Abstract
Background
Recently, the attention of researchers to the study of the properties of human saliva, as a material with unique properties and diagnostic capabilities, has increased.
Research objective
to study the connection of the biochemical composition of saliva and blood plasma in the norm, depending on gender and age.
Methods
107 volunteers took part in the study, including 46 female (37.2 ± 3.9 years old) and 61 male (36.1 ± 2.8 years old). In all samples of saliva and blood plasma, 16 biochemical parameters were determined, including mineral and protein composition, enzyme activity. Non-parametric statistical methods were used to process the data.
Results
It has been shown that it is difficult to establish an unambiguous relation between biochemical parameters of saliva and blood plasma. The calculation of the Spearman correlation coefficients showed that only 7 of the 16 parameters demonstrate the presence of a weak correlation between the content in saliva and plasma.
Conclusion
In general, the determination of the composition of saliva may have an independent diagnostic value; in this case, drawing a parallel with the composition of serum and blood plasma is not advisable. Nevertheless, the use of saliva in clinical laboratory diagnostics is associated with the need to establish criteria for the norm and pathology for each biochemical parameter.
Keywords: Saliva, Blood plasma, Biochemical composition, Correlations, Gender, Age
1. Introduction
Human saliva is a material with unique properties and diagnostic capabilities.1,2 The study of saliva refers to non-invasive methods, which is its main advantage compared with other biological fluids.1, 2, 3, 4, 5 Saliva contains many biological molecules, including DNA, mRNA, miRNA, protein, metabolites, and the microbiota. Changes in their concentration in saliva can be used to identify systemic and oral diseases at early stages, as well as to assess the prognosis of the disease and control the response to treatment.6 In 2008, the term « Salivaomics» was proposed, which combines knowledge of various components in saliva, including genome, epigenome, transcriptome, proteome, metabolome, and microbiome.7,8
Numerous studies clearly show that the determination of biochemical parameters is informative when using saliva as a substrate.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 In particular, the important indicators are the acidity of the medium9,10 and the mineral composition of saliva.11 It is known that the concentration of inorganic ions plays a significant role in such vital processes as cardiac activity, acid-base balance, regulation of intracellular homeostasis.12, 13, 14, 15 The important role of calcium and inorganic phosphorus was shown in maintaining the equilibrium of the processes of mineralization and demineralization in the oral cavity.16,17 Saliva can be used to study oxidative stress along with blood, because saliva contains antioxidant enzymes (catalase, superoxide dismutase, glutathione peroxidase), antioxidant vitamins (A, E, C), lipid peroxidation products come from the blood into the saliva (diene and triene conjugates, Schiff bases).18, 19, 20, 21 The level of nitric oxide,22,23 as well as the concentration of uric acid, which belongs to the body's antioxidant system24,25 can give important information in saliva. However, the use of saliva in the diagnosis is combined in two directions: the detection of diseases of the oral cavity26, 27, 28, 29, 30, 31 and the identification of pathologies of the organism as a whole.32, 33, 34, 35, 36 In the first case, it is sufficient to determine the corresponding biochemical indicator for the control group and in the presence of oral diseases. In the second case, there is a need to compare the result with the reference value (serum or plasma). Often, a parallel is drawn between the dynamics of the biochemical index in saliva and blood. Nevertheless, the question of the relationship between the biochemical composition of saliva and blood still remains not fully studied,37, 38, 39, 40, 41 and the presence of a correlation between the content of individual components is unproven.42, 43, 44 In this connection, it is relevant to compare the biochemical composition of saliva and blood plasma to establish the presence/absence of a relationship between the composition of these biological fluids in the norm, taking into account age and gender of the subjects.
2. Materials and methods
2.1. Participants
Blood and saliva samples were taken from healthy male (n = 61) and female (n = 46) volunteers aged 20–59 years. The study group was divided into subgroups by age with a step of 10 years: 20–29 (29 men; 9 women), 30–39 (19 men; 11 women), 40–49 (9 men; 13 women) and 50–59 years (5 men; 13 women). The healthy individuals did not show any clinical or laboratory characteristics of autoimmune, cardiovascular or oncological disease. Samples were collected at the blood transfusion station, all volunteers are active blood donors, do not have bad habits, in that they do not smoke and do not drink alcohol. For two weeks before the experiment, the volunteers did not take any drugs. Particular attention was paid to the state of oral hygiene, while participants with inflammation and periodontal disease were excluded. All volunteers provided written informed consent.
2.2. Blood and saliva sampling
Blood samples (8.0 mL) were collected in tubes with a gel separator to obtain serum for biochemical measurements. Samples were transported at 4 °C to the laboratory within 30 min, and centrifuged under refrigeration at 1800×g for 10 min. Saliva samples were collected between 8 and 10 a.m. in sterile tubes by spitting.44,46 The participants in the experiment rinsed their mouths with distilled water for 10–15 min before collecting saliva. The volume of saliva was measured for determining the salivary flow rate (ml/min). Samples were centrifuged (7000×g for 10 min) to minimize the turbidity of saliva.47 Blood and saliva samples were analyzed immediately after centrifugation (without freezing).
2.3. Biochemical analysis of saliva and blood samples
The biochemical composition of the samples was established using the StatFax 3300 semi-automatic biochemical analyzer.48 The pH, mineral composition (calcium, phosphorus, magnesium, chlorides), the content of urea, total protein, albumin, uric acid and sialic acids, the activity of enzymes (aminotransferases (ALT, AST); alkaline phosphatase (ALP); lactate dehydrogenase (LDH); gamma-glutamyl transpeptidase (GGT); catalase) were determined in all samples.
2.4. Statistical methods
Statistical analysis of the data was performed using Statistica Trial (StatSoft) with the non-parametric method using the Wilcoxon test and the Mann-Whitney U test. The sample was described by calculating the median (Me) and interquartile range in the form of the 25th and 75th percentile [LQ; UQ]. Statistical relationships were studied by calculating the Spearman correlation coefficients. Differences were considered statistically significant at p < 0.05.
3. Results
At the first stage, the characterization of the distribution and homogeneity of the dispersions in the groups was carried out. According to the Shapiro-Wilk test, the content of all determined parameters does not correspond to the normal distribution (p < 0.05). The test for homogeneity of dispersions in groups (Bartlett test) allowed us to reject the hypothesis that dispersions are homogeneous in groups (p = 0.00017). Therefore, non-parametric statistical methods were used to process the data.
The minimum and maximum values, as well as a comparison of the medians and the interquartile range for each parameter are given in Table 1. It was shown that for most parameters, the ranges of the determined concentrations overlap, with the exception of total protein, albumin and sialic acids, for which the content in the blood plasma is an order of magnitude higher than in saliva (Table 1).
Table 1.
