Abstract
Numerous social-cognitive models posit that social behavior largely is driven by links between constructs in long-term memory that automatically become activated when relevant stimuli are encountered. Various response biases have been understood in terms of the influence of such “implicit” processes on behavior. This article reviews event-related potential (ERP) studies investigating the role played by cognitive control and conflict resolution processes in social-cognitive phenomena typically deemed automatic. Neurocognitive responses associated with response activation and conflict often are sensitive to the same stimulus manipulations that produce differential behavioral responses on social-cognitive tasks and that often are attributed to the role of automatic associations. Findings are discussed in the context of an overarching social cognitive neuroscience model in which physiological data are used to constrain social-cognitive theories.
Scientists who study interpersonal perception and social behavior have long been interested in the role played by covert, rapidly-occurring cognitive and affective processes (e.g., Markus & Zajonc, 1985). Borrowing largely from paradigms in cognitive psychology, social psychologists have developed clever experimental designs, many involving reaction time (RT) or memory measures, aimed at providing evidence for the influence of these processes on overt responses (e.g., see Fazio, 2001; Macrae, Bodenhausen, Schloersheidt, & Milne, 1999). Such paradigms have been important for establishing both the effects of environmental manipulations on social information processing and the limits of the social-cognitive system. However, use of measures such as RT and recall places frustrating limits on the inferences that can be drawn about social cognition. For example, the time it takes for a research participant to respond behaviorally following the onset of a target stimulus reflects a complex combination of perceptual, cognitive and motor operations (e.g., Coles, Henderikus, Smid, Scheffers, & Otten, 1995), only some of which may be of theoretical interest. Put more simply, behavioral measures represent the outcome of a set of cognitive (and other) processes performed on stimuli of interest, but are not, themselves, direct measures of those processes.
Fortunately, psychophysiologists have long known of methods, such as the event-related brain potential (ERP), which can more directly measure neural and other physiological responses that reflect cognitive and affective processes of interest to social cognition. Recent years have witnessed a resurgence of interest in using psychophysiological measures in social psychological research (for recent reviews, see Decety & Cacioppo, in press; Harmon-Jones & Beer, 2009; Harmon-Jones & Winkielman, 2007). Using physiological measures to understand social psychological phenomena is not a new idea, dating back at least to Rankin and Campbell’s (1955) measurement of electrodermal responses in White students interacting with Black (vs. White) experimenters as a way to understand covert racial attitudes (see also Porier & Lott, 1967). However, systematic integration at the social, cognitive and neural levels of analysis is a relatively new development in the field (see Cacioppo, Berntson, Sheridan, & McClintock, 2000; Ochsner & Lieberman, 2001). It is within this tradition that the work reviewed here is situated. In reviewing ERP studies of selective attention, Mangun and Hillyard (1995) explained how physiological information can be used to constrain theories about cognition. This idea is at the core of the research discussed here, in which my colleagues and I have used ERPs to investigate the role of conflict and control in social-cognitive processes.
Mechanisms of Social Cognition
A fundamental principle of social cognition is that the beliefs, concepts, and expectancies constituting an individual’s knowledge about the social world are represented in an associative memory network (Carlston & Smith, 1996). This principle has led to the development of theoretical models positing that exposure to a stimulus representing a social category (e.g., a person representing a racial group) spontaneously and effortlessly triggers evaluative and semantic constructs linked to that category (see Macrae & Bodenhausen, 2000; Wheeler & Petty, 2001), leading to facilitated responses (i.e., faster RT). Thus, findings in social cognition experiments relying on RT measures often are interpreted in terms of spreading activation (e.g., Collins & Loftus, 1975; Neely, 1977), the idea that cognitive constructs are linked together in memory such that activation of one node (i.e., via perception of a relevant stimulus) quickly and automatically increases the activation level of other, semantically- or affectively-related constructs. This basic idea has been used to explain participants’ responses in a large array of so-called priming tasks, including those designed to understand the automatic activation of attitudes (e.g., Fazio, Sanbonmatsu, Powell, & Kardes, 1986) and activation of stereotypical thoughts and prejudiced feelings about members of racial groups (e.g., Devine, 1989; Dovidio, Evans, & Tyler, 1986; Lepore & Brown, 1997; Fiske & Neuberg, 1990). In such studies, participants’ responses to relevant target stimuli (e.g., attitude words or traits associated with stereotypes) tend to be faster and more accurate when those targets are preceded or accompanied by other stimuli (i.e., primes) that represent a related category (i.e., congruent trials), compared to when primes represent an opposing or unrelated category (i.e., incongruent trials). Such congruency effects generally are attributed to the prime activating a semantic category and, through spreading of activation, constructs related to that category, making them relatively more accessible in memory than less-well-related constructs (see Higgins, 1996). Within this framework, priming effects are thought to reflect differences in the strength of automatic associations in long-term memory (e.g., Fazio et al., 1986).
Recently, however, a number of models have been proposed that posit a prominent role for cognitive control in regulating behavioral responses in social cognition (see Conrey et al., 2005; Payne, 2005; Sherman, 2009; Sherman et al., 2008). In general, such models assume that automatic associations are only part of what drives responses to social targets, and that the extent to which biases arising from automatically-activated associations are expressed behaviorally is determined by the application of controlled, self-regulatory processes. For example, Sherman and colleagues (e.g., Conrey et al., 2005; Sherman et al., 2008) recently have outlined a quadruple-process model (i.e., Quad model) that permits estimation of 4 distinct processes involved in the regulation of well-learned, prepotent responses in a number of domains: activation, the likelihood that an association is activated when a relevant stimulus is encountered; detection, the likelihood that an accurate or appropriate response to a target can be determined; overcoming bias, the likelihood that an activated association or response tendency can be overcome and replaced with a contextually-appropriate one; and guessing, which occurs when no association is activated and a correct response cannot be determined. Applied to the domain of racial stereotyping, the Quad model can be used to understand whether, for example, variability in stereotype-based, biased responses in a given task results from differences in the activation of automatic associations (e.g., Fazio, Jackson, Dunton, & Williams, 1995) versus differences in the ability to overcome prepotent response tendencies based on those associations (see Gonsalkorale, Sherman, & Klauer, 2009).
A core idea underlying effects attributed to overcoming bias is that stereotype-incongruent stimulus situations elicit response conflict, similar to that induced by incongruent trials in the Stroop color-naming task (e.g., MacLeod, 1991). For example, in the Weapons Identification Task (WIT) often used to assess race bias (see Payne, 2001), participants must attempt to quickly categorize (via button press) target objects, presented following white or black men’s faces, as either handguns or hand tools. Research consistently shows that on black face trials responses to guns are faster and more accurate than are responses to tools (e.g., Amodio et al., 2004, 2008; Payne, 2001, 2005; Payne et al., 2005). This pattern is thought to occur because exposure to a black man’s face is associated with a biased tendency to activate the “gun” response, simply on the basis of long-held, stereotypic associations between black men and violence (e.g., Correll, Park, Judd, & Wittenbrink, 2002; Devine & Elliot, 1995; Sagar & Schofield, 1980). On trials in which the target is actually a tool, this prepotent tendency to respond “gun” conflicts with the correct, goal-driven “tool” response (see also Correll et al., 2002). Thus, although constructs associated with the black stereotype are thought to become automatically activated upon exposure to a black face (e.g., Dovidio, Evans, & Tyler, 1986), overcoming the prepotent tendency to respond in a biased, stereotype-consistent manner requires control (Conrey et al., 2005; Payne, 2005; Sherman et al., 2008). Thus, it could be that expression of bias depends at least as much on whether or not control can be exerted (e.g., Amodio et al., 2004; Devine, 1989; Radvansky, Lynchard, & von Hippel, 2008; von Hippel, 2007) as on the strength of automatic evaluations or stereotypical associations (cf., Fazio et al., 1995; Greenwald, McGhee, & Schwartz, 1998).
