Summary
Long-chain polyunsaturated fatty acids (LC-PUFAs) influence human health in several areas, including cardiovascular disease, diabetes, fatty liver disease, and cancer. ELOVL2 encodes one of the key enzymes in the in vivo synthesis of LC-PUFAs from their precursors. Variants near ELOVL2 have repeatedly been associated with levels of LC-PUFA-derived metabolites in genome-wide association studies (GWAS), but the mechanisms behind these observations remain poorly defined. In this study, we found that rs953413, located in the first intron of ELOVL2, lies within a functional FOXA and HNF4α cooperative binding site. The G allele of rs953413 increases binding of FOXA1/FOXA2 and HNF4α to an evolutionarily conserved enhancer element, conferring allele-specific upregulation of the rs953413-associated gene ELOVL2. The expression of ELOVL2 was significantly downregulated by both FOXA1 and HNF4α knockdown and CRISPR/Cas9-mediated direct mutation to the enhancer element. Our results suggest that rs953413 regulates LC-PUFAs metabolism by altering ELOVL2 expression through FOXA1/FOXA2 and HNF4α cooperation.
Subject Areas: Health Sciences, Biological Sciences, Genetics, Molecular Genetics, Molecular Biology, Molecular Mechanism of Gene Regulation
Graphical Abstract
Highlights
-
•
rs953413 resides in an evolutionarily conserved enhancer region
-
•
rs953413 mediates the cooperative binding of FOXA and HNF4α to the enhancer region
-
•
The rs953413 locus plays a key role in regulating ELOVL2 expression
-
•
rs953413 is implicated in PUFA metabolism by regulating ELOVL2 expression
Health Sciences; Biological Sciences; Genetics; Molecular Genetics; Molecular Biology; Molecular Mechanism of Gene Regulation
Introduction
LC-PUFAs, including ω-3 and ω-6, are essential fatty acids to mammals that cannot be synthesized de novo. The ω-6 arachidonic acid, ω-3 eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) are obtained through the diet either directly or as their precursors, linoleic acid and alpha-linolenic acid. Bioconversion of LC-PUFAs from their precursors is mediated by desaturases encoded by fatty acid desaturase 1 (FADS1) and FADS2 in the FADS gene cluster and elongases encoded by ELOVL fatty acid elongase 2 (ELOVL2) and ELOVL5 (Nakamura and Nara, 2004, Zhang et al., 2016).
Disrupting any of the desaturases and elongases blocked the in vivo synthesis of LC-PUFAs, which significantly affected normal growth and development in mouse models (Fan et al., 2012, Moon et al., 2009, Stroud et al., 2009, Zadravec et al., 2011). Recently, the ELOVL2 enzyme, in addition to FADS2, was verified to be another key enzyme in the synthesis of DHA in vivo (Gregory et al., 2011, Pauter et al., 2014, Pauter et al., 2017). SNPs in the ELOVL2 locus are strongly associated with levels of LC-PUFA-derived metabolites in blood in many GWAS and meta-analyses (Table S1). Minor alleles of SNPs in the ELOVL2 locus were associated with higher levels of EPA and DPA and lower levels of DHA in plasma phospholipids, suggesting carriers of the minor alleles have lower efficiency in the bioconversion cascade from EPA to DPA and DHA catalyzed by the ELOVL2 enzyme (Lemaitre et al., 2011, Suhre et al., 2011). The identification of genetic determinants of plasma and tissue lipid levels is a key enabling factor for successful future personalized medicine interventions with EPA/DHA supplements (Chilton et al., 2017). The recent successful outcome of REDUCE-IT illustrates the value of evaluating the effects of EPA/DHA treatment on specific patient segments (hypertriglyceridemia and related lipoprotein abnormalities selected biochemically or genetically) with elevated risk that is believed to be at least partly attributable to an elevated level of the target of the intervention (Bhatt et al., 2019).
The identification of functional variants behind GWAS is hindered by the linkage disequilibrium (LD) in associated regions. To prioritize the variants in LD with reported SNPs in GWAS for functional validation, we developed an allele-specific (AS)-SNPs pipeline (Cavalli et al., 2016b). This pipeline utilizes the available chromatin immunoprecipitation coupled with massively parallel sequencing (ChIP-seq) data from the Encyclopedia of DNA Elements (ENCODE) project to detect variants located in regulatory regions and that showed allelic imbalance in chromatin binding by different transcription factors (TFs) (The Encode Project Consortium, 2012).
Liver is the most important organ for LC-PUFAs synthesis and ELOVL2 is also highly expressed in the liver. We hypothesized that the observed association of LC-PUFA profiles in blood to the ELOVL2 locus is mainly reflecting the differences in LC-PUFAs metabolism in the liver. Among all the AS-SNPs identified in human liver HepG2 cells, rs953413 located in the first intron of ELOVL2 showed significant allelic imbalance in chromatin binding. In the present study, we found that rs953413, located in an evolutionarily conserved enhancer element, directly regulates ELOVL2 expression by mediating the cooperative binding between FOXA and HNF4α factors and that it is an important contributor to the observed difference in LC-PUFAs levels among carriers of different alleles in GWAS.
Results
Prioritization of Candidate Regulatory Variations in the ELOVL2 Locus
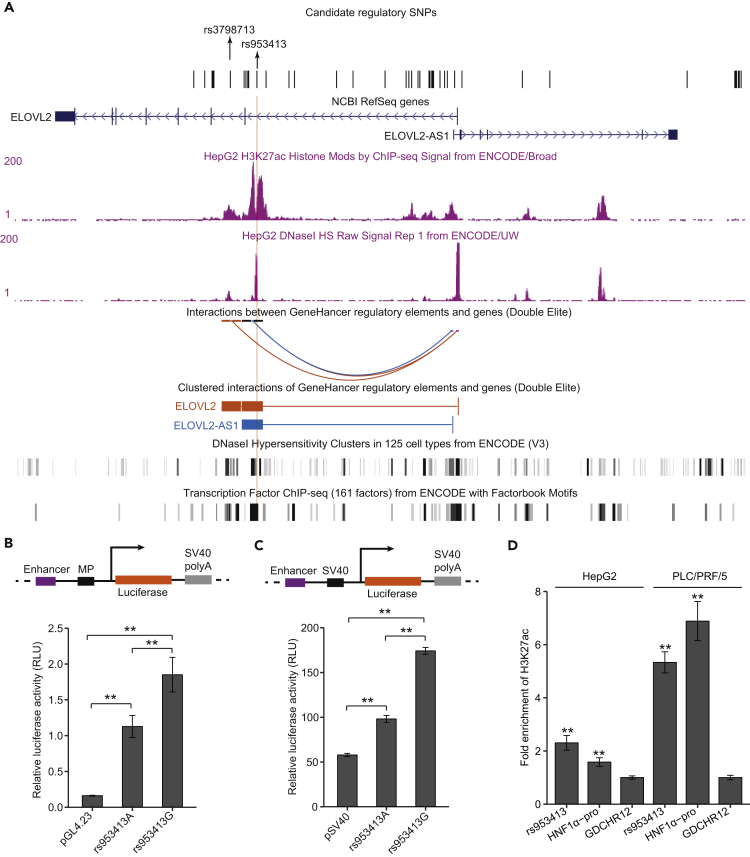
The reported SNPs in the ELOVL2 locus span a region of ∼90kb that totally covers the gene body of ELOVL2 and are observed to be in high LD in the European populations (Figure S1). The alleles of the reported SNPs in LD with the major G allele of rs953413 were generally associated with a more efficient synthesis of LC-PUFAs from their precursors, e.g. synthesis of DHA from EPA and DPA (Draisma et al., 2015, Illig et al., 2009, Lemaitre et al., 2011, Li et al., 2018, Suhre et al., 2011). To pinpoint the true causal variants in the ELOVL2 locus, fine mapping of variants in this region to a 99% credible set was carried out in a trans-ethnic meta-analysis in Chinese- and European-ancestry populations (Hu et al., 2016). A total of 123 potential regulatory variations were identified in the HaploReg database that are in LD (r2 > 0.8 in 1,000 Genomes Pilot I) with the SNPs in the credible set led by rs3798713 for EPA, rs9393915 for DPA, and rs953413 for DHA (Hu et al., 2016). As liver is the most important organ for in vivo synthesis of LC-PUFAs, we hypothesized that the observed association of LC-PUFA profiles in blood to the ELOVL2 locus is mainly reflecting the differences in LC-PUFAs metabolism in the liver. Only the potential regulatory variants that are annotated to be located in active promoter or enhancer regions in the liver that consist of 47 variations were kept for further consideration (Figure 1A; detailed information on variations is listed in Table S2).
Figure 1.
SNP rs953413 Locates in an Active Enhancer Region to Regulate Its Enhancer Activity
(A) Both rs953413 and rs3798713 are located in highly active DNase I hypersensitivity sites that are also enriched with H3K27ac signals in HepG2 cells. The candidate regulatory SNPs in the ELOVL2 locus selected from the credible set that are located in regulatory regions in the liver are listed.
(B and C) The G allele of rs953413 showed significant higher enhancer activity compared with the A allele in luciferase assay coupled with both the MP (B) and SV40 promoter (C). Error bars, s.d. n = 6 from two independent plasmid extractions and transfections with each transfection had three technical replicates.