Descriptive statistics of the biochemical composition of saliva and blood plasma.
| Indicator | Saliva, n = 107 |
Blood plasma, n = 107 |
Correlation coefficient | ||
|---|---|---|---|---|---|
| Me [LQ; UQ] | MIN-MAX | Me [LQ; UQ] | MIN-MAX | ||
| рН | 6.50 [6.32; 6.68] | 4.93–7.25 | 7.60 [7.50; 7.80] | 6.54–7.99 | 0.3015* |
| Calcium, mmol/L | 1.19 [0.88; 1.51] | 0.28–3.02 | 2.39 [2.08; 2.67] | 0.70–6.74 | 0.3213* |
| Phosphorus, mmol/L | 2.96 [2.39; 3.61] | 0.60–6.92 | 1.15 [0.95; 1.58] | 0.54–4.55 | 0.0990 |
| Chlorides, mmol/L | 20.73 [15.70; 24.42] | 4.04–41.48 | 70.77 [65.26; 76.29] | 58.85–92.35 | 0.0741 |
| Magnesium, mmol/L | 0.254 [0.192; 0.338] | 0.034–0.621 | 0.824 [0.730; 0.902] | 0.358–1.230 | −0.2481* |
| Protein, g/L | 0.47 [0.35; 0.65] | 0.04–1.41 | 60.00 [52.50; 67.50] | 25.00–102.50 | 0.0456 |
| Albumin, mmol/L | 0.259 [0.169; 0.394] | 0.038–1.624 | 40.69 [37.89; 44.38] | 28.36–52.59 | −0.1086 |
| Urea, mmol/L | 8.28 [5.48; 10.35] | 0.11–17.27 | 4.09 [3.17; 4.75] | 1.84–9.30 | 0.1977* |
| Uric acid, nmol/mL | 59.58 [32.11; 107.14] | 4.59–451.83 | 261.90 [216.67; 307.14] | 110.09–392.86 | 0.2315* |
| Sialic acids, mmol/L | 0.122 [0.098; 0.171] | 0.031–0.513 | 3.15 [2.54; 3.72] | 0.90–7.84 | −0.1570 |
| ALT, U/L | 3.38 [2.23; 4.38] | 0.85–14.54 | 8.15 [6.92; 10.00] | 2.69–16.62 | 0.4077* |
| AST, U/L | 3.92 [2.83; 5.67] | 0.67–19.33 | 8.08 [6.83; 9.50] | 3.17–17.08 | 0.1193 |
| AST/ALT | 1.30 [0.96; 1.59] | 0.31–3.20 | 0.94 [0.81; 1.19] | 0.52–3.43 | 0.0898 |
| ALP, U/L | 69.54 [47.81; 99.96] | 15.21–517.17 | 323.78 [271.63; 386.79] | 117.34–727.96 | 0.0002 |
| LDH, U/L | 1281.5 [905.6; 1911.0] | 193.6–2849.0 | 690.5 [596.6; 762.6] | 334.0–1154.4 | 0.3521* |
| GGT, U/L | 19.6 [17.5; 22.3] | 13.8–30.6 | 32.3 [27.1; 38.2] | 16.0–124.3 | 0.1032 |
| Catalase, ncat/L | 3.65 [2.84; 4.85] | 1.64–8.96 | 22.9 [17.3; 39.2] | 0.135–0.723 | 0.0641 |
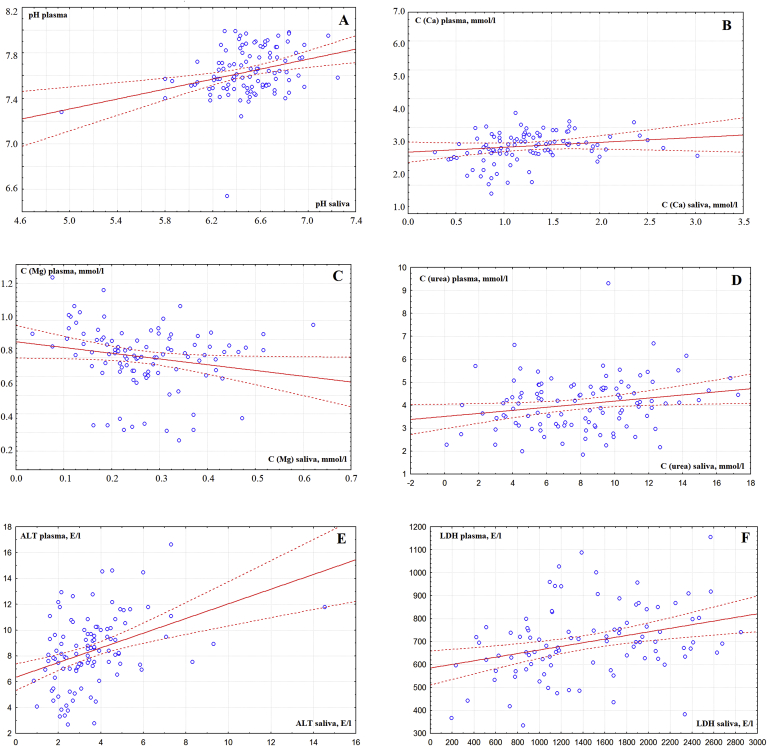
Additional calculations showed that, for each parameter, statistically significant differences were observed between blood plasma and saliva (p˂0.0001). The calculation of the correlation coefficients for Spearman showed that only 7 of the 16 parameters demonstrate the presence of a weak correlation between the content in saliva and plasma (Table 1). Moreover, all the identified correlation relationships are positive, except for the concentration of magnesium (Fig. 1A–F).
Fig. 1.
Correlations between the composition of blood plasma and saliva (A - pH, B - calcium, C - magnesium, D - urea, E - alanine aminotransferase, F − lactate dehydrogenase).
It was established that the correlation of the biochemical composition of saliva and blood plasma substantially depends on the sex of the volunteers (Table 2). So, for women, positive correlations were found for pH (R = 0.3460), uric acid (R = 0.3294), ALT (R = 0.3521) and negative correlation for sialic acids (R = -0.2521). While for men, positive correlations are noted for pH (R = 0.2382), calcium (R = 0.3452), phosphorus (R = 0.3031), ALT (R = 0.4078), LDH (R = 0.4698) and negative for magnesium (R = - 0.3183) and albumin (R = -0.3123).
Table 2.