ERP Measures of Conflict and Control
Two components of the stimulus-locked ERP have proven useful in our research for investigating the possibility that response conflict occurs in social-cognitive tasks.1 First, as described eloquently by Coles (1989; Coles et al., 1995), the lateralized readiness potential (LRP) indexes neural activity in pre-motor and motor areas of cortex (see Brunia, 1988; Requin, 1985) associated with preparing and generating behavioral responses. For example, as a participant prepares to make a left-hand response, the “readiness potential” (see Kornhuber & Deecke, 1965) will be largest over the right side of the scalp, its amplitude directly reflecting the magnitude of the activated response (see Coles, 1989). The LRP is particularly useful in sequential priming tasks for determining whether and to what extent a response is activated by the prime prior to the onset of the target (see Gratton et al., 1990). Moreover, in tasks involving 2 response options mapped to opposite hands, the polarity of the LRP reveals which response (e.g., correct or incorrect) is activated by the warning or prime stimulus.
An additional component, the N2 (or N200), consistently has been linked to the hypothesized conflict-monitoring function of the anterior cingulate cortex (see Botvinick, Braver, Barch, Carter, & Cohen, 2001; van Veen & Carter, 2002). The N2 generally has a frontal or fronto-central scalp distribution and is believed to reflect activity in medial-frontal cortical areas (Botvinick et al., 2001; van Veen & Carter, 2002). The N2 tends to be larger on trials involving conflict between competing response representations, such as incongruent Stroop trials (e.g., Liotti, Woldorff, Perez, & Mayberg, 2000) and incompatible flanker trials (e.g., Kopp, Rist, & Mattler, 1996). Moreover, N2 amplitude covaries with the degree of incorrect response activation measured via muscle movement (Kopp et al., 1996). The N2 also often is larger on trials requiring a low-frequency response (e.g., Nieuwenhuis, Yeung, van den Wildenberg, & Ridderinkhof, 2003), presumably because activating the correct response on such trials conflicts with the (prepotent) response activated by more frequently-occurring trials (see Jones, Cho, Nystrom, Cohen, & Braver, 2002).
Although not a measure of response activation or conflict, the parietal P3 (or P300) also can be useful when investigating whether various manipulations that affect response output have effects on stimulus evaluation, in addition to or instead of response generation. Among other processes, the P3 is known to be sensitive to the ease with which stimuli can be evaluatively categorized: larger P3 amplitude is associated with a greater change in evaluative categorization (see Cacioppo, Crites, Berntson, & Coles, 1993; Ito, Larsen, Smith, & Cacioppo, 1998) and longer P3 latency reflects more effortful evaluation (see Kutas, McCarthy, & Donchin, 1977; McCarthy & Donchin, 1981). Moreover, the fact that P3 appears to be largely insensitive to response-related processes (see Crites, Cacioppo, Gardner, & Berntson, 1995; Magliero, Bashore, Coles, & Donchin, 1984) means that measuring the P3 in conjunction with components related to response generation (e.g., LRP and N2) provides a way to test the extent to which observed behavioral effects arise from evaluative categorization, response generation, or both (see Gratton, Coles, & Donchin, 1992; Smid, Lamain, Hogeboom, Mulder, & Mulder, 1991; Smulders, Kok, Kenemans, & Bashore, 1995).
Finally, an additional stimulus-locked component, the negative slow wave (NSW), also known as the frontal slow wave because of its typical prominence at frontal and fronto-central scalp locations, can be useful for investigating the extent to which cognitive control processes are brought to bear during social-cognitive tasks. The NSW has been linked to implementation of cognitive control in that it generally is larger on trials in which conflict is successfully resolved, such as incongruent Stroop trials (e.g., West & Alain, 1999; see also Curtin & Fairchild, 2003). In addition, alcohol consumption reduces NSW amplitude (e.g., Bartholow, Dickter, & Sestir, 2006; Curtin & Fairchild, 2003), further implicating this component in cognitive control processes. When used in combination with one other, and/or in combination with response-locked components, such as the error-related negativity (ERN; see Amodio et al., 2004), these components (LRP, N2, P3, and NSW) can shed a great deal of light on the neural mechanisms underlying observed behavioral responses on social-cognitive tasks.
Recent years have witnessed a surge of interest in neurocognitive models of behavioral regulation and their application to social behavior. The dual-process model of cognitive control proposed by Botvinick et al. (2001) has been particularly influential. According to this model, an evaluative conflict-monitoring system, located in the anterior cingulate cortex (ACC) and nearby medial-frontal regions (see Botvinick et al., 2001; Taylor, Stern, & Gehring, 2007), monitors ongoing behaviors and identifies instances of response conflict or potential conflict (see Carter et al., 1998; Gehring & Fencsik, 2001; van Veen & Carter, 2002). Activity of the conflict monitoring system is thought to manifest at the scalp in the N2 and error-related negativity (ERN) components of the ERP (see Yeung, Botvinick, & Cohen, 2004). This activity alerts a second, regulatory system, relying on more anterior structures such as dorsolateral prefrontal cortex, which implements top-down control in service of activating an intended response and inhibiting unintended responses (e.g., Botvinick et al., 2001; Carter et al., 2000; Kerns et al., 2004). Regulatory control is believed to be reflected in the amplitude of the NSW component of the ERP (see Curtin & Fairchild, 2003; West & Alain, 1999, 2000).
Racial Categorization, Stereotypes, and Conflict
As reviewed previously, a number of recent studies have conceptualized responses on racial stereotyping tasks as derived from conflict and control processes (e.g., Amodio et al., 2004, 2008; Conrey et al., 2005; Correll et al., 2002; Payne, 2001, 2005; Payne et al., 2005). Evidence supporting this idea has come from studies showing, for example, that the ability to quickly associate black faces and positive words (as opposed to negative words) relies on overcoming the biased tendency to associate black with negative (Conrey et al., 2005). Similarly, the ability to correctly identify tools following black face primes in the WIT is positively associated with individual differences in aspects of executive control (Payne, 2005). Also, Klauer and colleagues (e.g., Klauer & Mierke, 2005; Klauer, Schmitz, Teige-Mocigemba, & Voss, in press) have shown that scores on the Implicit Association Test (IAT; Greenwald et al., 1998) are associated with measures of task-switching, a component of executive control (see Miyake et al., 2000). However, extant studies have not addressed a number of questions about the mechanisms and neural bases of conflict in such tasks, including the stages of processing at which conflict and/or other operations might occur to influence behavior.
Previous work (see Livingston & Brewer, 2002; Richeson & Trawalter, 2005) has shown that racial categorization is slowed by the presence of context information that is inconsistent with racial stereotypes. Based mainly on principles of spreading activation, this finding has been understood in terms of category membership being more difficult to evaluate when targets are presented in the context of category-inconsistent information (i.e., because activation between race categories and inconsistent concepts takes longer to spread). However, it could be that category-inconsistent information activates response channels opposing those needed to correctly categorize a target individual (i.e., produces response conflict), which also would slow execution of the correct categorization response.
This possibility was investigated in two recent experiments by Bartholow and Dickter (2008). Participants completed a modified Eriksen flanker task (see Eriksen & Eriksen, 1974) in which the target stimuli were men’s faces varying by race (white and black) and the peripheral flanker stimuli were words associated with stereotypes of whites and blacks (see Lepore & Brown, 1997; Wittenbrink, Judd, & Park, 1997). Compatible trials were defined as those in which the race of the target and the flanker words were stereotypically congruent (e.g., black with “violent”); incompatible trials were those in which target race and flanker words were stereotypically incongruent (e.g., black with “smart”). Participants were instructed to categorize the target as either white or black as quickly as possible by pressing one of two buttons, and to simply ignore the flanker words (described as distracters). Thus, unlike most traditional flanker tasks in which the flankers themselves are directly linked to a valid response, in this case the flankers were not mapped directly to any response and were only indirectly diagnostic of target categories. We also were interested in whether the relative probability of stereotype-congruent and –incongruent trials would lead to adjustments in processing strategy similar to that seen in studies using traditional flanker tasks (e.g., Bartholow et al., 2005; Gratton et al., 1992). This was achieved by creating blocks of “expect-compatible” trials (i.e., 80% compatible) and “expect-incompatible” trials (i.e., 20% compatible).
