Abstract

In recent years, metal–organic frameworks (MOFs) have been wildly studied as heterogeneous catalysts due to their diversity of structures and outstanding physical and chemical properties. Meanwhile, MOFs have also been regarded as promising templates for the synthesis of conductive and electrochemically active catalysts. However, in most of the studies, high-temperature annealing is needed to transform nonconductive or low-conductive MOFs to conductive materials for electrocatalyis, during which the unique structures and intrinsic active sites in MOFs can be easily destroyed. Therefore, in recent years, different strategies have been developed for improving the catalytic performances of MOF-based composites for electrochemical reactions with no need of post-treatment. This mini-review highlights the recent advances on MOF-based structures with improved conductivities and electrochemical activities for the application in electrocatalysis. Overall, the advanced MOF-based electrocatalysts include the highly conductive and electrochemically active pristine MOFs, MOFs combined with conductive substrates, and MOFs hybridized with active materials. Finally, we propose the direction for future works on MOF-based electrocatalysts.
Introduction
With the booming consumption of traditional fossil energy and increasingly serious environmental pollution, exploiting more efficient, clean, and sustainable energy conversion and storage systems is necessarily imminent. Hydrogen (H2), with high gravimetric energy density, zero contaminant, and renewability, has been regarded as one of the most appealing candidates for the next-generation energy carrier. Electrochemical hydrogen evolution reaction (HER), which gets rid of hydrocarbons as the source of hydrogen, is a promising and environmentally friendly way for hydrogen production.1 However, the sluggish kinetics of the electrochemical oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) that arose from its proton-coupled multielectron transfer seriously hinder their practical application. Additionally, the most efficient eletrocatalysts are all precious metal materials such as Pt and Ru, which are expensive, terrestrial scarce, and easily dissolved during the electrochemical processes.2 Therefore, it is necessary to develop stable and cheap alternatives or to reduce the usage of noble-metal-based electrocatalysts.
Metal–organic frameworks (MOFs), also called porous coordination polymers (PCPs), are considered to be one class of the most promising candidates for heterocatalysis due to their high specific surface area, regular channels, and abundant metal active sites. In addition, their designable and adjustable channel structure, channel size, and channel environment make MOFs the ideal templates for coated active guest molecules with desired functions.3 MOF-based materials can be defined as pristine MOFs, MOF composites, and MOF-derived materials. Due to the low conductivity and instability in acidic or alkaline media, most pristine MOFs and MOF composites cannot be used directly as electrocatalysts.4−6 Normally, MOFs have been regarded as promising templates for the synthesis of metals, metal compounds, and carbon-based porous materials after postcalcination treatment, which generally show high catalytic performances for water splitting and ORR.2 However, in the reported strategies, the intrinsic active sites in MOFs may be lost during the calcination treatment at high temperatures, and the ordered structure may also be destroyed completely.
For the application of MOF-based structures in electrocatalysts, the determination of catalytically active sites and the catalytic mechanisms is crucially important. The MOF is an extended coordination network through metal ions connecting to each other via organic linker molecules.5 As a result, for pristine MOFs, metal centers or ligands are currently considered to be the active sites for catalytic reactions. The catalytically active species can be easily introduced in the metal nodes or organic ligands, such as the incorporation of active metal sites with nitrogen-containing ligands (e.g., porphyrin- and bipyridine-based ligands) or the functionalizaiton of ligands with electron-donating or electron-withdrawing groups.7,8 In addition, the well-defined porous structures of pristine MOFs offer templates for the introduction of additional active species with controllable size, morphology, component, and location.7,9 Meanwhile, the synergistic effects among the functional units would further enhance the catalytic properties of the MOF-based catalysts.10 In addition to the active sites, the excellent electrocatalytic activities of MOF-based structures can also be due to their following aspects. The good permeability of MOF channels can result in rapid mass transport during electrochemical reactions. The substrate specificity of the catalytic sites improves the selectivity of the electrochemical response. These features render MOFs a class of ideal candidates for electrocatalysis.9,11
To improve the electrocatalytic activities of MOFs, three main strategies have been proposed: preparing conductive or electrochemically active MOFs, MOFs supported on conductive substrates, and MOFs hybridized with active materials. In the following sections, we summarize briefly the development of MOF-based composites for electrocatalysis, including hydrogen evolution reaction (HER), oxygen reduction reaction (ORR), and oxygen evolution reaction (OER) (Figure 1). It should be pointed out that, in the past years, there have been several excellent review papers dedicated to the MOF-derived materials, such as carbon, metal/carbon, and metal oxide materials for electrocatalysis.2,12 In this mini-review, we focus on the design and synthesis of pristine MOFs and their composites, and their direct application in electrocatalysis.9,13 We hope this review will inspire the related researchers with more interests and new ideas for future studies on MOF-based electrocatalysts.
Figure 1.

Schematic illustration of various fabrication strategies for MOF-based catalysts to enhance the conductivity and electrochemical catalytic activities of MOF composites.