Biochemical composition of saliva and blood plasma depending on gender.
| Indicator | Saliva |
Blood plasma |
||
|---|---|---|---|---|
| Female, n = 46 | Male, n = 61 | Female, n = 46 | Male, n = 61 | |
| Flow rate, mL/min | 0.82 [0.68; 0.96] | 0.88 [0.70; 0.99] | – | – |
| рН | 6.50 [6.32; 6.68] | 6.51 [6.32; 6.67] | 7.64 [7.51; 7.80] | 7.59 [7.48; 7.80] |
| Calcium, mmol/L | 1.12 [0.79; 1.45] | 1.21 [0.94; 1.57] | 2.18 [1.94; 2.61] | 2.45 [2.19; 2.75] |
| p = 0.0246 | ||||
| Phosphorus, mmol/L | 2.94 [2.33; 3.65] | 2.99 [2.46; 3.52] | 1.13 [0.96; 1.61] | 1.16 [0.88; 1.57] |
| Chlorides, mmol/L | 18.90 [15.42; 23.75] | 21.10 [16.18; 24.84] | 70.77 [65.38; 75.00] | 71.01 [65.15; 78.02] |
| Magnesium, mmol/L | 0.252 [0.180; 0.321] | 0.273 [0.214; 0.343] | 0.834 [0.749; 0.902] | 0.820 [0.719; 0.895] |
| Protein, g/L | 0.42 [0.30; 0.63] | 0.50 [0.37; 0.71] | 60.00 [52.50; 65.00] | 62.50 [52.50; 70.00] |
| Urea, mmol/L | 7.20 [4.57; 10.27] | 8.59 [5.61; 10.40] | 3.71 [2.90; 4.53] | 4.22 [3.44; 4.81] |
| p = 0.0295 | ||||
| Albumin, mmol/L | 0.207 [0.142; 0.325] | 0.300 [0.223; 0.413] | 40.30 [38.28; 42.64] | 41.72 [36.14; 45.32] |
| p = 0.0192 | ||||
| Uric acid, nmol/mL | 54.76 [29.82; 95.24] | 68.81 [33.33; 112.39] | 235.71 [173.81; 275.23] | 292.86 [250.00; 345.24] |
| p˂0.0001 | ||||
| Sialic acids, mmol/L | 0.110 [0.085; 0.146] | 0.128 [0.098; 0.195] | 3.18 [2.62; 4.14] | 3.06 [2.54; 3.44] |
| ALT, U/L | 3.31 [2.15; 4.31] | 3.38 [2.62; 4.46] | 7.92 [6.54; 9.46] | 8.62 [7.31; 10.85] |
| AST, U/L | 3.88 [2.17; 5.58] | 4.08 [3.08; 6.00] | 7.92 [6.58; 9.25] | 8.17 [7.00; 9.58] |
| AST/ALT | 1.21 [0.91; 1.57] | 1.36 [1.02; 1.62] | 0.96 [0.86; 1.22] | 0.91 [0.78; 1.18] |
| ALP, U/L | 71.71 [49.98; 99.96] | 67.36 [47.81; 93.44] | 305.31 [271.63; 343.33] | 347.68 [291.18; 404.18] |
| p = 0.0202 | ||||
| LDH, U/L | 1176.0 [822.2; 1886.0] | 1496.0 [978.5; 1975.5] | 698.0 [596.6; 757.7] | 673.1 [598.6; 781.4] |
| GGT, U/L | 19.4 [17.7; 23.0] | 19.7 [17.5;22.1] | 29.1 [26.8; 36.2] | 33.9 [28.2; 43.9] |
| Catalase, ncat/L | 3.42 [2.72; 4.69] | 3.82 [3.25; 4.92] | 22.7 [16.8; 38.4] | 23.3 [19.3; 39.5] |
In general, there is a similar trend in the ratio of concentrations of certain parameters in the blood and saliva depending on the gender. Thus, the level of most of the studied parameters is higher in the group of men (Table 2). For magnesium, sialic acid and LDH, plasma levels of men are lower, whereas in saliva, a similar trend is observed only for alkaline phosphatase.
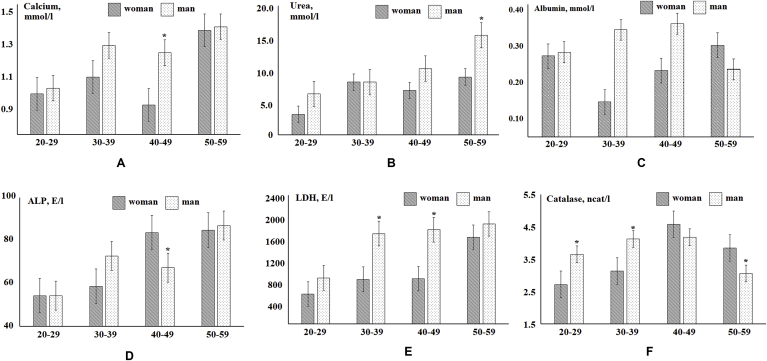
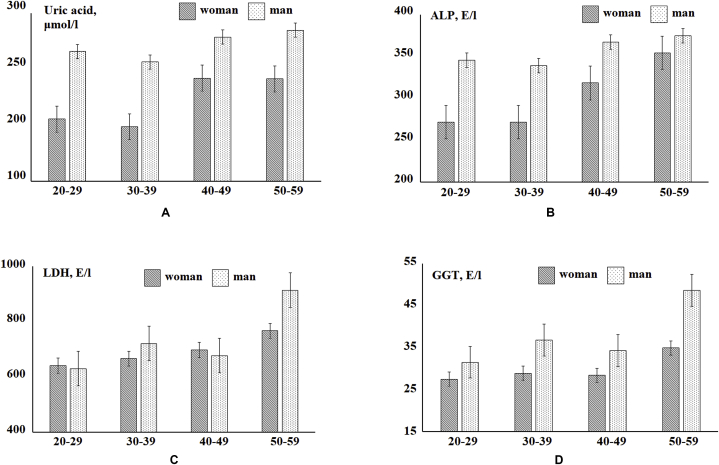
At the next stage, indicators of the biochemical composition of saliva and blood plasma, the values of which vary significantly depending on the age of the volunteers were revealed. For clarity, the study group was divided into subgroups by age with a step of 10 years (Fig. 2, Fig. 3). It has been established that with age, women's saliva statistically significantly increases the calcium content (R = 0.3440, Fig. 2A), urea (R = 0.3117, Fig. 2B), albumin (R = 0.3166, Fig. 2C), LDH (R = 0.3099, Fig. 2E) and catalase activity (R = 0.3006, Fig. 2F). In the saliva of men, enzyme activity also increases with age: ALP (R = 0.3074, Fig. 2D) and LDH (R = 0.3277, Fig. 2E). For the blood plasma of women, an increase in the concentration of uric acid (R = 0.3531, Fig. 3A), an increase in the activity of the enzymes ALP (R = 0.5516, Fig. 3B), LDH (R = 0.3255, Fig. 3C) and GGT (R = 0.3319, Fig. 3D) was noted. For a group of men, only an increase in LDH activity (R = 0.3582, Fig. 3C) with age was observed.
