Abstract
Background
Subfertility is a condition found in up to 15% of couples of reproductive age. Gamete micromanipulation, such as intracytoplasmic sperm injection (ICSI), is very useful for treating couples with compromised sperm parameters. An alternative method of sperm selection has been described; the spermatozoa are selected under high magnification (over 6000x) and used for ICSI. This technique, named intracytoplasmic morphologically selected sperm injection (IMSI), has a theoretical potential to improve reproductive outcomes among couples undergoing assisted reproduction techniques (ART). However, our previous version of this Cochrane Review was unable to find evidence that supported this possible beneficial effect. This is an update of Teixeira 2013.
Objectives
To identify, appraise, and summarise the available evidence regarding efficacy and safety of IMSI compared to ICSI in couples undergoing ART.
Search methods
We searched for randomised controlled trials (RCTs) in these electronic databases: the Cochrane Gynaecology and Fertility Group Specialised Register, CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, LILACS, and in these trial registers: ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform. We also handsearched the reference lists of included studies and similar reviews. We performed the last electronic search on 18 November 2019.
Selection criteria
We only considered RCTs that compared ICSI and IMSI; we did not include quasi‐randomised trials. We considered studies that permitted the inclusion of the same participant more than once (cross‐over or per cycle trials) only if data regarding the first treatment of each participant were available.
Data collection and analysis
Two review authors independently performed study selection, data extraction, and assessment of the risk of bias and quality of the evidence; we solved disagreements by consulting a third review author. We corresponded with study investigators to resolve any queries, as required.
Main results
The updated search retrieved 535 records; we included 13 parallel‐designed RCTs comparing IMSI and ICSI (four studies were added since the previous version), comprising 2775 couples (IMSI = 1256; ICSI = 1519).
We are uncertain if IMSI improves live birth rates (risk ratio (RR) 1.11, 95% confidence interval (CI) 0.89 to 1.39; 5 studies, 929 couples; I² = 1%), miscarriage rates per couple (RR 1.07, 95% CI 0.78 to 1.48; 10 studies, 2297 couples; I² = 0%, very‐low quality evidence), and miscarriage rate per pregnancy (RR 0.90, 95% CI 0.68 to 1.20; 10 studies, 783 couples; I² = 0%, very‐low quality evidence). We are uncertain if IMSI improves clinical pregnancy rates (RR 1.23, 95% CI 1.11 to 1.37; 13 studies, 2775 couples; I² = 47%, very‐low quality evidence). None of the included studies reported congenital abnormalities. We judged the evidence for all outcomes to be of very low‐quality. We downgraded the quality of the evidence due to limitations of the included studies (risk of bias), inconsistency of results, and a strong indication of publication bias.
Authors' conclusions
The current evidence from randomised controlled trials does not support or refute the clinical use of intracytoplasmic sperm injection (intracytoplasmic morphologically selected sperm injection (IMSI). We are very uncertain of the chances of having a live birth and of the risk of having a miscarriage. We found very low‐quality evidence that IMSI may increase chances of a clinical pregnancy, which means that we are still very uncertain about any real difference.
We did not find any trials reporting on the risk of congenital abnormalities. Well‐designed and sufficiently powered trials are still required.
Plain language summary
Regular (ICSI) versus ultra‐high magnification (IMSI) sperm selection for assisted reproduction
Background: Sperm micromanipulation, such as intracytoplasmic sperm injection (ICSI), is very useful for treating couples in which the male partner has reduced sperm concentration, motility, or both. In the past decade, a different approach for sperm selection has been described, which analyses sperm under ultra‐high powered magnification (6000x). Initial studies showed that intracytoplasmic morphologically selected sperm injection (IMSI), using sperm selected under high magnification, was associated with higher pregnancy rates than those selected with conventional ICSI in couples with repeated implantation failures. However, the evidence from our previous Cochrane Review was uncertain of the real beneficial effects of this intervention.
Search date: We updated our search of the medical literature in November 2019, looking for studies that evaluated the effectiveness and safety of IMSI (using 6000x magnification) compared to conventional ICSI (using 200x to 400x magnification) procedures.
Study characteristics: We found 13 randomised controlled trials (four more than in the previous version), evaluating 2775 couples, that compared regular ICSI with IMSI for assisted reproduction. These studies were funded by fertility centres and universities.
Key results: Based on the very low‐quality evidence that we found, we are uncertain of the benefit of IMSI over ICSI. The chance of having a live birth with IMSI was between 20% and 32%, compared to 25% with ICSI. For women with a 7% risk of miscarriage with regular ICSI, the risk with IMSI was between 5% and 10%. The clinical pregnancy rate with IMSI was between 35% and 44%, compared with 32% with ICSI.
Quality of the evidence: We downgraded the quality of the evidence because of limitations in the included studies (risk of bias), inconsistency of the observed effect across studies, and high risk of publication bias. There was no evidence concerning congenital abnormalities. We conclude that the current evidence is very limited for suggesting using IMSI instead of ICSI in clinical practice.
Summary of findings
for the main comparison.
| Regular (ICSI) compared with ultra‐high magnification (IMSI) for assisted reproduction | |||||
|
Patient or population: couples undergoing assisted reproduction treatment Setting: fertility clinics Intervention: sperm selection under ultra‐high magnification (IMSI) Comparison: sperm selection under regular magnification (ICSI) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| ICSI | IMSI | ||||
| Live birth per allocated couple | 243 per 1000 |
269 per 1000 (216 to 337) |
RR 1.11 (0.89 to 1.39) | 929 (5 studies) | ⊕⊝⊝⊝ very lowa,b |
| Miscarriage per allocated couple | 70 per 1000 |
75 per 1000 (54 to 103) |
RR 1.07 (0.78 to 1.48) | 2297 (10 studies) |
⊕⊝⊝⊝ very lowb,c |
| Miscarriage per clinical pregnancy | 230 per 1000 |
207 per 1000 (157 to 276) |
RR 0.90 (0.68 to 1.20) | 783 (10 studies) | ⊕⊝⊝⊝ very lowb,c |
| Clinical pregnancy per allocated couple | 320 per 1000 |
394 per 1000 (355 to 438) |
RR 1.23 (1.11 to 1.37) | 2775 (13 studies) | ⊕⊝⊝⊝ very lowc,d,e |
| Congenital abnormalities per live birth | No studies reported on this outcome | ||||
| The median control group risk across studies was used as the basis for the assumed risk. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICSI: intracytoplasmic sperm injection; IMSI: intracytoplasmic morphologically selected sperm injection; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High. We are very confident that the true effect lies close to that of the observed in this review Moderate. We are moderately confident in the effect estimate; although the true effect is likely to be close to the observed in this review, there is a possibility of being substantially different Low. Our confidence in the effect estimate is limited, since the true effect may be substantially different from that observed in this review Very low. We have very little confidence in the effect estimate, since the true effect is likely to be substantially different from that observed in this review | |||||
aThe quality of the evidence was downgraded one level because of limitations of the included studies – all studies had unclear risk of selection bias bDowngraded two levels due to very serious imprecision cDowngraded once due to the limitations of the included studies – high or unclear of risk of bias in most domains dDowngraded one level due to inconsistency across studies (I² = 47%) eDowngraded one level due to high risk of publication bias
Background
Description of the condition
Subfertility is a condition found in up to 15% of couples of reproductive age; until the late 1970s, there were few treatment options for those couples. Compromised semen parameters account for 20% to 30% of infertility cases; at least 30 million men worldwide are considered subfertile (Agarwal 2015). Since the first successful in vitro fertilisation (IVF) was described, the efficacy of subfertility treatment has greatly improved. However, for those men, IVF was still insufficient. During the 1980s, other assisted reproductive technology (ART) techniques were developed, which focused on gamete micromanipulation; yet, for all these techniques, spermatozoa had to be progressively motile and have the potential for an acrosome reaction, leaving infertility due to severe male factors inadequately treated. After the introduction of the intracytoplasmic sperm injection (ICSI), couples in which the men had severe male factor infertility could now achieve pregnancy (Palermo 1992).
Description of the intervention
For ICSI, after sperm preparation, an optical magnification of 200x to 400x is used to examine the sample. The best 'normal looking' motile spermatozoa are selected, based on their major morphology, and then injected into oocytes retrieved after ovarian stimulation. However, live birth rates still remain low, which may justify the search for novel interventions to improve the results (Gleicher 2019). In the early 2000s, an alternative approach to sperm selection was described (Bartoov 2002). This technique requires the analysis of minor morphological criteria using ultra‐high magnification (≥ 6000x) microscopy. When using this technique, the motile sperm fraction is examined, based on six subcellular organelles: acrosome, postacrosomal lamina, neck, mitochondria, tail, and nucleus. When the ICSI used high magnification to select the sperm, it was named intracytoplasmic morphologically selected sperm injection (IMSI (Bartoov 2003)).
How the intervention might work
With high magnification, some organelle malformations that are not detectable using standard magnification may be seen, and it is thought that sperm selection based on these small details will improve reproductive outcomes (Berkovitz 2006).
Why it is important to do this review
Initial reports had shown that IMSI was associated with higher pregnancy rates in couples with repeated implantation failures (Bartoov 2002; Bartoov 2003). High magnification became available as a new technique, adding costs to the treatment, without any proven evidence of benefit or safety. Our previous Cochrane Review evaluating this comparison concluded that there was no evidence of benefit for live birth or miscarriage (Teixeira 2013). However, the uncertainty of our previous findings, and the fact that IMSI continues to be used in clinical practice justify the need for an updated review.
Objectives
To identify, appraise, and summarise the available evidence regarding efficacy and safety of intracytoplasmic morphologically selected sperm injection (IMSI) compared to intracytoplasmic sperm injection (ICSI) in couples undergoing assisted reproductive technology (ART).
Methods
Criteria for considering studies for this review
Types of studies
We considered only truly randomised controlled trials (RCTs) to be eligible; we did not include quasi or pseudo‐randomised trials. We included cross‐over trials only if data regarding the first treatment of each couple were available.
Types of participants
Couples undergoing assisted reproductive technology (ART).
Types of interventions
Trials comparing intracytoplasmic morphologically selected sperm injection (IMSI), using high magnification (≥ 6000x), versus intracytoplasmic sperm injection (ICSI), using regular magnification (200x to 400x), for sperm selection.
Types of outcome measures
Primary outcomes
Live birth. However, we used ongoing pregnancy, defined as a clinical pregnancy that is less likely to result in a miscarriage (i.e. those with fetal heartbeat beyond 10 to 16 weeks of gestational age) as a surrogate in case live birth was not reported.
Miscarriage per allocated couple and per pregnancy
Secondary outcomes
Clinical pregnancy per allocated couple
Congenital abnormality per live birth
Although implantation rates were important outcomes for this review, we did not include them in the quantitative meta‐analysis, because of different denominators (transferred embryos). However, we reported them in the 'Characteristics of included studies' tables.
Search methods for identification of studies
We searched for all published and unpublished RCTs that compared IMSI to ICSI, in consultation with the Gynaecology and Fertility Group (CGF) Information Specialist. We did not limit searches by language or publication status.
Electronic searches
We searched the following electronic databases for relevant trials:
The CGF Specialised Register of Controlled Trials, PROCITE (searched 18 November 2019; Appendix 1);
CENTRAL; Ovid (searched, Issue October 2019, 18 November 2019; Appendix 2);
MEDLINE Ovid (1946 to 18 November 2019; Appendix 3);
Embase Ovid (1980 to 18 November 2019; Appendix 4);
PsycINFO Ovid (1806 to 18 November 2019; Appendix 5);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1961 to 18 November 2019; Appendix 6);
LILACS Web (Latin American and Caribbean Health Science Information database; 18 November 2019; Appendix 7)
We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying randomised trials (Lefebvre 2011). We combined the Embase, PsycINFO, and CINAHL searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN).