Pristine Metal–Organic Frameworks
The first successful demonstration on the use of pristine MOFs as electrocatalysts for ORR was the wildly used Cu(II)-based MOF (Cu-bipy-BTC, bipy = 2,2′-bipyridine, BTC = 1,3,5-tricarboxylate), which exhibited a high and stable catalytic activity (Eonset ≈ −0.1 V vs Ag/AgCl) with almost 4e– reduction of O2.14 However, the low conductivities of pristine MOFs largely limit their catalytic performances. For electrocatalysts, increasing the conductivities of MOFs is an effective but difficult strategy. A conductive pristine MOF was first reported in 2009 by Takaishi et al. which showed a conductivity of 6 × 10–4 S/cm at 300 K.15 Over the next decade, several conductive pristine MOFs were developed. Among the reported conductive MOFs in the early stage, the 2D graphene-like Ni3(HITP)2 (HITP = 2,3,6,7,10,11-hexaiminotriphenylene) MOF was used as a well-defined, tunable oxygen reduction electrocatalyst with an onset potential of 0.82 V (with j = −50 μA/cm2) in alkaline solution, as shown in Figure 2A–C.16 However, its ORR catalytic performance is still too low for real application.
Figure 2.
Structure of Ni3(HITP)2 (A) and its ORR performance: polarization curves (B) and Tafel slope (C). Reproduced with permission from ref (16). Copyright 2016, Nature Publishing Group. (D) The connection mode between Zn-ε-Keggin and BTB3– fragments and (E) the 3D (3,4)-connected framework of NENU-500. (F) The polarization curves of the prepared catalysts in 0.5 M H2SO4 aqueous solution. Reproduced with permission from ref (17). Copyright 2015, American Chemical Society.
Introducing redox or catalytic active sites in MOFs is an effective method to enhance the electrocatalytic performances of MOFs. The active sites may contain organic ligands, metal nodes, and redox-active metal complexes. The organic ligands with π–π* bonds or extension of the conjugated systems can be potentially used for the construction of electrochemically active MOFs, such as the derivatives of benzene, pyridine, imidazole, and thiophene. Incorporation of these organic ligands with redox metal ions is conducive to preparing electrocatalytically active MOFs. For example, Wang et al. synthesized a cobalt-containing zeolitic imidazolate framework (Co-ZIF-9) as an OER electrocatalyst by assembling redox cobalt centers with benzimidazolate linkers. The fabricated Co-ZIF-9 showed effective OER catalytic activity in a wide pH range.18 In another work, Gong et al. prepared two novel Co(II) coordination polymers based on 1,4-bis(3-pyridylaminomethyl)benzene with coordination geometries analogous to those of most active ruthenium-based OER catalysts.19 The two complexes exhibited efficient OER electrocatalytic activities with lowered overpotentials and enhanced current densities. However, the structures of these MOFs may collapse during the electrochemical oxidation–reduction processes.
Polyoxometalates (POMs), containing a large abundance of O moieties in the backbone, have been studied as hopeful electrocatalysts for HER. Meanwhile, the redox activity of POMs could further affect the catalytic process. Using POMs as metal nodes, the as-prepared MOFs generally possess the advantages of a 3D framework, large channels, high surface area, large number of O moieties, and higher stability. For example, Qin et al. presented a synthetic strategy to tailor two new POMOFs (NENU-500 and NENU-501) using ε-Keggin polymolybdate units as the nodes and benzene tribenzoate as the linkers (Figure 2D,E).17 The NENU-500 catalyst exhibited efficient HER property with an overpotential of 237 mV at 10 mA/cm2 and a Tafel slope of 96 mV/dec in acidic solution, which are comparable to other excellent non-noble metal electrocatalysts (Figure 2F). In addition, the NENU-500 showed fast electrode kinetics (Rct: 28 Ω) and excellent stability. The superior HER performance of NENU-500 was attributed to the rigid, stable open-pore network and the good redox properties of the POMs. This study paves a way to exploit high-porosity and stable POM-based MOFs for the application in novel HER electrocatalysts.