Fig. 2.
Biochemical composition of saliva depending on age and gender (* - differences from the previous age group are statistically significant).
Fig. 3.
Biochemical composition of blood plasma depending on age and gender.
For the rest of the studied parameters, no statistically significant differences in different age groups were revealed. However, for saliva samples, there is a significant variability of indicators in the transition from the younger age group to the older one, which must be taken into account when identifying the criteria of the norm and pathology for a correct statistical evaluation of the data obtained (Table 3). It should be noted that statistically significant differences in salivation rate were not found, however, there is a tendency for a slight decrease in this indicator with age, while for each age group, the salivation rate for men is slightly higher than for women (Table 3).
Table 3.
Biochemical composition of saliva depending on gender and age.
| Indicator | Gender | Age (20–29 years) | Age (30–39 years) | Age (40–49 years) | Age (50–59 years) |
|---|---|---|---|---|---|
| Flow rate, mL/min | F | 0.86 [0.72; 1.12] | 0.82 [0.68; 1.00] | 0.80 [0.69; 0.95] | 0.78 [0.65; 0.92] |
| M | 0.94 [0.79; 1.20] | 0.90 [0.85; 1.04] | 0.87 [0.72; 1.01] | 0.79 [0.65; 0.93] | |
| рН | F | 6.49 [6.33; 6.64] | 6.43 [6.30; 6.62] | 6.55 [6.29; 6.78] | 6.66 [6.34; 6.74] |
| M | 6.48 [6.37; 6.68] | 6.44 [6.26; 6.62] | 6.53 [6.45; 6.58] | 6.82 [6.54; 6.89] | |
| Calcium, mmol/L | F | 1.02 [0.78; 1.44] | 1.11 [0.62; 1.31] | 0.96 [0.76; 1.93] | 1.36 [1.04; 1.46] |
| M | 1.05 [0.89; 1.64] | 1.28 [1.05; 1.92] | 1.24 [0.90; 1.35] | 1.38 [1.27; 1.46] | |
| Phosphorus, mmol/L | F | 2.36 [1.16; 3.04] | 2.72 [2.35; 2.96] | 3.20 [1.95; 3.65] | 3.16 [2.66; 4.03] |
| M | 2.96 [2.58; 3.29] | 2.79 [2.22; 3.52] | 3.02 [2.66; 3.51] | 3.74 [3.28; 5.52] | |
| Chlorides, mmol/L | F | 13.33 [10.34; 24.27] | 19.78 [12.63; 21.57] | 16.26 [15.82; 23.75] | 23.46 [18.19; 25.68] |
| M | 18.53 [14.38; 24.62] | 22.07 [17.53; 25.19] | 20.83 [19.17; 25.83] | 20.29 [17.58; 31.16] | |
| Magnesium, mmol/L | F | 0.200 [0.167; 0.269] | 0.252 [0.207; 0.273] | 0.213 [0.111; 0.324] | 0.279 [0.209; 0.367] |
| M | 0.276 [0.192; 0.338] | 0.280 [0.243; 0.374] | 0.231 [0.183; 0.318] | 0.224 [0.209; 0.352] | |
| Protein, g/L | F | 0.28 [0.24; 0.55] | 0.36 [0.32; 0.56] | 0.41 [0.31; 0.66] | 0.59 [0.36; 0.70] |
| M | 0.45 [0.35; 0.78] | 0.45 [0.37; 0.72] | 0.55 [0.51; 0.65] | 0.46 [0.36; 0.53] | |
| Urea, mmol/L | F | 4.53 [3.59; 5.50] | 8.54 [5.65; 11.21] | 7.50 [4.80; 9.91] | 9.17 [6.94; 10.27] |
| M | 7.09 [5.61; 9.63] | 8.54 [5.48; 9.33] | 10.21 [9.50; 10.35] | 14.34 [8.70; 15.24] | |
| Albumin, mmol/L | F | 0.263 [0.109; 0.337] | 0.142 [0.094; 0.179] | 0.225 [0.183; 0.300] | 0.292 [0.188; 0.615] |
| M | 0.273 [0.173; 0.371] | 0.332 [0.244; 0.528] | 0.348 [0.270; 0.386] | 0.228 [0.207; 0.303] | |
| Uric acid, nmol/mL | F | 78.57 [51.61; 98.62] | 29.82 [6.88; 54.76] | 64.22 [32.11; 107.14] | 61.78 [36.16; 103.57] |
| M | 64.17 [30.34; 95.24] | 50.32 [35.71; 110.09] | 102.06 [24.74; 218.02] | 99.77 [58.49; 126.15] | |
| Sialic acids, mmol/L | F | 0.098 [0.055; 0.128] | 0.116 [0.098; 0.146] | 0.137 [0.092; 0.186] | 0.104 [0.092; 0.153] |
| M | 0.122 [0.104; 0.171] | 0.122 [0.085; 0.207] | 0.146 [0.110; 0.165] | 0.177 [0.134; 0.211] | |
| ALT, U/L | F | 2.23 [1.85; 3.38] | 3.62 [2.15; 3.77] | 4.12 [2.96; 5.00] | 2.46 [1.92; 4.31] |
| M | 3.38 [2.77; 4.46] | 3.23 [2.85; 4.54] | 2.38 [1.77; 4.08] | 2.81 [1.92; 4.27] | |
| AST, U/L | F | 4.00 [2.08; 5.58] | 2.83 [1.58; 5.00] | 4.88 [3.33; 5.88] | 3.67 [2.00; 6.17] |
| M | 3.92 [3.33; 5.33] | 4.67 [2.25; 7.17] | 4.67 [3.25; 5.67] | 3.33 [1.83; 6.42] | |
| AST/ALT | F | 1.65 [1.20; 1.86] | 0.97 [0.62; 1.57] | 1.03 [0.93; 1.31] | 1.30 [0.95; 1.47] |
| M | 1.31 [1.02; 1.45] | 1.49 [0.77; 1.62] | 1.67 [1.38; 1.96] | 1.46 [0.76; 2.07] | |
| ALP, U/L | F | 54.33 [49.98; 78.23] | 58.67 [39.11; 97.79] | 83.66 [45.63; 131.47] | 84.75 [60.84; 108.65] |
| M | 54.33 [34.77; 91.27] | 72.80 [47.81; 115.17] | 67.36 [60.84; 82.57] | 86.92 [61.93; 102.13] | |
| LDH, U/L | F | 863.9 [428.1; 1622.5] | 1094.0 [777.9; 1199.5] | 1102.0 [737.2; 1922.0] | 1746.5 [1205.5; 1980.5] |
| M | 1116.0 [881.0; 1654.0] | 1802.0 [1030.0; 2107.0] | 1864.0 [1292.0; 2068.0] | 1951.5 [1359.0; 2470.5] | |
| GGT, U/L | F | 18.80 [18.35; 20.85] | 18.40 [16.30; 20.70] | 19.55 [17.70; 23.00] | 22.20 [19.00; 24.25] |
| M | 19.60 [17.50; 20.90] | 20.30 [17.20; 23.10] | 21.20 [18.80; 23.50] | 19.25 [17.90; 23.05] | |
| Catalase, ncat/L | F | 2.72 [2.41; 3.13] | 3.13 [2.68; 3.96] | 4.58 [3.37; 5.50] | 3.84 [3.13; 4.69] |
| M | 3.65 [2.74; 4.77] | 4.12 [3.37; 4.94] | 4.18 [3.52; 4.77] | 3.06 [1.85; 4.86] |
4. Discussion
According to the results of the study, it was shown that it is difficult to establish the unambiguous connection between biochemical parameters, including mineral and protein composition, the activity of enzymes of saliva and blood plasma.