We also searched for relevant trial registrations in:
ClinicalTrials.gov (clinicaltrials.gov; searched 18 Noveber 2019; Appendix 8);
World Health Organization (WHO) International Clinical Trials Registry Platform search portal (apps.who.int/trialsearch/Default.aspx; searched 18 November 2019; Appendix 8).
Searching other resources
We handsearched reference lists of relevant trials and systematic reviews retrieved by the electronic searches, and contacted experts in the field to obtain additional trials. We also searched conference abstracts that were not covered in the CGF Specialised Register.
Data collection and analysis
Selection of studies
After DMT and AHM independently completed the initial screen of titles and abstracts retrieved by the search, we retrieved the full texts of all potentially eligible studies. Two review authors (DMT and AHM) independently examined these full‐text articles for compliance with the inclusion criteria, and select eligible studies. We corresponded with study investigators as required, to clarify study eligibility. We resolved disagreements by discussion. If any reports required translation, we described the process used for data collection. We documented the selection process with a PRISMA flow chart.
Data extraction and management
Two review authors (DMT and AHM) independently extracted data from eligible studies using a data extraction form designed and pilot‐tested by the authors. Any disagreements were resolved by discussion with all authors. Data extracted included study characteristics and outcome data. Where studies had multiple publications, the authors collated multiple reports of the same under a single study ID with multiple references.
We corresponded with study investigators for further data on methods or results, as required.
Assessment of risk of bias in included studies
Two review authors (DMT and AHM) independently assessed risk of bias in the included studies using the Cochrane 'Risk of bias' assessment tool to assess: selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias (Higgins 2011). We assigned judgements as recommended in the Cochrane Handbook for Systematic Reviews of Interventions, Section 8.5 (Higgins 2011). We resolved disagreements through discussion. We described all judgements fully and present the conclusions in the 'Risk of Bias' table, which we incorporated into the interpretation of review findings by means of sensitivity analyses.
With respect to within‐trial selective reporting, where identified studies failed to report the primary outcome of live birth, but did report interim outcomes, such as pregnancy, we assessed whether the interim values were similar to those reported in studies that also reported live birth.
Measures of treatment effect
For dichotomous data (e.g. live birth rates), we used the numbers of events in the control and intervention groups of each study to calculate the Mantel‐Haenszel risk ratio (RR). We preferred to use RR, because odds ratio (OR) is harder to understand and apply in practice. Misinterpretation of the OR, as if it is the same as the RR, will tend to overestimate the intervention effect, especially when events are common, and there is concern that this occurs quite frequently in published reports of individual studies and systematic reviews (Higgins 2011). However, had we observed a zero event count or prevalence less than 1%, we would have used the Peto fixed‐effect OR, because this method is found to be the least biased and most powerful, providing the best confidence interval (CI) coverage in these situations (Higgins 2011); the OR value in such situations is also very similar to RR, avoiding misinterpretations. We calculated the 95% CI to determine the precision of the estimates. Whenever estimates were statistically significant, we also determined the number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH).
Unit of analysis issues
The primary analysis was per couple randomised. Exceptions were miscarriage, where we also considered the number of clinical pregnancies as the denominator, and congenital abnormality, which we will analyse per live birth when it is measured. We counted the delivery of a multiple pregnancy (e.g. twins or triplets) as one live birth event.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis, when possible (i.e. including all randomised couples in analysis, in the groups to which they were randomised). We attempted to obtain missing data from the original trials. When these were unobtainable, we did not use imputation of individual values. We assumed that live birth did not occurred in couples with cycle cancellation.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the measure of the I². An I² value greater than 50% indicated substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication and other reporting biases, we aimed to minimise their potential impact by ensuring we conducted a comprehensive search for eligible studies, and by being alert for duplication of data. We used a funnel plot to explore the possibility of small study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
Using a fixed‐effect model, we combined the data from sufficiently similar studies that compared IMSI versus ICSI. An increased risk of a particular outcome associated with IMSI, which may be beneficial (e.g. live birth) or detrimental (e.g. miscarriage), was displayed graphically in the meta‐analysis to the right of the centre line, and a decreased risk to the left of the centre line.
Subgroup analysis and investigation of heterogeneity
When we detected a substantial heterogeneity (I² > 50%), we explored possible explanations in subgroup analyses (e.g. differing populations), sensitivity analyses (e.g. differing risk of bias), or both. We took statistical heterogeneity into account when interpreting the results, especially when there was any variation in the direction of effect.
We planned to perform the following subgroup analyses.
Sperm quality: studies including only couples where the male partner had poor sperm quality versus partners with good or unselected sperm quality.
Sperm source: ejaculate versus surgical.
Previously unsuccessful embryo transfers: studies only including women with repeated previously unsuccessful embryo transfers versus studies including any women (irrespective of the number of previous attempts)
Sensitivity analysis
We had planned to perform sensitivity analyses for the outcome, live birth or ongoing pregnancy, to determine whether the conclusions were robust, given our arbitrary decisions made regarding the eligibility and analysis. This analysis included consideration of whether the review conclusions would have differed if:
Eligibility had been restricted to studies with low risk of bias (defined as low risk of selection bias and no high risk of bias in any other domain);
Only studies reporting live birth without any imputation had been included.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro and Cochrane methods (GRADEpro GDT; Higgins 2011). This table evaluates the overall quality of the body of evidence for the main review outcomes (live birth, clinical pregnancy, miscarriage, and congenital abnormality) for the comparison IMSI versus ICSI. We assessed the quality of the evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness, and publication bias). Two review authors, working independently, made the judgements about evidence quality (high, moderate, low, or very low); they resolved disagreements discussion. They justified, documented, and incorporated judgments into the reporting of results for each outcome. The quality of the evidence was interpreted as follows (Balshem 2011): high: we are very confident that the true effect lies close to that of the observed in this review; moderate: we are moderately confident in the effect estimate, although the true effect is likely to be close to the observed in this review, there is a possibility of being substantially different; low: our confidence in the effect estimate is limited, since the true effect may be substantially different from that observed in this review; very low: we have very little confidence in the effect estimate, since the true effect is likely to be substantially different from the observed in this review.
We extracted study data, formatted our comparisons in data tables, and prepared a 'Summary of findings' table before writing the results and conclusions of our review.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The updated search retrieved 535 records. We considered 27 to be potentially eligible, and examined them for eligibility. Combining results of the updated search and the previous review, 13 trials (in 17 records) met our inclusion criteria, and we excluded 15 studies (in 17 records). Five studies are awaiting classification. There is one ongoing study. The study flow diagram is shown in Figure 1.
1.
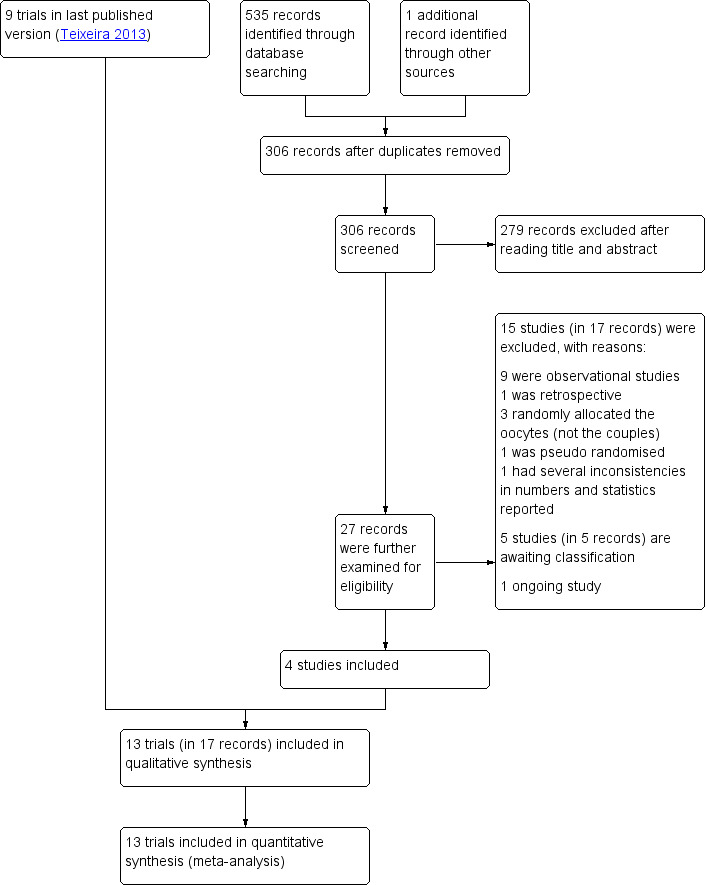
Study flow diagram
Included studies
Study design and setting
We included 13 RCTs (2775 couples) in this review. All were single‐centre studies, conducted in private assisted reproduction centres or academic centres, from Italy (Antinori 2008; Marci 2013), Turkey (Balaban 2011), Brazil (Figueira 2011; Setti 2011; Setti 2012a; Setti 2012b), Slovenia (Knez 2011; Knez 2012), Tunisia (Mahmoud 2011), France (Leandri 2013), USA (Check 2013), and Iran (Mangoli 2019).
Participants
The studies included 1256 couples in the intervention groups (intracytoplasmic morphologically selected sperm injection (IMSI)) and 1519 couples in the control groups (intracytoplasmic sperm injection (ICSI)). Seven studies included only couples in which the male partner had poor sperm quality (Antinori 2008; Knez 2011; Knez 2012; Leandri 2013; Mahmoud 2011; Mangoli 2019; Setti 2011); three included women with advanced maternal age (Figueira 2011; Setti 2012a; Setti 2012b); one study included only women with repeated implantation failure (Check 2013); and one study included couples who underwent assisted reproductive technology (ART), without specifying further details (Balaban 2011). One trial excluded couples with female factor infertility (Antinori 2008), and three excluded women with polycystic ovaries syndrome (PCOS) or endometriosis (Knez 2011; Knez 2012; Setti 2012b).
Interventions
All studies compared IMSI versus ICSI .
Outcomes
Five studies reported live birth (Balaban 2011; Check 2013; Leandri 2013; Mangoli 2019; Marci 2013);
All thirteen studies reported clinical pregnancy.
Nine studies reported miscarriage (Antinori 2008; Check 2013; Figueira 2011; Knez 2011; Leandri 2013; Marci 2013; Setti 2011; Setti 2012a; Setti 2012b).
No studies reported congenital abnormalities.
Excluded studies
We excluded 15 studies from the review, for the following reasons:
ten were not RCTs: nine were observational studies (Apryshko 2010; Bartoov 2003; Berkovitz 2005; Berkovitz 2006; Cassuto 2011; Ghazali 2017; Hazout 2005; Oliveira 2011; Wilding 2011), and one was a retrospective study (Gatimel 2016);
three randomly allocated the oocytes, not the couples (Braga 2011; De Vos 2013; Mauri 2011);
one was pseudo‐randomised (La Sala 2015);
in one, we could not determine if the study was adequately randomised. In addition, the number of women or couples enrolled was higher than the number of cycles reported, and we found several inconsistencies in numbers reported and statistical analysis performed (Karabulut 2019).
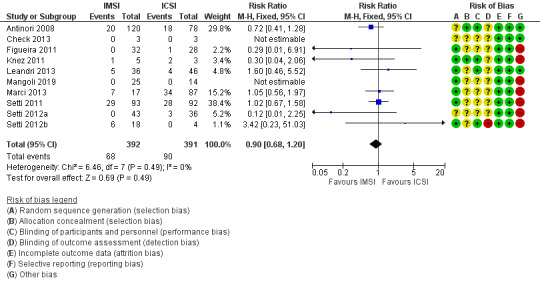
Risk of bias in included studies
See table Characteristics of included studies, Figure 2, and Figure 3 for detailed information.
2.