Metal complexes are another class of stable and active species, which are made from stable ligands and active metal ions. Owing to the stereospecificity and different electron atmosphere of metal ions in the complexes, metal complexes generally have enhanced redox selectivity and catalytic capacity. In addition, metal complexes can effectively retain the excellent properties of the ligands. Metalloporphyrins, a class of typical metal complexes, which retain the excellent electrical conductivity and stability of porphyrin ligands, offer a versatile platform for the design of electrocatalytic materials. Meanwhile, they generally exhibit high catalytic activities to catalyze a wide array of substrates under mild conditions. For instance, the Fe(III) porphyrins were found to be highly efficient molecular catalysts for ORR, which are structurally related to hemoproteins and exhibit strong interaction with O2 molecules. The robust PCN-223-Fe is constructed from Zr6 oxo clusters and Fe(III) porphyrin linkers. As an ORR electrocatalyst, PCN-223-Fe showed a large catalytic current under cathodic potentials and achieved high H2O/H2O2 selectivity with the number of transferred electrons approaching 4.20
In recent years, bimetallic and multimetallic MOFs, especially Fe-, Co-, and Ni-based MOFs, have been widely used as excellent electrocatalytic materials due to the synergy effects among metal ions. In addition, theoretical computations and experiments demonstrated that high valent 3p or transition metal cations can effectively strengthen the electrostatic interactions between the metal nodes and carboxylic groups, thus improving the stability of MOFs. Compared with the monometallic MOFs, the multimetallic MOFs have been proved to have enhanced electrical conductivities and OER catalytic performances.21,22 For example, the NiFe-MOF arrays synthesized by Zhao et al. showed an enhanced electrical conductivity (1 ± 0.2 × 10–3 S/m) and exhibited a lower OER overpotential for 10 mA/cm2 than Ni-MOF (240 mV vs 56 mV).22 Zhou’s group synthesized a kind of very stable bimetal–organic framework of Fe2M-BPTC (M = Co, Ni, and Zn, H3BPTC = biphenyl-3,4,5-tricarboxylic acid), which has a much lower OER overpotential for 10 mA/cm2 and a faster charge transfer rate than that of Fe3-BPTC.21 We also fabricated two kinds of FeNi-MOFs with excellent OER performance and stability.23,24 Li and coworkers prepared a series of Fe/Ni-based trimetallic MOFs (Fe/Ni/Co(Mn)-MIL-53) via a simple solvothermal process, and the MOFs exhibited high intrinsic OER electrocatalytic activity and stability.25 The optimized Fe/Ni2.4/Co0.4-MIL-53 MOF showed excellent OER catalytic performance with an overpotential of 236 mV at 20 mA/cm2, better than those of other proportions of trimetallic MOFs. The high OER catalytic performance can be attributed to its unique structure and porosity, as well as the synergy effect of the mixed metals. Unfortunately, the multimetallic MOFs are complicated, and the catalytic mechanism is still not clear. Therefore, more studies are needed in this field.
On the other hand, decreasing the size of the MOF particles to nanoscale or preparing ultrathin MOF nanosheets (NSs) can expose most of the active metal sites to the reactants, thus improving the electrocatalytic properties of MOFs. Recently, Tang et al. prepared ultrathin NiCo bimetallic MOFs nanosheets (NiCo-UMOFNs) with abundant coordinatively unsaturated metal sites as promising electrocatalysts for the OER in alkaline conditions, as shown in Figure 3A–C.26 The obtained NiCo-UMOFNs with thickness around 3 nm on a glassy carbon electrode exhibited higher OER electrocatalytic activity with a more negative onset potential of 1.42 V, a lower overpotential of 250 mV at 10 mA/cm2, and a smaller Tafel slope of 42 mV/dec than those of the bulk NiCo-MOFs and the monometallic Ni- or Co-UMOFNs. The enhanced electrocatalytic properties of the NiCo-UMOFNs can be ascribed to the ultrathin nanosheet structure for facile mass transport and fast electron transfer, the rich available coordinatively unsaturated metal sites with high catalytic activity, and the synergistic interaction between Co and Ni ions. Wang et al. synthesized various bimetal–MOF nanosheet (BMNSs) arrays on different conductive substrates by in situ transformation of the presynthesized layered double hydroxide (LDH) NS arrays, as shown in Figure 3D.27 The obtained ultrathin NiCo-BDC BMNSs exhibit excellent catalytic activity and durability toward OER with overpotential of only 230 mV at 10 mA/cm2 and Tafel slope of 42 mV/dec in 1 M KOH (Figure 3E–G).
Figure 3.
(A) AFM image of the as-prepared NiCo-UMOFNs. (B) Polarization curves of NiCo-UMOFNs, Ni-UMOFNs, Co-UMOFNs, RuO2, and bulk NiCo-MOFs in O2-saturated 1 M KOH solution at a scan rate of 5 mV/s. (C) The corresponding Tafel plots. Reproduced with permission from ref (26). Copyright 2016, Nature Publishing Group. (D) Schematic illustration of the fabrication of ultrathin BMNSs arrays on the 3D conductive matrix, (E) polarization curves, and (F) the corresponding Tafel slopes of the NiCo-BDC BMNS array, NiCo-BDC BMNS powder, and NiCo-LDH NS array. (G) The stability curves of the NiCo-BDC BMNS array compared with NiCo-BDC BMNS powder. Reproduced with permission from ref (27). Copyright 2019, Wiley-VCH.