It is known that the concentration of most electrolytes and trace elements in saliva is comparable to their concentration in serum and plasma.49, 50 However, many organic components are contained in saliva in concentrations much lower than in plasma, in particular, the concentration of albumin saliva is only 0.1–1% of its concentration in plasma. There are discrepancies with literature data, according to which the activity of ALP and ALT in saliva is almost 2 times lower than in blood, whereas the activity of AST is almost the same.49 According to our data, ALP activity in saliva is 4–5 times lower than in plasma, whereas for ALT and AST the concentration in saliva decreases proportionally by about 2–2.5 times (Table 1, Table 2). The conducted studies revealed a contradiction with the literature data, according to which the activity of ALP saliva practically does not depend on the sex and age of the subjects.51,52 It is shown that during the transition from the younger age group to the senior, ALP activity increases for men by 60.0%, for women by 56.0% (Table 3).
LDH activity in saliva was shown to be 3 times higher than according to literature data (360–430 U/L).53,54 Additionally, a statistically significant increase in LDH activity with age was revealed both in saliva and in blood plasma, regardless of the gender of the subjects, which is not mentioned in the literature.
Experimental studies have shown that saliva urea significantly and positively correlates with serum urea.55 With age, an increase in the urea content is observed (Table 3). It can be assumed that renal function, including glomerular filtration rate, slowly decreases after a certain age, which leads to a slight increase in urea concentration, which is more expressed in the group of men.
It should be noted that the pH value of saliva does not show a relationship with the age or gender of the subjects and only slightly differs between groups, which is consistent with the literature data.56,57 Specific gender differences in saliva pH values remain somewhat controversial, although they have been seen in several studies.58
It is known that the salivation rate gradually decreases with the age of volunteers.59,60 A number of studies show that the functions and anatomical features of the salivary glands also depend on gender.61,62 It was also found that women have a lower mean salivation rate than men at rest.63 A decrease in the rate of saliva with age can result from a response to hormonal changes during puberty, menstruation, pregnancy, and menopause.64 However, in a number of works no differences in salivation rate between age groups were found, which allows the authors to state that salivation rate and saliva composition do not depend on age in healthy people.65,66
In general, experimental data emphasize that gender is one of the most important biological variables that significantly affect the metabolic profile of blood plasma and other biological fluids, particularly urine.67, 68, 69 However, gender differences in the biochemical composition of saliva are inconclusive or controversial.70 Additional consideration of the age group of the surveyed shows that the male plasma metabolism forms a unique cluster regardless of age, whereas for women there are two age clusters: the first is between 30 and 49 years old, the second is over 50 years old.71,72 Therefore, it is necessary to take into account the gender and age characteristics of groups when conducting research.
However, this is not the only limitation when using saliva as a substrate in clinical laboratory diagnostics. Before using salivary metabolic profiles for clinical use, standard operating procedures should be developed, such as sample collection and storage conditions.73 The diurnal variability of the composition of saliva is of concern in connection with the establishment of the sampling protocol. For example, saliva secretion depends on circadian rhythms.45 Metabolic profiles correlate with circadian rhythms in various samples of biological fluids, especially in blood samples.74 As for saliva, such data is limited and reported in only a few studies (for example, the time dependence of several polyamines and the differences between morning and night).75 Several comparative studies of metabolites in saliva and blood have also been conducted.76 Daily fluctuations of miRNA and microbiota can alter salivary metabolic profiles.77 Gender-dependent differences in metabolic profiles of saliva were previously shown.78
Thus, saliva is a clinically informative biological fluid, but further development and testing of biomarkers of saliva is necessary for introduction into clinical laboratory diagnostics.79, 80, 81
The study has several limitations. So, the main attention was paid to the biochemical composition of saliva and serum, while the physicochemical properties and microbiological composition of biological fluids are undoubtedly important.2,82,83 We have previously shown that there are differences in gender and age of such physicochemical characteristics of saliva as surface tension and viscosity, which is reflected in its structural and mineralizing properties.48 The biochemical composition of saliva may vary for individuals as a result of differences in the microbiological composition of saliva. However, most authors agree that changes in the microflora of the oral cavity accompany a number of dental diseases.84,85 In this study, volunteers did not have inflammatory diseases of the oral cavity, which in a sense reduces the effect of this factor on the composition of saliva. Nevertheless, in future studies, we plan to pay attention to this issue.
5. Conclusions
Determining the composition of saliva may have independent diagnostic value, in this case, drawing a parallel with the composition of serum and blood plasma is not always necessary. Nevertheless, the use of saliva in clinical laboratory diagnostics is associated with the need to establish criteria for the norm and pathology for each biochemical parameter. A promising area of research, in our opinion, is an increase in the list of biomarkers identified in saliva, as well as testing the sensitivity and accuracy of their detection, increasing the sensitivity and reproducibility of the tests, and evaluating the cost-effectiveness of their introduction into routine clinical diagnosis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
All authors declare that there are no conflicts of interest.