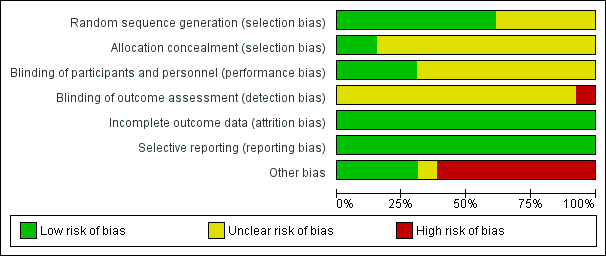
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
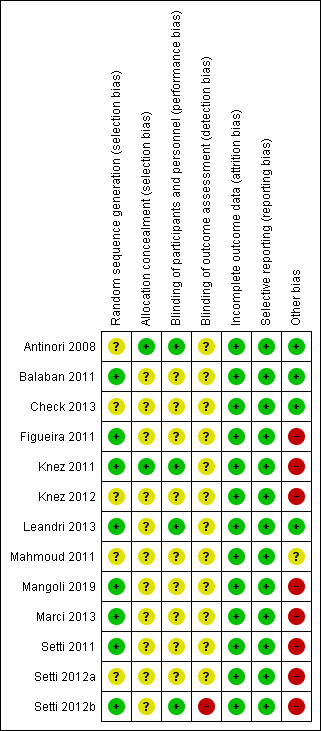
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
Eight of the thirteen studies were at low risk of selection bias related to sequence generation, as they used computer randomisation or a random numbers table (Balaban 2011; Figueira 2011; Knez 2011; Leandri 2013; Mangoli 2019; Marci 2013; Setti 2011; Setti 2012b). The other five studies did not describe the method used, and were at unclear risk of this bias.
Two studies were at low risk of selection bias related to allocation concealment, as they used sealed, opaque envelopes prepared by research nurses (Antinori 2008; Knez 2011). The other eleven studies did not describe the method used for allocation concealment, and we classified them to be at unclear risk of bias.
Blinding
Four studies were at low risk of performance bias as the couples and personnel were blinded to the intervention performed (Antinori 2008; Knez 2011; Leandri 2013;Setti 2012b); the other nine studies did not report blinding and we judged them to be at unclear risk of bias in this domain.
One study was at high risk of detection bias, as primary study team was not blinded to the intervention performed (Setti 2012b). All other twelve studies did not report blinding of outcome assessment and we judged them to be at unclear risk of detection bias.
Incomplete outcome data
We considered all thirteen studies to be at low risk of bias in this domain, as they stated that all allocated women or couples were analysed.
Selective reporting
We considered all thirteen studies to be at low risk of selective reporting bias, because clinical pregnancy was reported by all included studies. Although eight studies did not report live birth, and none reported congenital malformations, we believe these studies might not have been designed to evaluate these outcomes, as they require longer follow‐up to be properly assessed.
Other potential sources of bias
We deemed eight studies to be at high risk of other bias: four because there were substantial differences for the mean number of oocytes retrieved, or embryos transferred between groups, or both (Figueira 2011; Setti 2011; Setti 2012a; Setti 2012b); one because reported miscarriage rates were quite different than the calculated rates (Marci 2013); two because different interventions were performed for the study and control groups (Knez 2011; Knez 2012); and one because the number of embryos transferred for each group was not reported, and demographic characteristics between groups were not compared (Mangoli 2019). We considered one study to be at unclear risk of bias, because there was insufficient information to compare the number of oocytes retrieved or embryos transferred, or both, per woman (Mahmoud 2011). We judged four studies to be at low risk of other potential sources of bias (Antinori 2008; Balaban 2011; Check 2013; Leandri 2013).
Effects of interventions
See: Table 1
1. IMSI versus ICSI for assisted reproduction
Primary outcomes
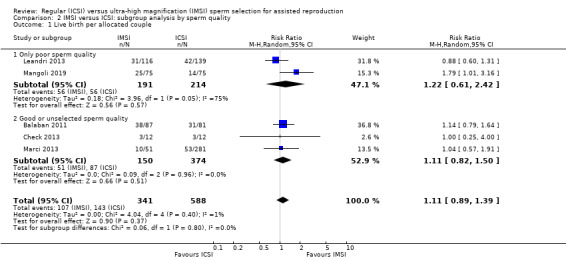
1.1 Live birth (effectiveness)
We are uncertain whether IMSI improves live birth (risk ratio (RR) 1.11, 95% confidence interval (CI) 0.89 to 1.39; 5 studies, 929 couples; I² = 1%; very low‐quality evidence; Analysis 1.1; Figure 4). This suggests that if the chance of having a live birth with ICSI is assumed to be 24%, the chance following IMSI, would be between 21% and 33%.
1.1. Analysis.

Comparison 1 Ultra‐high (IMSI) versus regular magnification (ICSI), Outcome 1 Live birth per allocated couple.
4.
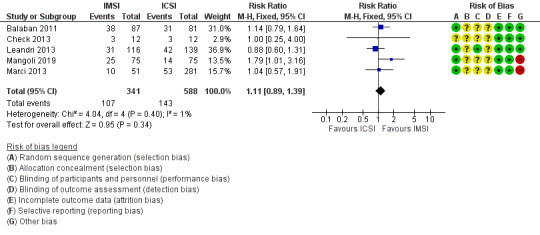
Forest plot of comparison: Ultra‐high (IMSI) versus regular magnification (ICSI), outcome: 1.1 Live birth per allocated couple.
There were no studies at low risk of selection bias, and therefore, we did not perform a sensitivity analysis.
1.2 Miscarriage per allocated couple (adverse events)
We are uncertain whether IMSI reduces miscarriage rate per couple (RR 1.07, 95% CI 0.78 to 1.48; 10 studies, 2297 couples; I² = 0%, very low‐quality evidence; Analysis 1.2). This suggests that if the chance of having a miscarriage with ICSI is assumed to be 7%, the chance following IMSI, would be between 5% and 10%. Sensitivity analysis restricting the eligibility to studies with low risk of bias would make the estimates even more uncertain and imprecise (RR 0.93, 95% CI 0.09 to 9.58; 1 study, 57 couples; I² = not applicable).
1.2. Analysis.

Comparison 1 Ultra‐high (IMSI) versus regular magnification (ICSI), Outcome 2 Miscarriage per allocated couple.
1.3 Miscarriage per clinical pregnancy (adverse events)
We are uncertain whether IMSI reduces miscarriage rate per clinical pregnancy (RR 0.90, 95% CI 0.68 to 1.20; 10 studies, 783 couples; I² = 0%, very low‐quality evidence; Analysis 1.3; Figure 5). This suggests that if the chance of having a miscarriage with ICSI is assumed to be 23%, the chance following IMSI, would be between 16% and 28%. Sensitivity analysis restricting the eligibility to studies with low risk of bias would make the estimates even more uncertain and imprecise (RR 0.30, 95% CI 0.04 to 2.06; 1 study, 8 couples; I² = not applicable).
1.3. Analysis.
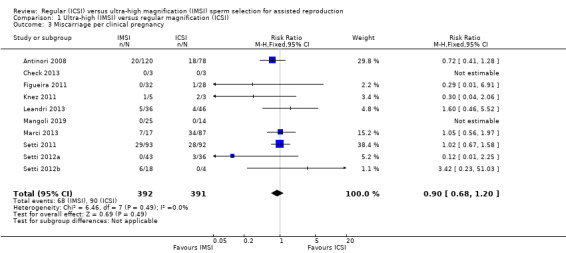
Comparison 1 Ultra‐high (IMSI) versus regular magnification (ICSI), Outcome 3 Miscarriage per clinical pregnancy.
5.
Forest plot of comparison: Ultra‐high (IMSI) versus regular magnification (ICSI), outcome: 1.3 Miscarriage per clinical pregnancy
Secondary outcomes
1.4 Clinical pregnancy (effectiveness)
Due to the very low quality evidence, we are uncertain whether IMSI was associated with an increase in clinical pregnancy rates (RR 1.23, 95% CI 1.11 to 1.37; 13 studies, 2775 couples; I² = 47%; very low‐quality evidence; Analysis 1.4; Figure 6). This suggests that if the chance of having a clinical pregnancy with ICSI is assumed to be 32%, the chance following IMSI would be between 35% and 44%. The resulting number needed to treat for an additional beneficial outcome (NNTB) was 12 (95% CI 7 to 45).
1.4. Analysis.

Comparison 1 Ultra‐high (IMSI) versus regular magnification (ICSI), Outcome 4 Clinical pregnancy per allocated couple.
6.
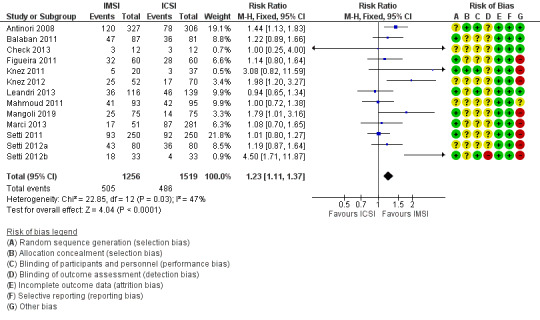
Forest plot of comparison: Ultra‐high (IMSI) versus regular magnification (ICSI), outcome: 1.4 Clinical pregnancy per allocated couple
Subgroup analysis (separating the studies by those that included only couples with poor sperm quality and those that included couples with good or unselected sperm quality) did not reduce the observed heterogeneity (Analysis 2.2). Sensitivity analysis restricting the eligibility to studies with low risk of bias changed this estimate, making the evidence of benefit highly uncertain and imprecise (RR 3.08, 95% CI 0.82 to 11.59; 1 study, 57 couples; I² = not applicable). The asymmetric funnel plot suggested a small studies effect, and therefore, we strongly suspected publication bias (Figure 7).
2.2. Analysis.

Comparison 2 IMSI versus ICSI: subgroup analysis by sperm quality, Outcome 2 Clinical pregnancy per allocated couple.
7.

Funnel plot of comparison: Ultra‐high (IMSI) versus regular magnification (ICSI), outcome: 1.4 Clinical pregnancy per allocated couple
1.5 Congenital abnormalities (adverse events)
None of the included studies reported congenital abnormalities.
Discussion
Summary of main results
High magnification led to inconclusive results for live birth or miscarriage and was associated with a small improvement in clinical pregnancy rates. However, all evidence was of very low‐quality, and thus, we have very little confidence in these results. The true effects are likely to be substantially different from those observed in this review. There is no available evidence on the impact on congenital abnormalities. See Table 1 for further details.
Overall completeness and applicability of evidence
The objectives of this review were addressed by the included studies. Six studies included only couples with poor sperm quality, and six included couples with good or unselected sperm quality; however, these subgroup analyses did not add information to the main analysis (Analysis 2.2). No study sorted the couples according to the sperm source ‐ ejaculate or surgical, and only one evaluated women with previously unsuccessful embryo transfers. However, the quality of the pooled evidence does not allow robust conclusions, and we are uncertain about the true effect of intracytoplasmic morphologically selected sperm injection (IMSI) on the studied reproductive outcomes. Because of this, the review findings still do not support the use of IMSI in clinical practice.
Quality of the evidence
We considered the evidence to be very low‐quality (see Table 1). Issues, such as risk of bias in the included studies, inconsistency, and strong suspicion of publication bias contributed to our decision to downgrade the quality of the evidence.
For live birth, we deemed the evidence to be very low‐quality because we assessed all studies as unclear risk of selection bias. Evidence was downgraded another two levels due to serious imprecision (95% confidence interval (CI) was compatible with both appreciable harm and no effect; and small number of events).
For miscarriage, we deemed the evidence to be very low‐quality because we considered seven of the ten studies that reported this outcome at high risk of bias: five related to differences in the mean number of oocytes retrieved or embryos transferred between groups, one reported inconsistent numbers for miscarriage rates, and one was due to the impossibility of ensuring that groups were comparable. There was also very serious imprecision in the estimate: there were only 158 miscarriages across both groups, and the 95% CI was compatible with both appreciable harm and no effect.
