Abstract

A fascinating way to originate a mechanically stable metallogel of ferric ions with metal-coordinating organic ligand triethylenetetramine through direct mixing of their water solutions in a stoichiometric ratio is achieved under ambient conditions. The rheological study established the mechanical property of the Fe(III) metallogel. A cashew-shaped microstructure of the metallogel was observed by FESEM analysis. The electrical property of the Fe(III) metallogel was also carefully scrutinized. The semiconducting features like the Schottky barrier diode property of the Fe(III) metallogel were explored. The catalytic role of the Fe(III) metallogel was also critically explored. The Fe(III) metallogel shows an excellent catalytic property toward the synthesis of aryl thioethers via a C–S coupling reaction under mild reaction conditions without the use of any organic solvent.
Introduction
Over past decades, through the development of new functional materials and their imminent applications in a wide range of science and commercial industries,1,2 gels earn a strong position in scientific research. Gels are semisolid 3D scaffolds where the solvents are immobilized by the gelator networks.1,3 The semisolid-like architecture is the outcome of self-assembly phenomena between small molecules in different solvents directing the gelation process.1 Low-molecular weight gelators (LMWGs) produce 3D gel structures that trap the solvent, and from this, we derive the semisolid viscoelastic material noted as the supramolecular gel.4 Several supramolecular interactions including hydrogen bonding,5 hydrophobic interactions,6−9 metal coordination,10−13 electrostatic interactions,14−16 aromatic π-ring-mediated interactions,6−9 van der Waals forces,5 and π-system-based stacking17,18 are key factors for developing functional supramolecular gels. Hydrogels have potential applications in science and cost-effective industries ranging from chemistry to physics and biology to pharmacy including cellular encapsulation,19 tissue engineering,20 and material science covering oil recovery,21 sensors,22 electro-optics/photonics,23 cosmetics,24 structure-directing agents,25 catalysis,26 drug delivery,27 therapeutic delivery,28 electrochemical applications,29 photolithography,30 biomedical applications,31 power source,32 electronic devices,33 etc. Among these types of gel materials, metallogels belong to an important class describing the assembly of metal ions and organic ligands via metal–ligand interactions to obtain a stable gel network.34 Due to their diverse applications, the metallogel has become an interesting aspect in conduction,35 proton conductivity,34,36 magnetism,37 redox,38 catalytic properties,39−41 sensing,42,43 etc.
The metallogelation process is manifested by different approaches including coordination polymeric networks,44−46 metal coordination complexes,7 organometallics,47 cross-linked coordination polymers,48 and metal–ligand interaction-based gelation.49 Transition-metal ions like Co2+,50−52 Ni2+,49,50 Cu2+,49,53 Cd2+,54,55 Fe2+/3+,38,36,56−59 and Zn2+53,60 are used for metallogelation with multiple extraordinary applications in science and technology.61 The Fe(III) metallogel has been gaining special attention due to its diverse uses in magnetism,38 semiconducting properties,57 self-healing ability,36 etc.
During the last few years, our group has been involved in metallogel-based research on fabrication of semiconducting devices.57 Besides, we have an aim to approach the multi-functional behavior of the metallogel system.62 Powered with the knowledge of transition-metal ion-directed catalysis toward synthetic organic chemistry,63 Fe(III)-metallogel-based catalysis in organic synthesis has been targeted. Through this present endeavor, we have successfully shown the multifunctional applications of the metallogel system.
Here, we have introduced a triethylenetetramine (TETA)-based metallogel of Fe(III) (Fe@TETA). Fe@TETA shows the semiconducting application of a Schottky barrier diode device. Interestingly, Fe@TETA exhibits an excellent catalytic role for the synthesis of thioethers via a C–S coupling reaction. Though the transition-metal-catalyzed aryl–S cross-coupling reactions are well established,64−66 the development of a mostly cost-effective catalytic method for the aryl–S coupling reaction is still a challenging task due to the enormous applications of C–S coupled products in the pharmaceutical industry and biological aspects. The synthesized Fe@TETA metallogel-based catalysis for the synthesis of aryl thioethers is a comparatively64−66 more industrial and environmentally friendly faster pathway that does not need to use any supporting ligand/additive and any organic solvent. This is one of the significant studies to establish the metallogel-based Schottky barrier diode device along with the metallogel-catalyzed, solvent-free aryl–S cross-coupling reactions to get aryl thioethers by a structurally flexible multifunctional soft metallogel system. In this current work, the instantaneous gel formation of Fe(III) with triethylenetetramine (TETA) in water is described (Figure 1).
Figure 1.
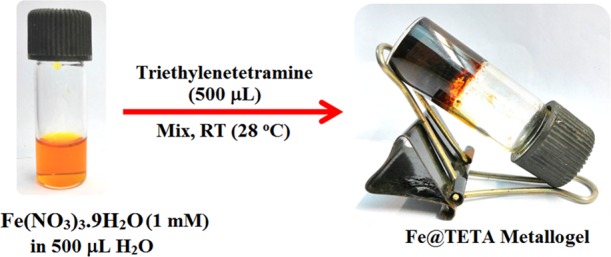
Synthetic strategy of the Fe(III) metallogel and the inversion vial test photograph of Fe@TETA.
The critical gelation concentration was found to be 404 mg mL–1. To determine the minimum critical gel concentration (MGC) of the Fe@TETA metallogel, the concentration of Fe(NO3)3·9H2O was varied from 1 to 404 mg mL–1, and effectively, the nonflowing gel was observed only at a minimum concentration of 404 mg mL–1, which was determined to be the critical gelation concentration (Figure 2). The gel to sol temperature (Tgel) of the Fe@TETA metallogel was found to be moderately excellent, and it was recorded at 130 °C. (See the Supporting Information for the gelation process of the Fe@TETA metallogel with a Fe(NO3)3·9H2O salt in various solvents, Table S1.)
Figure 2.

Images showing the gelation of the Fe@TETA metallogel at the minimum critical gelation concentration (MGC) (404 mg mL–1).
Results and Discussion
Rheological Analysis
The rheological measurements confirm the viscoelastic semisolid nature of the Fe@TETA hydrogel (Figure 3). The storage modulus (G′) represents the amount of energy stored in the system during the application of shear on the viscoelastic region. The loss modulus (G″) represents the amount of energy dissipated under the application of oscillatory stress. For the gel state, the storage modulus (G′) is considerably higher than the loss modulus (G″).
Figure 3.

Angular frequency-dependent storage modulus (G′) and loss modulus (G″) of the Fe@TETA metallogel.
The storage modulus (G′) and loss modulus (G″) of the samples are expressed as
| 1 |
| 2 |
(Here, σ0 is the shear stress amplitude, γ0 is the strain amplitude, σ0/γ0 is the amplitude ratio, and δ is the phase angle.)
Rheological results support that the G′ of the Fe@TETA metallogel is significantly higher than G″ (i.e., G′ > 104) at a certain gel concentration (Figure 3). The observed rheological data (i.e., G′ ≫ G″) establishes the stability of the gel structural property of the Fe@TETA hydrogel as well as the solid-like behavior. The oscillatory stress measurements of the Fe@TETA metallogel were also obtained (Figure 4). The value was constant over an oscillatory stress range of 100–102 rad/s.
Figure 4.