MOF-Conductive Material Composites
A simple and efficient strategy to improve the conductivity of MOFs is to hybridize MOFs with secondary highly conductive supports, like graphene, conductive polymers, carbon nanotubes (CNTs), and so on. Graphene and graphene oxide (GO) have been used widely as substrates for immobilizing and/or hybridizing MOFs due to their high electrical conductivity, high specific surface area, and very efficient charge transfer. There are two hybrid ways between graphene and MOFs: graphene just as a support or graphene as an integral component of the framework. The former is a simple and widely used method. However, although this approach can improve the electronic conductivity of MOFs at a macroscopic level, the local charge transport in MOFs is still limited by the pore aperture-defined size-exclusion effects.28 To resolve such a problem, Jahan et al. inserted GO into the copper MOF as an integral component, in which GO acts as both struts to link MOF nodes and a good electron transfer mediator, as shown in Figure 4A.28 The as-prepared GO-MOFs showed good performance as a trifunctional catalyst (HER, OER, and ORR) with smaller overpotentials, higher current densities, and better stability in acid media compared to pure MOFs (Figure 4B). The remarkable electrocatalytic properties and stability of the GO-MOF composites can be ascribed to the special porous scaffold structure, improved conductivity, and synergistic effects between GO and MOF. This study not only confirms that the incorporation of GO into the MOF is an efficient strategy to enhance the stability and electrocatalytic performance of MOFs but also offers a new path to design and synthesize nonprecious metal catalysts for energy conversion. Later, in a work reported by Jin and co-workers, CNTs were used as an integral component and hybridized with Co-MOF acting as struts to link MOF nodes.20 In the fabricated Co-MOF@CNTs, CNTs can not only effectively improve the conductivity and the stability of the composite under harsh oxidative environment but also enhance the bifunctional electrocatalytic performance due to the synergistic catalysis of active centers Co(II), organic ligands, and CNTs (Figure 4C–F).29 The Co-MOF@CNTs (5 wt %) exhibit high OER and ORR catalytic activities comparable to RuO2 and 20 wt % Pt/C catalysts, and the stability is comparable to most of the reported excellent cobalt-based catalysts. Hybridizing MOFs with conductive polymers is also an effective method to increase the conductivity of MOFs. There are the following kinds of MOF–polymer composites: polymer polymerized inside the pores of MOFs, MOFs grown inside or on the surface of a polymer, MOFs cross-linked with polymers through the organic ligands, etc. Such proposed catalyst systems based on conductive polymers and MOFs have potential electrocatalysis applications, though few systems have been reported.2
Figure 4.
(A) Chemical structures of Cu-MOF, GO, and the secondary building units of Cu-MOF. (B) Polarization curves of Pt/C (GO 2, 4, 6, and 8 wt %) Cu-MOF, and Cu-MOF in N2-saturated 0.5 M H2SO4 with scan rate of 2 mV/s. Reproduced with permission from ref (28). Copyright 2013, Wiley-VCH. (C) ORR LSV curves of the CNTs, Co-MOF, Co-MOF@CNTs (5 wt %), and 20 wt % Pt/C at 5 mV/s with rotation rate of 1600 rpm. (D) The preparation of Co-MOF@CNTs. (E) LSV curves of OER on CNTs, Co-MOF, Co-MOF@CNTs (15 wt %), Co-MOF@CNTs (10 wt %), Co-MOF@CNTs (5 wt %), and Co-MOF@CNTs (1 wt %) in 1.0 M KOH at 5 mV/s. (f) Durability test for the Co-MOF@CNTs (5 wt %) over 1000 cycles. Reproduced with permission from ref (29). Copyright 2016, Elsevier.
Nowadays, loading MOFs on conductive substrates such as nickel foam (NF), carbon cloth, and glass substrates coated with tin-doped indium oxide, by solvothermal, electrochemical, or direct deposition methods, is another widely used strategy to enhance the conductivity and electrocatalytic performances of MOFs. For example, the OER catalytic performance of Fe/Ni2.4/Co0.4-MIL-53 can be further enhanced by direct growth on porous NF with high conductivity.25 When the ultrathin CoNi-MOF nanosheets are loaded on copper foam, the overpotential of OER at 10 mA/cm2 decreases from 250 mV to 189 mV.26
MOF-Active Species Composites
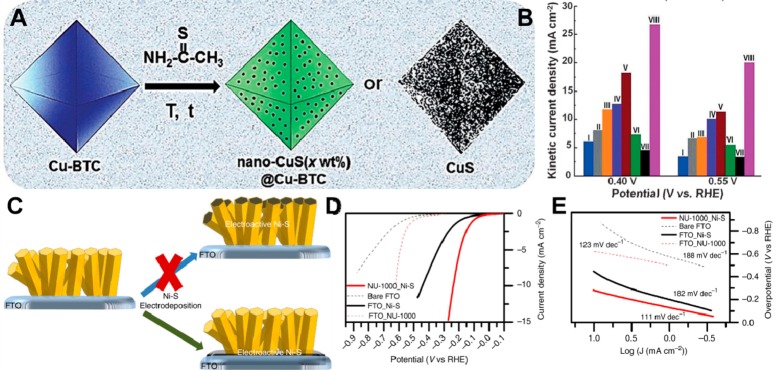
Although efforts have been made to hybridize MOFs or their composites with conductive CNTs and GO as electrocatalysts, their performances are not very satisfactory. To develop efficient MOF-based electrocatalysts, loading active species into MOF channels has attracted great interest, especially metal and metal oxide (sulfide, phosphide, or nitride) nanoparticles (NPs) with excellent electrochemical catalytic activities. In such composites, MOFs not only act as support and/or template to control the shape and size of NPs but also offer immobilization, protection, and selectivity to improve the stability of NPs. On the other hand, the confined NPs generally show excellent electrocatalytic activities and meanwhile can improve the conductivity of the composites. Recently, we successfully encapsulated small Pd nanoclusters with diameter of 2–3 nm into the channel of MOF-74 (Pd@MOF-74).3 Without high-temperature treatment, the prepared Pd@MOF-74 was directly used as a bifunctional electrocatalyst for HER and ORR. To develop efficient MOF-based ORR electrocatalysts, Cho et al. loaded nanosized copper sulfide into the pores of Cu-BTC (nano-CuS(x wt %)@Cu-BTC), and the composite has low surface resistivity and high ORR catalytic activity, as shown in Figure 5A,B.30,31 The ORR performance of nano-CuS(x wt %)@Cu-BTC is closely dependent on the conductivity and porosity of the material, which can be adjusted by using different amounts of nano-CuS. With the amount of nano-CuS increasing, the electrical conductivity increases, but porosity decreases. All the synthesized nano-CuS(x wt %)@Cu-BTC samples demonstrated significantly higher electrocatalytic ORR activities compared to Cu-BTC and nano-CuS(99 wt %). The optimal CuS (28 wt %)@Cu-BTC catalyst showed an onset potential of 0.91 V vs RHE, quasi-four-electron transfer pathway, and a kinetic current density of 11.3 mA/cm2 at 0.55 V vs RHE.