There was very low‐quality evidence for clinical pregnancy because all studies were at risk of bias (see Assessment of risk of bias in included studies; Figure 3), and publication bias was strongly suspected; funnel plot analysis suggested a small studies effect (Figure 7). We also found substantial heterogeneity, which added inconsistency to our estimates.
There was no evidence from randomised controlled trials (RCT) on the effect of IMSI on congenital abnormalities.
Potential biases in the review process
We did not identify any potential bias in the review process. However, we acknowledge a potential risk of bias regarding the source of the included studies. Eight of the thirteen included studies were performed in private fertility centres; although not often declared, this might be interpreted as a possible conflict of interest, since researchers involved might be interested in proving the success of new techniques. In addition to all the other limitations of the studies that built this body of evidence, this is something to consider with even more caution when interpreting the findings and applying them to clinical practice and future research.
Agreements and disagreements with other studies or reviews
Over the past five years, we have observed a lack of scientific interest on the topic; only a few studies comparing IMSI and intracytoplasmic sperm injection (ICSI) were published; none of them supports the use of IMSI in clinical practice.
Three other reviews evaluated the effect of IMSI (Duran‐Retamal 2019; He 2018; Setti 2010). Setti 2010 included studies recovered from a single database (MEDLINE) and supplemented evidence from a single RCT with non‐RCT studies. They reported a significant improvement in clinical pregnancy rates and a decrease in the risk of miscarriage with IMSI, while we did not observe this effect. The findings of the two more recent reviews are similar to ours: inconclusive results for live birth and miscarriage rates with IMSI; these authors did not support the routine use of IMSI either (Duran‐Retamal 2019; He 2018).
The benefit of IMSI on live birth and miscarriage is only seen when data from observational studies are pooled for meta‐analysis. However, supplementing data from RCTs with non‐RCTs must be cautiously interpreted: it may represent an exchange of undesirable uncertainty for unacceptable error (Higgins 2011).
The updated results are also similar to our previous review (Teixeira 2013). We included four new studies, but the results are similar to our previous findings; we found inconclusive results for live birth and miscarriage, and we are still very uncertain about the beneficial effects of IMSI on clinical pregnancy.
Authors' conclusions
Implications for practice.
The current evidence from randomised controlled trials does not support or refute the clinical use of intracytoplasmic morphologically selected sperm injection (IMSI). We are very uncertain of the chances of having a live birth and of the risk of having a miscarriage. We found very low‐quality evidence that IMSI increases clinical pregnancy, which means that we are still very uncertain about any real difference. We did not find any trials reporting on the risk of congenital abnormalities with IMSI.
Implications for research.
More studies are needed to evaluate the effect of IMSI on live birth, clinical pregnancy, miscarriage, and congenital abnormalities. Future research should focus on appropriate design, minimising the risk of selection bias, adequately performing the same interventions for control and study groups, and avoiding the use of interventions that may possibly interfere with the results. In addition, it is of great importance to try to include more women and follow them for a longer period of time (to evaluate live birth and congenital abnormalities). Only then will we have sufficiently powered studies to build the body of evidence, and draw more robust conclusions on the real effect IMSI.
What's new
| Date | Event | Description |
|---|---|---|
| 26 February 2020 | Amended | Author initials updated |
History
Protocol first published: Issue 10, 2012 Review first published: Issue 7, 2013
| Date | Event | Description |
|---|---|---|
| 11 December 2019 | New citation required but conclusions have not changed | The addition of new studies did not lead to changes in conclusions |
| 18 November 2019 | New search has been performed | Updated search and analysis, added four new studies (Check 2013; Leandri 2013; Mangoli 2019; Marci 2013) |
Acknowledgements
We would like to thank Rui Ferriani for contributing to previous version of the review. We acknowledge the important help provided by Helen Nagels and Elena Kostova, Managing Editors from the Cochrane Gynaecology and Fertility Group; by Marian Showell, Information Specialist for the Cochrane Gynaecology and Fertility Group; and by Anne Lethaby, Debbie Blake, Eleonora Uphoff, and Harry Siristatidis who kindly reviewed the article and made great contributions to its final version.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group (CGF) Specialised Register of Controlled Trials search strategy
Searched 18 November 2019
Procite platform
Keywords CONTAINS "IMSI" or "intracytoplasmic morphologically selected sperm injection" or Title CONTAINS "IMSI" or "intracytoplasmic morphologically selected sperm injection" (35 records)
Appendix 2. CENTRAL search strategy
2019, Issue 10
Searched 18 November 2019
Ovid platform
1 icsi.tw. (2742) 2 intracytoplasmic sperm injection$.tw. (1192) 3 exp Sperm Injections, Intracytoplasmic/ (570) 4 conventional intracytoplasmic injection$.tw. (1) 5 regular magnification.tw. (0) 6 or/1‐5 (3168) 7 intracytoplasmic morphologically selected sperm injection$.tw. (44) 8 IMSI.tw. (53) 9 MSOME.tw. (14) 10 motile sperm organelle morphology examination$.tw. (10) 11 high magnification.tw. (58) 12 or/7‐11 (97) 13 6 and 12 (50)
Appendix 3. MEDLINE search strategy
Searched from 1946 to 18 November 2019
Ovid platform
1 icsi.tw. (8082) 2 intracytoplasmic sperm injection$.tw. (6957) 3 exp Sperm Injections, Intracytoplasmic/ (6397) 4 conventional intracytoplasmic injection$.tw. (1) 5 regular magnification.tw. (2) 6 or/1‐5 (11562) 7 intracytoplasmic morphologically selected sperm injection$.tw. (89) 8 IMSI.tw. (104) 9 MSOME.tw. (61) 10 motile sperm organelle morphology examination$.tw. (49) 11 high magnification.tw. (1528) 12 or/7‐11 (1631) 13 6 and 12 (138) 14 randomized controlled trial.pt. (494657) 15 controlled clinical trial.pt. (93427) 16 randomized.ab. (461485) 17 placebo.tw. (208670) 18 clinical trials as topic.sh. (189189) 19 randomly.ab. (322062) 20 trial.ti. (208356) 21 (crossover or cross‐over or cross over).tw. (82595) 22 or/14‐21 (1282142) 23 exp animals/ not humans.sh. (4643847) 24 22 not 23 (1178241) 25 13 and 24 (35)
Appendix 4. Embase search strategy
Searched from 1980 to 18 November 2019
Ovid platform
1 exp intracytoplasmic sperm injection/ (20186) 2 icsi.tw. (15685) 3 intracytoplasmic sperm injection$.tw. (9333) 4 conventional intracytoplasmic injection$.tw. (1) 5 regular magnification.tw. (5) 6 or/1‐5 (23288) 7 intracytoplasmic morphologically selected sperm injection$.tw. (167) 8 IMSI.tw. (258) 9 MSOME.tw. (146) 10 motile sperm organelle morphology examination$.tw. (109) 11 high magnification.tw. (1998) 12 or/7‐11 (2225) 13 6 and 12 (310) 14 Clinical Trial/ (949847) 15 Randomized Controlled Trial/ (576975) 16 exp randomization/ (85027) 17 Single Blind Procedure/ (37233) 18 Double Blind Procedure/ (164707) 19 Crossover Procedure/ (61280) 20 Placebo/ (329912) 21 Randomi?ed controlled trial$.tw. (216419) 22 Rct.tw. (34863) 23 random allocation.tw. (1954) 24 randomly allocated.tw. (33713) 25 allocated randomly.tw. (2477) 26 (allocated adj2 random).tw. (810) 27 Single blind$.tw. (23720) 28 Double blind$.tw. (197271) 29 ((treble or triple) adj blind$).tw. (1051) 30 placebo$.tw. (294125) 31 prospective study/ (565420) 32 or/14‐31 (2107582) 33 case study/ (65448) 34 case report.tw. (385407) 35 abstract report/ or letter/ (1070864) 36 or/33‐35 (1511824) 37 32 not 36 (2055758) 38 13 and 37 (78)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 18 November 2019
Ovid platform
1 intracytoplasmic sperm injection$.tw. (56) 2 icsi.tw. (71) 3 conventional intracytoplasmic injection$.tw. (0) 4 regular magnification.tw. (0) 5 or/1‐4 (91) 6 intracytoplasmic morphologically selected sperm injection$.tw. (0) 7 IMSI.tw. (3) 8 MSOME.tw. (0) 9 motile sperm organelle morphology examination$.tw. (0) 10 high magnification.tw. (34) 11 or/6‐10 (37) 12 5 and 11 (0)
Appendix 6. CINAHL search strategy
Searched from 1961 to 18 November 2019
EBSCO platform
S11 S4 AND S10 20 S10 S5 OR S6 OR S7 OR S8 OR S9 189 S9 TX high magnification 138 S8 TX motile sperm organelle morphology examination* 6 S7 TX MSOME 6 S6 TX IMSI 41 S5 TX intracytoplasmic morphologically selected sperm injection* 14 S4 S1 OR S2 OR S3 1,535 S3 TX ICSI 1,141 S2 TX intracytoplasmic injection* 891 S1 TX intracytoplasmic sperm injection* 887
Appendix 7. LILACS search strategy
Search results for Literatura Latino‐Americana e do Caribe em Ciências da Saúde (LILACS), searched 18 November 2019
Web platform
((intracytoplasmic sperm injection$) OR (icsi) OR (conventional intracytoplasmic injection$) OR (regular magnification)) AND ((intracytoplasmic morphologically selected sperm injection$) OR (IMSI) OR (MSOME) OR (motile sperm organelle morphology examination$) OR (high magnification))
3 records
Appendix 8. Clinical Trial Registers search strategy
Search results for ClinicalTrial.gov, searched 18 November 2019
Web platform
(imsi) OR (msome) OR (motile sperm organelle morphology examination) OR ((high magnification) AND (sperm))
6 records
Search results for Current Controlled Trials,18 November 2019:
(imsi) OR (msome) OR (motile sperm organelle morphology examination) OR ((high magnification) AND (sperm))
0 records
Search results for World Health Organization International Clinical Trials Registry Platform, 18 November 2019:
IMSI OR MSOME
6 records
Data and analyses
Comparison 1. Ultra‐high (IMSI) versus regular magnification (ICSI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth per allocated couple | 5 | 929 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.89, 1.39] |
| 2 Miscarriage per allocated couple | 10 | 2297 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.81, 1.51] |
| 3 Miscarriage per clinical pregnancy | 10 | 783 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.68, 1.20] |
| 4 Clinical pregnancy per allocated couple | 13 | 2775 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.11, 1.37] |
Comparison 2. IMSI versus ICSI: subgroup analysis by sperm quality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth per allocated couple | 5 | 929 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.89, 1.39] |
| 1.1 Only poor sperm quality | 2 | 405 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.61, 2.42] |
| 1.2 Good or unselected sperm quality | 3 | 524 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.82, 1.50] |
| 2 Clinical pregnancy per allocated couple | 13 | 2775 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.09, 1.47] |
| 2.1 Only poor sperm quality | 7 | 1905 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.04, 1.62] |
| 2.2 Good or unselected sperm quality | 6 | 870 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.99, 1.56] |
2.1. Analysis.
Comparison 2 IMSI versus ICSI: subgroup analysis by sperm quality, Outcome 1 Live birth per allocated couple.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Antinori 2008.