Oscillatory stress measurements of the Fe@TETA metallogel.
The strain sweep measurements of the Fe@TETA metallogel performed at a constant frequency of 1 rad/s. Here, Fe(III) acts as cross-linker and strengthening agent into the gel network. The metal probably increases the additional stability to the metallogel.
Microstructural and Infrared Spectral Study
The microstructural feature of the Fe@TETA metallogel was scrutinized by FESEM structural analysis. The morphology of Fe@TETA showed the agglomerated cashew-like structure of the metallogel (Figure 5a,b). The structural patterns of the metallogel, observed in FESEM, were generated through immediate mixing of Fe(NO3)3·9H2O and TETA ligands in a water medium. The elemental mapping (Figure 5c–g) evidences C, N, O, and Fe elements present in the gel network (see the Supporting Information for detailed FESEM images and EDX spectra, Figures S1 and S2). The detailed infrared spectral study of the Fe@TETA metallogel and pure triethylenetetramine is given in the Supporting Information (Figure S3).
Figure 5.
Fe (III) metallogel: (a, b) FESEM microstructural image of Fe@TETA; (c–g) elemental mapping of Fe@TETA showing C, N, O, and Fe elements present in the gel.
Device Fabrication
Here, in this report, we have fabricated multiple metal–semiconductor (MS) junction devices to perform an electrical study. The devices were fabricated as sandwich-like structures in the form of ITO/Fe@TETA/Al. In this regard, on top of the precleaned ITO-coated glass substrate, the thin film of our synthesized metallogel (Fe@TETA) was deposited by a doctor-blade method. To evaporate the solvent part completely, the as-deposited thin film was dried in a vacuum oven at 80 °C for several minutes subsequently. Using a surface profiler, the thicknesses of the developed thin films were measured as ∼1 μm. The aluminum was deposited as a metal electrode in the vacuum coating unit of HINDHIVAC (model no: 12A4D) under a pressure of 10–6 Torr. Using a shadow mask, the effective area of the device was maintained as 7.065 × 10–6 m2.
Employing a two-probe technique, the current–voltage (I–V) characteristics of the devices were recorded with the help of a source meter made by Keithley (model no: 2635B). All the measurements and study were carried out under a N2-filled glovebox and laboratory temperature of 26 °C.
Optical Characterization
UV–vis analysis was performed here to analyze the optical characterization of the synthesized Fe@TETA metallogel. The solid-state absorbance spectra of the Fe@TETA metallogel (inset, Figure 6) were recorded by preparing the thin films of well dispersed complexes in DMF in the range of 200–600 nm. The direct optical band gap was estimated using Tauc’s equation (eq 3).67
| 3 |
where α, Eg, h, and ν stand for the absorption coefficient, band gap, Planck’s constant, and frequency of light, respectively. The exponent “n” is the electron transition process-dependent constant. “A” is a constant, which is considered as 1 for an ideal case. Considering n = 1/2,67 the direct optical band gap of the Fe@TETA metallogel was calculated from the extrapolation of the linear region of the plot (αhν)2 versus hν (Figure 6) to an α = 0 absorption as 2.84 eV.
Figure 6.

UV–vis absorption spectra (inset) and Tauc’s plot of the Fe@TETA metallogel.
Electrical Characterization
The obtained optical band gap of the Fe@TETA metallogel suggests it as a semiconductor material. Hence, we have fabricated a metal (Al)–semiconductor (Fe@TETA) (MS) junction thin-film device and studied its electrical parameters by analyzing the charge transport behavior.
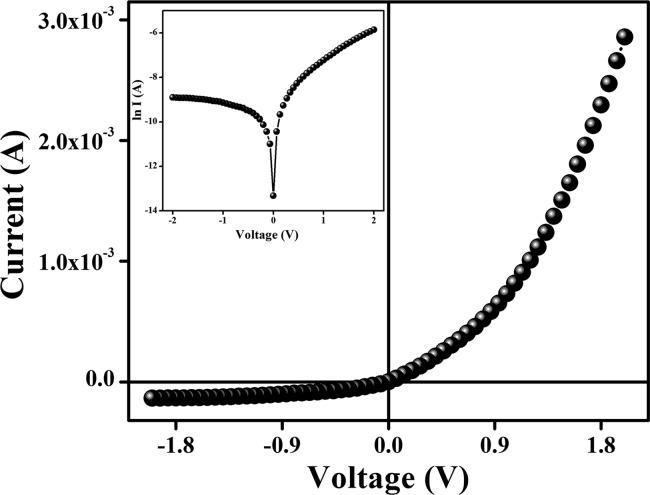
The I–V characteristic of our complex-based devices was recorded at a corresponding applied bias voltage sequentially within the limit of ±2 V and is presented in Figure 7. The electrical conductivity of our complex-based device was estimated as 1.003 × 10–4 S m–1, typical of a semiconductor. Moreover, the representative I–V characteristic of the Al/Fe@TETA interface (Figure 7) represents a nonlinear rectifying behavior, similar to that of the Schottky barrier diode (SBD). The rectification ratio (Ion/Ioff) of our fabricated SBD at ±2 V was obtained as 21.04.
Figure 7.
I–V characteristic curve for ITO/Fe@TETA/Al-structured thin-film devices.
Using thermionic emission theory, the I–V characteristic of the Fe@TETA-based SBDs was analyzed. To extract important diode parameters, here, we have employed Cheung’s method.67,69 The obtained I–V curve was quantitatively analyzed by considering the following standard equations:67,68
| 4 |
| 5 |
where I0, k, T, V, A, η, and A* stand for the saturation current, electronic charge, Boltzmann constant, temperature in Kelvin, forward bias voltage, effective diode area, ideality factor, and effective Richardson constant, respectively. The effective Richardson constant for our fabricated Fe@TETA metallogel-based devices was considered as 32 AK–2 cm–2.
The ideality factor, barrier potential height, and series resistance of our devices were also determined by using eqs 6–8, which were extracted from Cheung’s work69,70
| 6 |
| 7 |
| 8 |
From the intercept and slope of the dV/d ln I versus I plot (Figure 8), the ideality factor (η) and the series resistance (RS) for our fabricated device were determined. For our fabricated SBD, the value of the ideality factor (η) was estimated as 1.39, which depicts a deviation from the ideal value (∼1). The existence of interface states and series resistance at the junction and the presence of inhomogeneities of the Schottky barrier height may be the main reason of this deviation.71,72 As the value of the ideality factor of our synthesized complex-based device is more or less near the ideal value (∼1), it depicts the fewer number of recombination of interfacial charge carriers and generation of better homogeneity at the barrier of Schottky junctions.67 From this, it may be concluded that our synthesized complex-based SBD possesses less carrier recombination at the junction, that is, better barrier homogeneity.
Figure 8.