Figure 5.
(A) Synthesis process of nano-CuS(x wt %)@Cu-BTC and nano-CuS (99 wt %). (B) ORR activities of Cu-BTC (I), nano-CuS(1.4 wt %)@Cu-BTC (II), nano-CuS(5.3 wt %)@Cu-BTC (III), nano-CuS(8.8 wt %)@Cu-BTC (IV), nano-CuS(28 wt %)@Cu-BTC (V), nano-CuS(56 wt %)@Cu-BTC (VI), nano-CuS(99 wt %) (VII), and commercial 20 wt % Pt/C (VIII) at 0.40 and 0.55 V. Reproduced with permission from ref (30 and 31). Copyright 2016, Wiley-VCH. (C) Electrodeposition of Ni–S film to create the NU-1000_Ni–S hybrid. (D) Polarization curves and (E) the corresponding Tafel slopes of bare FTO, FTO_NU-1000, FTO_Ni–S, and NU-1000_Ni–S. Reproduced with permission from ref (32). Copyright 2015, Nature Publishing Group.
Depositing active materials on the surface of MOFs is also an efficient strategy to prepare MOFs with high electrocatalytic activities. For example, although Hod et al. failed to coat uniform Ni–S film on the surface of Zr-MOF, the Ni–S electrodeposited at the bottom of the NU-1000 rods also showed enhanced electrocatalytic HER performance than the films with only NU-1000 or Ni–S, as shown in Figure 5C–E.32 The MOFs can not only facilitate the exposure of the Ni–S surface but also modify the local environment of the active Ni–S catalyst. He et al. prepared a bifunctional electrocatalyst by coating various CoIII and CoII contents onto the surface of MIL-101(Cr) with oxidative or reductive treatment during the impregnation.33,34 The electrocatalytic activities of the Co/MIL-101(Cr) composites are closely dependent on the contents of CoIII and CoII on the surface. A higher content of CoIII is favorable to adsorb O on the surface of electrocatalyst, thus improving the OER activity. On the contrary, increasing the content of CoII would be more preferential to absorb O2 on the surface of the electrocatalyst, thus improving the ORR activity accordingly. With the CoIII/CoII ratio of ∼0.89, the Co/MIL-101(Cr) catalyst exhibits the highest overall bifunctional OER and ORR activities with ΔE value of ∼1.16 V.
Conclusions and Outlook
This mini-review highlights the latest developed strategies for the fabrication of MOFs and MOF composites for the application in electrocatalysis, by taking advantage of their unique structures and excellent physical and chemical properties. It is well-known that pristine MOFs and MOF composites generally have low electric conductivity and are unstable in strongly acidic and alkaline solutions, which are the disadvantages of MOFs for their application in the field of electrocatalysis. Therefore, it is very important to explore stable and electrochemically active MOFs. To this end, several strategies have been proposed in recent years to synthesize functional MOFs:
-
(1)
Highly conductive pristine MOFs with large surface area and abundant metal active sites have been developed. The conductivity of these MOFs is close to that of graphite, and meanwhile the coordinated redox centers or catalytic actively organic ligands and metal nodes can effectively enhance the electrocatalytic properties of MOFs. However, it is still a great challenge to design novel pristine MOFs with ideal electrocatalytic performances for real applications.
-
(2)
By reducing the size of MOFs to nanoscale or constructing ultrathin nanostructures, more metal centers can be exposed and available to the reactants, thus significantly improving the electrochemical activity of MOFs.
-
(3)
Hybridizing MOFs with highly conductive materials is a facile, efficient method to develop highly conductive MOF-based composites for electrocatalysis. The conductive materials can be used as support to grow MOFs or can be encapsulated into the channels of MOFs as an integral component of MOFs. Such a strategy paves a way to exploit highly conductive and stable MOF composites for the application in electrocatalysis.