Behavioral data from both experiments were consistent with the notion that stereotype-inconsistent flanker words interfered with target categorization, producing a “compatibility effect” (i.e., faster responses on compatible than incompatible trials) similar to that often seen in more traditional flanker tasks (e.g., Coles et al., 1985; Eriksen & Schultz, 1979; Gratton et al., 1988). Moreover, the compatibility effect was significantly larger in the 80% compatible blocks (Ms = 13 ms and 12 ms in Experiments 1 and 2, respectively) than in the 20% compatible blocks (Ms = 3 ms and 2 ms, respectively), suggesting that participants strategically controlled their attention to the flankers (cf., Gratton et al., 1992). Specifically, when flanker words were likely to be predictive of target race (based on stereotypic associations), participants appeared to attend to and extract information from the entire stimulus array. This was less likely to occur in the 20% compatible condition, in which flanker words were unlikely to provide valid information concerning target race.
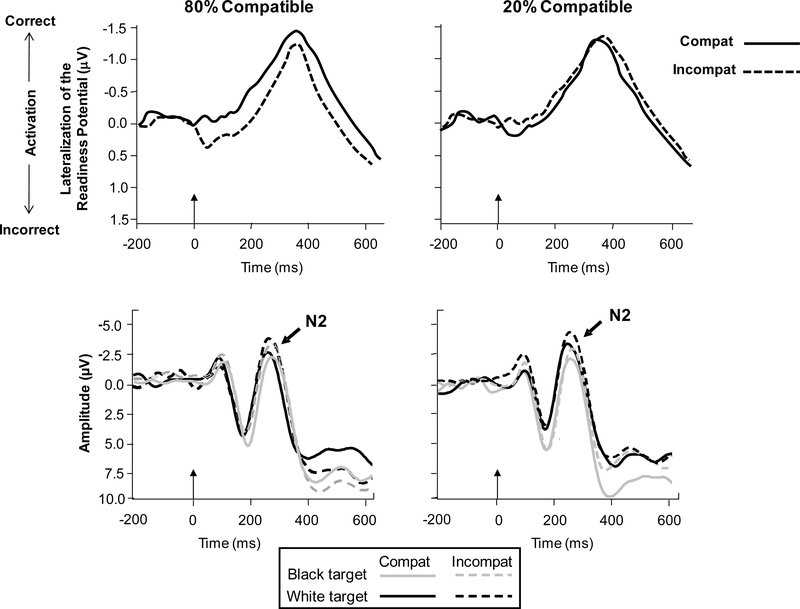
The ERP data (Experiment 2) further supported the hypothesis that stereotype-inconsistent flankers elicited response conflict. Figure 1 (top panel) shows the LRP (i.e., response activation) elicited by compatible and incompatible trials as a function of block type. In blocks in which compatible trials were more probable, incompatible trials produced a positive deflection in the LRP around 50 ms post-stimulus, indicative of a tendency to initially activate the incorrect categorization response, prior to activation and execution of the correct response. The presence of this “error dip” on high-conflict trials has been described as evidence of consecutive activation of opposing response tendencies within individual trials (see Gratton et al., 1988, 1992; Spencer & Coles, 1999), in this case indicating that initial response activation was driven by the flankers when compatible trials were more probable (cf., Gratton et al., 1992). The N2 amplitude data (bottom panel of Figure 1) further indicate that conflict was enhanced by the presence of stereotype-inconsistent flankers, in that the N2 was enhanced for incompatible relative to compatible trials.
Figure 1.
Top panel: Lateralized readiness potential (LRP) waveforms derived from electrodes C3 and C4 for compatible (Compat) and incompatible (Incompat) trials in the 80% compatible and 20% compatible blocks (from Bartholow & Dickter, 2008). The arrow at time 0 indicates stimulus array onset. The formula used to derive the LRP is applied with reference to the correct response hand in each condition, such that negative (upward) deflections reflect preferential activation of the correct response, whereas positive (downward) deflections indicate preferential activation of the incorrect response. The positive “dip” occurring around 50 ms post-stimulus for incompatible trials in the 80% compatible condition indicates initial activation of the incorrect categorization response prior to activation and execution of the correct response. Bottom panel: ERP waveforms measured at electrode FCz depicting the N2 component as a function of target race and trial type.
To the extent that the behavioral compatibility effects in this study were related to conflicting response activations on incompatible trials, there should be some association between the size of the compatibility effect in RT and the size of the “error dip” (essentially, an analogue to the compatibility effect) in the LRP. Correlational analyses supported this prediction, showing that these variables were significantly associated in the 80% compatible blocks (r = .45, p < .05) but not in the 20% compatible blocks (r = −.28, p > .10). In contrast, differences in RT across compatible and incompatible trials were uncorrelated with P3 latency data (rs < .20, ps > .40). This lack of association suggests that any potential differences in evaluation difficulty between compatible and incompatible trials were not responsible for observed differences in RT. Moreover, the P3 latency data did not show any compatibility effects (Fs < 1), suggesting that evaluative categorization of the stimulus arrays was not affected by flanker compatibility in this paradigm (see also Smid et al., 1991).
Taken together, the data from these experiments support an important role for conflict during response output in determining slowed responses on stereotype-incongruent trials in reaction-time tasks. These findings indicate that preparation of responses can be influenced at a very early stage by peripheral, nontarget information, to the extent that an individual expects that information to be useful in aiding appropriate responding to the target. However, relying on such peripheral stimuli produces conflict (and, thus, less efficient responding) when it suggests a response opposed to the one required by the target. Moreover, although connections between the flankers and targets can be attributed to highly accessible, largely automatic associations (i.e., the activation parameter proposed by Sherman et al., 2008), the ERP data from these experiments suggest that the purely associative account implied by a spreading activation model of response facilitation and inhibition (e.g., Fazio et al., 1995) is insufficient to explain the stereotype congruency effects seen here and in other, similar studies (e.g., Amodio et al., 2004; Conrey et al., 2005; Payne, 2001, 2005).
Controlling (and Failing to Control) Race Bias
A consistent trend in research on intergroup attitudes in recent decades suggests a shift away from negative and toward more positive evaluations of racial outgroup members (see Schuman, Steeh, Bobo, & Krysan, 1997). However, despite trend toward positive overtly-expressed racial attitudes, the content of stereotypes about racial minority groups remains quite negative (see Devine & Elliot, 1995; Dovidio & Gaertner, 1998; Fiske, Cuddy, Glick, & Xu, 2002). Research using more indirect methods (e.g., so-called implicit measures) continues to show that minority racial group categories generally are associated with more negative than positive evaluations (e.g., Fazio et al., 1995; Greenwald & Banaji, 1995; Greenwald, Oakes, & Hoffman, 2003; Payne, 2001). This discrepancy between negative, implicit associations and overt expression of positive attitudes suggests that people often make effortful attempts to control the expression of race bias. Such control attempts can be considered a component of a more general skill set associated with self-regulation, which often requires implementation of top-down control over well-learned, prepotent response tendencies in favor of alternative responses considered more appropriate in a given context (e.g., MacDonald, Cohen, Stenger, & Carter, 2000).
Recent work by Amodio and his colleagues (Amodio et al., 2004, 2006, 2008) has been instrumental in applying aspects of the neurocognitive control framework outlined previously (e.g., Botvinick et al., 2001) to understanding race bias expression and control. These researchers have demonstrated that response errors indicative of race bias are associated with heightened activation of the neural conflict monitoring system, producing enhanced amplitude of the ERN. Moreover, the amplitude of the ERN correlates with estimates of controlled processing on subsequent race bias trials (e.g., more accurate target detection), providing initial evidence of the role of the conflict monitoring system in control of race bias.
A recent experiment by Bartholow, Dickter and Sestir (2006, Experiment 2) extended these efforts by testing the role of the regulatory system in race bias control. In particular, this study was aimed at determining how temporary impairment of regulatory control would affect the expression of bias and the neural processes relevant to its control. To accomplish this, Bartholow et al. (2006) had participants consume one of three beverages (a moderate alcohol dose, a higher alcohol dose, or an alcohol placebo) prior to engaging in a go-stop task designed to measure inhibition and expression of bias. Considerable research indicates that alcohol consumption specifically targets cognitive control resources required for inhibition of prepotent responses, but has virtually no effect on the activation and implementation of responses (see Easdon & Vogel-Sprott, 2000; Fillmore & Vogel-Sprott, 1999, 2000; Mulvihill, Skilling, & Vogel-Sprott, 1997). Moreover, increased inhibition errors (e.g., responding on “no-go” trials) following alcohol consumption are thought to stem from alcohol’s impairment of cognitive control, rather than potential effects of the drug on motivation or other aspects of information processing (e.g., Abroms, Fillmore, & Marczinski, 2003). Thus, comparing placebo and alcohol effects provides a relevant and interesting manipulation of the extent to which regulative control can be implemented when bias control is required.