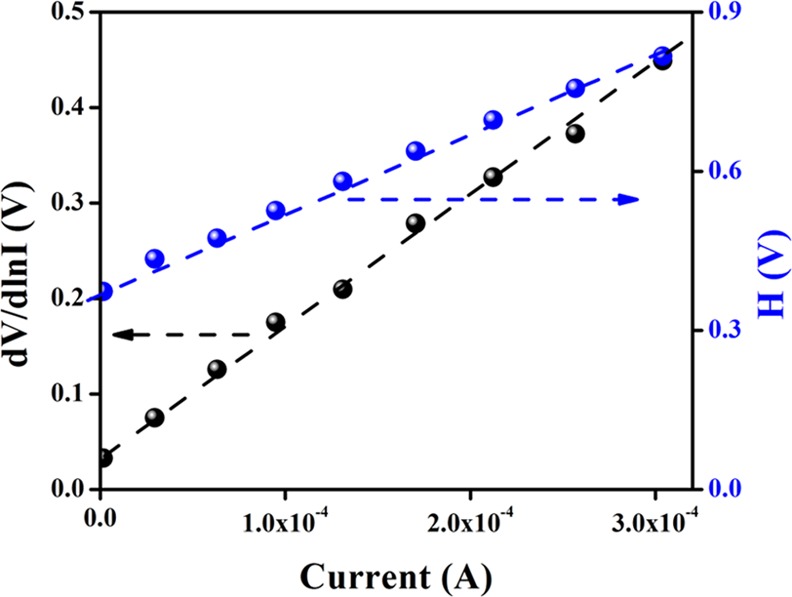
dV/d ln I vs I and H vs I curves for the synthesized Fe@TETA-based thin-film device.
Using the just calculated ideality factor (η), the value of the barrier height (ØB) was determined from the intercept of the H(I) versus I plot (Figure 8) [eq 10]. The series resistance (RS) of the device can also be determined from the slope of this plot. The measured ideality factor (η), potential height (ϕB), and series resistance (RS) for our devices are listed in Table 1. The series resistance obtained from both processes shows good consistency.
Table 1. Schottky Device Parameters of Compound-Based SBD.
| on/off | conductivity (S m–1) | ideality factor | barrier height (eV) | RS from dV/d ln I (Ω) | RS from H (Ω) |
|---|---|---|---|---|---|
| 21.04 | 1.003 × 10–4 | 1.39 | 0.28 | 1346.02 | 1451.37 |
For a better understanding of the charge transport phenomena at the MS junction, it requires an analysis of the I–V curves in detail. The characteristic I–V curve in the logarithmic scale reveals that it can be differentiated into two slopes (Figure 9), which have been marked as regions I and II.
Figure 9.
log I vs log V curves for the Fe@TETA-based thin-film device.
In region I, the current follows the relation I ∝ V and the value of the slope is ∼1. This region is referred to as the ohmic regime. In region II, the current is proportional to V2 (Figure 9). Here, the value of the slope is approximately 2. This is the characteristic of a trap-free space charge-limited current (SCLC) regime.67,73 In this region, the injected carriers are more than the background carriers. Therefore, the injected carriers spread and generate a space charge field. Hence, in this region, the currents are controlled by this space charge field and are known as SCLC.67,73 Therefore, we have adopted the SCLC theory to calculate the device performance of this region.
Using the Mott-Gurney equation, the effective carrier mobility has been estimated by following the SCLC model from a higher voltage region of the I versus V2 plot (Figure 10)67,70,73
| 9 |
where I, ε0, εr, and μeff present the current, permittivity of free space, relative dielectric constant of the synthesized material, and effective dielectric constant, respectively.
Figure 10.
I vs V2 curves for the Fe@TETA-based thin-film device.
To measure the relative dielectric constant, we have drawn the capacitance against the frequency of the synthesized material in a film format at a constant bias potential (Figure 11).
Figure 11.
Capacitance vs frequency graph for determination of the dielectric constant.
The dielectric permittivity of the complex was calculated from the saturated values of capacitance at the higher frequency regime (Figure 11) using the following equation:67
| 10 |
where C, D, and A refer to the capacitance (at saturation), thickness of the film that has been considered as ∼1 μm, and effective area, respectively. Using the above formula, the relative dielectric constant of our synthesized material was estimated as 2.06 × 10–1. To analyze charge transport across the junction, a few more key parameters such as the transit time (τ) and diffusion length (LD) were also estimated. Using the slope of the SCLC region (region II) in a logarithmic representation of the forward I–V curve (Figure 9), τ was evaluated from eq 11(67)
| 11 |
| 12 |
| 13 |
where D is the diffusion coefficient determined using the Einstein–Smoluchowski equation (eq 12).67 When a metal semiconductor junction is formed, the diffusion length (LD) of charge carriers plays an influential role in device performance and is further extracted from eq 13. All the parameters estimated in the SCLC region are listed below in Table 2. The higher mobility implies a higher transport rate; also, it depicts the generation of a higher number of charge carriers. The diffusion length reveals the path length of the charge carriers before being recombined. The diode parameters of our synthesized complex-based SBD demonstrate that these kinds of materials can pave the way for a very promising future in device application.
Table 2. Charge-Conducting Parameters of the Synthesized Complex-Based Thin-Film Devices.
| εr | μeff (m2 V–1 s–1) | τ (s) | μeffτ (m2 V–1) | D | LD (m) |
|---|---|---|---|---|---|
| 2.06 × 10–1 | 5.02 × 10–5 | 5.92 × 10–9 | 2.97 × 10–13 | 1.29 × 10–6 | 1.23 × 10–7 |
Our synthesized Fe@TETA metallogel-based fabricated SBD shows recognizable device performance. Here, we have compared a few of the charge transport parameters of our synthesized material-based device with our other previously reported values and presented them in Table 3.
Table 3. Comparison Table Showing Charge Transport Parameters of the Fe@TETA Metallogel.
| sample name/formula | electrical conductivity | barrier height | ideality factor | ref |
|---|---|---|---|---|
| Fe(III) metallohydrogel (Fe@MEA) | 1.0 × 10–12 S m–1 | 0.72 eV | 1.33 | (57) |
| Mn(II) metallogel (Mn@OX) | 1.27 × 10–4S m–1 | 0.22 eV | 2.19 | (74) |
| Cd(II) metallogel (CdA-OX) | 5.35 × 10–4 S m–1 | 0.32 eV | 1.8 | (75) |
| Co(II) metallohydrogel (CoMEA) | 6.36 × 10–4 S m–1 | 0.59 eV | 1.16 | (62) |
| monoethanolamine-based Mg(OH)2 metallogel (Mg@MEA) | 1.43 × 10–5 S m–1 | 0.38 eV | 1.89 | (76) |
| Fe(III) metallogel (Fe@TETA) | 1.01 × 10–4 S m–1 | 0.28 eV | 1.39 | present work |
Fe@TETA-Catalyzed Formation of Aryl Thioethers
Fe@TETA acts as an economically viable catalyst toward the aryl–S coupling reaction. Thiophenol along with its derivative and different aryl halides is used to explore the possibility of Fe@TETA as a catalyst toward C–S cross-coupling reactions under solvent-free conditions. Initially, bromobenzene and thiophenol reacted in a Fe@TETA metallogel with zinc dust for 12 h in an open single-reaction vessel while maintaining the temperature at 80 °C. Diphenylsulfane as the coupled product (Table 4, entry 1) was synthesized with an 88% yield maintaining the optimized reaction strategy. To explore the catalytic role of Fe@TETA in versatile C–S coupling reactions, different aromatic halides including 4-bromobenzonitrile, 1-iodo-4-methoxybenzene, 2-bromopyridine, and 9-bromophenanthrene (Table 4, entries 2–5) are reacted, and Fe@TETA efficiently catalyzed the C–S coupling reactions to isolate aryl sulfanes (Table 2) such as 4-(phenylthio)benzonitrile (entry 2, yield 89%), (4-methoxyphenyl)(phenyl)sulfane (entry 3, yield 87%), 2-(phenylthio)pyridine (entry 4, yield 88%), and benzene-1,2,4-triyltris(phenylsulfane) (entry 5, yield 90%) through an optimized reaction condition of using a single-pot reaction at 80 °C under open aerial conditions. Following a similar methodology, Fe@TETA also acts as the catalyst for the C–S coupling reactions of aryl halides with the versatile derivatives of thiophenols (Table 5, entries 1–5).