-
(4)
MOFs combined with active materials are helpful to stabilize the active species and improve the conductivity and electrocatalytic activity of the composites. The advantage of this method is that MOFs can be used to hybridize various dimensional electrocatalytically active materials, including nanoparticles (0 D), nanotubes (1 D), films (2 D), and 3 D scaffold materials.
In summary, although there are still problems with MOFs and MOF-based composites in electrocatalytic application, their unique structures and excellent properties make them promising electrocatalysts. Exploiting more effective methods to design and fabricate novel MOFs and MOF-based composites with excellent electrocatalytic activity and stability will be crucial for their practical application in electrochemical fields.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 21575134, 21633008, 21605006, 21773224), the National Key Research and Development Plan (2016YFA0203200), and K. C. Wong Education Foundation.
Biographies

Fuqin Zheng received her B.S. degree in 2011 from Yanshan University. Currently, she is pursuing a Ph.D. under the supervision of Prof. Wei Chen at Changchun Institute of Applied Chemistry, Chinese Academy of Sciences. Her scientific interests focus on the design and synthesis of metal–organic framework based materials and their applications in energy storage and conversion.

Ziwei Zhang received her B.S. degree from Northeast Normal University in 2017. Currently, she is pursuing a Ph.D. under the supervision of Prof. Wei Chen at Changchun Institute of Applied Chemistry, Chinese Academy of Sciences. Her research mainly focuses on the design and synthesis of electrochemical catalysts and sensing materials.

Chunmei Zhang received her B.S. degree in 2013 from College of Chemistry and Chemical Engineering, Inner Mongolia University. She got her Ph.D. in electrochemistry from Changchun Institute of Applied Chemistry, Chinese Academy of Sciences in 2018. She is currently working in Institute of Materials Science and Devices, Suzhou University of Science and Technology. Her current research focuses on gas sensing materials, electrochemical catalysts, and electrochemical sensing materials based on unique nanostructured materials, such as graphene-based materials, hollow carbon sphere-supported materials, and black phosphorus-based materials.

Wei Chen received his Ph.D. in electrochemistry from Xiamen University in 2003. Following his graduate studies, he began working as a postdoctoral associate in the area of synthesis and property studies of metal nanoclusters at the University of California, Santa Cruz. He is currently a full professor at Changchun Institute of Applied Chemistry, Chinese Academy of Sciences. His research interests include electroanalytical chemistry, surface electrochemistry, electrocatalysis, photoelectrocatalysis, and the controlled synthesis, characterization, and application of nanomaterials in energy storage and conversion.
The authors declare no competing financial interest.
References
- Bakuru V. R.; Dmello M. E.; Kalidindi S. B. Metal-Organic Frameworks for Hydrogen Energy Applications: Advances and Challenges. ChemPhysChem 2019, 20 (10), 1177–1215. 10.1002/cphc.201801147. [DOI] [PubMed] [Google Scholar]
- Mahmood A.; Guo W.; Tabassum H.; Zou R. Metal-Organic Framework-Based Nanomaterials for Electrocatalysis. Adv. Energy Mater. 2016, 6 (17), 1600423. 10.1002/aenm.201600423. [DOI] [Google Scholar]
- Zheng F.; Zhang C.; Gao X.; Du C.; Zhuang Z.; Chen W. Immobilizing Pd nanoclusters into electronically conductive metal-organic frameworks as bi-functional electrocatalysts for hydrogen evolution and oxygen reduction reactions. Electrochim. Acta 2019, 306, 627–634. 10.1016/j.electacta.2019.03.175. [DOI] [Google Scholar]
- Sun L.; Campbell M. G.; Dinca M. Electrically Conductive Porous Metal–Organic Frameworks. Angew. Chem., Int. Ed. 2016, 55 (11), 3566–3579. 10.1002/anie.201506219. [DOI] [PubMed] [Google Scholar]