The task required participants to respond (via button press) as quickly as possible to stereotype-related words following white and black men’s faces (i.e., primes). On 25% of the trials, however, a visual cue following word onset signaled participants to withhold their response. Trials of interest were those in which the target word was consistent with stereotypes associated with the race of the prime, as these represent prepotent response mappings and therefore should require more regulatory control to successfully inhibit. Therefore, these trials were predicted to be the most difficult for intoxicated participants to manage.
Behavioral data from this study (numbers of inhibition errors on stop trials) are shown in Figure 2A. Analyses of these data showed that whereas errors on stereotype-inconsistent (SI) trials were not significantly affected by beverage type, errors on stereotype-consistent (SC) trials increased significantly as a linear function of alcohol dose. Moreover, only in the highest-dose alcohol condition were inhibition failures more likely on SC than SI trials. These data are consistent with the idea that alcohol specifically impairs regulation of prepotent responses (see also Curtin & Fairchild, 2003), in this case, those associated with the expression of race bias.
Figure 2.
Panel A: Mean numbers of inhibition errors on stereotype-consistent (SC) and stereotype-inconsistent (SI) trials as a function of beverage group (from Bartholow et al., 2006). Whereas errors on SI trials did not differ across beverage groups, errors on SC trials increased linearly with alcohol dose. Panel B: ERP waveforms showing the negative slow wave (NSW) component measured at electrode Cz on successfully inhibited “stop” trials as a function of trial type for those in the placebo and high-dose alcohol groups. Time zero epresents the onset of the stop signal.
Two hypotheses pertaining to the activation of regulatory control were investigated. First, it was hypothesized that if the NSW reflects implementation of cognitive control required for inhibition, the component should be larger on stop trials than on go trials. This hypothesis was confirmed, but only for placebo participants. That is, participants who consumed alcohol showed similar NSW amplitudes on both go and stop trials. Second, to the extent that inhibition of prepotent, race-biased responses requires greater implementation of regulatory control than does inhibition of responses not associated with bias, the NSW should be larger for SC stop trials than for SI stop trials. Here again, however, this prediction applies only to the placebo group. Indeed, as shown in Figure 2B, placebo participants showed larger NSW on SC than SI trials, but high-dose alcohol participants did not.
To the extent that the NSW reflects the implementation of control in order to overcome prepotent response tendencies, NSW amplitude should correlate with the number of inhibition failures on SC trials. Specifically, as the amplitude of the NSW becomes larger (i.e., more negative), inhibition errors on SC trials should decrease, leading to positive correlations. Indeed, this is the pattern we found, with stronger positive correlations at frontal electrodes, particularly left-frontal locations (e.g., r = .56, p < .01) and weaker associations at more posterior locations, particularly right-parietal electrodes (e.g., r = .17, ns) (see Bartholow et al., 2006, Table 1).
Within the context of the current review, an important question about the findings reported by Bartholow et al. (2006) is whether alcohol effects on expression of bias reflect differences in the activation of automatic associations, the ability to regulate responses based on those associations, or some combination. Although Bartholow et al. argued that their findings reflected effects on regulation of biased responding, Sherman et al. (2008) recently addressed this question more directly by analyzing the inhibition error data from this study using formulas in their Quad model. Their analyses indicated that, compared to the placebo beverage, the alcohol beverage impaired the parameter associated with overcoming bias, but had no effect on the activation, detection, or guessing parameters. Thus, consistent with the argument put forth by Bartholow et al. (2006), these findings indicate that alcohol has no effect on the activation of stereotypic associations, but rather interferes with the ability to regulate relevant responses once those associations are activated.
Findings from other, previous studies have demonstrated that race-biased responding can occur despite the detection of conflict inherent in trying to overcome prepotent, stereotypic associations by the evaluative, conflict-monitoring system (e.g., Amodio et al., 2004, 2006, 2008; see also Payne et al., 2005). The findings reported by Bartholow et al. (2006) importantly extended this work by demonstrating that successful control over race-biased response tendencies requires intact regulatory control mechanisms, which are significantly impaired by the acute effects of alcohol (see also Casbon, Curtin, Lang, & Patrick, 2003; Curtin & Fairchild, 2003). More recent work in our lab (Bartholow, Henry, Lust, & Saults, 2009) indicates that alcohol also can interfere with the conflict monitoring system (see also Ridderinkhof et al., 2002), and that this effect is due, at least in part, to alcohol’s reduction of the distress that typically accompanies the experience of conflict. More generally, findings from this study provide additional evidence of the important role played by cognitive control in social cognition, and underscore that a focus on automatic associations cannot fully account for why and under what circumstances biases in overt behavior will be observed.
Response Conflict and Affective Congruency Effects
Although recent models of control in social cognition have focused largely on understanding expression of race bias (e.g., Conrey et al., 2005; Payne, 2005), similar ideas have been debated in the more general area of attitude activation (e.g., Klinger et al., 2000), often studied in the lab using various affective priming tasks (see Fazio, 2001; Klauer & Musch, 2003). Affective priming (or affective congruency) effects occur when a valenced target stimulus is categorized more quickly as positive or negative (i.e., an evaluative decision task) when it is preceded by an evaluatively-congruent prime compared to an evaluatively-incongruent prime. First demonstrated by Fazio et al. (1986), this basic finding has been replicated many times (see Fazio, 2001; Klauer & Musch, 2003). Early explanations of the affective congruency effect proposed spreading activation as the likely mechanism, wherein target responses are presumed to be facilitated on congruent trials because evaluative categorization of targets is eased by the prime pre-activating the appropriate evaluative construct (e.g., Fazio et al., 1986; see also De Houwer & Hermans, 1994; Hermans et al., 1994). Some more recent studies similarly have focused on the possibility that affective congruency effects occur at the evaluative categorization stage of processing (see Abrams, Klinger, & Greenwald, 2002; Klauer, Musch, & Eder, 2005).
However, other researchers have begun to conceptualize the effect in terms of conflict at the response output stage of processing. The response conflict model of affective priming holds that both primes and targets activate response tendencies (see Wentura & Rothermund, 2003). On congruent trials primes activate the same response tendency needed to correctly categorize the target, whereas on incongruent trials primes and targets activate opposing response tendencies. Thus, responses on congruent trials are facilitated because the correct target response is partially pre-activated by the prime. In contrast, on incongruent trials the response activated by the prime conflicts with the correct target response, thus slowing its execution. Although evidence from several behavioral studies is consistent with this model (e.g., De Houwer, Hermans, Rothermund, & Wentura, 2002; Gawronski, Deutsch, & Seidel, 2005; Klauer & Musch, 2002; Klinger, Burton, & Pitts, 2000; Wentura, 1999), direct evidence that primes activate response tendencies, and that such activation produces conflict on incongruent trials, has been lacking.
In a recent experiment, Bartholow, Riordan, Saults, and Lust (2009) used ERPs to directly test the hypothesis that incongruent trials in affective priming elicit response conflict. This hypothesis rests on two assumptions: (1) that primes activate response channels prior to target onset, and (2) that this activation generates conflict on incongruent relative to congruent trials. A further assumption is that this conflict slows response execution on incongruent trials. To test these assumptions, we measured the LRP and N2 components while participants completed an evaluative decision task. Any preferential response activation following prime onset (and prior to target onset) would be evident in the amplitude and polarity of the LRP. To the extent that such response activation conflicts with the response required by a target, the N2 should be enhanced following target onset.