Table 4. Results of Fe@TETA Metallogel-Catalyzed Coupling of Aryl Halides and Thiophenola.

Reaction conditions: 0.5 mmol of aryl halide, 0.5 mmol of thiophenol, and 1.5 equiv of Zn powder at an 80 °C temperature for 12 h. (See the Supporting Information for 1H NMR and 13C NMR spectra, Figures S4–S13.)
Table 5. Results of Fe@TETA Metallogel-Catalyzed Coupling of Aryl Halides and Thiophenolsa.

Reaction conditions: 0.5 mmol of aryl halide, 0.5 mmol of thiophenol, and 1.5 equiv of Zn powder at an 80 °C temperature for 12 h. (See the Supporting Information for 1H NMR and 13C NMR spectra, Figures S14–S23.)
When 4-methoxybenzenethiol reacts with 1-iodo-4-methylbenzene, 4-bromobenzonitrile and 1-chloro-4-iodobenzene also offer aryl thioethers, that is, (4-methoxyphenyl)(p-tolyl)sulfane (Table 5, entry 1, yield 88%), 4-(4-methoxyphenylthio)benzonitrile (Table 5, entry 2, yield 84%), and (4-chlorophenyl)(4-methoxyphenyl)sulfane (Table 5, entry 3, yield 87%) by using the Fe@TETA catalyst (10 mol %). Likewise, thiophenol derivatives involving 4-methylbenzenethiol and 4-chlorobenzenethiol are employed to judge the catalytic efficiency of Fe@TETA for the formation of aryl thioethers with 4-bromobenzonitrile to produce 4-(p-tolylthio)benzonitrile (Table 5, entry 4, yield 89%) and 4-(4-chlorophenylthio)benzonitrile (Table 5, entry 5, yield 85%), respectively, as aryl sulfides following the optimized reaction condition.
Previously, Bolm et al. reported65 a critical reaction process for iron-catalyzed C–S coupling reactions, which needs a high temperature of 135 °C and long reaction time of 24 h with organic solvent toluene and supporting ligands (Scheme 1). Anilkumar and co-workers also reported66 the iron-catalyzed C–S coupling reaction in the presence of supporting ligands and external base at a temperature of 130 °C for a 24 h reaction time (Scheme 1). In comparison, our method of the Fe@TETA-metallogel catalyzed C–S coupling reaction for the synthesis of aryl thioethers is really noteworthy as neither it needs to use any supporting ligands/additives nor it requires any solvent to perform the reaction. Moreover, the presence of the Fe@TETA metallogel catalyst is able to complete the coupling reaction within 12 h at a comparatively65,66 lower temperature (80 °C). Thus, the Fe@TETA metallogel-based catalytic method offers a solvent-free synthetic strategy of aryl thioethers through a C–S coupling reaction.
Scheme 1. Iron-Catalyzed C–S Coupling Reaction for the Synthesis of Aryl Thioethers.

Reusability of the Fe@TETA Catalyst
The Fe@TETA metallogel was examined to estimate its recycling abilities (entry 1 of Table 4). After the completion of each Fe@TETA-catalyzed reaction on entry 1 of Table 4, Fe@TETA was collected by filtration and a subsequent drying process.
The Fe@TETA metallogel was then reused further to produce thioether up to four cycles. After the first run, 98% catalyst was recovered, whereas the recovery rate gradually lowers from the second, third, to fourth runs (Scheme 2).
Scheme 2. Recycling of the Catalyst using Entry 1 of Table 4.

Conclusions
In summary, a triethylenetetramine (TETA)-directed iron(III) metallogel was synthesized by instantaneous mixing of TETA and Fe(III) salt in water at ambient conditions. FESEM microstructural analyses of the Fe@TETA metallogel explored the cashew-shaped architecture of the metallogel. The rheological investigations of the Fe@TETA metallogel show that the metallogel material is mechanically stable. The electrical experiment (i.e., I–V characteristics) explores the usefulness of the Fe@TETA metallogel as a Schottky barrier diode device. Remarkably, the I–V characteristics of the Fe@TETA-mediated SBD exhibit a considerable rectification ratio. The diode parameters of Fe@TETA are also calculated, and they depict the formation of a Fe@TETA-based electronic device to act as a Schottky barrier diode. Here, we have also explored the Fe(III)-metallogel-catalyzed C–S coupling reaction of aryl halides with aromatic thiols to synthesize aryl thioethers following an optimized reaction protocol. This C–S coupling reaction highlights the effective use of the Fe(III) metallogel as an economically viable catalyst for the aryl–S coupling reaction.
Experimental Section
Chemicals, Instruments, and Measurements
Iron(III) nitrate nonahydrate (≥99.95% trace-metal basis) and triethylenetetramine (≥97.0% (T)) were bought from Merck and utilized. Other chemicals from Sigma-Aldrich Company were used as received. Solvents were obtained from commercial sources and distilled before use. Double-distilled water was used where necessary. UV–vis data was collected by a SHIMADZU UV-3101PC spectrophotometer. Rheological analysis was done using a rheometer of TA Instrument. FESEM images were collected using Carl Zeiss SUPRA 55VP FESEM. EDX studies and elemental mapping were done by a ZEISS EVO 18 instrument. Solid-state infrared spectroscopy was done by a Shimadzu FTIR-8400S spectrometer between 400 and 4000 cm–1 using the KBr pellet method. The gel destruction temperature of the nonthermoreversible-type Fe(III) metallogel was documented by a sophisticated digital melting-point measurement apparatus.
1H NMR spectra were collected on a 400 MHz spectrometer from CDCl3 solutions. Chemical shifts are expressed in parts per million (δ), and the signals are reported as s (singlet), d (doublet), t (triplet), and m (multiplet), and coupling constants (J) are given in hertz. 13C {1H} NMR spectra were recorded at 100 MHz in CDCl3 solutions. Chemical shifts are referenced to CDCl3 (δ = 7.26 for 1H and δ = 77.16 for 13C {1H} NMR) as an internal standard.
A Keithley 2635B source meter interfaced with a computer was used to perform I–V characteristics of the Fe@TETA metallogel material-based thin-film device.
Synthesis of the Iron(III) Metallogel (Fe@TETA)
The Fe(III) metallogel was synthesized by mixing 500 μL of a water solution of Fe(NO3)3·9H2O (0.404 g, 1 mM) and 500 μL of pure triethylenetetramine (TETA) under ambient conditions. The image of the insertion vial test of the Fe@TETA given in Figure 1 shows the stability of the hydrogel against gravitation.