- Xue Y.; Zheng S.; Xue H.; Pang H. Metal-organic framework composites and their electrochemical applications. J. Mater. Chem. A 2019, 7 (13), 7301–7327. 10.1039/C8TA12178H. [DOI] [Google Scholar]
- Noh H.; Kung C.-W.; Otake K.-i.; Peters A. W.; Li Z.; Liao Y.; Gong X.; Farha O. K.; Hupp J. T. Redox-Mediator-Assisted Electrocatalytic Hydrogen Evolution from Water by a Molybdenum Sulfide-Functionalized Metal–Organic Framework. ACS Catal. 2018, 8 (10), 9848–9858. 10.1021/acscatal.8b02921. [DOI] [Google Scholar]
- Liang Z.; Qu C.; Xia D.; Zou R.; Xu Q. Atomically Dispersed Metal Sites in MOF-Based Materials for Electrocatalytic and Photocatalytic Energy Conversion. Angew. Chem., Int. Ed. 2018, 57 (31), 9604–9633. 10.1002/anie.201800269. [DOI] [PubMed] [Google Scholar]
- Deng H.; Doonan C. J.; Furukawa H.; Ferreira R. B.; Towne J.; Knobler C. B.; Wang B.; Yaghi O. M. Multiple Functional Groups of Varying Ratios in Metal-Organic Frameworks. Science 2010, 327 (5967), 846–850. 10.1126/science.1181761. [DOI] [PubMed] [Google Scholar]
- Liu W.; Yin X.-B. Metal–organic frameworks for electrochemical applications. TrAC, Trends Anal. Chem. 2016, 75, 86–96. 10.1016/j.trac.2015.07.011. [DOI] [Google Scholar]
- Wang H.; Zhu Q.-L.; Zou R.; Xu Q. Metal-Organic Frameworks for Energy Applications. Chem. 2017, 2 (1), 52–80. 10.1016/j.chempr.2016.12.002. [DOI] [Google Scholar]
- Cheng M.; Lai C.; Liu Y.; Zeng G.; Huang D.; Zhang C.; Qin L.; Hu L.; Zhou C.; Xiong W. Metal-organic frameworks for highly efficient heterogeneous Fenton-like catalysis. Coord. Chem. Rev. 2018, 368, 80–92. 10.1016/j.ccr.2018.04.012. [DOI] [Google Scholar]
- Wu H. B.; Lou X. W. Metal-organic frameworks and their derived materials for electrochemical energy storage and conversion: Promises and challenges. Sci. Adv. 2017, 3 (12), eaap9252 10.1126/sciadv.aap9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z.; Qu C.; Guo W.; Zou R.; Xu Q. Pristine Metal–Organic Frameworks and their Composites for Energy Storage and Conversion. Adv. Mater. 2018, 30 (37), 1702891. 10.1002/adma.201702891. [DOI] [PubMed] [Google Scholar]
- Mao J.; Yang L.; Yu P.; Wei X.; Mao L. Electrocatalytic four-electron reduction of oxygen with Copper (II)-based metal-organic frameworks. Electrochem. Commun. 2012, 19, 29–31. 10.1016/j.elecom.2012.02.025. [DOI] [Google Scholar]
- Takaishi S.; Hosoda M.; Kajiwara T.; Miyasaka H.; Yamashita M.; Nakanishi Y.; Kitagawa Y.; Yamaguchi K.; Kobayashi A.; Kitagawa H. Electroconductive Porous Coordination Polymer Cu[Cu(pdt)2] Composed of Donor and Acceptor Building Units. Inorg. Chem. 2009, 48 (19), 9048–9050. 10.1021/ic802117q. [DOI] [PubMed] [Google Scholar]
- Miner E. M.; Fukushima T.; Sheberla D.; Sun L.; Surendranath Y.; Dinca M. Electrochemical oxygen reduction catalysed by Ni3(hexaiminotriphenylene)2. Nat. Commun. 2016, 7, 10942. 10.1038/ncomms10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J.-S.; Du D.-Y.; Guan W.; Bo X.-J.; Li Y.-F.; Guo L.-P.; Su Z.-M.; Wang Y.-Y.; Lan Y.-Q.; Zhou H.-C. Ultrastable Polymolybdate-Based Metal–Organic Frameworks as Highly Active Electrocatalysts for Hydrogen Generation from Water. J. Am. Chem. Soc. 2015, 137 (22), 7169–7177. 10.1021/jacs.5b02688. [DOI] [PubMed] [Google Scholar]
- Wang S.; Hou Y.; Lin S.; Wang X. Water oxidation electrocatalysis by a zeolitic imidazolate framework. Nanoscale 2014, 6 (17), 9930–9934. 10.1039/C4NR02399D. [DOI] [PubMed] [Google Scholar]
- Gong Y.; Shi H.-F.; Hao Z.; Sun J.-L.; Lin J.-H. Two novel Co(ii) coordination polymers based on 1,4-bis(3-pyridylaminomethyl)benzene as electrocatalysts for oxygen evolution from water. Dalton Trans. 2013, 42 (34), 12252–12259. 10.1039/c3dt50697e. [DOI] [PubMed] [Google Scholar]
- Usov P. M.; Huffman B.; Epley C. C.; Kessinger M. C.; Zhu J.; Maza W. A.; Morris A. J. Study of Electrocatalytic Properties of Metal–Organic Framework PCN-223 for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2017, 9 (39), 33539–33543. 10.1021/acsami.7b01547. [DOI] [PubMed] [Google Scholar]
- Wang X.-L.; Dong L.-Z.; Qiao M.; Tang Y.-J.; Liu J.; Li Y.; Li S.-L.; Su J.-X.; Lan Y.-Q. Exploring the Performance Improvement of the Oxygen Evolution Reaction in a Stable Bimetal–Organic Framework System. Angew. Chem., Int. Ed. 2018, 57 (31), 9660–9664. 10.1002/anie.201803587. [DOI] [PubMed] [Google Scholar]