(D) The rs953413 region is enriched with H3K27ac signal in both HepG2 and PLC/PRF/5 cells compared with the negative control region GDCHR12 in ChIP-qPCR. The HNF1α-pro is employed as the positive control. Error bars, s.d. n = 3 technical replicates. In B–D, **p < 0.01 evaluated using two-tailed Student's t tests.
See also Figure S2.
To further prioritize the identified regulatory variants, we intersected the 47 regulatory variants with SNPs in the ELOVL2 locus that showed allelic imbalance in chromatin binding identified by the AS-SNPs pipeline in HepG2 cells. A total of three SNPs in the ELOVL2 locus including rs953413, rs3798713, and rs17675073 were identified to be AS-SNPs in HepG2 cells that showed allelic imbalance in chromatin binding for FOXA1 and FOXA2 (Table S3) (Cavalli et al., 2016a). As both rs953413 and rs3798713 were identified to be lead SNPs in the calculated credible set and showed allelic imbalance in chromatin binding for FOXA1 and FOXA2, they were selected for further functional validation.
rs953413 Resides in a Liver-Specific Enhancer
The ELOVL2 locus has copy number variation (CNV) in HepG2 cells, which has two copies of chromosome bearing the G allele of rs953413 (Lopez-Terrada et al., 2009, Zhou et al., 2018). To verify that the observed allelic imbalance in rs953413 and rs3798713 is not only caused by the CNV and to identify candidate causal TFs, we employed a more stringent strategy that filtered out PCR duplicates and tolerated no mismatch other than the SNP itself to each TF that mapped to these two regions in ChIP-seq experiments in HepG2 cells. Among all the TFs that have peaks overlapping or nearby rs953413 and rs3798713, only FOXA1 and FOXA2 showed reproducible allelic imbalance in chromatin binding with a preference for the G alleles of rs953413 and rs3798713 (Table S3). In accordance with occupation by multiple TFs, both the rs953413 region and rs3798713 region are highly enriched with H3K27ac signals and are also located in active DNase I hypersensitivity sites in HepG2 cells (Figure 1A). Additionally, both the rs953413 region and rs3798713 region are observed to directly interact with the promoter region of ELOVL2 identified by Capture Hi-C in GeneHancer, suggesting that these regions may be actively involved in ELOVL2 regulation (Figure 1A) (Fishilevich et al., 2017).
We next performed luciferase assay to test the enhancer activity of the two selected regions. As rs17675073 is in proximity to rs3798713, we amplified a 287-bp fragment encompassing both SNPs and inserted upstream of the minimal promoter (MP) to test its ability to drive luciferase expression. This region did show enhancer activity compared with the control plasmid without insertion. However, neither of the SNPs showed significant differences between alleles conditional on the other SNP (Figure S2). This region was then discarded for further analysis. For the rs953413 region, the enhancer activity was tested with a 529-bp fragment with rs953413 located in the middle. Both alleles of rs953413 were observed to have strong enhancer activity in cooperation with the MP in driving luciferase expression. The G allele of rs953413 had a significantly higher enhancer activity compared with the A allele (Figure 1B). This difference in enhancer activity between alleles of rs953413 was further validated with the SV40 promoter in the other luciferase construct (Figure 1C).
The meta-analysis carried out in the CHARGE Consortium provided us with the comprehensive association results for ω-3 fatty acids in plasma phospholipid in the ELOVL2 locus (Lemaitre et al., 2011). rs953413 and its proxies (r2 > 0.8 in 1,000 Genomes phase 3) generally showed stronger association with EPA, DPA, and DHA levels compared with other SNPs (Figure S3). In addition, the rs953413 region showed tissue-specific H3K27ac signals with enrichment of H3K27ac binding only observed in HepG2, A549, and PANC-1 cells from all the available cell lines in the ENCODE project (Figure S4). The enrichment of H3K27ac over the rs953413 region was further validated with ChIP followed by quantitative real time (ChIP-qPCR) in liver cancer cell lines HepG2 and PLC/PRF/5 (Figure 1D). The enrichment of H3K27ac signal together with enrichment of TFs binding to the rs953413 region only in liver cells support the idea that the rs953413 region shows liver-specific enhancer feature (Figure S4). Interestingly, although not coming to genome-wide significant level, carriers of the G allele of rs953413 were observed to have higher expression of ELOVL2 (p = 1.4 × 10−4) in liver tissues from the GTEx project (Figure S5) (Battle et al., 2017). This observation coupled with the preferential binding of FOXA1 and FOXA2 to the G allele of rs953413 suggests that rs953413 may regulate ELOVL2 expression by modulating the enhancer activity of the rs953413 region in liver, which further affects the synthesis of LC-PUFAs.
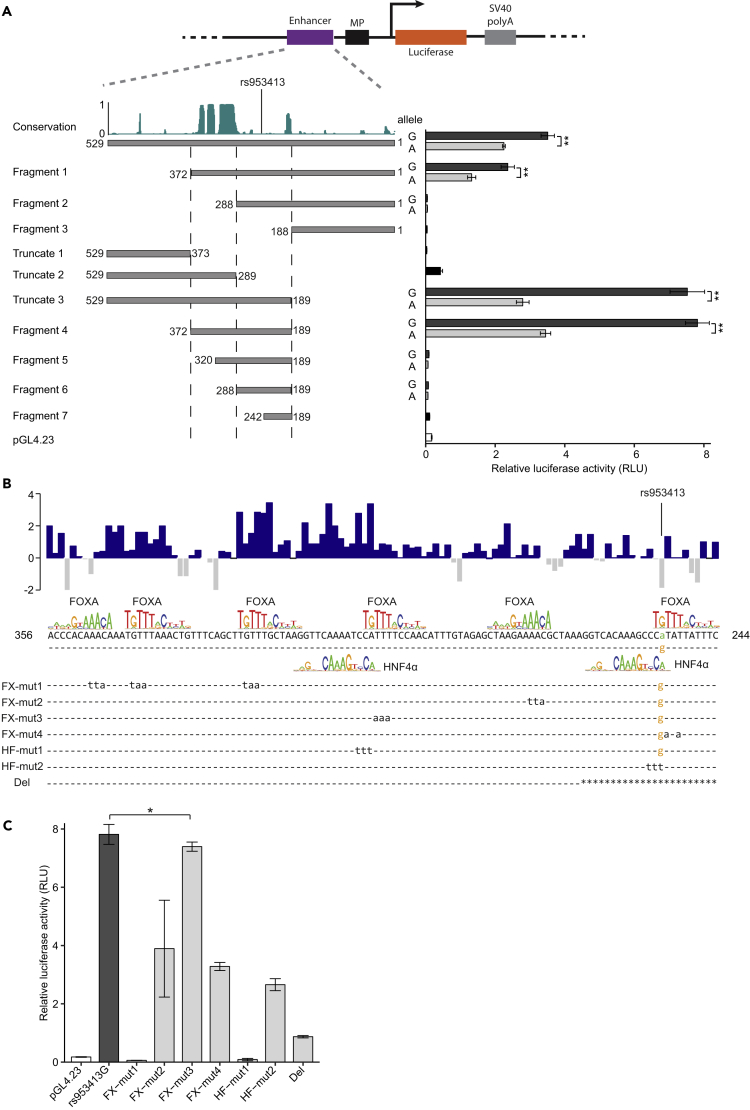
The rs953413 Region Is Evolutionarily Conserved
To identify the causal TFs mediating the difference in enhancer activity in the rs953413 region, we first isolated the minimal enhancer (ME) element for this region. A series of truncation and deletion luciferase constructs were made with both genotypes of the original 529-bp fragments as templates and inserted upstream of the MP sequence as in Figure 1B. The ME element was mapped to a 184-bp fragment (Fragment 4) that showed strong enhancer activity while keeping the sequence at minimum (Figure 2A). This ME element includes a region of around 80-bp in length that is highly conserved in vertebrates and is essential for the observed enhancer activity as only luciferase constructs containing the whole conserved region (the original 529-bp fragment, Fragment 1, 4, and Truncate 3) showed strong enhancer activity. The sequences surrounding rs953413 are less conserved in vertebrates but are also essential for the enhancer activity as the luciferase construct containing only the evolutionarily conserved region (Truncate 2) had drastically decreased enhancer activity compared with luciferase construct containing both the conserved element and the rs953413 surrounding sequence (Truncate 3) (Figure 2A).
Figure 2.
Fine Mapping of the ME Element of the rs953413 Region to an Evolutionarily Conserved Region that Is Enriched with Putative Binding Sites for Both FOXA and HNF4α
(A) The ME element is fine mapped to an evolutionarily conserved region encompassing rs953413 (Fragment 4) by luciferase assay with a series of truncation and deletion constructs from the original 529-bp fragment. The Conservation track showed the phastCons score for 100 species from the ENCODE project.
(B) The ME element was predicted to have multiple conserved binding sites for both FOXA and HNF4α. The basewise conservation score for 100 species from the ENCODE project is displayed on top of the sequence. The luciferase constructs with mutations introduced into the predicted motif sequences are generated by site-directed mutagen and listed at the bottom. The introduced mutations for each mutation construct are listed as lower-case letters with dash line standing for identical nucleotides. For the deletion construct (Del), the deleted region is marked with star character.