In addition, to investigate the extent to which participants can strategically control their responses, we manipulated the proportion of congruent and incongruent trials across trial blocks (see also Klauer, Rossnagel, & Musch, 1997; Spruyt et al., 2007). To the extent that response facilitation on congruent relative to incongruent trials is driven by automatic spreading of activation, congruent trial responses should be faster than incongruent trial responses regardless of contextual factors such as relative probability (see Spruyt et al., 2007). In contrast, strategic control of response activation would be evident by differential behavioral responses -- and patterns of response activation and conflict in the ERP -- when the probability of congruent trials is relatively low compared to when it is high (see Bartholow et al., 2005; Gratton et al., 1992).
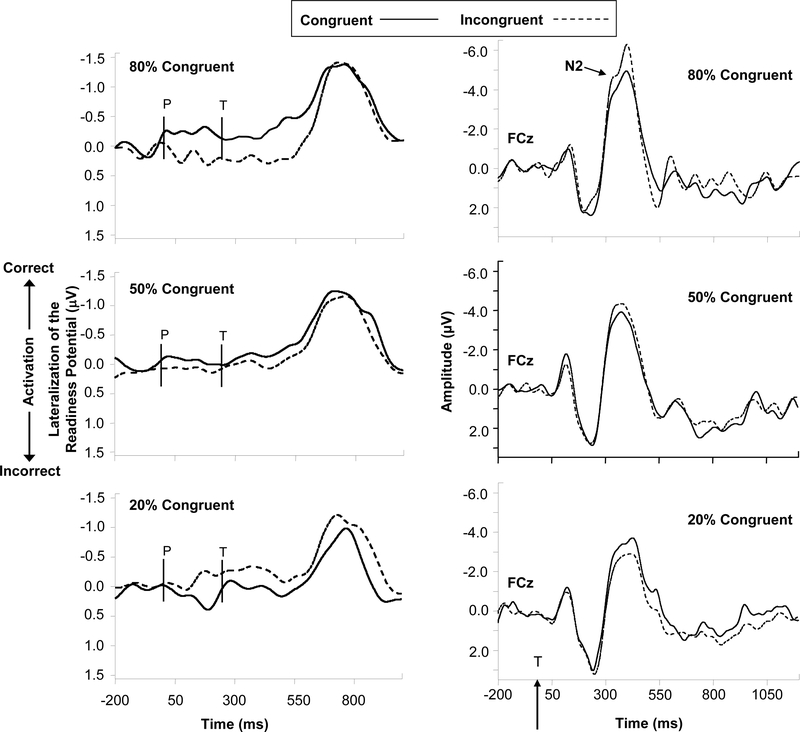
The behavioral data from this experiment produced significant Congruence x Probability interactions for both reaction time (RT) and error rates, replicating previous studies showing that the size of the affective congruency effect varies along with the proportion of congruent trials (e.g., Klauer et al., 1997; Spruyt et al., 2007). Of greater interest here, the LRP and N2 amplitude data also were modulated by congruence probability in a manner consistent with the response conflict hypothesis. As shown in Figure 3 (left panel), when congruent trials were highly probable participants began to preferentially activate the congruent target response before the target appeared (e.g., activating the “positive” response to positive primes), as evinced by the relatively negative amplitude of the LRP for congruent trials compared to incongruent trials. Due to this preferential response activation, incongruent targets elicited heightened response conflict, seen in the amplitude of the N2 component following target onset (see Figure 3, right panel). These effects were smaller, though still evident, when congruence probability was .50. Interestingly, though, when congruent trials were highly improbable participants appeared to activate the incongruent response at prime onset, leading to enhanced conflict for congruent relative to incongruent trials.
Figure 3.
ERP waveforms showing the lateralized readiness potential (LRP) measured from C3 and C4 electrodes (left panel) and the N2 component measured at FCz (right panel) as a function of target congruence and the probability of congruent trials (from Bartholow et al., 2009). Vertical marks on the LRP waveform labeled “P” and “T” represent the time of prime and target onset, respectively. Of primary interest here was the amplitude of the LRP between prime onset and target onset, which indicates relative response activation elicited by the primes. The vertical arrow (and “T”) on the timeline for the N2 represent target onset. Conflict monitoring reflected in the N2 increases as a function of the extent to which primes preferentially activate the response opposite the one required by the target.
Moreover, covariance analyses showed that variation in behavioral responses (RT) was dependent on variations in both LRP and N2 amplitude. For example, when the RT data were re-analyzed using LRP amplitude (difference in LRP for compatible vs. incompatible trials in 80% vs. 20% compatible blocks) as a covariate the original Congruence x Probability interaction was nonsignificant, but was qualified by a 3-way interaction involving the LRP covariate. The form of this interaction was probed by testing the Congruence x Probability interaction separately for participants with relatively large versus relatively small differences in LRP response activation across congruence probability levels (median split). Participants with large differences in their LRPs showed considerable differences in behavioral congruency effects across probability conditions (Ms = 79.1, 5.4, and −48.4 ms in the 80%, 50% and 20% congruent blocks, respectively). In contrast, participants with small differences in LRP response activation across probability conditions showed smaller and less differentiated behavioral congruency effects (Ms = 29, 31, and 20 ms in the 80%, 50%, and 20% congruent blocks, respectively). Taken together, these data indicate that differences in the activation of neural response channels across conditions produces corresponding differences in response output.
Given that the N2 is known to be highly sensitive to stimulus and/or response probability (see Nieuwenhuis et al., 2003), an alternative interpretation of the N2 effects is that they could be driven by simple infrequency effects. However, careful consideration of the N2 effects suggests that this was not the case here. Specifically, despite the fact that congruent trials were just as infrequent in the 20% congruent condition as incongruent trials were in the 80% congruent condition, the N2 was larger on incongruent trials in the 80% congruent condition than it was on congruent trials in the 20% congruent condition (p = .03), and the overall amplitude of the N2 was smaller in the 20% congruent condition compared to the 80% congruent condition.
The findings from this study are important in three primary respects. First, the N2 and LRP data are consistent with the notion that responses to attitude-related stimuli can be strategically controlled and are not predestined on the basis of automatic spreading of activation (cf., Fazio et al., 1986). Second, response activation and conflict are not driven simply by whether primes and targets share an evaluative category. Rather, conflict varied here as a function of whether the response required by the target was predictable from the prime. Third, this study showed that behavioral affective congruency effects can be predicted from neural measures of response activation and conflict, providing direct evidence of the involvement of these processes in affective priming.
Conclusions
The findings of the studies reviewed in this article all point to an important role for cognitive control and response conflict resolution in understanding behavioral responses typically observed in a number of laboratory paradigms used to study social cognition. More generally, the data from these studies are consistent with a number of recent models in which self-regulatory control processes assume a prominent role in explaining social behaviors, particularly in the domains of racial stereotyping and evaluations/attitudes (e.g., Conrey et al., 2005; Payne, 2005; Sherman et al., 2008). Perhaps the primary importance of these and related findings (e.g., Klauer & Teige-Mocigemba, 2007; Radvansky et al., 2008; Teige-Mocigemba & Klauer, 2007; von Hippel, 2007) lies in their implications for understanding individual differences in the expression of many forms of bias (e.g., in responses to racial outgroups; in evaluations more generally) in terms of the ability (and/or motivation) to exert control over one’s responses rather than differences in the strength of automatic associations, a prominent feature of many previous models (e.g., Fazio et al., 1986; Fazio et al., 1995; Greenwald et al., 1998).
Beyond their implications for recent models of bias expression, the findings reviewed here more generally underscore the usefulness of physiological responses for constraining theories about cognitive and social-cognitive processes (cf., Mangun & Hillyard, 1995). The psychophysiological measures used in these and other studies (for reviews see Bartholow & Amodio, 2009; Bartholow & Dickter, 2007, in press) provide a means to covertly assess neurocognitive responses with known links to psychological processes, allowing researchers to determine whether stimulus conditions of interest evoke differences in the involvement of those processes. Even with carefully planned experimental designs, it often is difficult to separate the contribution of multiple, sometimes overlapping processes to behavioral outcomes with behavioral measures alone (see Coles et al., 1995). However, augmenting behavioral response measures with ERPs (and/or other measures of physiological response) provides an opportunity to more comprehensively assess the mental and sensory operations that lead to theoretically relevant differences in human behavior.