| Methods | Randomised controlled trial conducted in a private assisted reproduction centre (Italy). Period of enrolment not reported. | |
| Participants | Inclusion criteria: at least 2 previous diagnoses of severe oligoasthenospermia; 3 years of primary infertility; absence of female factor. | |
| Interventions | Couples were randomised into 2 groups: Intervention: oocytes injected with spermatozoon selected under ultra‐high magnification (IMSI): on the basis of MSOME criteria, the examination and spermatozoa selection for IMSI procedure was performed in real time using an inverted light microscope equipped with high‐power Nomarski optics, enhanced by digital imaging to achieve a magnification up to 6300x, and the Eppendorf Micromanipulation System (Transfer‐Man NK2, Eppendorf, Germany). Only spermatozoa with normal head dimension (length 4.75 ± 0.28 μm; width 3.28 ± 0.20 μm), and shape, with no, or maximum 1 vacuole (0.78 ± 0.18 μm) microinjected; spermatozoa with abnormal head size were excluded (such spermatozoa were identified by superimposing a transparent celluloid form representing the correct spermatozoon size on the examined gametes). 2 spermatozoa for each oocyte were selected for insemination, using the classical ICSI technique. Control: oocytes injected with spermatozoon selected under regular magnification (ICSI): no further details. |
|
| Outcomes | Clinical pregnancy, miscarriage, and implantation rates | |
| Notes | We considered 2 publications to be related to the same study; although the number of couples was not the same, the only difference we observed was that the newer publication had evaluated more couples; the authors did not answer our e‐mails to resolve these data queries. Live birth and congenital abnormalities not reported Implantation rate: 23.0% (IMSI) vs 16.6% (ICSI); P value not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes prepared by a research nurse |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All study participants were blinded to treatment assignment for the duration of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not suspected |
| Other bias | Low risk | None |
Balaban 2011.
| Methods | Randomised controlled trial conducted in a private assisted reproduction centre (Turkey) between February and July 2009. | |
| Participants | Eligibility criteria: unselected women undergoing assisted reproduction treatment | |
| Interventions | Couples were randomised into 2 groups: Intervention: oocytes injected with spermatozoon selected under ultra‐high magnification (IMSI). The procedure was performed in real time using an inverted microscope (Olympus IX‐71; Japan) with actual digitally enhanced magnification, as determined by a 0.01 mm Olympus objective micrometer, at 6300x. Normal‐shaped nuclei were defined as smooth, symmetric, having an oval configuration, with mean length limits of 4.75 ± 0.28 μm and mean width limits of 3.28 ± 0.20 μm, with a homogeneous nuclear chromatin mass with no regional nuclear disorders, and containing no more than one small vacuole with a borderline diameter of 0.78 ± 0.18 μm. Mean time needed for IMSI = 21 minutes. Control: oocytes injected with spermatozoon selected under regular magnification (ICSI): not specified; the mean time needed for regular ICSI = 14 minutes. |
|
| Outcomes | Duration of ICSI procedure; 2‐pronuclei fertilisation rate; embryos with 4 blastomeres on day 2 post fertilisation; embryos with 8 blastomeres on day 3 post fertilisation; grades 1 and 2 embryos on transfer day; clinical pregnancy; live birth; implantation; and multiple pregnancy rate | |
| Notes | Miscarriage and congenital abnormalities not reported Implantation rate: 66/228 = 28.9% (IMSI) vs 42/215 = 19.5% (ICSI); P = 0.02 (however, study authors reported that P = NS (not significant)). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not suspected |
| Other bias | Low risk | None |
Check 2013.
| Methods | Prospective randomised clinical trial performed in a fertility centre (USA). Period of enrolment not reported. | |
| Participants | Eligibility criteria: couples undergoing ART, with failure to have successful conception after 3 consecutive embryo transfers, and whose male partner had a DNA fragmentation index > 30%. Only women aged ≤ 39 years were randomised. | |
| Interventions | Couples were randomised into 2 groups: Intervention: sperm selection in the ICSI group was analysed under high magnification Control: sperm selection in the ICSI group was analysed under normal magnification |
|
| Outcomes | Live birth, miscarriage, pregnancy, and implantation rates | |
| Notes | Since randomisation was performed only for women aged ≤ 39 years, we only extracted data regarding this group of women Authors were contacted for further information regarding period of enrolment, eligibility criteria and results, but no answer was received by the completion of the present update. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not suspected |
| Other bias | Low risk | None suspected |
Figueira 2011.
| Methods | Randomised controlled trial conducted in a private assisted reproduction centre (Brazil) between May and December 2009 | |
| Participants | Eligibility criteria: women undergoing assisted reproduction treatment in conjunction with pre‐implantation genetic screening for aneuploidy, as a result of advanced maternal age; sperm concentration > 1,000,000/mL and sperm motility > 20%; at least 6 oocytes available on oocyte retrieval | |
| Interventions | Couples were randomised into 2 groups: Intervention: oocytes injected with spermatozoon selected under ultra‐high magnification (IMSI): sperm morphology selection was assessed using an inverted Nikon Diaphot microscope (Eclipse TE 300; Nikon, Tokyo) equipped with high‐power DIC (Nomarski). The total calculated magnification was 6600x. The sperm cells exhibiting normally shaped nuclei (smooth, symmetric, and oval configuration) and normal nuclear chromatin content (if it contained no more than 1 vacuole, which occupied < 4% of the nuclear area) were selected for injection. Control: oocytes injected with spermatozoon selected under regular magnification (ICSI): sperm morphology selection was assessed using an inverted Nikon Diaphot microscope (Eclipse TE 300; Nikon, Tokyo) with a Hoffmann modulation contrast system under 400x magnification |
|
| Outcomes | Sperm nuclear morphology at high‐magnification ICSI; incidence of aneuploidy in derived embryos; clinical pregnancy rate | |
| Notes | All embryos were submitted to pre‐implantation genetic diagnosis and aneuploidy screening on day 3. Only the embryos found to be chromosomally normal were considered for embryo transfer, and a maximum of 3 embryos were transferred on day 4. The cycle was cancelled if normal embryos were absent after FISH. Live birth and congenital abnormalities not reported Implantation rate: 55.6% (IMSI) vs 40.9% (ICSI); P = 0.59 Study authors were contacted to clarify information about the 4 different included studies, from the same groups of authors (Figueira 2011; Setti 2011; Setti 2012a; Setti 2012b). All questions on methods of randomisation, patient overlapping, and data per woman were clarified. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated balanced table in sets of 10 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not suspected |
| Other bias | High risk | The number of oocytes retrieved and embryos transferred were significantly different between groups (P < 0.01). In the article, study authors reported that differences were not significant for oocytes retrieved (P = 0.20) and embryos transferred (P = 0.17). |
Knez 2011.
| Methods | Randomised controlled trial conducted in an academic setting (Slovenia) between October 2009 and June 2010 | |
| Participants | Eligibility criteria: all embryos arrested after prolonged 5‐day embryo culture to the blastocyst stage in their previous conventional ICSI attempts; poor semen quality characterised by the incidence of teratozoospermia by less than 14% of morphologically normal sperm according to the Strict Kruger Criteria, oligozoospermia by a sperm concentration of < 20 million/mL and asthenozoospermia by < 50% of motile sperm according the WHO criteria; women without PCOS or endometriosis, and aged < 42 years | |
| Interventions | Couples were randomised into 2 groups: Intervention: oocytes injected with spermatozoon selected under ultra‐high magnification (IMSI): sperm were selected in dishes with a glass bottom (GWSt 1000; Will Co., Wells BV, Amsterdam, The Netherlands) and monitored under an inverted microscope with a heated stage equipped with DIC (Nikon ECLIPSE TE2000‐S, Japan). Approximately 5 elongated droplets of SpermSlow medium (Origio, Denmark) were placed on the bottom of a glass dish to immobilise the sperm. A smaller droplet of prepared sperm was placed near each SpermSlow droplet. Then, the connections were made between the sperm and the SpermSlow droplets for sperm to swim into the SpermSlow droplets and to bind to the HA. All droplets were covered with paraffin oil (Origio, Denmark). For observation under 6000x magnification, a droplet of immersion oil was inserted underneath the glass dish (under the SpermSlow droplet). One droplet of SpermSlow with bound sperm was monitored by the immersion objective, DIC, and Nikon Digital Sight DS‐Ri1 camera. The single (mature) sperm that was bound to the HA and had the best morphology was chosen, aspirated in the microinjection pipette, scored in 3‐dimensions, and evaluated, according to the morphology and head vacuoles, at 6000x magnification. Control: oocytes injected with spermatozoon selected under regular magnification (ICSI): the sperm selection for microinjection was performed at a magnification of 200x to 400x. Sperm with severe head‐shape defects clearly seen under the magnification (pin, amorphous, tapered, round, and multinucleated head) were excluded from microinjection into the oocyte. |
|
| Outcomes | Fertilisation, blastocyst, implantation, and pregnancy rates | |
| Notes | Live birth and congenital abnormalities not reported Implantation rate: 6/35 = 17.1% (IMSI) vs 3/44 = 6.8% (ICSI); P = 0.17 Study author was contacted to clarify information about the 2 different included studies (Knez 2011; Knez 2012). All questions on methods of randomisation, patient overlapping, and data per woman were clarified. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers (unrestricted randomisation list) |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes prepared by a research nurse |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All study participants were blinded as regards their treatment assignment for the duration of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not suspected |
| Other bias | High risk | ‐ Study authors did not report the number of oocytes retrieved in the groups. The number of transferred embryos per woman was not significantly different between groups. ‐ Sperm selection for IMSI group was performed using hyaluronic acid, and this intervention was not applied to ICSI group; this means different interventions between the study groups. |
Knez 2012.
| Methods | Randomised controlled trial conducted in an academic setting (Slovenia) between January and October 2011. | |
| Participants | Eligibility criteria: at least 6 mature oocytes available upon oocyte retrieval; isolated teratozoospermia, which was determined as having < 14% of morphologically normal spermatozoa according to the Kruger strict criteria, > 15 million spermatozoa per millilitre, and at least 40% motile spermatozoa; women without PCOS or endometriosis | |
| Interventions | Couples were randomised into 2 groups: Intervention: oocytes injected with spermatozoon selected under ultra‐high magnification (IMSI): a single spermatozoon bound to the HA and with the best morphology was chosen, aspirated in the microinjection pipette, scored in 3 dimensions, and evaluated, according to the morphology and head vacuoles, at 6000x magnification Control: oocytes injected with spermatozoon selected under regular magnification (ICSI): spermatozoa without severe head shape defects clearly seen under the microscope (pin, amorphous, tapered, round, and multinucleated head) were selected at magnification 200x to 400x |
|
| Outcomes | Fertilisation, blastocyst, implantation, and pregnancy rates | |
| Notes | Live birth, miscarriage, and congenital abnormalities not reported Implantation rate not reported Study author was contacted to clarify information about the 2 different included studies (Knez 2011; Knez 2012). All questions on methods of randomisation, patient overlapping, and data per woman were clarified. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not suspected |
| Other bias | High risk | ‐ Study authors did not provide the number of transferred embryos per woman, nor the SD for the number of oocytes retrieved (mean 11.0 with IMSI vs 9.8 with ICSI). ‐ Sperm selection for IMSI group was performed using hyaluronic acid and this intervention was not applied to ICSI group; this means different interventions between the study groups. |
Leandri 2013.
| Methods | Randomised controlled trial conducted in seven fertility centres (France) between September 2008 and December 2011 | |
| Participants | Eligibility criteria: couples undergoing first ICSI attempt, due to male infertility, with use of fresh ejaculated spermatozoa with at least 3 million spermatozoa in the ejaculate, and fewer than 1 million motile spermatozoa recovered after density gradient with whatever sperm morphology | |
| Interventions | Couples were randomised into 2 groups: Intervention: IMSI was performed. Sperm preparation was placed in a glass‐bottomed dish and examined by Nomarski interference contrast microscopy with a Leica DFC‐280 camera mounted on a Leica DMI 6000 microscope with an immersion objective lens 100x and camera magnification 1x. Spermatozoa were divided into 3 groups, according to morphology, and then injected into oocytes. Control: conventional ICSI, with sperm selection for microinjection at a magnification of 400x |
|
| Outcomes | Fertilisation, implantation, clinical pregnancy, and live birth rates | |
| Notes |
Implantation rate: 24% (IMSI) vs 23% (ICSI); P = NS Study was registered as protocol on ClinicalTrials.gov as NCT01780649. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All couples were blinded regarding their treatment assignment for the duration of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected |
| Selective reporting (reporting bias) | Low risk | Not suspected |
| Other bias | Low risk | None suspected |
Mahmoud 2011.