(C) The enhancer activity of the rs953413 region is significantly decreased by mutations introduced into the predicted binding sites for HNF4α and FOXA factors (as illustrated in B) in luciferase assay. Luciferase construct Fragment 4 bearing the G allele of rs953413 was employed as template for mutation. For A and C, error bars, s.d. n = 6 technical replicates from two independent plasmid extractions and transfections with each transfection had three technical replicates. *p < 0.05; **p < 0.01 calculated by two-tailed Student's t tests.
We then searched for TFs predicted to bind differently to the rs953413 region with the ME element as template and using the TRAP tool with position weight matrices (PWMs) from both the JASPAR database and literature as references (Mathelier et al., 2014, Thomas-Chollier et al., 2011). In accordance with the observed allelic imbalance in chromatin binding for FOXA1 and FOXA2 in ChIP-seq, we identified multiple highly conserved FOXA motifs together with two conserved HNF4α motifs in the ME element (Figure 2B). The predicted motifs are located in evolutionarily conserved sequences denoted by the basewise conservation in 100 vertebrates from the ENCODE project (Figure 2B). The variant rs953413 is predicted to be located in two closely located motifs bound by FOXA and HNF4α indicative of cooperative binding by these TFs (Jolma et al., 2015).
We next examined the effect of the predicted TF binding sites on the enhancer activity of the rs953413 region by site-directed mutagenesis. Fragment 4 bearing the G allele of rs953413 and containing the whole ME element was employed as the template for construction of mutants (Figure 2B). We observed that introducing mutations at any of the predicted TF binding sites significantly decreased the enhancer activity of the rs953413 region (Figure 2C). The enhancer activity was completely depleted when mutations were introduced to the predicted binding sites in highly conserved region (constructs FX-mut1 and HF-mut1). Notably, introducing mutations to the predicted binding sites overlapping with rs953413 (constructs FX-mut4, HF-mut2, and Del) also severely decreased the enhancer activity, suggesting that rs953413 is functional in determining the enhancer activity of this region (Figures 2B and 2C).
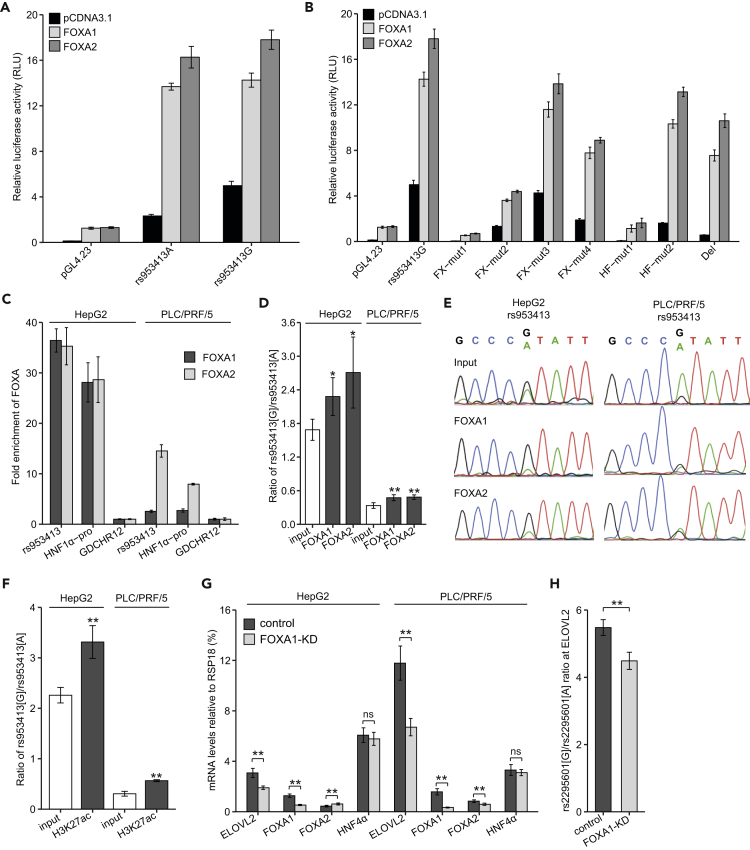
FOXA1 and FOXA2 Regulates ELOVL2 Expression by Binding to the rs953413 Region
We next examined if both FOXA1 and FOXA2 are directly involved in the regulation of the enhancer activity of the rs953413 region. In accordance with our motif prediction, the minimal region that responds to both FOXA1 and FOXA2 induction is mapped to the luciferase construct containing the whole ME element (Fragment 4) from a series of deletion constructs in luciferase assay (Figure S6). Both alleles of rs953413 were highly induced by both FOXA1 and FOXA2 overexpression (Figure 3A). The difference in enhancer activity between alleles of rs953413 was significantly compromised by FOXA overexpression probably due to presence of multiple conserved FOXA binding sites in this region. The responses to both FOXA1 and FOXA2 overexpression were significantly decreased by introducing mutations to the predicted FOXA binding sites in luciferase construct Fragment 4 bearing the G allele of rs953413. Introducing mutations to the predicted HNF4α binding sites also made this region less responsive to both FOXA1 and FOXA2 overexpression with significantly decreased enhancer activities compared with the wild type (Figure 3B). Especially, the response to FOXA1/FOXA2 overexpression was completely depleted in luciferase construct HF-mut1 with one of the predicted HNF4α binding sites being mutated, suggesting that binding of both FOXA1/FOXA2 and HNF4α to the rs953413 region is essential for the enhancer activity.
Figure 3.
FOXA Factors Directly Bind to the ME Element and Confer Allelic Imbalance in ELOVL2 Expression Induced by rs953413
(A) The enhancer activity of the rs953413 region is highly induced by both FOXA1 and FOXA2 overexpression in luciferase assay.
(B) The response to either FOXA1 or FOXA2 overexpression is significantly compromised by the mutations introduced into the predicted binding sites for HNF4α and FOXA factors in luciferase assay. In A and B, error bars, s.d. n = 6 technical replicates from two independent plasmid extractions and transfections with each transfection had three technical replicates.
(C) Both FOXA1 and FOXA2 are highly enriched in the rs953413 region verified by ChIP-qPCR in both HepG2 and PLC/PRF/5 cells. Error bars, s.d.
(D and E) FOXA1 and FOXA2 favor binding to the G allele of rs953413 as determined by ChIP followed by AS-qPCR (D) and ChIP followed by PCR amplification and Sanger sequencing (E) in both HepG2 and PLC/PRF/5 cells. The input for ChIP was used as the control. *p < 0.05 and **p < 0.01 calculated by two-tailed Student's t tests. Error bars, s.d. In C and D, n = 4 for HepG2 cells and n = 3 for PLC/PRF/5 cells.
(F) The G allele of rs953413 is also significantly more enriched with H3K27ac signal compared with the A allele determined by ChIP followed by AS-qPCR. n = 3. Error bars, s.d. **p < 0.01 calculated by two-tailed Student's t tests.
(G) The expression of ELOVL2 is significantly downregulated by FOXA1 knockdown in HepG2 and PLC/PRF/5 cells.
(H) The allelic imbalance in ELOVL2 expression is significantly decreased by FOXA1 knockdown in HepG2 cells. The raw ratios are shown here without correcting for the CNV. In G and H, **p < 0.01 and ns, not significant, calculated by two-tailed Student's t tests. Error bars, s.d. n = 8 technical replicates.
See also Figures S6–S8.
We next assessed the in vivo binding of both FOXA1 and FOXA2 to the rs953413 region through ChIP-qPCR. In accordance with the enrichment of ChIP-seq signals for both FOXA1 and FOXA2 in this region in HepG2 cells from the ENCODE project, the rs953413 region was observed to be highly enriched with FOXA1 and FOXA2 binding in parallel with the positive control region HNF1α-pro after normalization with the negative control region GDCHR12 in both HepG2 and PLC/PRF/5 cells (Figure 3C). To address whether FOXA1/FOXA2 showed allele-specific DNA binding at rs953413 in vivo, we conducted ChIP followed by allele-specific qPCR (AS-qPCR). The allele-specific amplification of each allele was achieved with primers designed following mismatch amplification mutation assay (MAMA) and validated with genomic DNA from samples bearing different genotypes at rs953413 as templates (Figure S7) (Cha et al., 1992, Li et al., 2004). In accordance with the observed preference for the G allele in ChIP-seq experiments, both FOXA1 and FOXA2 were identified to be preferentially bound to the G allele of rs953413 in ChIP followed by AS-qPCR in both HepG2 and PLC/PRF/5 cells (Figure 3D). This allelic imbalance in chromatin binding for both FOXA1 and FOXA2 at the rs953413 region was further confirmed with Sanger sequencing analysis of the rs953413-containing region in chromatin fragments immunoprecipitated with antibody against both FOXA1 and FOXA2 compared with that in the input genomic DNA as control (Figure 3E). Additionally, the G allele of rs953413 was significantly more enriched with H3K27ac signal compared with the A allele in ChIP samples from Figure 1D in both HepG2 and PLC/PRF/5 cells, suggesting more of FOXA binding in this region leading to stronger enhancer activity (Figure 3F).
