with the Vps34 complex catalyzing PI3P synthesis at autophagosome initiation sites. The presence of the AtTSPO-PI(4,5)P2-PIP2;7 complex in ER/Golgi membranes and the phagophore containing PI(4,5)P2 and Atg8 may be close enough to allow lipid-protein and protein-protein interactions.
Discussion
Herein, we investigated the potential physiological role and evolutionary significance of the N-terminal extension in plant TSPO. We demonstrate that stress-induced plant TSPO protects plant tissues against water loss by depleting the PM of a major water channel and the signaling lipid PI(4,5)P2. These functions require the plant-specific polybasic N-terminus absent in bacterial and animal TSPO. We provide evidence suggesting a molecular link between Arabidopsis AtTSPO, PI(4,5)P2, and the highly expressed PM aquaporin PIP2;7 that underpins responses to dehydration. Additionally, the findings reveal cell-type-dependent functional evolution among TSPOs.
Involvement in stress responses appears to be a common feature among TSPOs across kingdoms (Vanhee et al., 2011a, Batoko et al., 2015, Li et al., 2016, Guilarte et al., 2016), but the molecular mechanism and cellular components are yet to be described in any cell type. Herein, we show that AtTSPO reduces aquaporin PIP2;7 levels at the cell surface to reduce water loss, and this function requires the plant-specific N-terminal extension of AtTSPO. Interestingly, expressing the mouse mitochondrial TSPO homolog (mTSPO) in plant cells did not affect PIP2;7 levels, but a chimeric version (AtTSPONter-mTSPO) containing the plant-specific N-terminus of AtTSPO did lower PIP2;7 abundance at the PM. Thus, acquisition of the conserved N-terminal extension through evolution has endowed plant TSPO with unique cellular responses and adaptation to osmotic stress.
The AtTSPO N-terminal extension binds the signaling lipid PI(4,5)P2 in vitro and in vivo, and this may be a prerequisite for physical and functional interactions with aquaporin PIP2;7. Cells expressing AtTSPO accumulated a PI(4,5)P2 biosensor at the Golgi membrane rather than the PM, as did control cells and cells expressing a truncated version of AtTSPO devoid of its N-terminal extension. Thus, in the presence of AtTSPO, Golgi membranes become enriched in free PI(4,5)P2, possibly at the expense of the PM. We did not find any significant difference in overall PM levels between cells expressing AtTSPO and control cells (Figure S11), but PI(4,5)P2 depletion was specifically observed in PM fractions from cells expressing AtTSPO compared with controls and cells expressing N-terminally truncated AtTSPO (Figure 5B). This suggest that expression of AtTSPO has a target effect on PM PI(4,5)P2 levels. Phospholipid transport proteins function at organelle contact sites (Wang et al., 2014, Wang et al., 2019, Yen et al., 2018). Transient apposition between organelles can alter PI levels by presenting membrane-bound phosphatases and/or phosphoinositide-specific transporters to their respective substrates (Wang et al., 2019). Recent studies identified proteins mediating PI(4,5)P2 removal from the PM of eukaryotic cells (Cockcroft and Raghu, 2018, Wang et al., 2019), but whether AtTSPO is enriched in organelle contact sites and/or whether it transports lipid molecules remains unknown. Biosensor experiments demonstrated a substantial amount of free PI4P in the PM of plant cells expressing AtTSPO, suggesting PI(4,5)P2 depletion in the presence of AtTSPO may reflect mislocalization or inhibition of PM-bound PI4P5K enzyme activity. However, AtTSPO did not affect subcellular localization of a functional heterologously expressed PI4P5K (Figure S9). We cannot exclude the possibility that endogenous plant PIP5K may act differently and AtTSPO may affect its localization resulting in PI(4,5)P2 remodeling. So far, we have no evidence suggesting that AtTSPO is involved directly in the translocation of any of its lipid ligand and whether any of these interactions could modulate the structure-function of AtTSPO. We favor the possibility that expression of AtTSPO induces the enrichment of PI(4,5)P2 in the Golgi membrane by enhancing the enzymatic phosphorylation of its precursor PI4P within this lipid bilayer.