- Duan J.; Chen S.; Zhao C. Ultrathin metal-organic framework array for efficient electrocatalytic water splitting. Nat. Commun. 2017, 8, 15341. 10.1038/ncomms15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F.; Xiang D.; Li P.; Zhang Z.; Du C.; Zhuang Z.; Li X.; Chen W. Highly Conductive Bimetallic Ni–Fe Metal Organic Framework as a Novel Electrocatalyst for Water Oxidation. ACS Sustainable Chem. Eng. 2019, 7 (11), 9743–9749. 10.1021/acssuschemeng.9b01131. [DOI] [Google Scholar]
- Zheng F.; Zhang Z.; Xiang D.; Li P.; Du C.; Zhuang Z.; Li X.; Chen W. Fe/Ni bimetal organic framework as efficient oxygen evolution catalyst with low overpotential. J. Colloid Interface Sci. 2019, 555, 541–547. 10.1016/j.jcis.2019.08.005. [DOI] [PubMed] [Google Scholar]
- Li F.-L.; Shao Q.; Huang X.; Lang J.-P. Nanoscale Trimetallic Metal–Organic Frameworks Enable Efficient Oxygen Evolution Electrocatalysis. Angew. Chem., Int. Ed. 2018, 57 (7), 1888–1892. 10.1002/anie.201711376. [DOI] [PubMed] [Google Scholar]
- Zhao S.; Wang Y.; Dong J.; He C.-T.; Yin H.; An P.; Zhao K.; Zhang X.; Gao C.; Zhang L.; Lv J.; Wang J.; Zhang J.; Khattak A. M.; Khan N. A.; Wei Z.; Zhang J.; Liu S.; Zhao H.; Tang Z. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1 (12), 16184. 10.1038/nenergy.2016.184. [DOI] [Google Scholar]
- Wang B.; Shang J.; Guo C.; Zhang J.; Zhu F.; Han A.; Liu J. A General Method to Ultrathin Bimetal-MOF Nanosheets Arrays via In Situ Transformation of Layered Double Hydroxides Arrays. Small 2019, 15 (6), 1804761. 10.1002/smll.201804761. [DOI] [PubMed] [Google Scholar]
- Jahan M.; Liu Z.; Loh K. P. A Graphene Oxide and Copper-Centered Metal Organic Framework Composite as a Tri-Functional Catalyst for HER, OER, and ORR. Adv. Funct. Mater. 2013, 23 (43), 5363–5372. 10.1002/adfm.201300510. [DOI] [Google Scholar]
- Fang Y.; Li X.; Li F.; Lin X.; Tian M.; Long X.; An X.; Fu Y.; Jin J.; Ma J. Self-assembly of cobalt-centered metal organic framework and multiwalled carbon nanotubes hybrids as a highly active and corrosion-resistant bifunctional oxygen catalyst. J. Power Sources 2016, 326, 50–59. 10.1016/j.jpowsour.2016.06.114. [DOI] [Google Scholar]
- Cho K.; Han S.-H.; Suh M. P. Copper–Organic Framework Fabricated with CuS Nanoparticles: Synthesis, Electrical Conductivity, and Electrocatalytic Activities for Oxygen Reduction Reaction. Angew. Chem., Int. Ed. 2016, 55 (49), 15301–15305. 10.1002/anie.201607271. [DOI] [PubMed] [Google Scholar]
- Wei J.; Liang Y.; Hu Y.; Kong B.; Zhang J.; Gu Q.; Tong Y.; Wang X.; Jiang S. P.; Wang H. Hydrothermal Synthesis of Metal–Polyphenol Coordination Crystals and Their Derived Metal/N-doped Carbon Composites for Oxygen Electrocatalysis. Angew. Chem., Int. Ed. 2016, 55 (40), 12470–12474. 10.1002/anie.201606327. [DOI] [PubMed] [Google Scholar]
- Hod I.; Deria P.; Bury W.; Mondloch J. E.; Kung C.-W.; So M.; Sampson M. D.; Peters A. W.; Kubiak C. P.; Farha O. K.; Hupp J. T. A porous proton-relaying metal-organic framework material that accelerates electrochemical hydrogen evolution. Nat. Commun. 2015, 6, 8304. 10.1038/ncomms9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X.; Yin F.; Li G. A Co/metal–organic-framework bifunctional electrocatalyst: The effect of the surface cobalt oxidation state on oxygen evolution/reduction reactions in an alkaline electrolyte. Int. J. Hydrogen Energy 2015, 40 (31), 9713–9722. 10.1016/j.ijhydene.2015.06.027. [DOI] [Google Scholar]
- Zaretski A. V.; Marin B. C.; Moetazedi H.; Dill T. J.; Jibril L.; Kong C.; Tao A. R.; Lipomi D. J.. Metal-assisted exfoliation (MAE): green process for transferring graphene to flexible substrates and templating of sub-nanometer plasmonic gaps. In Carbon Nanotubes, Graphene, and Emerging 2d Materials for Electronic and Photonic Devices Viii; Razeghi M., Ghazinejad M., Bayram C., Yu J. S., Eds.; 2015, International Society For Optics and Photonics, Washington State, USA. [Google Scholar]