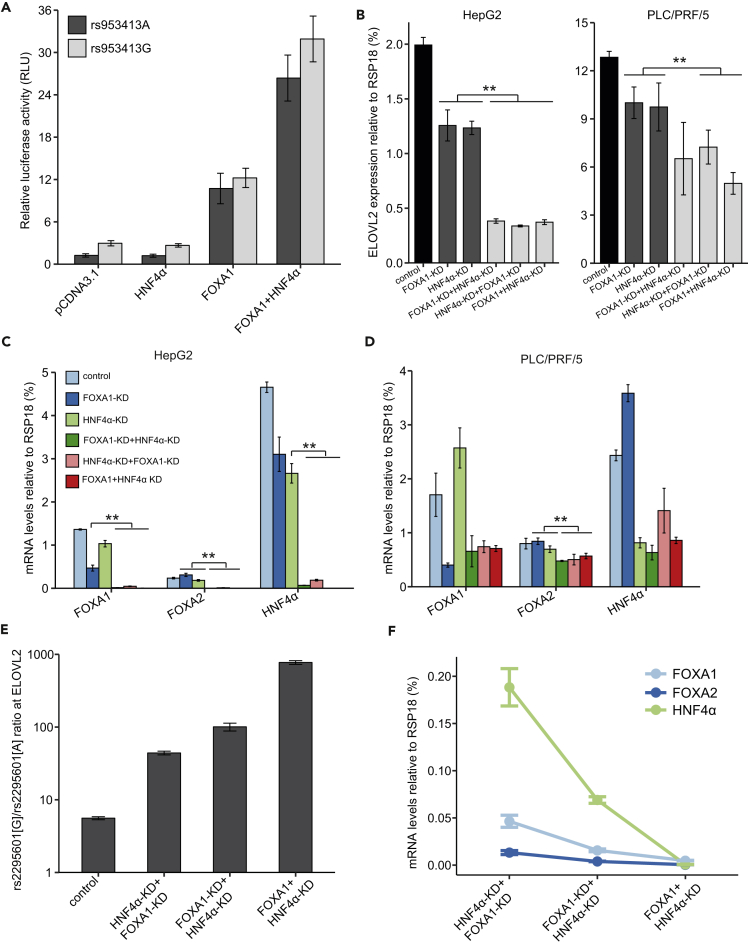
To determine if FOXA1 and FOXA2 are directly involved in regulation of ELOVL2 expression, we next performed lentiviral-mediated FOXA1 knockdown through short hairpin RNA (shRNA) and artificial microRNA (amiRNA) in HepG2 and PLC/PRF/5 cells (Liang et al., 2012). Knockdown of FOXA1 significantly decreased the expression of ELOVL2 in both cells after normalization to reference genes RSP18, ACTB, and GAPDH, respectively (Figures 3G and S8). The expression of FOXA2 is significantly increased by FOXA1 knockdown in HepG2 cells, whereas it is observed to be significantly downregulated by FOXA1 knockdown in PLC/PRF/5 cells, indicative of cell heterogeneity between these two cells. The A allele of exonic SNP rs2295601 of ELOVL2 is on the same haplotype as the A allele of rs953413 evaluated with the data from the 1,000 Genomes Project (Machiela and Chanock, 2015). The efficiency and specificity of AS-qPCR assay targeting rs2295601 was validated with genomic DNA from the same set of samples for rs953413 AS-qPCR as templates (Figure S7). We have evidence that FOXA1/FOXA2 favor binding to the G allele of rs953413 (Figures 3D and 3E). We therefore evaluated whether this allele-specific binding caused allelic imbalance in ELOVL2 expression with rs2295601 AS-qPCR in HepG2 cells, which is heterozygous at this location, whereas PLC/PRF/5 was identified to be homozygous with the G allele. This analysis identified that the G allele of rs2295601 in linkage with the G allele of rs953413 was selectively transcribed in HepG2 cells. Knockdown of FOXA1 significantly diminished the allelic imbalance in ELOVL2 expression (Figure 3H).
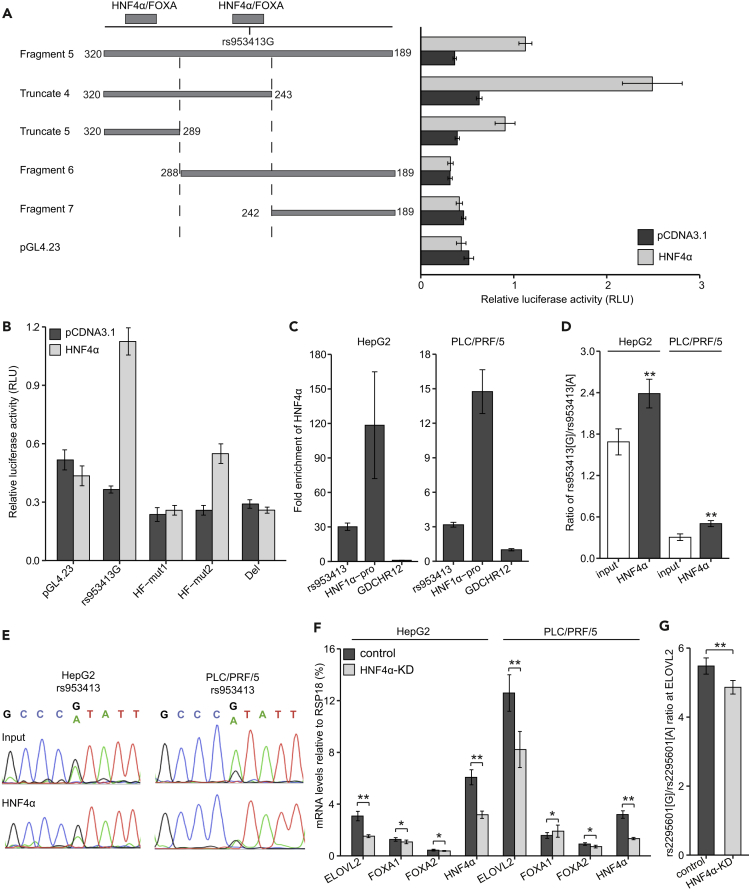
HNF4α Regulates ELOVL2 Expression through Interacting with the rs953413 Region
We next assessed whether HNF4α is also directly involved in ELOVL2 regulation through interacting with the rs953413 region. We observed that luciferase construct (Fragment 5) encompassing both of the predicted HNF4α binding sites was highly induced by HNF4α overexpression (Figure 4A). To refine the HNF4α responsive regions, truncation and deletion luciferase constructs were further constructed and subjected to HNF4α overexpression in luciferase assay. In accordance with our prediction, only constructs containing the predicted HNF4α-binding sites (Fragment 5, Truncate 4 and 5) were induced by HNF4α overexpression. To map the sequences needed for HNF4α induction, we introduced mutations to either of the predicted HNF4α sites with Fragment 5 as template that led to drastically diminished HNF4α induction (Figure 4B). Intriguingly, the Del construct (as illustrated in Figure 2B) with the predicted binding sites for HNF4α and FOXA factors surrounding rs953413 being removed completely abolished the HNF4α induction, suggesting rs953413 is directly located in one functional HNF4α-binding site.
Figure 4.
HNF4α regulates ELOVL2 Expression by Directly Binding to the ME Element and Confers Allelic Imbalance in ELOVL2 Expression by rs953413
(A) Both of the predicted HNF4α binding sites in the ME element are essential for the maximal induction by HNF4α in luciferase assay.
(B) Disruption either of the predicted HNF4α-binding sites significantly decreased the enhancer activity induced by HNF4α in luciferase assay. In A and B, error bars, s.d. n = 6 technical replicates from two independent plasmid extractions and transfections with each transfection had three technical replicates.
(C) The rs953413 region is highly enriched with HNF4α binding compared with GDCHR12 in ChIP-qPCR. Error bars, s.d.
(D and E) HNF4α favors binding to the G allele of rs953413 relative to the A allele as determined by ChIP followed by AS-qPCR (D) and ChIP followed by PCR amplification and Sanger sequencing (E) in both HepG2 and PLC/PRF/5 cells. The input for ChIP was used as the control. **p < 0.01 calculated by two-tailed Student's t tests. Error bars, s.d. In C and D, n = 4 for HepG2 cells and n = 3 for PLC/PRF/5 cells.
(F) The expression of ELOVL2 is significantly decreased by HNF4α knockdown in both HepG2 and PLC/PRF/5 cells.
(G) The allelic imbalance in ELOVL2 expression is significantly decreased by HNF4α knockdown in HepG2 cells. The raw ratios are shown here without correcting for the CNV. In F and G, error bars, s.d. n = 8 technical replicates.
See also Figures S7 and S9.