Phosphatase-mediated hydrolysis of PI(4,5)P2 generates second messengers such as DAG and PA involved in ABA signaling. Expression of AtTSPO stimulates PLC activity. Because the kit used to measure this activity does not specifically report on PI-PLC, it may be that expression of AtTSPO enhances PLC in general, including NPC activity. It was shown that overexpression of NPC4 renders plants more sensitive to ABA and more tolerant to hyperosmotic stress than WT plants (Peters et al., 2010). NPC-produced DAG is converted to PA, and its derived lipids positively modulate ABA response and promote plant tolerance to drought and salt stresses. Interestingly, the ABA-dependent increase in PLC activity requires full-length AtTSPO (Figure 5C), suggesting that ABA may alter PM levels of PI(4,5)P2 via plant TSPO. Both PI-PLC and NPC promote responses to ABA and tolerance to hyperosmotic stress, but there is no evidence of protein kinase C enzymes or inositol triphosphate (IP3) receptors in plants. PLC-dependent signaling is essentially mediated by PA generated from DAG by DAG kinase (Hong et al., 2016). PA effector proteins in plants include ABI1, an ABA co-receptor protein and negative regulator of this stress hormone-signalling pathway, and the Gα subunit of heterotrimeric G protein. This relates to opening of stomata and water loss, because binding to ABI1 promotes stomatal closure and Gα binding to PA mediates ABA inhibition of stomatal opening (Mishra et al., 2006). Interestingly, stress- and ABA-dependent induction of AtTSPO is highest in guard cells. Circumstantial evidence (Ma et al., 2015) suggests a positive correlation between PI(4,5)P2 abundance in the PM, increased active aquaporins, and increased osmotic water permeability. Consistently, AtTSPO expression reduced levels of both PI(4,5)P2 and PIP2;7 at the PM and reduced water loss in transgenic plants expressing AtTSPO or its stabilized point mutant AtTSPOH91A, compared with WT plants. It is not yet known whether PI(4,5)P2 directly or indirectly modulates the structure-function properties of PM aquaporins, although other channels responding to osmotic stress are regulated by PI(4,5)P2 (Liu et al., 2005).
Free PI(4,5)P2 is normally found in the PM, as demonstrated by cognate biosensor subcellular localization and biochemical analyses (Simon et al., 2014). Whether PI(4,5)P2 signaling occurs in intracellular compartments of eukaryotic cells remains contentious, although evidence supports specific and essential functions for intracellular PI(4,5)P2 (Tan et al., 2015). Levels of PI(4,5)P2 in intracellular compartments are low, presumably due to lack of free PI(4,5)P2, which is bound to effectors associated with biosynthetic kinases (PI4P5Ks). The present work suggests that AtTSPO expression triggers accumulation of free PI(4,5)P2 at the Golgi membrane, but exactly how this intracellular PI(4,5)P2 signaling platform is generated remains unclear. The effect of expressed AtTSPO on the relative level of PIP2;7 at the PM could indirectly also affect the level of PI(4,5)P2 in addition to enhancing PLC activity. It may be that induction of AtTSPO at Golgi/ER binds PIP2;7 and prevents it from reaching the PM. The lack of the aquaporin at the PM may affect PI(4,5)2 levels along with other effects of the osmotic stress on PLC or PIP5K activity.
Generation of PI(4,5)P2 requires recruitment of PIP kinases, particularly PI4P5Ks, to the target membrane, typically via the same effectors that bind PI(4,5)P2. In addition to PI3P and PI5P, PI(4,5)P2 contributes to initiation, trafficking of membrane precursors, and fusion of autophagosomes with the lytic compartment (Tan et al., 2016). Nucleation of the autophagosome initiation membrane requires the vacuolar protein sorting (VPS) 34 complex, which generates PI3P. The VPS34 complex is recruited to autophagosome initiation sites by ATG14/Barkor, which binds PI3P via a Barkor/ATG14 autophagosome-targeting sequence (BATS) domain, and this domain also binds PI4P5K and PI(4,5)P2 (Tan et al., 2016). PI(4,5)P2 signaling at autophagosome initiation membranes could facilitate targeting of the AtTSPO containing complex to this site. Binding of PI(4,5)P2 was not required for autophagy-dependent degradation of AtTSPO, because the N-terminally truncated protein cannot bind PI(4,5)P2 but was still translocated to the vacuole (Figure 5A). However, binding of PI(4,5)P2 is necessary for AtTSPO to interact with aquaporin PIP2;7. This interaction could occur because autophagosomes are initiated near AtTSPO-containing organelles, allowing the cytosolic N-terminus of AtTSPO to contact the autophagosome initiation membrane enriched in PI(4,5)P2 and PI3P (Figure 7C). How exactly the AtTSPO-containing complex is extracted and translocated to the autophagosomal membrane remains unknown.