References
- 1.Chojnowska S., Baran T., Wilińska I., Sienicka P., Cabaj-Wiater I., Knaś M. Human saliva as a diagnostic material. Adv Med Sci. 2018;63(1):185–191. doi: 10.1016/j.advms.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Kubala E., Strzelecka P., Grzegocka M. A review of selected studies that determine the physical and chemical properties of saliva in the field of dental treatment. BioMed Res Int. 2018 doi: 10.1155/2018/6572381. Article ID 6572381, 13 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereira Lima D., Garcia Diniz D., Adas S., Moimaz S. Saliva: reflection of the body. Int J Infect Dis. 2010;14:184–188. doi: 10.1016/j.ijid.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Chiappin S., Antonelli G., Gatti R., De Palo E.F. Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clin Chim Acta. 2007;383:30–40. doi: 10.1016/j.cca.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Zhang A., Sun H., Wang X. Saliva metabolomics opens door to biomarker discovery, disease diagnosis, and treatment. Appl Biochem Biotechnol. 2012;168:1718–1727. doi: 10.1007/s12010-012-9891-5. [DOI] [PubMed] [Google Scholar]
- 6.Spielmann N., Wong D.T. Saliva: diagnostics and therapeutic perspectives. Oral Dis. 2011;17:345–354. doi: 10.1111/j.1601-0825.2010.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y., Sun J., Lin C., Abemayor E., Wang M.B., Wong D.T. The emerging landscape of salivary diagnostics. OHDM. 2014;13(2):200–210. [PubMed] [Google Scholar]
- 8.Wong D.T. Salivaomics. J Am Dent Assoc. 2012;143:19S–24S. doi: 10.14219/jada.archive.2012.0339. [DOI] [PubMed] [Google Scholar]
- 9.Cohen M., Khalaila R. Saliva pH as a biomarker of exam stress and a predictor of exam performance. J Psychosom Res. 2014;77(5):420–425. doi: 10.1016/j.jpsychores.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Ramya A.S., Uppala D., Majumdar S., Surekha Ch, Deepak K.G.K. Are salivary amylase and pH – prognostic indicators of cancers? J Oral Biol Craniofacial Res. 2015;5(2):81–85. doi: 10.1016/j.jobcr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey P., Reddy N.V., Rao V.A., Saxena A., Chaudhary C.P. Estimation of salivary flow rate, pH, buffer capacity, calcium, total protein content and total antioxidant capacity in relation to dental caries severity, age and gender. Contemp Clin Dent. 2015:S65–S71. doi: 10.4103/0976-237X.152943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues V.P., Franko M.M., Marques C.P.C. Salivary levels of calcium, phosphorus, potassium, albumin and correlation with serum biomarkers in hemodialysis patients. Arch Oral Biol. 2016;62:58–63. doi: 10.1016/j.archoralbio.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Mori M., Iwata T., Satori T., Ohira S.-I., Itabashi H., Tanaka K. Ion-exclusion/cation-exchange chromatographic determination of common inorganic ions in human saliva by using an eluent containing zwitterionic surfactant. J Chromatogr A. 2008;1213:125–129. doi: 10.1016/j.chroma.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Badita D.G., Stanescu, Stroescu A.B. Salivary and serum biochemical alterations in patients with acute viral hepatitis. Rev Chim (Bucharest) 2018;69(3):547–571. [Google Scholar]
- 15.Shirzaiy M., Heidari F., Dalirsani Z., Dehghan J. Estimation of salivary sodium, potassium, calcium, phosphorus and urea in type II diabetic patients. Diabetes & Metabolic Syndrome: Clin Res Rev. 2015;9(4):332–336. doi: 10.1016/j.dsx.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Meyer-Lueckel H., Chatzidakis A.J., Kielbassa A.M. Effect of various calcium/phosphates ratios of carboxymethylcellulose-based saliva substitutes on mineral loss of bovine enamel in vitro. J Dent. 2007;35:851–857. doi: 10.1016/j.jdent.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka T., Kobayashi T., Takii H. Optimization of calcium concentration of saliva with phosphoryl oligosaccharides of calcium (POs-Ca) for enamel remineralization in vitro. Arch Oral Biol. 2013;58:174–180. doi: 10.1016/j.archoralbio.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Zygula A., Kosinski P., Zwierzchowska A. Oxidative stress markers in saliva and plasma differ between diet-controlled and insulin-controlled gestational diabetes mellitus. Diabetes Res Clin Pract. 2019;148:72–80. doi: 10.1016/j.diabres.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Karlík M., Valkovič P., Hančinová V., Krížová L., Tóthová L., Celec P. Markers of oxidative stress in plasma and saliva in patients with multiple sclerosis. Clin Biochem. 2015;48(1-2):24–28. doi: 10.1016/j.clinbiochem.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Reznick A.Z., Shehadeh N., Shafir Y., Nagler R.M. Free radicals related effects and antioxidants in saliva and serum of adolescents with Type 1 diabetes mellitus. Arch Oral Biol. 2006;51:640–648. doi: 10.1016/j.archoralbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Tartaglia G.M., Gagliano N., Zarbin L., Tolomeo G., Sforza C. Antioxidant capacity of human saliva and periodontal screening assessment in healthy adults. Arch Oral Biol. 2017;78:34–38. doi: 10.1016/j.archoralbio.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Mobarak E.H., Abdallah D.M. Saliva nitric oxide levels in relation to caries experience and oral hygiene. J Adv Res. 2011;2(4):357–362. [Google Scholar]
- 23.Rocha B.S., Lundberg J.O., Radi R., Laranjinha J. Role of nitrite, urate and pepsin in the gastroprotective effects of saliva. Redox Biology. 2016;8:407–414. doi: 10.1016/j.redox.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenberg E., Shtahl S., Geller R. Serum and salivary oxidative analysis in complex regional pain syndrome. Pain. 2008;138:226–232. doi: 10.1016/j.pain.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Korde (Choudhari) Sh, Sridharan G., Gadbail A., Poornima V. Nitric oxide and oral cancer: a review. Oral Oncol. 2012;48(6):475–483. doi: 10.1016/j.oraloncology.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Roi A., Rusu L.C., Roi C.I., Luca R.E., Boia S., Munteanu R.I. A new approach for the diagnosis of systemic and oral diseases based on salivary biomolecules. Dis Markers. 2019 doi: 10.1155/2019/8761860. Article ID 8761860, 11 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim E.-H., Joo J.-Y., Lee Y.J. Grading system for periodontitis by analyzing levels of periodontal pathogens in saliva. PloS One. 2018;13(11) doi: 10.1371/journal.pone.0200900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y., Zhu M., Li Z. Mass spectrometry-based metabolomics profiling identifies alterations in salivary redox status and fatty acid metabolism in response to inflammation and oxidative stress in periodontal disease. Free Radic Biol Med. 2014;70:223–232. doi: 10.1016/j.freeradbiomed.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 29.Giannobile W.V., Wong D.T.W. Salivary diagnostics: oral health and beyond! J Dent Res. 2011;90(10):1153–1154. doi: 10.1177/0022034511420436. [DOI] [PubMed] [Google Scholar]