We next performed ChIP-qPCR assays with antibody against HNF4α in both HepG2 and PLC/PRF/5 cells. In accordance with the data from the ENCODE project, we confirmed HNF4α chromatin binding at the rs953413 region and at the positive HNF1α-pro region after normalization with GDCHR12 (Figure 4C). To address whether HNF4α showed allele-specific DNA binding at rs953413 in vivo, we conducted ChIP followed by rs953413 AS-qPCR. This analysis showed that HNF4α was preferentially bound to the G allele of rs953413 in both HepG2 and PLC/PRF/5 cells (Figure 4D). Consistent with this finding, Sanger sequencing analysis of the rs953413-containing region showed that the G allele was enriched in chromatin fragments immunoprecipitated with antibody against HNF4α compared with the input genomic DNA as control in both cells (Figure 4E). We next performed lentiviral-mediated HNF4α knockdown through shRNA and amiRNA to test its effect on ELOVL2 expression (Liang et al., 2012). Knockdown of HNF4α resulted in significantly decreased expression of ELOVL2 after normalization to reference genes RSP18, ACTB, and GAPDH, respectively (Figures 4F and S9). We then performed rs2295601 AS-qPCR to evaluate if the allelic imbalance in ELOVL2 expression was affected by HNF4α knockdown. After HNF4α knockdown, the allelic imbalance in ELOVL2 expression was also observed to be significantly decreasing (Figure 4G).
Cooperation between HNF4α and FOXA Factors Determine the Enhancer Activity of the rs953413 Region
As both HNF4α and FOXA factors are bound to the rs953413 region and essential for its enhancer activity, we hypothesized that HNF4α and FOXA factors may cooperate with each other in a complex to determine the enhancer activity of the rs953413 region, which further regulates ELOVL2 expression. To test this hypothesis, we first carried out luciferase assay overexpressing both HNF4α and FOXA factors together with luciferase construct Fragment 4 containing the whole ME element. Compared with overexpression of HNF4α alone or either FOXA1 or FOXA2 alone, the enhancer activities of both alleles of rs953413 were significantly increased by overexpression of HNF4α together with either FOXA1 or FOXA2, indicative of cooperation between these TFs in determining the enhancer activity of the rs953413 region (Figures 5A and S10).
Figure 5.
Cooperation between HNF4α and FOXA Factors Determines the Enhancer Activity of the rs953413 Region and ELOVL2 Expression
(A) The enhancer activity of the rs953413 region was significantly increased by simultaneous overexpression of HNF4α and FOXA1 compared with overexpression of only HNF4α or FOXA1 in luciferase assay. Error bars, s.d. n = 6 technical replicates from two independent plasmid extractions and transfections with each transfection had three technical replicates.
(B–D) Double knockdown of both HNF4α and FOXA1 led to a significant decrease in ELOVL2 expression compared with knockdown only HNF4α or FOXA1 (B). This double knockdown also significantly downregulated FOXA2 expression in both HepG2 cells (C) and PLC/PRF/5 cells (D).
(E and F) The allelic imbalance in ELOVL2 expression shown in E is gradually increased by the gradual decreases in expression of HNF4α and FOXA1/FOXA2 in different double-knockdown HepG2 cells as shown in F. In B–F, **p < 0.01 calculated by two-tailed Student's t tests. Error bars, s.d. n = 4 technical replicates.
See also Figures S10 and S11.
To determine the effect of the cooperation between HNF4α and FOXA factors on ELOVL2 expression, we generated HepG2 and PLC/PRF/5 cells with both HNF4α and FOXA1 being knocked down. This double knockdown was achieved with either sequential or simultaneous transduction of lentivirus for HNF4α and FOXA1 knockdown. The double knockdown cells produced with simultaneous knockdown were named FOXA1 + HNF4α KD. FOXA1-KD + HNF4α-KD denotes cells first transduced with lentivirus for FOXA1 knockdown followed by HNF4α knockdown and vice versa for HNF4α-KD + FOXA1-KD. To be comparable, the control cells and cells with knockdown of only FOXA1 or HNF4α were also subjected to two rounds of lentiviral transduction. Compared with knockdown of only HNF4α or FOXA1, this double knockdown strategy led to significant downregulation of not only HNF4α and FOXA1 but also FOXA2 in both cells after normalization to reference genes RSP18, ACTB, and GAPDH, respectively (Figures 5B–5D and S11). It is noteworthy that the degree of knockdown for all the three TFs is much stronger compared with single knockdown in HepG2 cells (Figure 5C). As HNF4α and FOXA1/FOXA2 are all members of the hepatocyte nuclear factor family, the cross-regulation of one another and the synergy in gene regulation has been well studied (Lau et al., 2018). We investigated ChIP-seq signals for histone modifications (H3K4me3 and H3K27ac) in HepG2 cells indicating promoters and enhancers over the gene bodies of all the three TFs. We found that ChIP-seq signals for all the three TFs are enriched over the active chromatin regions of each TF's gene body indicative of coregulation of all the three TFs by themselves (Figure S12). Coupling to the knockdown of all the three TFs, the expression of ELOVL2 was significantly downregulated compared with knockdown of only HNF4α or FOXA1 in both HepG2 and PLC/PRF/5 cells (Figures 5B and S11). In addition, contrary to the observed decrease in allelic imbalance in ELOVL2 expression after knockdown of only HNF4α or FOXA1 in HepG2 cells, the triple knockdown led to a significant increase in the allelic imbalance in ELOVL2 expression (Figure 5E). Interestingly, the degree of allelic imbalance in ELOVL2 expression was tightly associated with the degree of downregulation of the three TFs, indicating that the G allele of rs953413 is preferentially bound by these TFs when the availability of these TFs is limited, which further determines the allelic imbalance in ELOVL2 expression (Figure 5F).
The rs953413 Region Works as a Key Enhancer Region for ELOVL2 Regulation
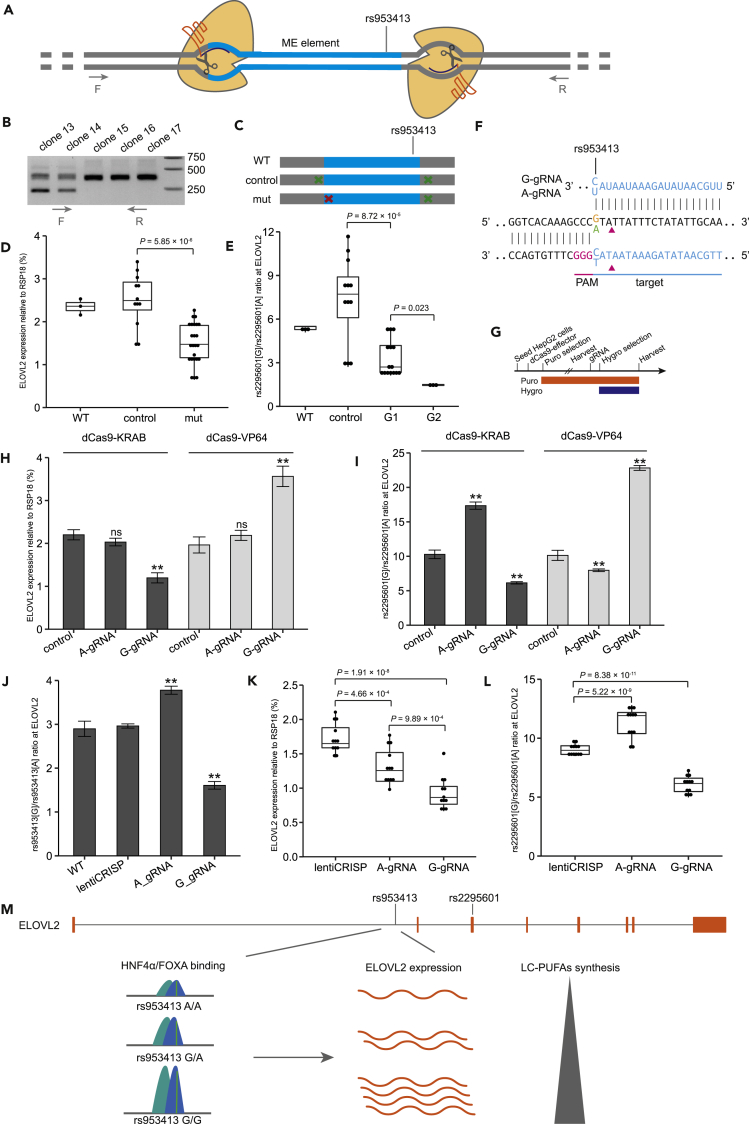
To provide more direct evidence that the rs953413 region is involved in ELOVL2 regulation, we first conducted CRISPR/Cas9-mediated mutagenesis of this region in HepG2 cells. Two guide RNAs were designed to cut the flanking sequences on both sides of the ME element to delete the whole rs953413 region (Figure 6A). After selecting multiple colonies, we did not get single colonies homozygous with each allele of the ME element being deleted due to the existence of CNV in this region. However, we observed that the expression of ELOVL2 together with the allelic imbalance in ELOVL2 expression was significantly different among different single colonies with different mutation profiles in the rs953413 region (Figure 6B). To determine the causal relationship between the mutations introduced into the rs953413 region with ELOVL2 expression, we selected nineteen single colonies with different mutation profiles for further characterization. Sanger sequencing on direct PCR product amplified from the rs953413 region was carried out from both sides to determine the mutation pattern for each colony together with multiple (from 5 to 26) TA clones of PCR product to determine the genotype of each colony. From all the selected colonies, we successfully identified the genotypes of twelve colonies, with the others either having multiple alleles detected or missing the A allele of rs953413 detected.
Figure 6.
CRISP/Cas9-mediated Mutation of the ME Element Impairs ELOVL2 Expression in an Allele-Specific Manner
(A) Schematic illustrating CRISP/Cas9-mediated mutation of the ME element by double gRNA. The ME element is highlighted in blue color. The binding sites for the primer pair used to validate mutations are indicated.