| Methods | Randomised controlled trial conducted in a private assisted reproduction centre (Tunisia) between April 2009 and November 2010. | |
| Participants | Eligibility criteria: oligoasthenozoospermia based on WHO references values. Teratozoospermia evaluated by the strict criteria of Kruger sperm morphology; healthy woman aged < 37 years | |
| Interventions | Couples were randomised into 2 groups: Intervention: oocytes injected with spermatozoon selected under ultra‐high magnification (IMSI): 6600x magnification, using Leica 6800 station Control: oocytes injected with spermatozoon selected under regular magnification (ICSI) |
|
| Outcomes | Fertilisation rate, percentage of good quality embryos, and the rates of clinical pregnancy and implantation | |
| Notes | Live birth, miscarriage, and congenital abnormalities not reported Implantation rate: 19.2% (IMSI) vs 17.2% (ICSI); P = NS Study authors were not contacted because we were unable to obtain their contact details. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not suspected |
| Other bias | Unclear risk | Study authors did not report the number of oocytes retrieved and embryos transferred. |
Mangoli 2019.
| Methods | Randomised controlled trial conducted in an academic assisted reproduction centre (Iran). Period of enrolment not reported. | |
| Participants |
Eligibility criteria: couples with male factor infertility and healthy woman, < 38 years old, with basal follicle‐stimulating hormone (FSH) < 10 IU/mL, body mass index 25 kg/m² to 30 kg/m², with a minimum of six mature oocytes. Exclusion criteria: PCOS, endometriosis, at least 3 years of primary infertility, and one previous failed ICSI treatment cycle |
|
| Interventions | Couples were randomised into 2 groups: Intervention: oocytes injected with spermatozoon selected under 6000x magnification (IMSI) Control: oocytes injected with spermatozoon selected under regular magnification (ICSI) |
|
| Outcomes | Chemical pregnancy, clinical pregnancy, live birth, and implantation rates | |
| Notes | Implantation rates were only reported by group and as percentages. We were unable to contact authors to obtain absolute numbers for calculating implantation rates for IMSI compared to ICSI. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not suspected |
| Other bias | High risk | Demographic characteristics of all patients submitted to IMSI were not compared to all patients submitted do ICSI. Data were only presented by subgroups. The number of embryos transferred for each group (IMSI or ICSI) was not reported and thus, we were unable to determine if they were comparable. |
Marci 2013.
| Methods | Randomised controlled trial conducted in an academic assisted reproduction centre (Italy) between January 2009 and March 2012. | |
| Participants | Eligibility criteria: unselected infertile couples undergoing ART | |
| Interventions | Couples were randomised into 2 groups: Intervention: oocytes injected with spermatozoon selected under ultra‐high magnification (IMSI). A high magnification objective lens (60x) in combination with Hoffman’s contrast on a standard injection microscope (Olympus IX71) and a 1.6x magnification enhancer. Real‐time digital image enhancement (Octax CytoScreen TM system, Medical Technology Vertriebs‐GmbH, Germany), with a total on‐screen magnification of about 5400x. Analysis and selection of motile spermatozoa were performed according to the MSOME criteria described by Bartoov 2002. Control: oocytes injected with spermatozoon selected under regular magnification (ICSI) |
|
| Outcomes | Fertilisation, implantation, clinical pregnancy, ongoing pregnancy, live birth, and miscarriage rates | |
| Notes |
Implantation rate: 16.67% (IMSI) vs 16.83% (ICSI); P = 0.22 Miscarriage rate was reported. However, the numbers were substantially different from the calculated miscarriage rate (clinical pregnancy ‐ live birth ‐ ongoing pregnancy); authors were contacted to obtain absolute numbers, but did not receive an answer by the completion of the present update. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not suspected |
| Other bias | High risk | Miscarriage rates reported are substantially different from calculated ones |
Setti 2011.
| Methods | Randomised controlled trial conducted in a private assisted reproduction centre (Brazil). Period of enrolment not reported. | |
| Participants | Eligibility criteria: first IVF treatment; abnormal semen parameters according to WHO, except for azoospermia; use of fresh semen sample; absence of a known female factor for infertility; and at least 6 oocytes available on retrieval | |
| Interventions | Couples were randomised into 2 groups: Intervention: oocytes injected with spermatozoon selected under ultra‐high magnification (IMSI): sperm selection was examined at high magnification with an inverted microscope (Eclipse TE 300; Nikon, Tokyo, Japan) equipped with high‐power DIC optics (Nomarski). The total calculated magnification was 6600x. Control: oocytes injected with spermatozoon selected under regular magnification (ICSI): sperm morphology selection was assessed using an inverted Nikon Diaphot microscope (Eclipse TE 300; Nikon, Tokyo) with a Hoffmann modulation contrast system under 400x magnification |
|
| Outcomes | Clinical pregnancy, implantation rate, and miscarriage | |
| Notes | Live birth and congenital abnormalities not reported Implantation rate: 158/664 = 23.8% (IMSI) vs 28/156 = 25.4% (ICSI); P = 0.60 Study authors were contacted to clarify information about the 4 different included studies, from the same groups of authors (Figueira 2011; Setti 2011; Setti 2012a; Setti 2012b). All questions on methods of randomisation, patient overlapping, and data per woman were clarified. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not suspected |
| Other bias | High risk | The number of oocytes retrieved and embryos transferred were significantly different between groups (P < 0.01 for both). In the article, study authors reported that differences were non‐significant (P = 0.29 for oocytes retrieved and P = 0.27 for embryos transferred). |
Setti 2012a.
| Methods | Randomised controlled trial conducted in a private assisted reproduction centre (Brazil) between May 2009 and December 2010. | |
| Participants | Eligibility criteria: women undergoing assisted reproduction treatment in conjunction with pre‐implantation genetic screening for aneuploidy, as a result of advanced maternal age; no severe spermatogenic alteration; at least 6 oocytes available on oocyte retrieval. | |
| Interventions | Couples were randomised into 2 groups: Intervention: oocytes injected with spermatozoon selected under ultra‐high magnification (IMSI): sperm selection was examined at high magnification using a similar inverted microscope equipped with high‐power DIC optics (Nomarski). The total calculated magnification was 6600x. The sperm cells exhibiting normally shaped nuclei (smooth, symmetric, and oval configuration) and normal nuclear chromatin content (if it contained no more than 1 vacuole, which occupied < 4% of the nuclear area) were selected for injection. Control: oocytes injected with spermatozoon selected under regular magnification (ICSI): sperm morphology selection was assessed using an inverted Nikon Diaphot microscope (Eclipse TE 300; Nikon, Tokyo, Japan) with a Hoffmann modulation contrast system under 400x magnification |
|
| Outcomes | Clinical pregnancy, implantation rate, miscarriage, and gender incidence | |
| Notes | All embryos were submitted to pre‐implantation genetic diagnosis and aneuploidy screening. On the morning of day 3 of embryo development, 1 cell per embryo was biopsied by laser zona drilling using a 1.48 mm infrared diode laser (Octax Laser Shot System, MTG, Bruckberg, Germany). Following the biopsies, the embryos were returned to the culture medium. The removed blastomere nuclei were spread using 0.1 mol/L HCl and 0.01% Tween 20 (Sigma, Dorset, UK). Briefly, the blastomeres were placed on a slide in a drop of HCl‐Tween spreading solution and observed until the cell had lysed. The slides were then air dried and dehydrated before FISH analysis was performed. All embryos were analysed for chromosomes X, Y, 13, 16, 18, 21, and 22. For the purpose of this study, the blastomeres were classified as normal when 2 sexual and 2 of each tested autosomal chromosomes were present. Embryo transfer was performed on day 5 using a soft catheter with transabdominal ultrasound guidance. Only the embryos found to be chromosomally normal were considered for embryo transfer, and up to a maximum of 3 embryos were transferred. Live birth and congenital abnormalities not reported Implantation rate: 46.1% (IMSI) vs 41.6% (ICSI); P = 0.59 Study authors were contacted to clarify information about the 4 different included studies, from the same groups of authors (Figueira 2011; Setti 2011; Setti 2012a; Setti 2012b). All questions on methods of randomisation, patient overlapping, and data per woman were clarified. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not suspected |
| Other bias | High risk | The number of oocytes retrieved and embryos transferred were significantly different between groups (P = 0.01). In the article, study authors reported these differences as not significant. |
Setti 2012b.
| Methods | Prospective randomised clinical trial performed in a private fertility centre (Brazil). Period of enrolment not reported. | |
| Participants | Eligibility criteria: women of good physical and mental health; undergoing ICSI as a result of advanced maternal age (≥ 37 years old); with regular menstrual cycles of 25 to 35 days; normal basal FSH and LH concentrations; body mass index < 30 kg/m²; presence of both ovaries and intact uterus; absence of PCOS, endometriosis, and gynaecological or medical disorders; and a negative result in screening for sexually transmitted diseases. All male partners were normozoospermic, according to the WHO reference values (2010). No woman had received any hormone therapy for at least 60 days preceding the study. | |
| Interventions | Couples were randomised into 2 groups: Intervention: sperm selection in the IMSI group was analysed under high magnification using an inverted Nikon Diaphot microscope equipped with high‐power DIC optics. The total calculated magnification was 6600x. An aliquot of the sperm cell suspension was transferred to a microdroplet of modified human tubal fluid medium containing 8% polyvinyl pyrrolidone (Irvine Scientific, Santa Ana, CA, USA) in a sterile glass dish (FluoroDish; World Precision Instrument, Sarasota, FL, USA). The dish was placed on a microscope stage above an Uplan Apo 100 oil/1.35 objective lens previously covered by a droplet of immersion oil. The sperm cells that were selected for injection exhibited normally shaped nuclei (smooth, symmetric, and oval configuration) and normal nuclear chromatin content (if it contained no more than 1 vacuole that occupied < 4% of the nuclear area). Control: sperm selection in the ICSI group was analysed under 400x magnification using an inverted Nikon Eclipse TE 300 microscope |
|
| Outcomes | Fertilisation rate, high‐quality embryo rate on day 3, blastocyst formation rate, number of transferred embryos, implantation rate, miscarriage rate, and pregnancy rate | |
| Notes | Live birth and congenital abnormalities not reported Implantation rate: 38.3% (IMSI) vs 12.1% (ICSI). P = 0.03 This article was withdrawn from one journal and republished in another one later on. Authors were contacted for more information regarding the reasons for this. Authors were also contacted to clarify information about the 4 different included studies, from the same groups of authors (Figueira 2011; Setti 2011; Setti 2012a; Setti 2012b). All questions on methods of randomisation, patient overlapping and data per woman were clarified. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Authors stated allocation sequence was concealed, but no further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The allocation sequence was concealed from the participant. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Primary study team was not blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up |
| Selective reporting (reporting bias) | Low risk | Not suspected |
| Other bias | High risk | Although there was no significant difference in the number of oocytes retrieved, and despite the total number of embryos being higher in the ICSI group, more embryos per woman were transferred in the IMSI group. This article has been withdrawn and republished in another journal. |
DIC: differential interference contrast; FISH: fluorescent in situ hybridisation; FSH: follicle‐stimulating hormone; HA: hyaluronate; HCl: hydrochloric acid; ICSI: intracytoplasmic sperm injection; IMSI: intracytoplasmic morphologically selected sperm injection; IVF: in vitro fertilisation; LH: luteinising hormone; MSOME: motile sperm organella morphology examination; PCOS: polycystic ovaries syndrome; SD: standard deviation; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Apryshko 2010 | Not an RCT (observational study) |
| Bartoov 2003 | Not an RCT (observational study) |
| Berkovitz 2005 | Not an RCT (observational study) |
| Berkovitz 2006 | Not an RCT (observational study) |
| Braga 2011 | Oocytes (not women or couples) were allocated |
| Cassuto 2011 | Not an RCT (observational study) |
| De Vos 2013 | Oocytes (not women or couples) were randomly allocated |
| Gatimel 2016 | Not an RCT (retrospective study) |
| Ghazali 2017 | Not an RCT (observational study) |
| Hazout 2005 | Not an RCT (observational study) |
| Karabulut 2019 | It was not possible to determine if study was adequately randomised. In addition, the number of women enrolled was higher than the number of cycles reported, and we found several inconsistencies in numbers reported and statistical analyses performed. |
| La Sala 2015 | Quasi‐randomised (according to patients' ID number). The published article was retracted because the study was not conducted with the appropriate ethical oversight. |
| Mauri 2011 | Oocytes (not women or couples) were randomly allocated |
| Oliveira 2011 | Not an RCT (observational study) |
| Wilding 2011 | Not an RCT (observational study) |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Kim 2014.