General Experimental Procedure for the Synthesis of Diphenylsulfide (Entry 1, Table 4)
The mixture of thiol (0.50 mmol), bromobenzene (0.50 mmol), zinc (0.750 mmol), and the Fe@TETA metallogel (10 mol %) was taken in a reaction vessel (tube) and stirred with a magnetic stir bar, and the mixture was allowed to stir for 12 h at ∼80 °C. The movement of the reaction was examined by TLC. The reaction mixture was extracted by chloroform (25 mL) after completion of each reaction. The Fe@TETA metallogel was washed with deionized water due to its compatibility with the aqueous solvent, and the catalyst was filtered from the reaction mixture. The Fe@TETA metallogel catalyst was allowed to dry for reuse. The organic phase was dried over anhydrous Na2SO4, and the concentrated pure coupling product diphenylsulfane was obtained through column chromatography with an 88% yield (Table 4, entry 1).
Acknowledgments
B.D. thankfully acknowledges WBSTBD (Govt. of West Bengal, India) for the research project (sanction letter no. 14(Sanc.)/ST/P/S&T/15G-18/2018 dated January 29, 2019) for financial support. P.P.R. acknowledges the support of the RUSA 2.0 program of Jadavpur University.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03194.
Gelation process of the Fe@TETA metallogel with Fe(NO3)3·9H2O salt in various solvents, microstructural study and EDX spectral analysis, infrared spectral study of the Fe@TETA metallogel, characterization data of the isolated compounds, and NMR spectra of isolated compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Sangeetha N. M.; Maitra U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. 10.1039/b417081b. [DOI] [PubMed] [Google Scholar]
- Sutar P.; Suresh V. M.; Maji T. K. Tunable emission in lanthanide coordination polymer gels based on a rationally designed blue emissive gelator. Chem. Commun. 2015, 51, 9876–9879. 10.1039/C5CC02709H. [DOI] [PubMed] [Google Scholar]
- Chen F.; Wang Y.-M.; Guo W.; Yin X.-B. Color-tunable lanthanide metal–organic framework gels. Chem. Sci. 2019, 10, 1644–1650. 10.1039/C8SC04732D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das U. K.; Banerjee S.; Dastidar P. Remarkable Shape-Sustaining, Load-Bearing, and Self-Healing Properties Displayed by a Supramolecular Gel Derived from a Bis-pyridyl-bis-amide of L-Phenyl Alanine. Chem. Asian J. 2014, 9, 2475–2482. 10.1002/asia.201402053. [DOI] [PubMed] [Google Scholar]
- Weiss R. G.; Terech P.. Molecular Gels:Materials with Self-Assembled Fibrillar Networks; Springer: Dordrecht, 2005. [Google Scholar]
- Dastidar P. Supramolecular gelling agents: can they be designed?. Chem. Soc. Rev. 2008, 37, 2699–2715. 10.1039/b807346e. [DOI] [PubMed] [Google Scholar]
- Tam A. Y.-Y.; Yam V. W.-W. Recent advances in metallogels. Chem. Soc. Rev. 2013, 42, 1540–1567. 10.1039/c2cs35354g. [DOI] [PubMed] [Google Scholar]
- Tomasini C.; Castellucci N. Peptides and peptidomimetics that behave as low molecular weight gelators. Chem. Soc. Rev. 2013, 42, 156–172. 10.1039/C2CS35284B. [DOI] [PubMed] [Google Scholar]
- Meazza L.; Foster J. A.; Fucke K.; Metrangolo P.; Resnati G.; Steed J. W. Halogen-bonding-triggered supramolecular gel formation. Nat. Chem. 2013, 5, 42–47. 10.1038/nchem.1496. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Mynar J. L.; Yoshida M.; Lee E.; Lee M.; Okuro K.; Kinbara K.; Aida T. High-Water-Content Mouldable Hydrogels by Mixing Clay and a Dendritic Molecular Binder. Nature 2010, 463, 339–343. 10.1038/nature08693. [DOI] [PubMed] [Google Scholar]
- Holten-Andersen N.; Harrington M. J.; Birkedal H.; Lee B. P.; Messersmith P. B.; Lee K. Y. C.; Waite J. H. PH-Induced MetalLigand Cross-Links Inspired by Mussel Yield Self-Healing Polymer Networks with Near-Covalent Elastic Moduli. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 2651–2655. 10.1073/pnas.1015862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnworth M.; Tang L.; Kumpfer J. R.; Duncan A. J.; Beyer F. L.; Fiore G. L.; Rowan S. J.; Weder C. Optically Healable Supramolecular Polymers. Nature 2011, 472, 334–337. 10.1038/nature09963. [DOI] [PubMed] [Google Scholar]
- Cordier P.; Tournilhac F.; Soulié-Ziakovic C.; Leibler L. Self Healing and Thermoreversible Rubber from Supramolecular Assembly. Nature 2008, 451, 977–980. 10.1038/nature06669. [DOI] [PubMed] [Google Scholar]
- Asoh T.-A.; Kikuchi A. Rapid Fabrication of Reconstructible Hydrogels by Electrophoretic Microbead Adhesion. Chem. Commun 2012, 48, 10019–10021. 10.1039/c2cc35634a. [DOI] [PubMed] [Google Scholar]
- Wang X.; Liu F.; Zheng X.; Sun J. Water-Enabled Self-Healing of Polyelectrolyte Multilayer Coatings. Angew. Chem., Int. Ed. 2011, 50, 11378–11381. 10.1002/anie.201105822. [DOI] [PubMed] [Google Scholar]
- Wang H.; Hansen M. B.; Löwik D. W. P. M.; van Hest J. C. M.; Li Y.; Jansen J. A.; Leeuwenburgh S. C. G. Oppositely Charged Gelatin Nanospheres as Building Blocks for Injectable and Biodegradable Gels. Adv. Mater. 2011, 23, H119–H124. 10.1002/adma.201003908. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Wu Q.; Sun Y.; Bai H.; Shi G. Three Dimensional Self-Assembly of Graphene Oxide and DNA into Multifunctional Hydrogels. ACS Nano 2010, 4, 7358–7362. 10.1021/nn1027104. [DOI] [PubMed] [Google Scholar]
- Burattini S.; Greenland B. W.; Merino D. H.; Weng W.; Seppala J.; Colquhoun H. M.; Hayes W.; Mackay M. E.; HamLey I. W.; Rowan S. J. A Healable Supramolecular Polymer Blend Based on Aromatic π-π Stacking and Hydrogen-Bonding Interactions. J. Am. Chem. Soc. 2010, 132, 12051–12058. 10.1021/ja104446r. [DOI] [PubMed] [Google Scholar]