(B) Representative gel image showing PCR amplification of the rs953413 region in selected individual clones displayed varieties of mutations introduced by the CRISP/Cas9 system.
(C) Individual clones are grouped by the position of mutations introduced.
(D) Clones with mutations introduced into the ME element of the rs953413 region (mut, n = 8) had significantly decreased expression level of ELOVL2 compared with clones without key mutations introduced (control, n = 4). WT denotes the ELOVL2 expression level in normal HepG2 cells.
(E) The allelic imbalance in ELOVL2 expression is decreased in a dosage dependent manner by mutations introduced into the ME element bearing the G allele of rs953413. For D and E, each clone had three technical replicates.
(F) Schematic illustrating AS-gRNA targeting rs953413 locus.
(G) Experimental time line for lentiviral transduction and antibiotic selection of HepG2 cells with lentiviral dCas9-effector and AS-gRNA.
(H–L) (H and I) Changes in ELOVL2 expression (H) and the coupled changes in allelic imbalance in ELOVL2 expression (I) following transduction of dCas9-KRAB and dCas9-VP64 together with AS-gRNA. In H and I, **p < 0.01 and ns, not significant, calculated by two-tailed Student's t tests. Error bars, s.d. n = 4 technical replicates (J–L). Each AS-gRNA preferentially mutated respective allele of rs953413 when coupled with wild-type Cas9 (J), which significantly downregulated ELOVL2 expression (K) in an allele-specific manner (L). In J–L, **p < 0.01 calculated by two-tailed Student's t tests. Error bars, s.d. n = 4 in J and n = 12 technical replicates in K and L.
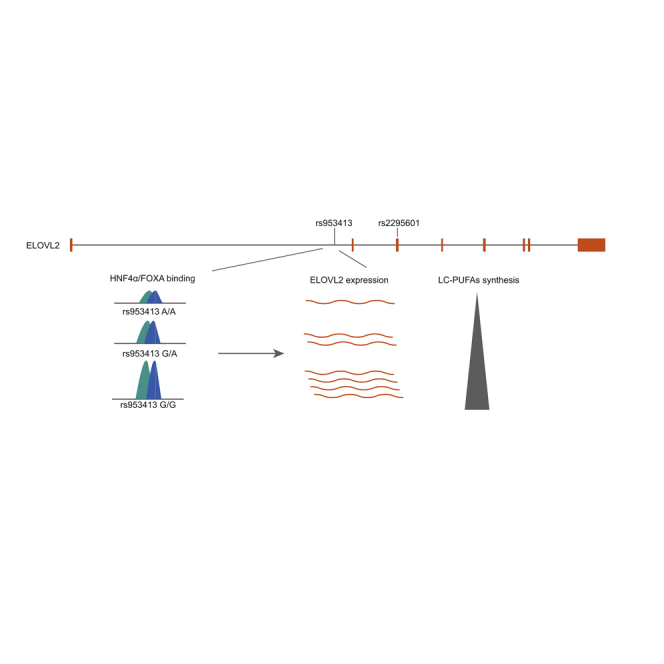
(M) Graphic representation of the regulatory relationships between HNF4α/FOXA, rs953413, and ELVOL2. HNF4α/FOXA binds to the evolutionarily conserved rs953413 region in the intron of ELOVL2 and regulates the expression of ELVOL2. The G allele of rs953413 results in increased HNF4α/FOXA binding and thus upregulates ELVOL2 expression, which consequently leads to increased synthesis of LC-PUFAs.
See also Figures S16–S19.
Our previous findings suggest that the predicted binding sites for FOXA factors are essential for the enhancer activity of the rs953413 region as mutation construct FX-mut1 completely lost the enhancer activity and its response to both FOXA1 and FOXA2 induction in luciferase assay (Figure 3B). The twelve colonies can generally be categorized into two groups based on the location of the mutations introduced. The alleles of the control group only have mutations flanking the ME element and the mut group have mutations to the ME element that also disrupted the predicted binding sites for FOXA factors as in mutation construct FX-mut1 (Figure 6C). Four colonies were categorized as control (Figure S13) and eight colonies fell into the category of mut with five colonies having critical mutations in one copy of the ME element bearing the G allele of rs953413 (G1; Figure S14). One colony had mutations in both copies of ME element bearing the G allele of rs953413 (G2; Figure S15A) and two colonies had all the alleles of the ME elements mutated (all; Figure S15B). In accordance with our previous results, the colonies with mutations in the predicted FOXA-binding sites (mut) had significantly decreased expression of ELOVL2 compared with the control group (control) after normalization with reference genes RSP18, ACTB, and GAPDH, respectively (Figures 6D and S16). Accordingly, the allelic imbalance in ELOVL2 expression detected by rs2295601 AS-qPCR was significantly decreased in both group G1 and G2 compared with the control group in a dosage-dependent manner (Figure 6E). In addition, the two colonies grouped with all the copies of the ME elements being mutated (all) had relatively more conserved FOXA binding sites in the ME elements bearing the G allele of rs953413 compared with that of the A allele of rs953413 that led to significantly increased allelic imbalance in ELOVL2 expression (Figure S17). This suggested that the ME element encompassing rs953413 is a key regulatory region for ELOVL2 expression.
The double gRNA strategy failed to directly mutate rs953413. We searched the rs953413 region again and designed allele-specific gRNA (AS-gRNA) that directly targets rs953413 to provide complementary evidence that rs953413 is essential for ELOVL2 expression (Figure 6F). As rs953413 is the first nucleotide 3′ adjacent to PAM, which is part of the seed sequence essential for efficient gRNA binding, the designed AS-gRNA targeting rs953413 in theory should show high allelic preference in binding to the rs953413 region (Semenova et al., 2011). To prove our hypothesis, each AS-gRNA targeting rs953413 was coupled with the catalytically inactive Cas9 (dCas9) fused with either the activating VP64 domain (dCas9-VP64) or the suppressive KRAB domain (dCas9-KRAB) to be delivered into HepG2 cells by lentiviral transduction to test its effect on ELOVL2 expression in trans (Ho et al., 2017) (Figure 6G). The AS-gRNA targeting the G allele of rs953413 (G-gRNA) potently suppressed ELOVL2 expression when coupled with the suppressive dCas9-KRAB, whereas the ELOVL2 expression was significantly increased by G-gRNA coupled with dCas9-VP64 after normalization with reference genes RSP18, ACTB, and GAPDH, respectively (Figures 6H and S18). The AS-gRNA targeting the A allele of rs953413 (A-gRNA) is less effective in regulating ELOVL2 expression, which was only observed to significantly increase ELOVL2 expression when coupled with dCas9-VP64 after normalization with GAPDH (Figure S18B). However, the rs2295601 AS-qPCR clearly showed that both AS-gRNA displayed high allelic preference in affecting ELOVL2 expression (Figure 6I). When coupled with dCas9-KRAB, each AS-gRNA preferentially suppressed the transcription of ELOVL2 located on the same chromosome with target allele of rs953413. While coupling with dCas9-VP64, the ELOVL2 transcript in linkage with respective allele of rs953413 was selectively activated by AS-gRNA.
We next coupled each AS-gRNA with wild-type Cas9 to directly introduce mutations to rs953413 to determine its effect on ELOVL2 expression in HepG2 cells. The allelic imbalance in mutating rs953413 by each AS-gRNA was determined with rs953413 AS-qPCR, which can specifically quantify the relative amount of mutations introduced into each allele of rs953413. In accordance with our previous observations, the A allele of rs953413 was preferentially mutated by A-gRNA coupled with wild-type Cas9, whereas the G allele of rs953413 was preferentially mutated by G-gRNA compared with cells transduced with lentiCRISP v2 virus as control (Figure 6J). The expression of ELOVL2 was observed to be significantly downregulated by mutations introduced to rs953413 by each AS-gRNA after normalization with reference genes RSP18, ACTB, and GAPDH, respectively (Figures 6K and S19). Accordingly, preferentially introducing mutations to either allele of rs953413 significantly decreased the expression of its linked ELOVL2 transcript determined by rs2295601 AS-qPCR (Figure 6L). These observations together with the results from double gRNA clearly demonstrated that rs953413 determines the enhancer activity of the identified ME element which further regulates ELOVL2 expression.
Discussion
We here demonstrate that rs953413 mediates the cooperative binding of HNF4α and FOXA1/FOXA2 to the evolutionarily conserved enhancer region, which acts as a key cis-regulatory element for ELOVL2 expression. The G allele of rs953413 is preferentially bound by the complex formed by these TFs and increases the expression of ELOVL2 that further increases the amount of LC-PUFAs synthesized (Figure 6M).