Structural analysis suggests that lipid binding by the N-terminal extension of AtTSPO may induce protein conformational changes. Interaction with anionic lipids stabilized helical structure within the 34–49 segment, close to the first transmembrane region of AtTSPO. Conformational changes induced by PI(4,5)P2 binding may therefore expose residues that interact with PIP2;7 within the lipid bilayer. The 34–49 segment contains key arginine/lysine residues involved in PI(4,5)P2 binding in vitro, and their mutation dramatically decreased AtTSPO interaction with PIP2;7 in vivo.
In conclusion, in response to osmotic stress, Arabidopsis TSPO is induced at the Golgi membrane, triggering depletion of PI(4,5)P2 at the PM and its subsequent enrichment at the Golgi membrane. Moreover, AtTSPO binds PI(4,5)P2 via its polybasic plant-specific N-terminus as a requirement for its interaction with the PM aquaporin PIP2;7. Given its involvement in key cellular processes at the PM, mechanisms controlling the localization and/or abundance of the multifunctional PI(4,5)P2 at the PM are of great interest for understanding cellular signaling and its implications. This work demonstrates how stress causes PI(4,5)P2 remodeling through expression of plant TSPO.
This underlying molecular mechanism illuminates functional divergence among TSPOs; the plant-specific N-terminal extension rich in polybasic residues determines the involvement of TSPO in osmotic stress responses.
Limitations of the Study
We found that both AtTSPO and the mouse TSPO bind PI3P in vitro. However, it is not clear yet whether this observation could be extended to bacterial TSPO, in which case it would be interesting to investigate if this has any conserved physiological meaning in vivo. The role of AtTSPO in osmotic response is intriguing and could be analyzed further in a cell-type-dependent manner, for example by expressing the relatively stable mutant variant of AtTSPO in the guard cells. It will be equally interesting to know if there is difference in the composition of the lipid at the PM vs the punctate structures enriched in AtTSPO or if different PIP5Ks are activated at this location.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank M. Boutry (LIBST, UCLouvain, Belgium) for sharing purified TEV enzyme and anti-PMA antibodies, M. Ghislain (LIBST, UCLouvain, Belgium) for anti-FLAG antibodies, K. Moonens (VIB, Center for Structural Biology, VUB, Belgium) for assistance with MST, D. Masquelier for preparing AtTSPONter-mTSPO, and A. Jurkiewicz, J. Nader, and M.C. Eloy for technical assistance. We acknowledge Y. Jaillais (Laboratoire Reproduction et Développement des Plantes, ENS Lyon, France), T. Munnik (Swammerdam Institute for Life Sciences, University of Amsterdam, The Netherlands), S. Assmann (Department of Biology, Pennsylvania State University, USA), and I. Heilmann (Institute for Biochemistry and Biotechnology, Martin-Luther-University Halle-Wittenberg) for providing reagents and genetic materials. Transgenic Arabidopsis plants expressing mVenus-AtTSPOΔN were generated by S. Guillon (UCLouvain). SL received a PhD fellowship from the Ministère de la Recherche Française. PJ received a PhD fellowship from UCLouvain-FSR. HA received a PhD fellowship from UCLouvain-ADRI. This work was partly funded by the Wallonia-Brussels Federation Joint Research Action (ARCgrant#11/16-036 to H.B.), and the Belgian Funds for Scientific Research (FNRS; CR grant#19516174 and PDRgrant# 6794930 and T.0050.18 to H.B.). H.B. is a senior research associate of FRS-FNRS.