- Smith L. J.; Taimoory S. M.; Tam R. Y.; Baker A. E. G.; Mohammad N. B.; Trant J. F.; Shoichet M. S. Diels-Alder Click-Cross-Linked Hydrogels with Increased Reactivity Enable 3D Cell Encapsulation. Biomacromolecules 2018, 19, 926–935. 10.1021/acs.biomac.7b01715. [DOI] [PubMed] [Google Scholar]
- Drury J. L.; Mooney D. J. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. 10.1016/S0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- a Ballabh A.; Trivedi D. R.; Dastidar P. New Series of Organogelators Derived from a Combinatorial Library of Primary Ammonium Monocarboxylate Salts. Chem. Mater. 2006, 18, 3795–3800. 10.1021/cm0605015. [DOI] [Google Scholar]; b Bhattacharya S.; Krishnan-Ghosh Y. First report of phase selective gelation of oil from oil/water mixtures. Possible implications toward containing oil spills. Chem. Communed. 2001, 185–186. 10.1039/b007848o. [DOI] [Google Scholar]; c Jadhav S. R.; Vemula P. K.; Kumar R.; Raghavan S. R.; John G. Sugar-derived phase-selective molecular gelators as model solidifiers for oil spills. Angew. Chem. Int. Ed. 2010, 49, 7695–7698. 10.1002/anie.201002095. [DOI] [PubMed] [Google Scholar]; d Angew. Chem. 2010, 122, 7861–7864, 10.1002/ange.201002095. [DOI] [Google Scholar]
- a Murata K.; Aoki M.; Nishi T.; Ikeda A.; Shinkai S. New cholesterol-based gelators with light-and metal-responsive functions. J. Chem. Soc., Chem. Commun. 1991, 1715–1718. 10.1039/c39910001715. [DOI] [Google Scholar]; b de Jong J. J. D.; Lucas L. N.; Kellogg R. M.; van Esch J. H.; Feringa B. L. Reversible Optical Transcription of Supramolecular Chirality into Molecular Chirality. Science 2004, 304, 278–281. 10.1126/science.1095353. [DOI] [PubMed] [Google Scholar]
- a Kato T. Self-Assembly of Phase-Segregated Liquid Crystal Structures. Science 2002, 295, 2414–2418. 10.1126/science.1070967. [DOI] [PubMed] [Google Scholar]; b Molecular wire encapsulated into pi organogels: efficient supramolecular light-harvesting antennae with color-tunable emission. Angew. Chem., Int. Ed. 2007, 46, 6260–6265, 10.1002/anie.200701925. [DOI] [PubMed] [Google Scholar]; b1 Angew. Chem. 2007, (), 6376–6381. [Google Scholar]; c Ajayaghosh A.; Vijayakumar C.; Praveen V. K.; Babu S. S.; Varghese R. Self-Location of Acceptors as “Isolated” or “Stacked” Energy Traps in a Supramolecular Donor Self-Assembly: A Strategy to Wavelength Tunable FRET Emission. J. Am. Chem. Soc. 2006, 128, 7174–7175. 10.1021/ja0621905. [DOI] [PubMed] [Google Scholar]
- a Wynne A.; Whitefield M.; Dixon A. J.; Anderson S. An effective, cosmetically acceptable, novel hydro-gel emollient for the management of dry skin conditions. J. Dermatol. Treat. 2009, 13, 61–66. 10.1080/095466302317584403. [DOI] [PubMed] [Google Scholar]; b Jenning V.; Gysler A.; Schäfer-Korting M.; Gohla S. H. Vitamin A loaded solid lipid nanoparticles for topical use: occlusive properties and drug targeting to the upper skin. Eur. J. Pharm. Biopharm. 2000, 49, 211–218. 10.1016/S0939-6411(99)00075-2. [DOI] [PubMed] [Google Scholar]
- a van Bommel K. J. C.; Friggeri A.; Shinkai S. Shinkai, S. Organic templates for the generation of inorganic materials. Angew. Chem., Int. Ed. 2003, 42, 980–999. 10.1002/anie.200390284. [DOI] [PubMed] [Google Scholar]; a1 Angew. Chem. 2003, 115, 1010–1030. [Google Scholar]; b Basit H.; Pal A.; Sen S.; Bhattacharya S. Two-Component Hydrogels Comprising Fatty Acids and Amines: Structure, Properties, and Application as a Template for the Synthesis of Metal Nanoparticles. Chem. – Eur. J. 2008, 14, 6534–6545. 10.1002/chem.200800374. [DOI] [PubMed] [Google Scholar]; c Ray S.; Das A. K.; Banerjee A. Smart oligopeptide gels: in situ formation and stabilization of gold and silver nanoparticles within supramolecular organogel networks. Chem. Commun. 2006, 2816–2818. 10.1039/b605498f. [DOI] [PubMed] [Google Scholar]; d Gundiah G.; Mukhopadhyay S.; Tumkurkar U. G.; Govindaraj A.; Maitra U.; Rao C. N. R. Hydrogel route to nanotubes of metal oxides and sulfates. J. Mater. Chem. 2003, 13, 2118–2122. 10.1039/b304007k. [DOI] [Google Scholar]
- Escuder B.; Rodríguez-Llansola F.; Miravet J. F. Supramolecular gels as active media for organic reactions and catalysis. New J. Chem. 2010, 34, 1044–1054. 10.1039/b9nj00764d. [DOI] [Google Scholar]
- Yang Z.; Liang G.; Wang L.; Xu B. Using a Kinase/Phosphatase Switch to Regulate a Supramolecular Hydrogel and Forming the Supramolecular Hydrogel in Vivo. J. Am. Chem. Soc. 2006, 128, 3038–3043. 10.1021/ja057412y. [DOI] [PubMed] [Google Scholar]
- Buwalda S. J.; Vermonden T.; Hennink W. E. Hydrogels for Therapeutic Delivery: Current Developments and Future Directions. Biomacromolecules 2017, 18, 316–330. 10.1021/acs.biomac.6b01604. [DOI] [PubMed] [Google Scholar]
- Hosbein K. N.; England A. H.; Price C. A.; Clare T. L. Measuring Sheet Resistances of Dielectrics Using Co-Planar Hydrogel Electrochemical Cells with Practical Applications to Characterize the Protective Quality of Paints on Sculptures. Electroanalysis 2017, 29, 1377–1387. 10.1002/elan.201600765. [DOI] [Google Scholar]
- Mehta S.; Jin T.; Stanciulescu I.; Grande-Allen K. J. Engineering Biologically Extensible Hydrogels using Photolithographic Printing. ActaBiomaterialia. 2018, 75, 52–62. [DOI] [PubMed] [Google Scholar]
- Caló E.; Khutoryanskiy V. V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. 10.1016/j.eurpolymj.2014.11.024. [DOI] [Google Scholar]
- Schroeder T. B. H.; Guha A.; Lamoureux A.; VanRenterghem G.; Sept D.; Shtein M.; Yang J.; Mayer M. An electric-eel-inspired soft power source from stacked hydrogels. Nature 2017, 552, 214–218. 10.1038/nature24670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.; Yuk H.; Zhang T.; Parada G. A.; Koo H.; Yu C.; Zhao X. Stretchable Hydrogel Electronics and Devices. Adv. Mater. 2016, 28, 4497–4505. 10.1002/adma.201504152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S.; Schön E.-M.; Cativiela C.; Díaz Díaz D.; Banerjee R. Proton-Conducting Supramolecular Metallogels from the Lowest Molecular Weight Assembler Ligand: A Quote for Simplicity. Chem. – Eur. J. 2013, 19, 9562–9568. 10.1002/chem.201204542. [DOI] [PubMed] [Google Scholar]