As members of the hepatocyte nuclear factor family, HNF4α and FOXA1/FOXA2 are all essential TFs for normal liver function and their expression profile also showed high tissue specificities with a preference for the liver, pancreas, and kidney (Lau et al., 2018). In accordance with the enhancer activity tightly regulated by the cooperation between FOXA1/FOXA2 and HNF4α, the rs953413 region showed tissue-specific enhancer features with both active chromatin marker H3K27ac and different TFs enriched mainly in liver HepG2 cells (Figure S4). As liver is the most important organ for LC-PUFAs synthesis, the rs953413 region is supposed to be a key enhancer region for ELOVL2 regulation in the liver, which further determines the systemic LC-PUFAs profiles. In accordance with the G allele of rs953413 upregulating ELOVL2 expression in our study, SNPs in LD with the G allele of rs953413 were generally associated with a more efficient conversion of DHA from its precursors in GWAS (Draisma et al., 2015, Illig et al., 2009, Lemaitre et al., 2011, Li et al., 2018, Suhre et al., 2011). The expression of ELOVL2 in other tissues might be regulated by different mechanisms other than the rs953413 region illustrated by the observation that the A allele of rs953413 was associated with higher expression of ELOVL2 in transformed fibroblasts (p = 1.5 × 10−6) from the GTEx project (Figure S5) (Battle et al., 2017). Additionally, it should be noted that an independent association between SNPs indexed by rs2281591 in the ELOVL2 locus with DPA levels was observed in conditional analyses in both European- and Chinese-ancestry populations (Hu et al., 2016, Lemaitre et al., 2011). As these SNPs are not in LD with rs953413, this independent association signal suggests that there are other functional variants in this locus involved in ELOVL2 regulation.
Previous studies have shown that ω-3 PUFAs, particularly DHA, may influence human health by exerting beneficial effects on many diseases such as cardiovascular disease, diabetes, cancer, depression, nonalcoholic fatty liver disease (NAFLD), and rheumatoid arthritis (Jump et al., 2018, Lopez-Vicario et al., 2014, Shahidi and Ambigaipalan, 2018). Recently, rs2236212, an intronic SNP of ELOVL2, was shown to be associated with NALFD in obese subjects (Zusi et al., 2019). Patients and animal models of nonalcoholic steatohepatitis (NASH), a severe form of NAFLD, showed marked decrease in hepatic ω-3 PUFAs levels, which may play a role in the development and progression of NASH (Jump et al., 2018, Lopez-Vicario et al., 2014). Dietary intervention with ω-3 PUFAs DHA alone or EPA/DHA have shown indications of improvement in biomarkers related to NASH. The identified TFs in this study, including FOXA1/FOXA2 and HNF4α, are tightly involved in NAFLD progression and are significantly downregulated in NAFLD (Lake et al., 2016, Moya et al., 2012, Wang et al., 2017, Weiss et al., 2017). This implies that downregulation of ELOVL2 together with its upstream regulators FOXA1/FOXA2 and HNF4α impairs hepatic DHA synthesis, which may play a role in the pathogenesis of NAFLD.
As a key enzyme in the in vivo synthesis of DHA, dysregulation of ELOVL2 may also be involved in impairment of the systemic inflammatory process and diabetes progression (Bellini et al., 2018, Cruciani-Guglielmacci et al., 2017, Talamonti et al., 2017, Tikhonenko et al., 2010). However, further studies are needed to investigate the key TFs regulating ELOVL2 in other cell types. It is interesting to note that HNF4α, FOXA1, and FOXA2 have been extensively studied and appears to be involved in the progression of diabetes, and variations nearby HNF4α and FOXA2 have been reported to be associated with diabetes related traits in GWAS (Lau et al., 2018, MacArthur et al., 2017). ELOVL2 may be an important downstream target in diabetes caused by dysregulation of these TFs.
In conclusion, we show that rs953413 affects LC-PUFAs levels by altering ELOVL2 expression through FOXA1/FOXA2 and HNF4α cooperation. The results provide important mechanistic insights to the transcriptional machinery regulating ELOVL2 in the liver and thereby circulating levels of PUFAs. Further studies are needed to elucidate the role of this pathway in diseases, liver diseases in particular.
Limitations of the Study
Due to lack of detailed genotype and phenotype data at individual level in reported GWAS in this locus, the exact effect of rs953413 and other variations conditional on rs953413 on the reported phenotypes cannot be determined. As the exonic SNP rs2295601 is homozygous in PLC/PRF/5 cells, the changes in allelic imbalance in ELOVL2 expression upon modulating the rs953413 locus cannot be determined in this cell line.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The study was supported by grants from AstraZeneca, The Swedish Diabetes Foundation (DIA 2015-064, 2017-269), The Family Ernfors Fund, and EXODIAB to CW and the Borgström Foundation to MC. Sequencing was performed at the SNP&SEQ platform of SciLifeLab, Uppsala. Funding for open access charge: The Swedish Diabetes Foundation (2017-269).
Author Contributions
C.W. conceived and oversaw this study. G.P. performed the experiments. G.P. and C.W. analyzed the data and wrote the manuscript with input from M.C., B.C., S.S., and C.K.. All authors approved the manuscript.
Declaration of Interests
B.C., S.S., and C.K. are employees of AstraZeneca and may own stock or stock options. The remaining authors declare no competing interests.
Published: January 9, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.100808.
Supplemental Information
3
References
- Battle A., Brown C.D., Engelhardt B.E., Montgomery S.B. Genetic effects on gene expression across human tissues. Nature. 2017;550:204–213. doi: 10.1038/nature24277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini L., Campana M., Rouch C., Chacinska M., Bugliani M., Meneyrol K., Hainault I., Lenoir V., Denom J., Veret J. Protective role of the ELOVL2/docosahexaenoic acid axis in glucolipotoxicity-induced apoptosis in rodent beta cells and human islets. Diabetologia. 2018;61:1780–1793. doi: 10.1007/s00125-018-4629-8. [DOI] [PubMed] [Google Scholar]
- Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., Doyle R.T., Juliano R.A., Jiao L., Granowitz C. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- Cavalli M., Pan G., Nord H., Wallen Arzt E., Wallerman O., Wadelius C. Allele-specific transcription factor binding in liver and cervix cells unveils many likely drivers of GWAS signals. Genomics. 2016;107:248–254. doi: 10.1016/j.ygeno.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Cavalli M., Pan G., Nord H., Wallerman O., Wallen Arzt E., Berggren O., Elvers I., Eloranta M.L., Ronnblom L., Lindblad Toh K. Allele-specific transcription factor binding to common and rare variants associated with disease and gene expression. Hum. Genet. 2016;135:485–497. doi: 10.1007/s00439-016-1654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha R.S., Zarbl H., Keohavong P., Thilly W.G. Mismatch amplification mutation assay (MAMA): application to the c-H-ras gene. PCR Methods Appl. 1992;2:14–20. doi: 10.1101/gr.2.1.14. [DOI] [PubMed] [Google Scholar]
- Chilton F.H., Dutta R., Reynolds L.M., Sergeant S., Mathias R.A., Seeds M.C. Precision nutrition and omega-3 polyunsaturated fatty acids: a case for personalized supplementation approaches for the prevention and management of human diseases. Nutrients. 2017;9:1165. doi: 10.3390/nu9111165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciani-Guglielmacci C., Bellini L., Denom J., Oshima M., Fernandez N., Normandie-Levi P., Berney X.P., Kassis N., Rouch C., Dairou J. Molecular phenotyping of multiple mouse strains under metabolic challenge uncovers a role for Elovl2 in glucose-induced insulin secretion. Mol. Metab. 2017;6:340–351. doi: 10.1016/j.molmet.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draisma H.H.M., Pool R., Kobl M., Jansen R., Petersen A.-K., Vaarhorst A.A.M., Yet I., Haller T., Demirkan A., Esko T. Genome-wide association study identifies novel genetic variants contributing to variation in blood metabolite levels. Nat. Commun. 2015;6:7208. doi: 10.1038/ncomms8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y.Y., Monk J.M., Hou T.Y., Callway E., Vincent L., Weeks B., Yang P., Chapkin R.S. Characterization of an arachidonic acid-deficient (Fads1 knockout) mouse model. J. Lipid Res. 2012;53:1287–1295. doi: 10.1194/jlr.M024216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishilevich S., Nudel R., Rappaport N., Hadar R., Plaschkes I., Iny Stein T., Rosen N., Kohn A., Twik M., Safran M. GeneHancer: genome-wide integration of enhancers and target genes in GeneCards. Database. 2017;2017:1–17. doi: 10.1093/database/bax028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory M.K., Gibson R.A., Cook-Johnson R.J., Cleland L.G., James M.J. Elongase reactions as control points in long-chain polyunsaturated fatty acid synthesis. PLoS One. 2011;6:e29662. doi: 10.1371/journal.pone.0029662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S.M., Hartley B.J., Flaherty E., Rajarajan P., Abdelaal R., Obiorah I., Barretto N., Muhammad H., Phatnani H.P., Akbarian S. Evaluating synthetic activation and repression of neuropsychiatric-related genes in hiPSC-derived NPCs, neurons, and astrocytes. Stem Cell Reports. 2017;9:615–628. doi: 10.1016/j.stemcr.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Li H.X., Lu L., Manichaikul A., Zhu J.W., Chen Y.D.I., Sun L., Liang S., Siscovick D.S., Steffen L.M. Genome-wide meta-analyses identify novel loci associated with n-3 and n-6 polyunsaturated fatty acid levels in Chinese and European-ancestry populations. Hum. Mol. Genet. 2016;25:1215–1224. doi: 10.1093/hmg/ddw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illig T., Gieger C., Zhai G., Römisch-Margl W., Wang-Sattler R., Prehn C., Altmaier E., Kastenmüller G., Kato B.S., Mewes H.-W. A genome-wide perspective of genetic variation in human metabolism. Nat. Genet. 2009;42:137–141. doi: 10.1038/ng.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolma A., Yin Y., Nitta K.R., Dave K., Popov A., Taipale M., Enge M., Kivioja T., Morgunova E., Taipale J. DNA-dependent formation of transcription factor pairs alters their binding specificity. Nature. 2015;527:384–388. doi: 10.1038/nature15518. [DOI] [PubMed] [Google Scholar]