Author Contributions
Conceptualization: O.L, J.-J.L, and H.B.; Methodology and Investigation, P.J., L.S., H.A., O.L., J.-J.L., and H.B.; Writing—Original Draft, P.J., J.-J.L., and H.B.; Review & Editing, P.J., O.L., J.-J.L., and H.B.; Funding Acquisition J.-J.L. and H.B.; Supervision, J.-J.L. and H.B.
Declaration of Interests
The authors have no conflict of interest to declare.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100889.
Supplemental Information
Figure numbering in this section corresponds to the numbering used in the article. The dataset relates to main Figures 1–5 and S2–S8.
References
- Augustine J.J., Bodziak K.A., Hricik D.E. Use of sirolimus in solid organ transplantation. Drugs. 2007;67:369–391. doi: 10.2165/00003495-200767030-00004. [DOI] [PubMed] [Google Scholar]
- Baba T., Toth D.J., Sengupta N., Kim Y.J., Balla T. Phosphatidylinositol 4,5bisphosphate controls Rab7 and PLEKMH1 membrane cycling during autophagosome lysosome fusion. EMBO J. 2019;38:e102837. doi: 10.15252/embj.2018100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae K.R., Shim H.J., Balu D., Kim S.R., Yu S.W. Translocator protein 18 kDa negatively regulates inflammation in microglia. J. Neuroimmune Pharmacol. 2014;9:424–437. doi: 10.1007/s11481-014-9540-6. [DOI] [PubMed] [Google Scholar]
- Banati R.B., Middleton R.J., Chan R., Hatty C.R., Kam W.W., Quin C., Graeber M.B., Parmar A., Zahra D., Callaghan P. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat. Commun. 2014;5:5452. doi: 10.1038/ncomms6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batoko H., Veljanovski V., Jurkiewicz P. Enigmatic Translocator protein (TSPO) and cellular stress regulation. Trends Biochem. Sci. 2015;40:497–503. doi: 10.1016/j.tibs.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Braestrup C., Squires R.F. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc. Natl. Acad. Sci. U S A. 1977;74:3805–3809. doi: 10.1073/pnas.74.9.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Raghu P. Phospholipid transport protein function at organelle contact sites. Curr. Opin. Cell Biol. 2018;53:52–60. doi: 10.1016/j.ceb.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S.R., Rodriguez P.L., Finkelstein R.R., Abrams S.R. Abscisic acid: emergence of a core signaling network. Annu. Rev. Plant Biol. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- Davey M.E., de Bruijn F.J. A homologue of the tryptophan-rich sensory protein TspO and FixL regulate a novel nutrient deprivation-induced Sinorhizobium meliloti locus. Appl. Environ.Microbiol. 2000;66:5353–5359. doi: 10.1128/aem.66.12.5353-5359.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A., Fujimoto A., Sato S., Uno T., Kanda Y., Asami K., Tanaka Y., Kita A., Satoh R., Sugiura R. Chemical genomics approach to identify genes associated with sensitivity to rapamycin in the fission yeast Schizosaccharomyces pombe. Genes Cells. 2015;20:292309. doi: 10.1111/gtc.12223. [DOI] [PubMed] [Google Scholar]
- Fan J., Lindemann P., Feuilloley M.G., Papadopoulos V. Structural and functional evolution of the translocator protein (18 kDa) Curr. Mol. Med. 2012;12:369–386. doi: 10.2174/1566524011207040369. [DOI] [PubMed] [Google Scholar]
- Gatliff J., East D., Crosby J., Abeti R., Harvey R., Craigen W., Parker P., Campanella M. TSPO interacts with VDAC1 and triggers a ROS-mediated inhibition of mitochondrial quality control. Autophagy. 2014;10:2279–2296. doi: 10.4161/15548627.2014.991665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatliff J., East D.A., Singh A., Alvarez M.S., Frison M., Matic I., Ferraina C., Sampson N., Turkheimer F., Campanella M. A role for TSPO in mitochondrial Ca. Cell Death Dis. 2017;8:e2896. doi: 10.1038/cddis.2017.