- 30.Kaur J., Jacobs R., Huang Y., Salvo N., Politis C. Salivary biomarkers for oral cancer and pre-cancer screening: a review. Clin Oral Invest. 2018;22(2):633–640. doi: 10.1007/s00784-018-2337-x. [DOI] [PubMed] [Google Scholar]
- 31.Liu J., Saliva Duan Y. A potential media for disease diagnostics and monitoring. Oral Oncol. 2012;48:569–577. doi: 10.1016/j.oraloncology.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Zian Z., Bakkach J., Barakat A., Nourouti N.G., Mechita M.B. Salivary biomarkers in systemic sclerosis disease. BioMed Res Int. 2018 doi: 10.1155/2018/3921247. Article ID 3921247, 7 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortha N., Uppala D., Kothia N.R., Majumdar S., Kotina S., Sravya K. A systematic review of saliva on its diagnostic utility. J NTR Univ Health Sci. 2018;7:115–119. [Google Scholar]
- 34.Abd-Elraheem S.E., Mansour H.H. Salivary changes in type 2 diabetic patients. Diabetes Metab Syndrome: Clin Res Rev. 2017;11(2):637–641. doi: 10.1016/j.dsx.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 35.Tong P., Yuan C., Sun X., Yue Q., Wang X., Zheng S. Identification of salivary peptidomic biomarkers in chronic kidney disease patients undergoing haemodialysis. Clin Chim Acta. 2019;489:154–161. doi: 10.1016/j.cca.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Nam Y., Kim Y-Young, Chang J-Youn, H-Seop Kho. Salivary biomarkers of inflammation and oxidative stress in healthy adults. Arch Oral Biol. 2019;97:215–222. doi: 10.1016/j.archoralbio.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 37.Barnes V.M., Kennedy A.D., Panagakos F. Global metabolomic analysis of human saliva and plasma from healthy and diabetic subjects, with and without periodontal disease. PloS One. 2014;9 doi: 10.1371/journal.pone.0105181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riis J.L., Bryce C.I., Ha T. Adiponectin: serum-saliva associations and relations with oral and systemic markers of inflammation. Peptides. 2017;91:58–64. doi: 10.1016/j.peptides.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Ialongo C. Preanalytic of total antioxidant capacity assays performed in serum, plasma, urine and saliva. Clin Biochem. 2017;50(6):356–363. doi: 10.1016/j.clinbiochem.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 40.Byrne M.L., O'Brien-Simpson N.M., Reynolds E.C. Acute phase protein and cytokine levels in serum and saliva: a comparison of detectable levels and correlations in a depressed and healthy adolescent sample. Brain Behav Immun. 2013;34:164–175. doi: 10.1016/j.bbi.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Sun Y., Liu S., Qiao Z. Systematic comparison of exosomal proteomes from human saliva and serum for the detection of lung cancer. Anal Chim Acta. 2017;982:84–95. doi: 10.1016/j.aca.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez-Botran R., Miller J.J., Burns V.E., Newton T.L. Correlations among inflammatory markers in plasma, saliva and oral mucosal transudate in postmenopausal women with past intimate partner violence. Brain Behav Immun. 2011;25:314–321. doi: 10.1016/j.bbi.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williamson S., Munro C., Pickler R., Grap M.J., Elswick R.K. Comparison of biomarkers in blood and saliva in healthy adults. Nurs Res Pract. 2012 doi: 10.1155/2012/246178. Article ID 246178, 4 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cullen T., Thomas A.W., Webb R., Hughes M.G. The relationship between interleukin-6 in saliva, venous and capillary plasma, at rest and in response to exercise. Cytokine. 2015;71(2):397–400. doi: 10.1016/j.cyto.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 45.Bel’skaya L.V., Kosenok V.K., Sarf E.A. Chronophysiological features of the normal mineral composition of human saliva. Arch Oral Biol. 2017;82:286–292. doi: 10.1016/j.archoralbio.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 46.Veerman E.C., van den Keybus P.A., Vissink A., Nieuw Amerongen A.V. Human glandular salivas: their separate collection and analysis. Eur J Oral Sci. 1996;104:346–352. doi: 10.1111/j.1600-0722.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 47.Dos Santos D.R., Souza R.O., Dias L.B. The effects of storage time and temperature on the stability of salivary phosphatases, transaminases and dehydrogenase. Arch Oral Biol. 2018;85:160–165. doi: 10.1016/j.archoralbio.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 48.Bel’skaya L.V., Sarf E.A., Solonenko A.P. Morphology of dried drop patterns of saliva from a healthy individual depending on the dynamics of its surface tension. Surfaces. 2019;2(2):395–414. [Google Scholar]
- 49.Noskov V.B. Saliva in clinical laboratory diagnostics (literature review) Klin Lab Diagn. 2008;6:14–17. [PubMed] [Google Scholar]
- 50.Wang D., Du X., Zheng W. Alteration of saliva and serum concentrations of manganese, copper, zinc, cadmium and lead among career welders. Toxicol Lett. 2008;176:40–47. doi: 10.1016/j.toxlet.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chapple I.L.C., Socransky S.S., Dibart S., Glenwright I.L.D., Mathews J.B. Chemiluminescent assay to alkaline phosphatases in human gingival cervical fluid: investigations with an experimental gingivitismodel and studies on the source of the enzyme within cervical fluid. J Clin Periodontol. 1996;23:587–594. doi: 10.1111/j.1600-051x.1996.tb01829.x. [DOI] [PubMed] [Google Scholar]
- 52.Todorovic T., Dozic I., Vicente-Barrero M. Salivary enzymes and periodontal disease. Med Oral Patol Oral Cir Bucal. 2006;11(2):E115–E119. [PubMed] [Google Scholar]
- 53.Nagler R.M., Lischinsky S., Diamond E., Klein I., Reznick A.Z. New insights into salivary lactate dehydrogenase of human subjects. J Lab Clin Med. 2001;137(5):363–369. doi: 10.1067/mlc.2001.114710. [DOI] [PubMed] [Google Scholar]
- 54.Avezov K., Reznick A.Z., Aizenbud D. LDH enzyme activity in human saliva: the effect of exposure to cigarette smoke and its different components. Arch Oral Biol. 2014;59:142–148. doi: 10.1016/j.archoralbio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Peng C.-H., Xia Y.-C., Wu Y., Zhou Z.-F., Cheng P., Xiao P. Influencing factors for saliva urea and its application in chronic kidney disease. Clin Biochem. 2013;46:275–277. doi: 10.1016/j.clinbiochem.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 56.Arregger A.L., Cardoso E.M., Tumilasci O., Contreras L.N. Diagnostic value of salivary cortisol in end stage renal disease. Steroids. 2008;73(1):77–82. doi: 10.1016/j.steroids.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Sreebny L.M. Saliva in health and disease: an appraisal and update. Int Dent J. 2000;50(3):140–161. doi: 10.1111/j.1875-595x.2000.tb00554.x. [DOI] [PubMed] [Google Scholar]