Acknowledgments
This article is based on an address presented upon receipt of the Award for a Distinguished Early Career Contribution to Psychophysiology at the 47th annual meeting of the Society for Psychophysiological Research, Savannah, GA, October 2007.
Portions of the research reviewed in this article were supported by research grants from ABMRF/The Foundation for Alcohol Research and the University of Missouri Research Board. Preparation of this article was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (R21 AA017282).
I would like to thank a number of individuals who have contributed in important ways to the research on which this article is based. First and foremost, I am forever indebted to Gabriele Gratton and Monica Fabiani, for the training in psychophysiology I received from them, for the influence their ideas continue to have on my work, and for setting an excellent example of balancing the demands of academic and family life. I also am grateful to number of colleagues, especially David Amodio, Eddie Harmon-Jones, Hart Blanton and Tiffany Ito, for their influence on my intellectual development and for their friendship. Finally, the research presented here could not have been accomplished without the help of numerous current and former graduate and undergraduate research assistants, especially Cheryl Dickter, Marc Sestir, Monica Riordan, Sarah Lust, and Erika Henry. Finally, I offer a very special thanks to Susan E. O’Neill for her constant support, friendship and love.
Footnotes
Other components also are useful for investigating conflict in social cognition, especially the response-locked error-related negativity (ERN). However, the work reviewed here did not involve measurement of response-locked components (but see Bartholow et al., 2005; Bartholow, Henry, Lust, & Saults, 2009). For excellent examples of the use of the ERN as a measure of social-cognitive conflict, see work by Amodio and colleagues (e.g., Amodio et al., 2004, 2006; Amodio, Harmon-Jones, & Devine, 2008).
References
- Abrams RL, Klinger MR, & Greenwald AG (2002). Subliminal words activate semantic categories (not automated motor responses). Psychonomic Bulletin & Review, 9, 100–106. [DOI] [PubMed] [Google Scholar]
- Abroms BD, Fillmore MT, & Marczinski CA (2003). Alcohol-induced impairment of behavioral control: effects on alteration and suppression of prepotent responses. Journal of Studies on Alcohol, 64, 687–695. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Devine PG, & Harmon-Jones E. (2008). Individual differences in the regulation of intergroup bias: The role of conflict monitoring and neural signals for control. Journal of Personality and Social Psychology, 94, 60–74. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Harmon-Jones E, Devine PG, Curtin JJ, Hartley SL, & Covert AE (2004). Neural signals for the detection of unintentional race bias. Psychological Science, 15, 88–93. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Kubota JT, Harmon-Jones E, & Devine PG (2006). Alternative mechanisms for regulating racial responses according to internal vs. external cues. Social Cognitive and Affective Neuroscience, 1, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow BD, & Dickter CL (2008). A response conflict account of the effects of stereotypes on racial categorization. Social Cognition, 26, 273–291. [Google Scholar]
- Bartholow BD, Dickter CL, & Sestir MA (2006). Stereotype activation and control of race bias: Cognitive control of inhibition and its impairment by alcohol. Journal of Personality and Social Psychology, 90, 272–287. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Henry EA, Lust SA, & Saults JS (2009). Alcohol impairs error processing in anterior cingulate cortex via reduced negative affect, not impaired error detection. Manuscript submitted for publication. [Google Scholar]
- Bartholow BD, Pearson MA, Dickter C, Sher KJ, Fabiani M, & Gratton G. (2005). Strategic control and medial frontal negativity: Beyond errors and response conflict. Psychophysiology, 42, 33–42. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Riordan MA, Saults JS, & Lust SA (2009). Psychophysiological evidence of response conflict and strategic control of responses in affective priming. Journal of Experimental and Social Psychology, 45, 655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, & Cohen JD (2001). Conflict monitoring and cognitive control. Psychological Review, 108, 624–52. [DOI] [PubMed] [Google Scholar]
- Brunia CHM (1988). Movement and stimulus preceding negativity. Biological Psychology, 26, 165–178. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Sheridan JF, & McClintock MK (2000). Multi-level integrative analyses of human behavior: Social neuroscience and the complementing nature of social and biological approaches. Psychological Bulletin, 126, 829–843. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Crites SL Jr., Berntson GG, & Coles MGH (1993). If attitudes affect how stimuli are processed, should they not affect the event-related brain potential? Psychological Science, 4, 108–112. [Google Scholar]
- Carlston DE, & Smith ER (1996). Principles of mental representation In: Higgins ET & Kruglanski AW (Eds.) Social psychology: Handbook of basic principles. New York: Guilford. [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998). Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280, 747–749. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, et al. (2000). Parsing executive processes: Strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences, USA 97, 1944–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casbon TS, Curtin JJ, Lang AR, & Patrick CJ (2003). Deleterious effects of alcohol intoxication: Diminished cognitive control and its behavioral consequences. Journal of Abnormal Psychology, 112, 476–487. [DOI] [PubMed] [Google Scholar]
- Coles MGH (1989). Modern mind-brain reading: Psychophysiology, physiology, and cognition. Psychophysiology, 26, 251–269. [DOI] [PubMed] [Google Scholar]
- Coles MGH, Gratton G, Bashore TR, Eriksen CW, & Donchin E. (1985). A psychophysiological investigation of the continuous flow model of human information processing. Journal of Experimental Psychology: Human Perception and Performance, 11, 529–553. [DOI] [PubMed] [Google Scholar]
- Coles MGH, Smid HGOM, Scheffers MK, & Otten LJ (1995). Mental chronometry and the study of human information processing In Rugg MD & H Coles MG (Eds.), Electrophysiology of mind: Event-related brain potentials and cognition (pp. 86–131). New York: Oxford University Press. [Google Scholar]
- Collins AM, & Loftus EF (1975). A spreading activation theory of semantic processing. Psychological Review, 82, 407–428. [Google Scholar]
- Conrey FR, Sherman JW, Gawronski B, Hugenberg K, & Groom C. (2005). Separating multiple processes in implicit social cognition: The Quad-Model of implicit task performance. Journal of Personality and Social Psychology, 89, 469–487. [DOI] [PubMed] [Google Scholar]
- Correll J, Park B, Judd CM, & Wittenbrink B. (2002). The police officer’s dilemma: Using ethnicity to disambiguate potentially threatening individuals. Journal of Personality and Social Psychology, 83, 1314–1329. [PubMed] [Google Scholar]
- Crites SL Jr., Cacioppo JT, Gardner WL, & Berntson GG (1995). Bioelectrical echoes from evaluative categorization: II. A late positive brain potential that varies as a function of attitude registration rather than attitude report. Journal of Personality and Social Psychology, 68, 997–1013. [DOI] [PubMed] [Google Scholar]
- Curtin JJ & Fairchild BA (2003). Alcohol and cognitive control: Implications for regulation of behavior during response conflict. Journal of Abnormal Psychology, 112, 424–436. [DOI] [PubMed] [Google Scholar]
- Decety J, & Cacioppo JT (in press). The Handbook of Social Neuroscience. New York: Oxford University Press. [Google Scholar]
- De Houwer J, & Hermans D. (1994). Differences in the affective processing of words and pictures. Cognition and Emotion, 8, 1–20. [Google Scholar]
- De Houwer J, Hermans D, Rothermund K, & Wentura D. (2002). Affective priming of semantic categorization responses. Cognition and Emotion, 16, 643–666. [Google Scholar]
- Devine PG (1989). Stereotypes and prejudice: Their automatic and controlled components. Journal of Personality and Social Psychology, 56, 5–18. [Google Scholar]
- Devine PG, & Elliot AJ (1995). Are racial stereotypes really fading? The Princeton trilogy revisited. Personality and Social Psychology Bulletin, 11, 1139–1150. [Google Scholar]