| Methods | Comparative prospective study performed in a private fertility centre (South Korea) between May 2013 and April 2014 |
| Participants | Eligibility criteria: patients with infertility related to teratozoospemia (strict morphologically normal sperm < 4%) and no or one previous conventional ICSI failure. |
| Interventions | Couples were randomised into three groups: Conventional ICSI group: spermatozoa were injected into oocytes under 200x magnification of TE 2000U (Nikon, Japan); IMSI group: spermatozoa were selected under high magnification observation (> 6000x) with the use of IMSI‐StrictTM (Hamilton Thorne, Inc., Beverly, MA, USA) Intervention I: IMSI was performed and spermatozoa were selected under high magnification observation (> 6000x) Intervention II: ICSI was performed and spermatozoa were selected with a HA‐bound of PICSI dish Control: conventional ICSI was performed under 200x magnification |
| Outcomes | Fertilization rates, embryonic development, implantation rates, and clinical pregnancy rates. |
| Notes | Study is awaiting classification to clarify if truly randomised. If included, we will only extract data from intervention group I (IMSI) and the control (ICSI) group. Authors were contacted for additional information, but we did not receive an answer by the completion of the present update. |
NCT02107521.
| Methods | Double‐blind, randomised trial conducted in a private fertility centre (Brazil) |
| Participants | Eligibility criteria: patients with ICSI indication, who have previously undergone at least one ICSI attempt, during which at least 2 blastocysts were transferred, with no implantation; regular menstrual cycles every 25 to 35 days; BMI < 35kg/m²; presence of both ovaries; no pelvic or clinically significant uterine anomalies, or both; normal cervical cytology; FSH within normal limits |
| Interventions | Couples were randomised into 2 groups: Intervention: sperm selection in the ICSI group was analysed under 6600x magnification Control: sperm selection in the ICSI group was analysed under 400x magnification |
| Outcomes | Implantation rate |
| Notes | According to ClinicalTrials.gov, study was terminated due to broken equipment. We contacted the authors, but we did not receive an answer by the completion of the present review. |
NCT02358733.
| Methods | Double‐blind randomised trial, performed in a private fertility centre (Spain) |
| Participants | Elegibility criteria: women undergoing ART, aged 37 years and older, with poor ovarian response |
| Interventions | Couples were randomised into 2 groups: Intervention: IMSI Control: ICSI |
| Outcomes |
Primary outcomes: clinical pregnancy rate Secondary outcomes: number of embryos obtained; percentage of cycles with embryo transfer |
| Notes | Authors were contacted for partial results, but informed us that study was terminated early and no partial data were available. |
Scarselli 2014.
| Methods | Randomised controlled trial conducted in a private assisted reproduction centre (Italy) between March and October 2013 |
| Participants | Elegibility criteria: couples undergoing ART and PGS; only couples with normozoospermic men were included |
| Interventions | Couples were randomised into 2 groups: Intervention: IMSI was performed with high resolution Normarski optics (6600X) Control: classical ICSI was carried out at 400X magnification |
| Outcomes | Euploid blastocyst rate, implantation rate |
| Notes | Implantation rate: IMSI ‐ 11/16 (69%) vs ICSI 5/11 (45%); P = 0.26. Authors were contacted to clarify randomisation and to provide results regarding outcomes of interest. We didn't receive a response by the time of completion of the present update. |
Setti 2012c.
| Methods | Prospective randomised clinical trial performed in a private fertility centre (Brazil). Period of enrolment not reported |
| Participants | Eligibility criteria: women undergoing ICSI as a result of unexplained fertility |
| Interventions | Couples were randomised into 2 groups: Intervention: sperm selection in the ICSI group was analysed under 6000x magnification Control: sperm selection in the ICSI group was analysed under 400x magnification |
| Outcomes | Fertilisation rate, high‐quality embryo rate, number of transferred embryos, implantation rate, and pregnancy rate |
| Notes | Miscarriage, live birth, and congenital abnormalities not reported Implantation rate: 39.6% (IMSI) vs 19.4% (ICSI); P = 0.019 Authors were contacted for further information regarding period of enrolment and eligibility criteria but no answer was received by the completion of the present update. |
ART: assisted reproduction technique; DNA: deoxyribonucleic acid; FSH: follicle‐stimulating hormone; ICSI: intracytoplasmic sperm injection; IMSI: intracytoplasmic morphologically selected sperm injection; IU: international units.
Characteristics of ongoing studies [ordered by study ID]
PACTR201411000928146.
| Trial name or title | IMSI versus ICSI in patients with severe male factor infertility and repeated ICSI failure |
| Methods | Randomised controlled trial, performed in an academic setting (Egypt) |
| Participants | Elegibility criteria: primary or secondary infertility, aged 18 to 37 years old, having good ovarian reserve (AMH > 1.5, AFC > 5, and basal FSH < 10 mIu/L), and at least one previously failed ICSI Male partner with severe oligospermia (sperm concentration < 5 x 106/mL) and severe teratospermia (normal form < 4%) |
| Interventions | Couples were randomised in two groups: Intervention: sperm selection performed at 10,000x magnification with a Nomarski‐contrast Nikson inverted microscope Control: sperm selection under regular (200x) magnification |
| Outcomes | Number of embryos, fertilisation rate, embryo grading, implantation rate, and pregnancy rate. |
| Starting date | January 2014 |
| Contact information | Prof. Mohmoud Al Abd: dr.mohmoudalabd@gmail.com |
| Notes | According to PACTR Registry, study is open to recruitment. Authors were contacted for further information, but we did not receive a response by the time of completion of the present update. |
Differences between protocol and review
Ongoing pregnancy was used as a surrogate for live birth.
Miscarriage was also presented as "per allocated couple".
Contributions of authors
Draft the protocol: WPM, all the other authors reviewed and approved before submission
Search for trials: DMT and AHM, other authors helped solve disagreements
Obtain copies of studies: DMT and WPM
Select which studies to include: DMT and AHM, other authors helped solve disagreements
Extract data from studies: DMT and AHM
Enter data into RevMan: DMT
Carry out the analysis: DMT and WPM
Interpret the analysis: All authors
Draft the final review: DMT, all the other authors reviewed and approved before submission
Sources of support
Internal sources
-
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil.
Scholarship
-
Hospital das Clínicas FMRP‐USP, Brazil.
Salary
-
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Scholarship
-
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil.
Scholarship
External sources
No sources of support supplied
Declarations of interest
The authors declare no conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Antinori 2008 {published data only}
- Antinori M, Licata E, Dani G, Cerusico F, Versaci C, d'Angelo D, et al. Intracytoplasmic morphologically selected sperm injection: a prospective randomized trial. Reproductive BioMedicine Online 2008;16(6):835‐41. [PUBMED: 18549694] [DOI] [PubMed] [Google Scholar]
- Antinori S, Licata E, Dani G, Cerusico F, Versaci C, Antinori M. A prospective randomized trial to verify the efficacy of IMSI procedure in daily IVF routine. Human Reproduction 2008;23 Suppl 1:i165. [Google Scholar]
Balaban 2011 {published data only}
- Balaban B, Yakin K, Alatas C, Oktem O, Isiklar A, Urman B. Clinical outcome of intracytoplasmic injection of spermatozoa morphologically selected under high magnification: a prospective randomized study. Reproductive Biomedicine Online 2011;22(5):472‐6. [PUBMED: 21324747] [DOI] [PubMed] [Google Scholar]
Check 2013 {published data only}
- Check JH, Bollendorf A, Summers‐Chase D, Yuan W, Horwath D. Isolating sperm by selecting those with normal nuclear morphology prior to intracytoplasmic sperm injection (ICSI) does not provide better pregnancy rates compared to conventional ICSI in women with repeated conception failure with in vitro fertilization. Clinical and Experimental Obstetrics & Gynecology 2013;40:15‐7. [PubMed] [Google Scholar]
Figueira 2011 {published data only}
- Figueira RCS, Braga DPAF, Pasqualotto EB, Pasqualotto FF, Iaconelli A, Borges E. The role of morphological nuclear integrity of the sperm cells in preimplantation genetic aneuploidy screening cycles outcome. Journal fur Reproduktionsmedizin und Endokrinologie 2010;7:250‐1. [Google Scholar]
- Figueira RDC, Braga DP, Setti AS, Iaconelli A Jr, Borges E Jr. Morphological nuclear integrity of sperm cells is associated with preimplantation genetic aneuploidy screening cycle outcomes. Fertility and Sterility 2011;95(3):990‐3. [PUBMED: 21130987] [DOI] [PubMed] [Google Scholar]
Knez 2011 {published data only}
- Knez K, Zorn B, Tomazevic T, Vrtacnik‐Bokal E, Virant‐Klun I. The IMSI procedure improves poor embryo development in the same infertile couples with poor semen quality: a comparative prospective randomized study. Reproductive Biology and Endocrinology 2011;9:123. [PUBMED: 21875440] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knez 2012 {published data only}
- Knez K, Tomazevic T, Zorn B, Vrtacnik‐Bokal E, Virant‐Klun I. Intracytoplasmic morphologically selected sperm injection improves development and quality of preimplantation embryos in teratozoospermia patients. Reproductive Biomedicine Online 2012;25(2):168‐79. [PUBMED: 22717245] [DOI] [PubMed] [Google Scholar]
Leandri 2013 {published data only}
- Leandri RD, Gachet A, Pfeffer J, Celebi C, Rives N, Carre‐Pigeon F, et al. Is intracytoplasmic morphologically selected sperm injection (IMSI) beneficial in the first ART cycle? a multicentric randomized controlled trial. Andrology 2013;1(5):692‐7. [CTG: NCT01780649] [DOI] [PubMed] [Google Scholar]
Mahmoud 2011 {published data only}
- Mahmoud K, Triki‐Hmam C, Terras K, Zhioua F, Hfaiedh T, Ben Aribia MH. How and in which indication the IMSI could improve outcomes?. Human Reproduction 2011;26 Suppl 1:i181. [Google Scholar]
Mangoli 2019 {published data only}
- Mangoli E, Khalili MA, Talebi AR, Agha‐Rahimi A, Soleimani M, Faramarzi A, et al. IMSI procedure improves clinical outcomes and embryo morphokinetics in patients with different aetiologies of male infertility. Andrologia 2019;51(8):e13340. [PUBMED: 31197867] [DOI] [PubMed] [Google Scholar]
Marci 2013 {published data only}
- Marci R, Murisier F, Lo Monte G, Soave I, Chanson A, Urner F, et al. Clinical outcome after IMSI procedure in an unselected infertile population: a pilot study. Reproductive Health 2013;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Setti 2011 {published data only}