- 58.Dawes C. Circadian rhythms in the flow rate and composition of unstimulated and stimulated human submandibular saliva. J Physiol. 1975;244:535–548. doi: 10.1113/jphysiol.1975.sp010811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boghori M., Aghamaali M., Sariri R., Mohamadpour F., Ghafouri H. Salivary enzymes and flow rate: markers of peptic ulcer. J Oral Biol Craniofac Res. 2014;4(1):24–29. doi: 10.1016/j.jobcr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ernstgard L. Influence of gender on the metabolism of alcohols in human saliva in vitro. Arch Oral Biol. 2009;54:737–742. doi: 10.1016/j.archoralbio.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 61.Mahesh D.R., Komali G., Jayanthi K., Dinesh D., Saikavitha T.V., Dinesh P. Evaluation of salivary flow rate, pH and buffer in pre, post &Post-Menopausal women on HRT. J Clin Diagn Res. 2014;8:233–236. doi: 10.7860/JCDR/2014/8158.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inoue H., Ono K., Masuda W. Gender difference in unstimulated whole saliva flow rate and salivary gland sizes. Arch Oral Biol. 2006;51:1055–1060. doi: 10.1016/j.archoralbio.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 63.Wang L.-H., Lin C.-Q., Yang L., Li R.-L., Chen L.-H., Chen W.-W. Gender differences in the saliva of young healthy subjects before and after citric acid stimulation. Clin Chim Acta. 2016;460(1):142–145. doi: 10.1016/j.cca.2016.06.040. [DOI] [PubMed] [Google Scholar]
- 64.Lukacs J.R., Largaespada L.L. Explaining sex differences in dental caries prevalence: saliva, hormones, and “life-history” etiologies. Am J Hum Biol. 2006;18(4):540–555. doi: 10.1002/ajhb.20530. [DOI] [PubMed] [Google Scholar]
- 65.Nagler R.M. Salivary glands and the aging process: mechanistic aspects, health-status and medicinal-efficacy monitoring. Biogerontology. 2004;5:223–233. doi: 10.1023/B:BGEN.0000038023.36727.50. [DOI] [PubMed] [Google Scholar]
- 66.Gupta A., Epstein J.B., Sroussi H. Hyposalivation in elderly patients. J Can Dent Assoc. 2006;72:841–846. [PubMed] [Google Scholar]
- 67.Audano M., Maldini M., De Fabiani E., Mitro N., Caruso Donatella. Gender-related metabolomics and lipidomics: from experimental animal models to clinical evidence. J Proteomics. 2018;178:82–91. doi: 10.1016/j.jprot.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Krumsiek J., Mittelstrass K., Do K.T. Gender-specific pathway differences in the human serum metabolome. Metabolomics. 2015;11(6):1815–1833. doi: 10.1007/s11306-015-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okemoto K., Maekawa K., Tajima Y., Tohkin M., Saito Y. Cross-classification of human urinary lipidome by sex, age, and body mass index. PloS One. 2016;11(12) doi: 10.1371/journal.pone.0168188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prodan A., Brand H.S., Ligtenberg A.J. Interindividual variation, correlations, and sex-related differences in the salivary biochemistry of young healthy adults. Eur J Oral Sci. 2015;123(3):149–157. doi: 10.1111/eos.12182. [DOI] [PubMed] [Google Scholar]
- 71.Jove M., Mate I., Naudi A. Is Human Aging, A metabolome-related matter of gender. J Gerontol A Biol Sci Med Sci. 2016;71(5):578–585. doi: 10.1093/gerona/glv074. [DOI] [PubMed] [Google Scholar]
- 72.Trabado S., Al-Salameh A., Croixmarie V. The human plasma-metabolome: reference values in 800 French healthy volunteers; impact of cholesterol, gender and age. PloS One. 2017;12(3) doi: 10.1371/journal.pone.0173615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawanishi N., Hoshi N., Masahiro S. Effects of inter-day and intra-day variation on salivary metabolomic profiles. Clin Chim Acta. 2019;489:41–48. doi: 10.1016/j.cca.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 74.Ladva C.N., Golan R., Greenwald R. Metabolomic profiles of plasma, exhaled breath condensate, and saliva are correlated with potential for air toxics detection. J Breath Res. 2017;12 doi: 10.1088/1752-7163/aa863c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomita A., Mori M., Hiwatari K. Effect of storage conditions on salivary polyamines quantified via liquid chromatography-mass spectrometry. Sci Rep. 2018;8:12075. doi: 10.1038/s41598-018-30482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsuruoka M., Hara J., Hirayama A. Capillary electrophoresis-mass spectrometry-based metabolome analysis of serum and saliva from neurodegenerative dementia patients. Electrophoresis. 2013;34:2865–2872. doi: 10.1002/elps.201300019. [DOI] [PubMed] [Google Scholar]
- 77.Hicks S.D., Khurana N., Williams J., Dowd Greene C., Uhlig R., Middleton F.A. Diurnal oscillations in human salivary microRNA and microbial transcription: implications for human health and disease. PloS One. 2018;13 doi: 10.1371/journal.pone.0198288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sugimoto M., Saruta J., Matsuki C. Physiological and environmental parameters associated with mass spectrometry-based salivary metabolomic profiles. Metabolomics. 2013;9:454–463. [Google Scholar]
- 79.Pfaffe T., Cooper-White J., Beyerlein P., Kostner K., Punyadeera C. Diagnostic potential of saliva. Current state and future applications. Clin Chem. 2011;57:675–687. doi: 10.1373/clinchem.2010.153767. [DOI] [PubMed] [Google Scholar]
- 80.Javaid M.A., Ahmed A.S., Durand R., Tran S.D. Saliva as a diagnostic tool for oral and systemic diseases. J Oral Biol Craniofac Res. 2016;6(1):67–76. doi: 10.1016/j.jobcr.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shipper R.G., Silletti E., Vingerhoeds M.H. Saliva as research material: biochemical, physicochemical and practical aspects. Arch Oral Biol. 2007;52:1114–1135. doi: 10.1016/j.archoralbio.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 82.Wade W.G. The oral microbiome in health and disease. Pharmacol Res. 2013;69(1):137–143. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 83.Zaura E., Nicu E.A., Krom B.P., Keijser B. Acquiring and maintaining a normal oral microbiome: current perspective. Front Cell Infect Microbiol. 2014;26(4):85. doi: 10.3389/fcimb.2014.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim E.-H., Joo J.-Y., Lee Y.J. Grading system for periodontitis by analyzing levels of periodontal pathogens in saliva. PloS One. 2018;13(11) doi: 10.1371/journal.pone.0200900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dawes C., Wong D.T.W. Role of saliva and salivary diagnostics in the advancement of oral health. J Dent Res. 2019;98(2):133–141. doi: 10.1177/0022034518816961. [DOI] [PMC free article] [PubMed] [Google Scholar]