- Dovidio JF, Evans N, & Tyler RB (1986). Racial stereotypes: The contents of their cognitive representations. Journal of Experimental Social Psychology, 22, 22–37. [Google Scholar]
- Dovidio JF, & Gaertner SL (1998). On the nature of contemporary prejudice: The causes, consequences, and challenges of aversive racism In Eberhardt JL & Fiske ST (Eds.), Confronting racism: The problem and the response (pp. 3–32). Thousand Oaks, CA: Sage. [Google Scholar]
- Easdon C, & Vogel-Sprott M. (2000). Alcohol and behavioral control: Impaired response inhibition and flexibility. Experimental and Clinical Psychopharmacology, 8, 387–394. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, & Eriksen CW (1974). Effects of noise letters upon the identification of target letter in a non-search task. Perception and Psychophysics, 16, 143–149. [Google Scholar]
- Eriksen CW, & Schultz DW (1979). Information processing in visual search: A continuous flow conception and experimental results. Perception and Psychophysics, 25, 249–263. [DOI] [PubMed] [Google Scholar]
- Fazio RH (2001). On the automatic activation of associated evaluations: An overview. Cognition and Emotion, 15, 115–141. [Google Scholar]
- Fazio RH, Jackson JR, Dunton BC, & Williams CJ (1995). Variability in automatic activation as an unobtrusive measure of racial attitudes: A bona fide pipeline? Journal of Personality and Social Psychology, 69, 1013–1027. [DOI] [PubMed] [Google Scholar]
- Fazio RH, Sanbonmatsu DM, Powell MC, & Kardes FR (1986). On the Automatic Activation of Attitudes. Journal of Personality and Social Psychology, 50, 229–238. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, & Vogel-Sprott M. (1999). An alcohol model of impaired inhibitory control and its treatment in humans. Experimental and Clinical Psychopharmacology, 7, 49–55. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, & Vogel-Sprott M. (2000). Response inhibition under alcohol: Effects of cognitive and motivational conflict. Journal of Studies on Alcohol, 61, 239–246. [DOI] [PubMed] [Google Scholar]
- Fiske ST, Cuddy AJ, Glick P, & Xu J. (2002). A model of (often mixed) stereotype content: Competence and warmth respectively follow from perceived status and competition. Journal of Personality and Social Psychology, 82, 878–902. [PubMed] [Google Scholar]
- Fiske ST, & Neuberg SL (1990). A continuum of impression formation, from category-based to individuating processes: Influences of information and motivation on attention and interpretation. Advances in Experimental Social Psychology, 23, 1–74. [Google Scholar]
- Gawronski B, Deutsch R, & Seidel O. (2005). Contextual influences on implicit evaluation: A test of additive versus contrastive effects of evaluative context stimuli in affective priming. Personality and Social Psychology Bulletin, 31, 1226–1236. [DOI] [PubMed] [Google Scholar]
- Gehring WJ & Fencsik DE (2001). Functions of the medial frontal cortex in the processing of conflict and errors. Journal of Neuroscience, 21, 9430–9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalkorale K, Sherman JW, & Klauer KC (2009). Aging and prejudice: Diminished regulation of automatic race bias among older adults. Journal of Experimental Social Psychology, 45, 410–414. [Google Scholar]
- Gratton G, Bosco CM, Kramer AF, Coles MG, Wickens CD, & Donchin E. (1990). Event-related brain potentials as indices of information extraction and response priming. Electroencephalography & Clinical Neurophysiology, 75, 419–432. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, & Donchin E. (1992). Optimizing the use of information: Strategic control of activation of responses. Journal of Experimental Psychology: General, 121, 480–506. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Sirevaag EJ, Eriksen CW, & Donchin E. (1988). Pre- and post-stimulus activation of response channels: A psychophysiological analysis. Journal of Experimental Psychology: Human Perception and Performance, 14, 331–344. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, & Banaji MR (1995). Implicit social cognition: Attitudes, self-esteem, and stereotypes. Psychological Review, 102, 4–27. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, McGhee DE, & Schwartz JLK (1998). Measuring individual differences in implicit cognition: The implicit association test. Journal of Personality and Social Psychology, 74, 1464–1480. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Oakes MA, & Hoffman H. (2003). Targets of discrimination: Effects of race on responses to weapons holders. Journal of Experimental Social Psychology, 39, 399–405. [Google Scholar]
- Harmon-Jones E, & Beer JS (Eds.) (2009). Methods in social neuroscience. Guilford Publications: New York. [Google Scholar]
- Harmon-Jones E, & Winkielman P. (Eds.) (2007). Social neuroscience: Integrating biological and psychological explanations of social behavior. Guilford Publications: New York. [Google Scholar]
- Hermans D, De Houwer J, & Eelen P. (1994). The affective priming effect: Automatic activation of evaluative information in memory. Cognition and Emotion, 8, 515–533. [Google Scholar]
- Higgins ET (1996). Knowledge activation: Accessibility, applicability, and salience In Higgins ET & Kruglanski AW (Eds.), Social psychology: Handbook of basic principles (pp. 133–168). New York: The Guilford Press. [Google Scholar]
- Ito TA, Larsen JT, Smith NK, & Cacioppo JT (1998). Negative information weighs more heavily on the brain: The negativity bias in evaluative categorizations. Journal of Personality and Social Psychology, 75, 887–900. [DOI] [PubMed] [Google Scholar]
- Jones AD, Cho RY, Nystrom LE, Cohen JD, & Braver TS (2002). A model of anterior cingulate activity, conflict monitoring, and control adjustments in choice-discrimination tasks. Cognitive, Affective, & Behavioral Neuroscience, 2, 300–317. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW III, Cho RY, Stenger VA, & Carter CS (2004). Anterior cingulate conflict monitoring predicts adjustments in control. Science, 303, 1023–1026. [DOI] [PubMed] [Google Scholar]
- Klauer KC, & Mierke J. (2005). Task-set inertia, attitude accessibility, and compatibility-order effects: New evidence for a task-set switching account of the Implicit Association Test. Personality and Social Psychology Bulletin, 31, 208–217. [DOI] [PubMed] [Google Scholar]
- Klauer KC, & Musch J. (2002). Goal-dependent and goal-independent effects of irrelevant evaluations. Personality and Social Psychology Bulletin, 28, 802–814. [Google Scholar]
- Klauer KC, & Musch J. (2003). Affective priming: findings and theories In Klauer C. and Musch J. (Eds.), The psychology of evaluation: affective processes in cognition and emotion (pp. 7–49). Philadelphia, PA: Lawrence Erlbaum. [Google Scholar]
- Klauer KC, Musch J, & Eder AB (2005). Priming of semantic classifications: Late and response-related, or earlier and more central? Psychonomic Bulletin & Review, 12, 897–903. [DOI] [PubMed] [Google Scholar]
- Klauer KC, Rossnagel C. & Musch J. (1997). List-context effects in evaluative priming. Journal of Experimental Psychology: Learning, Memory, and Cognition, 23, 246–255. [DOI] [PubMed] [Google Scholar]
- Klauer KC, Schmitz F, Teige-Mocigemba S. & Voss A. (in press). Understanding the role of executive control in the Implicit Association Test: Why flexible people have small IAT effects. Quarterly Journal of Experimental Psychology. [DOI] [PubMed] [Google Scholar]
- Klauer KC, & Teige-Mocigemba S. (2007). Controllability and resource-dependence in automatic evaluation. Journal of Experimental Social Psychology, 43, 648–655. [Google Scholar]
- Klinger MR, Burton P, & Pitts S. (2000). Mechanisms of unconscious priming: I: Response competition, not spreading activation. Journal of Experimental Psychology: Learning, Memory, & Cognition, 26, 441–455. [DOI] [PubMed] [Google Scholar]
- Kopp B, Rist F, & Mattler U. (1996). N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology, 33, 282–294. [DOI] [PubMed] [Google Scholar]
- Kornhuber HH, Deecke L. (1965). Hirnpotentialanderungen bei willkurbewegungen und passiven bewegungen des menschen: Bereitschaftspotential und reafferente potentiale. Pflugers Arch, 284, 1–17. [PubMed] [Google Scholar]
- Kutas M, McCarthy G. & Donchin E. (1977). Augmenting mental chronometry: The P300 as a measure of stimulus evaluation time. Science, 197, 792–795. [DOI] [PubMed] [Google Scholar]