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gookin T.E., Assmann S.M. Significant reduction of BiFC non-specific assembly facilitates in planta assessment of heterotrimeric G-protein interactors. Plant J. 2014;80:553–567. doi: 10.1111/tpj.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte T.R., Loth M.K., Guariglia S.R. TSPO finds NOX2 in microglia for redox homeostasis. Trends Pharmacol. Sci. 2016;37:334–343. doi: 10.1016/j.tips.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaumot D., Guillon S., Déplanque T., Vanhee C., Gumy C., Masquelier D., Morsomme P., Batoko H. The Arabidopsis TSPO-related protein is a stress and abscisic acidregulated, endoplasmic reticulum-Golgi-localized membrane protein. Plant J. 2009;60:242–256. doi: 10.1111/j.1365-313X.2009.03950.x. [DOI] [PubMed] [Google Scholar]
- Guo Y., Kalathur R.C., Liu Q., Kloss B., Bruni R., Ginter C., Kloppmann E., Rost B., Hendrickson W.A. Protein structure.Structure and activity of tryptophan-rich TSPO proteins. Science. 2015;347:551–555. doi: 10.1126/science.aaa1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C., Veljanovski V., Reinhardt H., Guillaumot D., Vanhee C., Chaumont F., Batoko H. The Arabidopsis abiotic stress-induced TSPO-related protein reduces cellsurface expression of the aquaporin PIP2;7 through protein-protein interactions and autophagic degradation. Plant Cell. 2014;26:4974–4990. doi: 10.1105/tpc.114.134080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G.R., Fischer M.J., Anderson K.E., Holdich J., Koteci A., Balla T., Irvine R.F. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science. 2012;337:727–730. doi: 10.1126/science.1222483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann I. Phosphoinositide signaling in plant development. Development. 2016;143:20442055. doi: 10.1242/dev.136432. [DOI] [PubMed] [Google Scholar]
- Hetherington A.M., Brownlee C. The generation of Ca(2+) signals in plants. Annu. Rev. Plant Biol. 2004;55:401–427. doi: 10.1146/annurev.arplant.55.031903.141624. [DOI] [PubMed] [Google Scholar]
- Hirayama T., Ohto C., Mizoguchi T., Shinozaki K. A gene encoding a phosphatidylinositol-specific phospholipase C is induced by dehydration and salt stress in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U S A. 1995;92:3903–3907. doi: 10.1073/pnas.92.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis J.C., Menon A.K. Lipid landscapes and pipelines in membrane homeostasis. Nature. 2014;510:48–57. doi: 10.1038/nature13474. [DOI] [PubMed] [Google Scholar]
- Hong S.H., Choi H.B., Kim S.U., McLarnon J.G. Mitochondrial ligand inhibits store-operated calcium influx and COX-2 production in human microglia. J. Neurosci. Res. 2006;83:1293–1298. doi: 10.1002/jnr.20829. [DOI] [PubMed] [Google Scholar]
- Hong Y., Zhao J., Guo L., Kim S.C., Deng X., Wang G., Zhang G., Li M., Wang X. Plant phospholipases D and C and their diverse functions in stress responses. Prog. Lipid Res. 2016;62:55–74. doi: 10.1016/j.plipres.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Hunt L., Mills L.N., Pical C., Leckie C.P., Aitken F.L., Kopka J., Mueller-Roeber B., McAinsh M.R., Hetherington A.M., Gray J.E. Phospholipase C is required for the control of stomatal aperture by ABA. Plant J. 2003;34:47–55. doi: 10.1046/j.1365-313x.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- Im Y.J., Perera I.Y., Brglez I., Davis A.J., Stevenson-Paulik J., Phillippy B.Q., Johannes E., Allen N.S., Boss W.F. Increasing plasma membrane phosphatidylinositol(4,5)bisphosphate biosynthesis increases phosphoinositide metabolism in Nicotiana tabacum. Plant Cell. 2007;19:1603–1616. doi: 10.1105/tpc.107.051367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischebeck T., Stenzel I., Heilmann I. Type B phosphatidylinositol-4-phosphate 5-kinases mediate Arabidopsis and Nicotiana tabacum pollen tube growth by regulating apical pectin secretion. Plant Cell. 2008;20:3312–3330. doi: 10.1105/tpc.108.059568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischebeck T., Stenzel I., Hempel F., Jin X., Mosblech A., Heilmann I. Phosphatidylinositol-4,5-bisphosphate influences Nt-Rac5-mediated cell expansion in pollen tubes of Nicotiana tabacum. Plant J. 2011;65:453–468. doi: 10.1111/j.1365-313X.2010.04435.x. [DOI] [PubMed] [Google Scholar]