- Jump D.B., Lytle K.A., Depner C.M., Tripathy S. Omega-3 polyunsaturated fatty acids as a treatment strategy for nonalcoholic fatty liver disease. Pharmacol. Ther. 2018;181:108–125. doi: 10.1016/j.pharmthera.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake A.D., Chaput A.L., Novak P., Cherrington N.J., Smith C.L. Transcription factor binding site enrichment analysis predicts drivers of altered gene expression in nonalcoholic steatohepatitis. Biochem. Pharmacol. 2016;122:62–71. doi: 10.1016/j.bcp.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau H.H., Ng N.H.J., Loo L.S.W., Jasmen J.B., Teo A.K.K. The molecular functions of hepatocyte nuclear factors - in and beyond the liver. J. Hepatol. 2018;68:1033–1048. doi: 10.1016/j.jhep.2017.11.026. [DOI] [PubMed] [Google Scholar]
- Lemaitre R.N., Tanaka T., Tang W., Manichaikul A., Foy M., Kabagambe E.K., Nettleton J.A., King I.B., Weng L.-C., Bhattacharya S. Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the charge Consortium. PLoS Genet. 2011;7:e1002193. doi: 10.1371/journal.pgen.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Kadura I., Fu D.-J., Watson D.E. Genotyping with TaqMAMA. Genomics. 2004;83:311–320. doi: 10.1016/j.ygeno.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Li Y., Sekula P., Wuttke M., Wahrheit J., Hausknecht B., Schultheiss U.T., Gronwald W., Schlosser P., Tucci S., Ekici A.B. Genome-wide association studies of metabolites in patients with CKD identify multiple loci and illuminate tubular transport mechanisms. J. Am. Soc. Nephrol. 2018;29:1513–1524. doi: 10.1681/ASN.2017101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G., He H., Li Y., Yu D. A new strategy for construction of artificial miRNA vectors in Arabidopsis. Planta. 2012;235:1421–1429. doi: 10.1007/s00425-012-1610-5. [DOI] [PubMed] [Google Scholar]
- Lopez-Terrada D., Cheung S.W., Finegold M.J., Knowles B.B. Hep G2 is a hepatoblastoma-derived cell line. Hum. Pathol. 2009;40:1512–1515. doi: 10.1016/j.humpath.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Lopez-Vicario C., Gonzalez-Periz A., Rius B., Moran-Salvador E., Garcia-Alonso V., Lozano J.J., Bataller R., Cofan M., Kang J.X., Arroyo V. Molecular interplay between Delta5/Delta6 desaturases and long-chain fatty acids in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2014;63:344–355. doi: 10.1136/gutjnl-2012-303179. [DOI] [PubMed] [Google Scholar]
- MacArthur J., Bowler E., Cerezo M., Gil L., Hall P., Hastings E., Junkins H., McMahon A., Milano A., Morales J. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog) Nucleic Acids Res. 2017;45:D896–D901. doi: 10.1093/nar/gkw1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiela M.J., Chanock S.J. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31:3555–3557. doi: 10.1093/bioinformatics/btv402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathelier A., Zhao X., Zhang A.W., Parcy F., Worsley-Hunt R., Arenillas D.J., Buchman S., Chen C.Y., Chou A., Ienasescu H. Jaspar 2014: an extensively expanded and updated open-access database of transcription factor binding profiles. Nucleic Acids Res. 2014;42:D142–D147. doi: 10.1093/nar/gkt997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon Y.-A., Hammer R.E., Horton J.D. Deletion of ELOVL5 leads to fatty liver through activation of SREBP-1c in mice. J. Lipid Res. 2009;50:412–423. doi: 10.1194/jlr.M800383-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya M., Benet M., Guzmán C., Tolosa L., García-Monzón C., Pareja E., Castell J.V., Jover R. Foxa1 reduces lipid accumulation in human hepatocytes and is down-regulated in nonalcoholic fatty liver. PLoS One. 2012;7:e30014. doi: 10.1371/journal.pone.0030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M.T., Nara T.Y. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu. Rev. Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- Pauter A.M., Olsson P., Asadi A., Herslof B., Csikasz R.I., Zadravec D., Jacobsson A. Elovl2 ablation demonstrates that systemic DHA is endogenously produced and is essential for lipid homeostasis in mice. J. Lipid Res. 2014;55:718–728. doi: 10.1194/jlr.M046151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauter A.M., Trattner S., Gonzalez-Bengtsson A., Talamonti E., Asadi A., Dethlefsen O., Jacobsson A. Both maternal and offspring Elovl2 genotypes determine systemic DHA levels in perinatal mice. J. Lipid Res. 2017;58:111–123. doi: 10.1194/jlr.M070862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova E., Jore M.M., Datsenko K.A., Semenova A., Westra E.R., Wanner B., van der Oost J., Brouns S.J., Severinov K. Interference by clustered regularly interspaced short palindromic repeat (CRISPR) RNA is governed by a seed sequence. Proc. Natl. Acad. Sci. U S A. 2011;108:10098–10103. doi: 10.1073/pnas.1104144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi F., Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018;9:345–381. doi: 10.1146/annurev-food-111317-095850. [DOI] [PubMed] [Google Scholar]
- Stroud C.K., Nara T.Y., Roqueta-Rivera M., Radlowski E.C., Lawrence P., Zhang Y., Cho B.H., Segre M., Hess R.A., Brenna J.T. Disruption of FADS2 gene in mice impairs male reproduction and causes dermal and intestinal ulceration. J. Lipid Res. 2009;50:1870–1880. doi: 10.1194/jlr.M900039-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhre K., Shin S.-Y., Petersen A.-K., Mohney R.P., Meredith D., Wägele B., Altmaier E., CARDIoGRAM. Deloukas P., Erdmann J. Human metabolic individuality in biomedical and pharmaceutical research. Nature. 2011;477:54–60. doi: 10.1038/nature10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamonti E., Pauter A.M., Asadi A., Fischer A.W., Chiurchiu V., Jacobsson A. Impairment of systemic DHA synthesis affects macrophage plasticity and polarization: implications for DHA supplementation during inflammation. Cell Mol. Life Sci. 2017;74:2815–2826. doi: 10.1007/s00018-017-2498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Encode Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Chollier M., Hufton A., Heinig M., O'Keeffe S., Masri N.E., Roider H.G., Manke T., Vingron M. Transcription factor binding predictions using TRAP for the analysis of ChIP-seq data and regulatory SNPs. Nat. Protoc. 2011;6:1860–1869. doi: 10.1038/nprot.2011.409. [DOI] [PubMed] [Google Scholar]
- Tikhonenko M., Lydic T.A., Wang Y., Chen W.Q., Opreanu M., Sochacki A., McSorley K.M., Renis R.L., Kern T., Jump D.B. Remodeling of retinal fatty acids in an animal model of diabetes A decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes. 2010;59:219–227. doi: 10.2337/db09-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Yao L.-J., Shen W., Ding K., Shi P.-M., Chen F., He J., Ding J., Zhang X., Xie W.-F. FOXA2 alleviates CCl4-induced liver fibrosis by protecting hepatocytes in mice. Sci. Rep. 2017;7:15532. doi: 10.1038/s41598-017-15831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T.S., Lupke M., Ibrahim S., Buechler C., Lorenz J., Ruemmele P., Hofmann U., Melter M., Dayoub R. Attenuated lipotoxicity and apoptosis is linked to exogenous and endogenous augmenter of liver regeneration by different pathways. PLoS One. 2017;12:e0184282. doi: 10.1371/journal.pone.0184282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadravec D., Tvrdik P., Guillou H., Haslam R., Kobayashi T., Napier J.A., Capecchi M.R., Jacobsson A. ELOVL2 controls the level of n-6 28:5 and 30:5 fatty acids in testis, a prerequisite for male fertility and sperm maturation in mice. J. Lipid Res. 2011;52:245–255. doi: 10.1194/jlr.M011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.Y., Kothapalli K.S.D., Brenna J.T. Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis. Curr. Opin. Clin. Nutr. Metab. Care. 2016;19:103–110. doi: 10.1097/MCO.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Ho S.S., Greer S.U., Spies N., Bell J.M., Zhang X., Zhu X., Arthur J.G., Byeon S., Pattni R. Haplotype-resolved and integrated genome analysis of ENCODE cell line HepG2. bioRxiv. 2018 doi: 10.1093/nar/gkz169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusi C., Mantovani A., Olivieri F., Morandi A., Corradi M., Miraglia Del Giudice E., Dauriz M., Valenti L., Byrne C.D., Targher G. Contribution of a genetic risk score to clinical prediction of hepatic steatosis in obese children and adolescents. Dig. Liver Dis. 2019;51:1586–1592. doi: 10.1016/j.dld.2019.05.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
3