- Lepore L, & Brown R. (1997). Category and stereotype activation: Is prejudice inevitable? Journal of Personality and Social Psychology, 72, 275–287. [Google Scholar]
- Liotti M, Woldorff MG, Perez III R, Mayberg HS (2000). An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia, 38, 701–711. [DOI] [PubMed] [Google Scholar]
- Livingston RW, & Brewer MB (2002). What are we really priming? Cue-based versus category-based processing of facial stimuli. Journal of Personality and Social Psychology, 82, 5–18. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, & Carter CS (2000). Dissociating the role of Dorsolateral Prefrontal and Anterior Cingulate Cortex in cognitive control. Science, 288, 1835–1838. [DOI] [PubMed] [Google Scholar]
- MacLeod CM (1991). Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin, 109, 163–203. [DOI] [PubMed] [Google Scholar]
- Macrae CN, & Bodenhausen GV (2000). Social cognition: Thinking categorically about others. Annual Review of Psychology, 51, 93–120. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Bodenhausen GV, Schloersheidt AM, & Milne AB (1999). Tales of the unexpected: Executive function and person perception. Journal of Personality and Social Psychology, 76, 200–213. [DOI] [PubMed] [Google Scholar]
- Magliero A, Bashore TR, Coles MGH, & Donchin E. (1984). On the dependence of P300 latency on stimulus evaluation processes. Psychophysiology, 21, 171–186. [DOI] [PubMed] [Google Scholar]
- Mangun GR, & Hillyard SA (1995). Attention: Mechanisms and models In Rugg MD & Coles MGH (Eds.), Electrophysiology of mind: Event-related potentials and cognition (pp. 40–85). New York: Oxford University Press. [Google Scholar]
- Markus H, & Zajonc RB (1985). The cognitive perspective in social psychology In Lindzey G. & Aronson E. (Eds.), Handbook of social psychology (3rd ed., pp. 137–229). New York: Random House. [Google Scholar]
- McCarthy G, & Donchin E. (1981). A metric of thought: A comparison of P300 latency and reaction time. Science, 21, 171–186. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager T. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Mulvihill LE, Skilling TA & Vogel-Sprott M. (1997). Alcohol and the ability to inhibit behavior in men and women. Journal of Studies on Alcohol, 58, 600–605. [DOI] [PubMed] [Google Scholar]
- Neely JH (1977). Semantic priming and retrieval from lexical memory: Roles of inhibition-less spreading activation and limited-capacity attention. Journal of Experimental Psychology: General, 106, 226–254. [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, & Ridderinkhof KR (2003). Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cognitive, Affective, and Behavioral Neuroscience, 3, 17–26. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, & Lieberman MD (2001). The emergence of social cognitive neuroscience. American Psychologist, 56, 717–734. [PubMed] [Google Scholar]
- Payne BK (2001). Prejudice and perception: The role of automatic and controlled processes in misperceiving a weapon. Journal of Personality and Social Psychology, 81, 181–192. [DOI] [PubMed] [Google Scholar]
- Payne BK (2005). Conceptualizing control in social cognition: How executive functioning modulates the expression of automatic stereotyping. Journal of Personality and Social Psychology, 89, 488–503. [DOI] [PubMed] [Google Scholar]
- Payne BK, Shimizu Y, & Jacoby LL (2005). Mental control and visual illusions: Toward explaining race-biased weapon misidentifications. Journal of Experimental Social Psychology, 41, 36–47. [Google Scholar]
- Porier GW, & Lott AJ (1967). Galvanic skin responses and prejudice. Journal of Personality and Social Psychology, 5, 253–259. [DOI] [PubMed] [Google Scholar]
- Radvansky GA, Lynchard NA, & von Hippel W. (2008). Aging and stereotype suppression. Aging, Neuropsychology, and Cognition, 16, 22–32. [DOI] [PubMed] [Google Scholar]
- Rankin RE, & Campbell DT (1955). Galvanic skin response to Negro and white experimenters. Journal of Abnormal and Social Psychology, 51, 30–33. [DOI] [PubMed] [Google Scholar]
- Requin J. (1985). Looking forward to moving soon: Ante factum selective processes in motor control In Posner MI & Marin OSM (Eds.), Attention and performance XI (pp. 147–167). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Richeson JA, & Trawalter S. (2005). On the categorization of admired and disliked exemplars of admired and disliked racial groups. Journal of Personality and Social Psychology, 89, 517–530. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, et al. (2002). Alcohol consumption impairs the detection of performance errors by mediofrontal cortex. Science, 298, 2209–2211. [DOI] [PubMed] [Google Scholar]
- Sagar HA, & Schofield JW (1980). Racial and behavioral cues in black and white children’s perceptions of ambiguously aggressive acts. Journal of Personality and Social Psychology, 19, 590–598. [DOI] [PubMed] [Google Scholar]
- Schuman H, Steeh C, Bobo L, & Krysan M. (1997). Racial attitudes in America: Trends and interpretations (revised edition). Cambridge: Harvard University Press. [Google Scholar]
- Sherman JW (2009). Controlled influences on implicit measures: Confronting the myth of process-purity and taming the cognitive monster In Petty RE, Fazio RH, & Brinol P. (Eds.), Attitudes: Insights from the new wave of implicit measures (pp. 391–426). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Sherman JW, Gawronski B, Gonsalkorale K, Hugenberg K, Allen TJ, & Groom CJ (2008). The self-regulation of automatic associations and behavioral impulses. Psychological Review, 115, 314–335. [DOI] [PubMed] [Google Scholar]
- Smid HGOM, Lamain W, Hogeboom MM, Mulder G, & Mulder LJM (1991). Psychophysiological evidence for continuous information transmission between visual search and response processes. Journal of Experimental Psychology: Human Perception and Performance, 17, 696–714. [DOI] [PubMed] [Google Scholar]
- Smulders FTY, Kok A, Kenemans JL, & Bashore TR (1995). The temporal selectivity of additive factor effects on the reaction process revealed in ERP component latencies. Acta Psychologica, 90, 97–109. [DOI] [PubMed] [Google Scholar]
- Spencer KM, & Coles MGH (1999). The lateralized readiness potential: relationship between human data and response activation in a connectionist model. Psychophysiology, 36, 364–370. [DOI] [PubMed] [Google Scholar]
- Spruyt A, De Houwer J, Hermans D, & Eelen P. (2007). Affective priming of non-affective semantic categorization responses. Experimental Psychology, 54, 44–53. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, & Gehring WJ (2007). Neural systems for error monitoring: Recent findings and theoretical perspectives. The Neuroscientist, 13, 162–172. [DOI] [PubMed] [Google Scholar]
- Teige-Mocigemba S, & Klauer KC (2007). ‘Automatic’ evaluation? Strategic effects on affective priming. Journal of Experimental Social Psychology, 44, 1414–1417. [Google Scholar]
- van Veen V, & Carter CS (2002). The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience, 14, 593–602. [DOI] [PubMed] [Google Scholar]
- von Hippel W. (2007). Aging, executive functioning, and social control. Current Directions in Psychological Science, 16, 240–244. [Google Scholar]
- Wentura D. (1999). Activation and inhibition of affective information: Evidence for negative priming in the evaluation task. Cognition and Emotion, 13, 65–91. [Google Scholar]
- Wentura D, & Rothermund K. (2003). The “meddling-in” of affective information: A general model of automatic evaluation effects In Musch J. & Klauer KC (Eds.), The psychology of evaluation: Affective processes in cognition and emotion (pp. 51–86). Mahwah, NJ: Erlbaum. [Google Scholar]
- West R, & Alain C. (1999). Event-related neural activity associated with the Stroop task. Cognitive Brain Research, 8, 157–164. [DOI] [PubMed] [Google Scholar]
- West R, & Alain C. (2000). Evidence for the transient nature of a neural system supporting goal-directed action. Cerebral Cortex, 10, 748–752. [DOI] [PubMed] [Google Scholar]
- Wheeler SC, & Petty RE (2001). The effects of stereotype activation on behavior: A review of possible mechanisms. Psychological Bulletin, 127, 797–826. [DOI] [PubMed] [Google Scholar]
- Wittenbrink B, Judd CM, & Park B. (1997). Evidence for racial prejudice at the implicit level and its relationship to questionnaire measures. Journal of Personality and Social Psychology, 72, 262–274. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, & Cohen JD (2004). The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review, 111, 931–959. [DOI] [PubMed] [Google Scholar]