- Jaremko L., Jaremko M., Giller K., Becker S., Zweckstetter M. Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science. 2014;343:13631366. doi: 10.1126/science.1248725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman E.E., Tieleman D.P., Testerink C., Munnik T., Rijkers D.T., Burger K.N., de Kruijff B. An electrostatic/hydrogen bond switch as the basis for the specific interaction of phosphatidic acid with proteins. J. Biol. Chem. 2007;282:11356–11364. doi: 10.1074/jbc.M609737200. [DOI] [PubMed] [Google Scholar]
- Kreps J.A., Wu Y., Chang H.S., Zhu T., Wang X., Harper J.F. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 2002;130:21292141. doi: 10.1104/pp.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavisse S., Guillermier M., Hérard A.S., Petit F., Delahaye M., Van Camp N., Ben Haim L., Lebon V., Remy P., Dollé F. Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J. Neurosci. 2012;32:10809–10818. doi: 10.1523/JNEUROSCI.1487-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Assmann S.M. Diacylglycerols induce both ion pumping in patch-clamped guard-cell protoplasts and opening of intact stomata. Proc. Natl. Acad. Sci. U S A. 1991;88:2127–2131. doi: 10.1073/pnas.88.6.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Kim Y.W., Jeon B.W., Park K.Y., Suh S.J., Seo J., Kwak J.M., Martinoia E., Hwang I. Phosphatidylinositol 4,5-bisphosphate is important for stomatal opening. Plant J. 2007;52:803–816. doi: 10.1111/j.1365-313X.2007.03277.x. [DOI] [PubMed] [Google Scholar]
- Li F., Liu J., Liu N., Kuhn L.A., Garavito R.M., Ferguson-Miller S. Translocator protein 18 kDa (TSPO): an old protein with new functions? Biochemistry. 2016;55:2821–2831. doi: 10.1021/acs.biochem.6b00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Liu J., Zheng Y., Garavito R.M., Ferguson-Miller S. Protein structure. Crystal structures of translocator protein (TSPO) and mutant mimic of a human polymorphism. Science. 2015;347:555–558. doi: 10.1126/science.1260590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Angelin A., Da Settimo F., Martini C., Taliani S., Zhu S., Wallace D.C. Genetic analysis of dTSPO, an outer mitochondrial membrane protein, reveals its functions in apoptosis, longevity, and Ab42-induced neurodegeneration. Aging Cell. 2014;13:507–518. doi: 10.1111/acel.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Li L., Luan S. An essential function of phosphatidylinositol phosphates in activation of plant shaker-type K+ channels. Plant J. 2005;42:433–443. doi: 10.1111/j.1365-313X.2005.02384.x. [DOI] [PubMed] [Google Scholar]
- Liu L., Zhang K., Sandoval H., Yamamoto S., Jaiswal M., Sanz E., Li Z., Hui J., Graham B.H., Quintana A. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015;160:177–190. doi: 10.1016/j.cell.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Shatil-Cohen A., Ben-Dor S., Wigoda N., Perera I.Y., Im Y.J., Diminshtein S., Yu L., Boss W.F., Moshelion M. Do phosphoinositides regulate membrane water permeability of tobacco protoplasts by enhancing the aquaporin pathway? Planta. 2015;241:741–755. doi: 10.1007/s00425-014-2216-x. [DOI] [PubMed] [Google Scholar]