- Feldner T.; Häring M.; Saha S.; Esquena J.; Díaz D. D.; Banerjee R. Supramolecular Metallogel That Imparts Self-Healing Properties to Other Gel Networks. Chem. Mater. 2016, 28, 3210–3217. 10.1021/acs.chemmater.6b01144. [DOI] [Google Scholar]
- Aiyappa H. B.; Saha S.; Wadge P.; Banerjee R.; Kurungot S. Fe(III) phytate metallogel as a prototype anhydrous, intermediate temperature proton conductor. Chem. Sci. 2015, 6, 603–607. 10.1039/C4SC02294G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin P.; Roubeau O.; Castro M.; Saadaoui H.; Colin A.; Clérac R. Multifunctional Gels from Polymeric Spin-Crossover Metallo-Gelators. Langmuir 2010, 26, 5184–5195. 10.1021/la903653d. [DOI] [PubMed] [Google Scholar]
- Mitsumoto K.; Cameron J. M.; Wei R.-J.; Nishikawa H.; Shiga T.; Nihei M.; Newton G. N.; Oshio H. A Multi-Redox Responsive Cyanometalate-Based Metallogel. Chem. – Eur. J. 2017, 23, 1502–1506. 10.1002/chem.201605542. [DOI] [PubMed] [Google Scholar]
- Xing B.; Choi M.-F.; Xu B. Design of Coordination Polymer Gels as Stable Catalytic Systems. Chem. – Eur. J. 2002, 8, 5028–5032. . [DOI] [PubMed] [Google Scholar]
- Huang J.; He L.; Zhang J.; Chen L.; Su C.-Y. Dynamic functionalised metallogel: An approach to immobilised catalysis with improved activity. J. Mol. Catal. A 2010, 317, 97–103. 10.1016/j.molcata.2009.11.001. [DOI] [Google Scholar]
- Díaz Díaz D.; Kühbeck D.; Koopmans R. J. Stimuli-responsive gels as reaction vessels and reusable catalysts. Chem. Soc. Rev 2011, 40, 427–448. 10.1039/C005401C. [DOI] [PubMed] [Google Scholar]
- Sarkar S.; Dutta S.; Chakrabarti S.; Bairi P.; Pal T. Redox-Switchable Copper(I) Metallogel: A Metal–Organic Material for Selective and Naked-Eye Sensing of Picric Acid. ACS Appl. Mater. Interfaces 2014, 6, 6308–6316. 10.1021/am501491u. [DOI] [PubMed] [Google Scholar]
- Lin Q.; Lu T.-T.; Zhu X.; Sun B.; Yang Q.-P.; Wei T.-B.; Zhang Y.-M. A novel supramolecular metallogel-based high-resolution anion sensor array. Chem. Commun. 2015, 51, 1635–1638. 10.1039/C4CC07814D. [DOI] [PubMed] [Google Scholar]
- López D.; Guenet J.-M. Behavior of a Self-Assembling Bicopper Complex in Organic Solutions. Macromolecules 2001, 34, 1076–1081. 10.1021/ma000839z. [DOI] [Google Scholar]
- Mukhopadhyay R. D.; Das G.; Ajayaghosh A. Stepwise control of host–guest interaction using a coordination polymer gel. Nat. Commun. 2018, 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishi-I T.; Iguchi R.; Snip E.; Ikeda M.; Shinkai S. [60]Fullerene Can Reinforce the Organogel Structure of Porphyrin-Appended Cholesterol Derivatives: Novel Odd-Even Effect of the (CH2)n Spacer on the Organogel Stability. Langmuir 2001, 17, 5825–5833. 10.1021/la0107749. [DOI] [Google Scholar]
- Bühler G.; Feiters M. C.; Nolte R. J. M.; Dötz K. H. A metal-carbene carbohydrate amphiphile as a low-molecular-mass organometallic gelator. Angew. Chem., Int. Ed. 2003, 42, 2494–2497. 10.1002/anie.200351134. [DOI] [PubMed] [Google Scholar]
- Terech P.; Schaffhauser V.; Maldivi P.; Guenet J. M. Living polymers in organic solvents. Langmuir 1992, 8, 2104–2106. 10.1021/la00045a007. [DOI] [Google Scholar]
- Wang X.; Duan P.; Liu M. Universal chiral twist via metal ion induction in the organogel of terephthalic acid substituted amphiphilic L-glutamide. ChemCommun. 2012, 48, 7501–7503. 10.1039/C2CC33246A. [DOI] [PubMed] [Google Scholar]
- Kelly N.; Gloe K.; Doert T.; Hennersdorf F.; Heine A.; März J.; Schwarzenbolz U.; Weigand J. J.; Gloe K. Self-assembly of [2+2] Co(II) metallomacrocycles and Ni(II) metallogels with novel bis(pyridylimine) ligands. J. Organomet. Chem. 2016, 821, 182–191. 10.1016/j.jorganchem.2016.04.021. [DOI] [Google Scholar]
- Yan L.; Liu C.; Shen L.; Li J.; Liu X.; Lv M.; Su C.; Ye Z. Visual Discrimination of 2-Picolinic Acid by a Supramolecular Metallogel. Chem. Lett. 2018, 47, 640–642. 10.1246/cl.180065. [DOI] [Google Scholar]
- Lee J. H.; Baek Y. E.; Kim K. Y.; Choi H.; Jung J. H. Metallogel of bis(tetrazole)-appended pyridine derivative with CoBr2 as a chemoprobe for volatile gases containing chloride atom. Supramol. Chem. 2016, 28, 870–873. 10.1080/10610278.2016.1142088. [DOI] [Google Scholar]
- Yu X.; Wang Z.; Li Y.; Geng L.; Ren J.; Feng G. Fluorescent and Electrochemical Supramolecular Coordination Polymer Hydrogels Formed from Ion-Tuned Self-Assembly of Small Bis-Terpyridine Monomer. Inorg. Chem. 2017, 56, 7512–7518. 10.1021/acs.inorgchem.7b01031. [DOI] [PubMed] [Google Scholar]
- Pandey V. K.; Dixit M. K.; Manneville S.; Bucher C.; Dubey M. A multi-stimuli responsive conductive sonometallogel: a mechanistic insight into the role of ultrasound in gelation. J. Mater. Chem. A 2017, 5, 6211–6218. 10.1039/C7TA00854F. [DOI] [Google Scholar]
- Banerjee S.; Adarsh N. N.; Dastidar P. A crystal engineering rationale in designing a CdII coordination polymer based metallogel derived from a C3 symmetric tris-amide-tris-carboxylate ligand. Soft Matter 2012, 8, 7623–7629. 10.1039/c2sm25717c. [DOI] [Google Scholar]
- Chen J.; Wang T.; Liu M. A hydro-metallogel of an amphiphilicL-histidine with ferric ions: shear-triggered self-healing and shrinkage. Inorg. Chem. Front. 2016, 3, 1559–1565. 10.1039/C6QI00238B. [DOI] [Google Scholar]
- Dhibar S.; Jana R.; Ray P. P.; Dey B. Monoethanolamine and Fe(III) based metallohydrogel: An efficient Schottky barrier diode. J. Mol. Liq. 2019, 289, 111126. 10.1016/j.molliq.2019.111126. [DOI] [Google Scholar]