- Setti AS, Figueira Rde C, Braga DP, Iaconelli A Jr, Borges E Jr. Intracytoplasmic morphologically selected sperm injection benefits for patients with oligoasthenozoospermia according to the 2010 World Health Organization reference values. Fertility and Sterility 2011;95(8):2711‐4. [PUBMED: 21458802] [DOI] [PubMed] [Google Scholar]
Setti 2012a {published data only}
- Iaconelli JA, Figueira RCS, Setti AS, Braga DPAF, Pasqualotto EE, Borges E Jr. Gender incidence on intracytoplasmic morphologically selected sperm injection approach: a prospective randomized study. Human Reproduction 2011;26 Suppl 1:i71. [Google Scholar]
- Setti AS, Figueira RC, Braga DP, Iaconelli A Jr, Borges E Jr. Gender incidence of intracytoplasmic morphologically selected sperm injection‐derived embryos: a prospective randomized study. Reproductive BioMedicine Online 2012;24(4):420‐3. [PUBMED: 22377154] [DOI] [PubMed] [Google Scholar]
Setti 2012b {published data only}
- Setti AS, Figueira RCS, Braga DPAF, Aoki T, Iaconelli Jr A, Borges Jr E. Intracytoplasmic morphologically selected sperm injection is beneficial in cases of advanced maternal age: A prospective randomized study. European Journal of Obstetrics Gynecology and Reproductive Biology 2013;171(2):286‐90. [PMID: 24103532] [DOI] [PubMed] [Google Scholar]
- Setti AS, Figueira RDC, Almeida Ferreira Braga DP, Iaconelli A, Borges E. IMSI is beneficial in cases of advanced maternal age: a prospective randomized study. Reproductive BioMedicine Online 2012 (Withdrawn). [DOI: 10.1016/j.rbmo.2012.10.020] [DOI] [PubMed]
References to studies excluded from this review
Apryshko 2010 {published data only}
- Apryshko VP, Yakovenko SA, Sivozhelezov VS, Yutkin EV, Rutman BK, Troshina MN, et al. IMSI based on Hoffman modulation contrast: 5 years experience. Reproductive BioMedicine Online 2010;20:S25. [Google Scholar]
Bartoov 2003 {published data only}
- Bartoov B, Berkovitz A, Eltes F, Kogosovsky A, Yagoda A, Lederman H, et al. Pregnancy rates are higher with intracytoplasmic morphologically selected sperm injection than with conventional intracytoplasmic injection. Fertility and Sterility 2003;80(6):1413‐9. [PUBMED: 14667877] [DOI] [PubMed] [Google Scholar]
Berkovitz 2005 {published data only}
- Berkovitz A, Eltes F, Yaari S, Katz N, Barr I, Fishman A, et al. The morphological normalcy of the sperm nucleus and pregnancy rate of intracytoplasmic injection with morphologically selected sperm. Human Reproduction 2005;20(1):185‐90. [PUBMED: 15471930] [DOI] [PubMed] [Google Scholar]
Berkovitz 2006 {published data only}
- Berkovitz A, Eltes F, Lederman H, Peer S, Ellenbogen A, Feldberg B, et al. How to improve IVF‐ICSI outcome by sperm selection. Reproductive Biomedicine Online 2006;12(5):634‐8. [PUBMED: 16790113] [DOI] [PubMed] [Google Scholar]
Braga 2011 {published data only}
- Braga DPAF, Setti AS, Figueira RC, Nichi M, Martinhago CD, Iaconelli A Jr, et al. Sperm organelle morphologic abnormalities: contributing factors and effects on intracytoplasmic sperm injection cycles outcomes. Urology 2011;78(4):786‐91. [PUBMED: 21820702] [DOI] [PubMed] [Google Scholar]
Cassuto 2011 {published data only}
- Cassuto NG, Hazout A, Benifla JL, Balet R, Larue L, Viot G. Decreasing birth defect in children by using high magnification selected spermatozoon injection. Fertility and Sterility 2011;1:S85. [Google Scholar]
De Vos 2013 {published data only}
- Vos A, Velde H, Bocken G, Eylenbosch G, Franceus N, Meersdom G, et al. Does intracytoplasmic morphologically selected sperm injection improve embryo development? A randomized sibling‐oocyte study. Human Reproduction 2013;28:617‐26. [DOI] [PubMed] [Google Scholar]
Gatimel 2016 {published data only}
- Gatimel N, Parinaud J, Leandri RD. Intracytoplasmic morphologically selected sperm injection (IMSI) does not improve outcome in patients with two successive IVF‐ICSI failures. Journal of Assisted Reproduction and Genetics 2016;33(3):349‐55. [PUBMED: 26754750] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghazali 2017 {published data only}
- Ghazali S, Talebi AR, Khalili MA, Aflatoonian A. Impact of morphological parameters at high magnification, nuclear structure and ultrastructure of spermatozoa on ART outcomes. Human Reproduction 2017;32(Suppl1):i249‐i250. [Google Scholar]
Hazout 2005 {published data only}
- Hazout A, Dumont‐Hassan M, Junca AM, Cohen Bacrie P, Tesarik J. High‐magnification ICSI overcomes paternal effect resistant to conventional ICSI. Reproductive Biomedicine Online 2005;12(1):19‐24. [PUBMED: 16454928] [DOI] [PubMed] [Google Scholar]
Karabulut 2019 {published data only}
- Karabulut S, Aksunger O, Korkmaz O, Gozel HE, Keskin I. Intracytoplasmic morphologically selected sperm injection, but for whom?. Zygote 2019;27(5):299‐304. [DOI] [PubMed] [Google Scholar]
La Sala 2015 {published data only}
- Sala GB, Nicoli A, Fornaciari E, Falbo A, Rondini I, Morini D, Valli B, Villani MT, Palomba S. Retraction Note: Intracytoplasmic morphologically selected sperm injection versus conventional intracytoplasmic sperm injection: a randomized controlled trial. Reproductive Biology and Endocrinology 2017;15:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala GB, Nicoli A, Fornaciari E, Falbo A, Rondini I, Morini D, et al. Intracytoplasmic morphologically selected sperm injection versus conventional intracytoplasmic sperm injection: a randomized controlled trial. Reproductive Biology and Endocrinology 2015 (retracted); Vol. 13, issue 1:97. [DOI] [PMC free article] [PubMed] [Retracted]
Mauri 2011 {published data only}
- Mauri AL, Petersen CG, Oliveira JB, Massaro FC, Baruffi RL, Franco JG Jr. Comparison of day 2 embryo quality after conventional ICSI versus intracytoplasmic morphologically selected sperm injection (IMSI) using sibling oocytes. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2011;150(1):42‐6. [PUBMED: 20171776] [DOI] [PubMed] [Google Scholar]
Oliveira 2011 {published data only}
- Oliveira JBA, Cavagna M, Petersen CG, Mauri AL, Massaro FC, Silva LFI, et al. Pregnancy outcomes in women with repeated implantation failures after intracytoplasmic morphologically selected sperm injection (IMSI). Reproductive Biology and Endocrinology 2011;9:99. [PUBMED: 21781299] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JBA, Petersen CG, Mauri AL, Massaro FC, Baruffi RLR, Franco JG Jr. Clinical outcomes of IMSI in previous ICSI failures. Fertility and Sterility 2010;1:S55. [Google Scholar]
Wilding 2011 {published data only}
- Wilding M, Coppola G, Matteo L, Palagiano A, Fusco E, Dale B. Intracytoplasmic injection of morphologically selected spermatozoa (IMSI) improves outcome after assisted reproduction by deselecting physiologically poor quality spermatozoa. Journal of Assisted Reproduction and Genetics 2011;28(3):253‐62. [PUBMED: 21072684] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kim 2014 {published data only}
- Kim JW, Jeong SY, Yang SH, Yoon SH, Jung JH, Lim JH. Are hyaluronic acid sperm selection and intracytoplasmic morphologically selected sperm injection effective in patients with teratozoospermia?. Fertility and Sterility 2014;102 Suppl(3):e349. [Google Scholar]
NCT02107521 {published data only}
- NCT02107521. IMSI in couples with previous implantation failures. clinicaltrials.gov/ct2/show/NCT02107521 (first posted 8 April 2014).
NCT02358733 {published data only}
- NCT02358733. Use of IMSI in poor responders to IVF. clinicaltrials.gov/ct2/show/NCT02358733 (first posted 9 February 2015).
Scarselli 2014 {published data only}
- Scarselli F, Casciani V, Lobascio AM, Colasante A, Spizzichino L, Biricik A, et al. IMSI selection in normozoospermic patients does not improve preimplantation genetic screening outcome. Human Reproduction 2014;29:i286‐7. [Google Scholar]
Setti 2012c {published data only}
- Setti AS, Braga DPAF, Figueira RCS, Colturato SS, Iaconelli A, Borges E. Intracytoplasmic morphologically selected sperm injection (IMSI) benefits in the presence of unexplained infertility: a prospective randomized study. Fertility and Sterility 2012;98 Suppl 1(3):S80. [Google Scholar]
References to ongoing studies
PACTR201411000928146 {published data only}
- PACTR201411000928146. IMSI versus ICSI in patients with severe male factor infertility and repeated ICSI failure [Comparative study between intracytoplasmic morphologically selected sperm injection (IMSI) versus intracytoplasmic sperm injection(ICSI) in patients with severe male factor infertility and repeated ICSI failure]. apps.who.int/trialsearch/Trial3.aspx?trialid=PACTR201411000928146 (first registered 06 November 2014).
Additional references
Agarwal 2015
- Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reproductive Biology and Endocrinology 2015;26(13):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI: 10.1016/j.jclinepi.2010.07.015; PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Bartoov 2002
- Bartoov B, Berkovitz A, Eltes F, Kogosowski A, Menezo Y, Barak Y. Real‐time fine morphology of motile human sperm cells is associated with IVF‐ICSI outcome. Journal of Andrology 2002;23(1):1‐8. [PUBMED: 11780915] [DOI] [PubMed] [Google Scholar]
Duran‐Retamal 2019
- Duran‐Retamal M, Morris G, Achilli C, Gaunt M, Theodorou E, Saab W, et al. Live birth and miscarriage rate following intracytoplasmic morphologically selected sperm injection vs intracytoplasmic sperm injection: an updated systematic review and meta‐analysis. Acta Obstetricia et Gynecologica Scandinavica [Epub 2019 August 30]. [DOI: 10.1111/aogs.13703; PUBMED: 31403712] [DOI] [PubMed]
Gleicher 2019
- Gleicher N, Kushnir VA, Barad DH. Worldwide decline of IVF birth rates and its probable causes. Human Reproduction Open 2019;2019(3):hoz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 1 September 2019. Hamilton (ON): McMaster University (developed by Evidence Prime).
He 2018
- He F, Wang MJ, Li SL, Zhang CY, Hu LN. IMSI versus ICSI for male factor infertility: A meta‐analysis. Zhonghua Nan Ke Xue 2018;24(3):254‐62. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Palermo 1992
- Palermo G, Joris H, Devroey P, Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992;340(8810):17‐8. [PUBMED: 1351601] [DOI] [PubMed] [Google Scholar]
Setti 2010
- Setti AS, Ferreira RC, Braga DPAF, Figueira RCS, Iaconelli A Jr, Borges E Jr. Intracytoplasmic sperm injection outcome versus intracytoplasmic morphologically selected sperm injection outcome: a meta‐analysis. Reproductive Biomedicine Online 2010;21(4):450‐5. [PUBMED: 20800549] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Teixeira 2013
- Teixeira DM, Barbosa MA, Ferriani RA, Navarro PA, Raine‐Fenning N, Nastri CO, et al. Regular (ICSI) versus ultra‐high magnification (IMSI) sperm selection for assisted reproduction. Cochrane Database of Systematic Reviews 2013, Issue CD010167. [DOI: 10.1002/14651858.CD010167.pub2; PUBMED: 23884963] [DOI] [PubMed] [Google Scholar]