- Marginedas-Freixa I., Hattab C., Bouyer G., Halle F., Chene A., Lefevre S.D., Cambot M., Cueff A., Schmitt M., Gamain B. TSPO ligands stimulate ZnPPIX transport and ROS accumulation leading to the inhibition of P. falciparum growth in human blood. Sci. Rep. 2016;6:33516. doi: 10.1038/srep33516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh J.A., Singh V.K., Jia Z., Forman-Kay J.D. Sensitivity of secondary structure propensities to sequence differences between alpha- and gamma-synuclein: implications for fibrillation. Protein Sci. 2006;15:2795–2804. doi: 10.1110/ps.062465306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra G., Zhang W., Deng F., Zhao J., Wang X. A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science. 2006;312:264–266. doi: 10.1126/science.1123769. [DOI] [PubMed] [Google Scholar]
- Morin D., Musman J., Pons S., Berdeaux A., Ghaleh B. Mitochondrial translocator protein (TSPO): from physiology to cardioprotection. Biochem. Pharmacol. 2016;105:1–13. doi: 10.1016/j.bcp.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Munnik T., Nielsen E. Green light for polyphosphoinositide signals in plants. Curr. Opin. Plant Biol. 2011;14:489–497. doi: 10.1016/j.pbi.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Nakashima A., Sato T., Tamanoi F. Fission yeast TORC1 regulates phosphorylation of ribosomal S6 proteins in response to nutrients and its activity is inhibited by rapamycin. J. Cell Sci. 2010;123:777–786. doi: 10.1242/jcs.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Zoghbi S.S., Hong J., Verma A., Pike V.W., Innis R.B., Fujita M. In vivo binding of protoporphyrin IX to rat translocator protein imaged with positron emission tomography. Synapse. 2010;64:649–653. doi: 10.1002/syn.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V., Baraldi M., Guilarte T.R., Knudsen T.B., Lacapère J.J., Lindemann P., Norenberg M.D., Nutt D., Weizman A., Zhang M.R. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Peters C., Li M., Narasimhan R., Roth M., Welti R., Wang X. Nonspecific phospholipase C NPC4 promotes responses to abscisic acid and tolerance to hyperosmotic stress in Arabidopsis. Plant Cell. 2010;22:2642–2659. doi: 10.1105/tpc.109.071720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R., Papadopoulos V., Rammes G., Baghai T.C., Fan J., Akula N., Groyer G., Adams D., Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010;9:971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- Sancak Y., Bar-Peled L., Zoncu R., Markhard A.L., Nada S., Sabatini D.M. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J.P., Chua N.H. Arabidopsis PLC1 is required for secondary responses to abscisic acid signals. Plant Cell. 2001;13:1143–1154. doi: 10.1105/tpc.13.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J.I., Kwak J.M., Allen G.J. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature. 2001;410:327–330. doi: 10.1038/35066500. [DOI] [PubMed] [Google Scholar]
- Seki M., Narusaka M., Ishida J., Nanjo T., Fujita M., Oono Y., Kamiya A., Nakajima M., Enju A., Sakurai T. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J. 2002;31:279–292. doi: 10.1046/j.1365-313x.2002.01359.x. [DOI] [PubMed] [Google Scholar]
- Simon M.L., Platre M.P., Assil S., van Wijk R., Chen W.Y., Chory J., Dreux M., Munnik T., Jaillais Y. A multi-colour/multi-affinity marker set to visualize phosphoinositide dynamics in Arabidopsis. Plant J. 2014;77:322–337. doi: 10.1111/tpj.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara T., Maeda T. Evolutionarily conserved regulation of TOR signalling. J. Biochem. 2013;154:1–10. doi: 10.1093/jb/mvt047. [DOI] [PubMed] [Google Scholar]