- Zhong J.-L.; Jia X.-J.; Liu H.-J.; Luo X.-Z.; Hong S.-G.; Zhang N.; Huang J.-B. Self-assembled metallogels formed from N,N′,N″-tris(4-pyridyl)trimesic amide in aqueous solution induced by Fe(III)/Fe(II) ions. Soft Matter 2016, 12, 191–199. 10.1039/C5SM01513H. [DOI] [PubMed] [Google Scholar]
- Arnedo-Sánchez L.; Nonappa; Bhowmik S.; Hietala S.; Puttreddy R.; Lahtinen M.; De Cola L.; Rissanen K. Rapid self-healing and anion selectivity in metallosupramolecular gels assisted by fluorine–fluorine interactions. Dalton Trans. 2017, 46, 7309–7316. 10.1039/C7DT00983F. [DOI] [PubMed] [Google Scholar]
- Adarsh N. N.; Dastidar P. A New Series of ZnII Coordination Polymer Based Metallogels Derived from Bis-pyridyl-bis-amide Ligands: A Crystal Engineering Approach. Cryst. Growth Des. 2011, 11, 328–336. 10.1021/cg101342g. [DOI] [Google Scholar]
- Dastidar P.; Ganguly S.; Sarkar K. Metallogels from Coordination Complexes, Organometallic, and Coordination Polymers. Chem. – Asian J. 2016, 11, 2484–2498. 10.1002/asia.201600814. [DOI] [PubMed] [Google Scholar]
- Dhibar S.; Dey A.; Jana R.; Chatterjee A.; Das G. K.; Ray P. P.; Dey B. A semiconducting supramolecular Co(II)-metallohydrogel: an efficient catalyst for single-pot aryl-S bond formation at room temperature. Dalton Trans. 2019, 48, 17388–17394. 10.1039/C9DT03373D. [DOI] [PubMed] [Google Scholar]
- a Lee C.-F.; Liu Y.-C.; Badsara S. S. Transition-Metal-Catalyzed C-S Bond Coupling Reaction. Chem. – Asian J. 2014, 9, 706–722. 10.1002/asia.201301500. [DOI] [PubMed] [Google Scholar]; b Davis H. J.; Mihai M. T.; Phipps R. J. Ion Pair-Directed Regiocontrol in Transition-Metal Catalysis: A Meta-Selective C-H Borylation of Aromatic Quaternary Ammonium Salts. J. Am. Chem. Soc. 2016, 138, 12759–12762. 10.1021/jacs.6b08164. [DOI] [PubMed] [Google Scholar]
- a Hartwig J. F.InHandbook of Organopalladium Chemistryfor Organic Synthesis; Vol 1Negishi E.-i. Ed., Wiley-Interscience: New York, 2002, 1051–1096; [Google Scholar]; b Ley S. V.; Thomas A. W. Modern synthetic methods for copper-mediated C(aryl)[bond]O, C(aryl)[bond]N, and C(aryl)[bond]S bond formation. Angew. Chem. 2003, 115, 5558–5607. 10.1002/ange.200300594. [DOI] [PubMed] [Google Scholar]; b1 Angew. Chem., Int. Ed. 2003, 42, 5400–5449, 10.1002/anie.200300594. [DOI] [PubMed] [Google Scholar]; c) Kunz K.; Scholz U.; Ganzer D. Renaissance of Ullmann and Goldberg Reactions - Progress in Copper Catalyzed C-N-C-O- and C-S-Coupling. Synlett 2003, 2428–2439. 10.1055/s-2003-42473. [DOI] [Google Scholar]; d Beletskaya I. P.; Cheprakov A. V. Copper in cross-coupling reactions: The post-Ullmann chemistry. Coord. Chem. Rev. 2004, 248, 2337–2364. 10.1016/j.ccr.2004.09.014. [DOI] [Google Scholar]; e Corbet J.-P.; Mignani G. Selected Patented Cross-Coupling Reaction Technologies. Chem. Rev. 2006, 106, 2651–2710. 10.1021/cr0505268. [DOI] [PubMed] [Google Scholar]
- Correa A.; Carril M.; Bolm C. Iron-Catalyzed S-Arylation of Thiols with Aryl Iodides. Angew. Chem., Int. Ed. 2008, 47, 2880–2883. 10.1002/anie.200705668. [DOI] [PubMed] [Google Scholar]
- Sindhu K. S.; Thankachan A. P.; Thomas A. M.; Anilkumar G. An efficient iron-catalyzed S-arylation of aryl and alkylthiols with aryl halides in the presence of water under aerobic conditions. Tetrahedron Lett. 2015, 56, 4923–4926. 10.1016/j.tetlet.2015.06.087. [DOI] [Google Scholar]
- Dey A.; Middya S.; Jana R.; Das M.; Datta J.; Layek A.; Ray P. P. Light induced charge transport property analysis of nanostructured ZnS based Schottky diode. J. Mater. Sci.: Mater. Electron. 2016, 27, 6325–6335. 10.1007/s10854-016-4567-5. [DOI] [Google Scholar]
- Rhoderick E. H.Metal-Semiconductor Contacts; Oxford University Press: London, 1978. [Google Scholar]
- Cheung S. K.; Cheung N. W. Extraction of Schottky diode parameters from forward current-voltage characteristics. Appl. Phys. Lett. 1986, 49, 85–87. 10.1063/1.97359. [DOI] [Google Scholar]
- Dey A.; Layek A.; Roychowdhury A.; Das M.; Datta J.; Middya S.; Das D.; Ray P. P. Investigation of charge transport properties in less defective nanostructured ZnO based Schottky diode. RSC Adv. 2015, 5, 36560–36567. 10.1039/C4RA16828C. [DOI] [Google Scholar]
- Gupta R. K.; Yakuphanoglu F. Photoconductive Schottky diode based on Al/p-Si/SnS2/Ag for optical sensor applications. Sol. Energy. 2012, 86, 1539–1545. 10.1016/j.solener.2012.02.015. [DOI] [Google Scholar]
- Miao X.; Tongay S.; Petterson M. K.; Berke K.; Rinzler A. G.; Appleton B. R.; Hebard A. F. High Efficiency Graphene Solar Cells by Chemical Doping. Nano Lett. 2012, 12, 2745–2750. 10.1021/nl204414u. [DOI] [PubMed] [Google Scholar]
- Blom P. W. M.; de Jong M. J. M.; van Munster M. G. Electric-field and temperature dependence of the hole mobility in poly(p-phenylene vinylene). Phys. Rev. B 1997, 55, R656–R659. 10.1103/PhysRevB.55.R656. [DOI] [Google Scholar]
- Dhibar S.; Dey A.; Dey A.; Majumdar S.; Ghosh D.; Ray P. P.; Dey B. Development of Supramolecular Semiconducting Mn(II)-Metallogel Based Active Device with Substantial Carrier Diffusion Length. ACS Appl. Electron. Mater. 2019, 1, 1899–1908. 10.1021/acsaelm.9b00410. [DOI] [Google Scholar]
- Dhibar S.; Dey A.; Majumdar S.; Ghosh D.; Mandal A.; Ray P. P.; Dey B. A supramolecular Cd(II)-metallogel: an efficient semiconductive electronic device. Dalton Trans. 2018, 47, 17412–17420. 10.1039/C8DT03773F. [DOI] [PubMed] [Google Scholar]
- Dhibar S.; Dey A.; Dey A.; Majumdar S.; Mandal A.; Ray P. P.; Dey B. The development of a rapid self-healing semiconducting monoethanolamine-based Mg(OH)2 metallogel for a Schottky diode application with a high ON/OFF ratio. New J. Chem. 2019, 43, 15691–15699. 10.1039/C9NJ03457A. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.