- Tan X., Thapa N., Choi S., Anderson R.A. Emerging roles of PtdIns(4,5)P2-beyond the plasma membrane. J. Cell Sci. 2015;128:4047–4056. doi: 10.1242/jcs.175208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X., Thapa N., Liao Y., Choi S., Anderson R.A. PtdIns(4,5)P2 signaling regulates ATG14 and autophagy. Proc. Natl. Acad. Sci. U S A. 2016;113:10896–10901. doi: 10.1073/pnas.1523145113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testerink C., Munnik T. Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci. 2005;10:368–375. doi: 10.1016/j.tplants.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Thoreen C.C., Kang S.A., Chang J.W., Liu Q., Zhang J., Gao Y., Reichling L.J., Sim T., Sabatini D.M., Gray N.S. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhee C., Guillon S., Masquelier D., Degand H., Deleu M., Morsomme P., Batoko H. A TSPO-related protein localizes to the early secretory pathway in Arabidopsis, but is targeted to mitochondria when expressed in yeast. J. Exp. Bot. 2011;62:497–508. doi: 10.1093/jxb/erq283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhee C., Zapotoczny G., Masquelier D., Ghislain M., Batoko H. The Arabidopsis multistress regulator TSPO is a heme binding membrane protein and a potential scavenger of porphyrins via an autophagy-dependent degradation mechanism. Plant Cell. 2011;23:785–805. doi: 10.1105/tpc.110.081570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A., Nye J.S., Snyder S.H. Porphyrins are endogenous ligands for the mitochondrial (peripheral-type) benzodiazepine receptor. Proc. Natl. Acad. Sci. U S A. 1987;84:22562260. doi: 10.1073/pnas.84.8.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Hawkins T.J., Richardson C., Cummins I., Deeks M.J., Sparkes I., Hawes C., Hussey P.J. The plant cytoskeleton, NET3C, and VAP27 mediate the link between the plasma membrane and endoplasmic reticulum. Curr. Biol. 2014;24:1397–1405. doi: 10.1016/j.cub.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Wang H., Ma Q., Qi Y., Dong J., Du X., Rae J., Wang J., Wu W.F., Brown A.J., Parton R.G. ORP2 delivers cholesterol to the plasma membrane in exchange for phosphatidylinositol 4, 5-bisphosphate (PI(4,5)P2) Mol. Cell. 2019;73:458–473.e7. doi: 10.1016/j.molcel.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Weisman R., Choder M. The fission yeast TOR homolog, tor1+, is required for the response to starvation and other stresses via a conserved serine. J. Biol. Chem. 2001;276:70277032. doi: 10.1074/jbc.M010446200. [DOI] [PubMed] [Google Scholar]
- Yakir-Tamang L., Gerst J.E. A phosphatidylinositol-transfer protein and phosphatidylinositol-4-phosphate 5-kinase control Cdc42 to regulate the actin cytoskeleton and secretory pathway in yeast. Mol. Biol. Cell. 2009;20:3583–3597. doi: 10.1091/mbc.E08-10-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeliseev A.A., Kaplan S. A novel mechanism for the regulation of photosynthesis gene expression by the TspO outer membrane protein of Rhodobacter sphaeroides 2.4.1. J. Biol. Chem. 1999;274:21234–21243. doi: 10.1074/jbc.274.30.21234. [DOI] [PubMed] [Google Scholar]
- Yeliseev A.A., Krueger K.E., Kaplan S. A mammalian mitochondrial drug receptor functions as a bacterial "oxygen" sensor. Proc. Natl. Acad. Sci. U S A. 1997;94:5101–5106. doi: 10.1073/pnas.94.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H.Y., Hoi K.K., Liko I., Hedger G., Horrell M.R., Song W., Wu D., Heine P., Warne T., Lee Y. PtdIns(4,5)P. Nature. 2018;559:423–427. doi: 10.1038/s41586-018-0325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Shan J., Krishnamoorthi R., Wang X. Activation of plant phospholipase Dbeta by phosphatidylinositol 4,5-bisphosphate: characterization of binding site and mode of action. Biochemistry. 2002;41:4546–4553. doi: 10.1021/bi0158775. [DOI] [PubMed] [Google Scholar]
- Zimmermann P., Hirsch-Hoffmann M., Hennig L., Gruissem W. GENEVESTIGATOR.Arabidopsis microarray database and analysis toolbox. Plant Physiol. 2004;136:2621–2632. doi: 10.1104/pp.104.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure numbering in this section corresponds to the numbering used in the article. The dataset relates to main Figures 1–5 and S2–S8.