ABSTRACT
Trichoderma species were known as biological control agents against phytopathogenic fungi because they produce a variety of chitinases. Chitinases are hydrolytic enzymes that break down glycosidic bonds in chitin, a major component of the cell walls of fungi. The present study shows that extracellular chitinase activity reached a maximum value of approximately 22 U/mL after 96 h of T. asperellum PQ34 strain culture. The optimal temperature and pH of enzyme are 40°C and 7, respectively, whereas the thermal and pH stability range from 25°C to 50°C and 4 to 10, respectively. Chitinase at 60 U/mL inhibited nearly completely in vitro growth of Colletotrichum sp. (about 95%) and Sclerotium rolfsii (about 97%). In peanut plants, 20 U/mL of chitinase significantly reduced the incidence of S. rolfsii infection compared to controls. The fungal infection incidence of seeds before germination and 30 days after germination was only 2.22% and 2.38%, while the control was 13.33% and 17.95%. Besides, chitinase from T. asperellum PQ34 can also prevent anthracnose that is caused by Colletotrichum sp. on both mango and chilli fruits up to 72 h after enzyme pre-treatment at 40 U/mL. In mango and chilli fruits infected with anthracnose, 40 U/mL dose of chitinase inhibited the growth of fungi after 96 h of treatment, the diameter of lesion was only 0.88 cm for mango and 1.45 cm for chilli, while the control was 1.67 cm and 2.85 cm, respectively.
KEYWORDS: Antifungal activity, chitinase, Colletotrichum sp, Sclerotium rolfsii, Trichoderma asperellum
1. Introduction
Genus Trichoderma (Hyphomycetes) is widely distributed in nature, especially in soil (Harman et al. 2004) and endophyte (Baiyee et al. 2019a). They have been used in the production of enzymes and biological controls of plant diseases (Samuels 1996). Trichoderma species excrete enzymes such as chitinases, β-glucanases and proteases; these enzymes are induced during interactions between Trichoderma and cell walls of phytopathogenic fungi (Wonglom et al. 2019; Baiyee et al. 2019b). Although other enzymes may be also involved in the complete degradation of cell walls of phytopathogenic fungi, chitinase is generally considered an important enzyme since its substrate, chitin, is the most abundant component in cell walls of many fungus species (Baek et al. 1999). Chitinases (E.C 3.2.2.14) are glycosyl hydrolases with the molecular weight ranging from 20 to 90 kDa. They are present in a wide range of organisms such as bacteria, fungi, yeasts, plants, actinomycetes, and arthropods (Hamid et al. 2013). Chitinases can degrade chitin into low molecular weight oligosaccharides (Kramer and Muthukrishnan 1997). Chitin (poly 1,4-β-N-acetylglucosamine) is the second most abundant polysaccharide (after cellulose) occurring in nature and is a long-chain polymer of N-acetylglucosamine (2-acetamido-2-deoxy-D-glucose) linked by β-1,4 bonds (Song et al. 2005). Chitin is the building material that gives strength to the exoskeletons of crustaceans, insects, and the cell wall of fungi (Elieh-Ali-Komi and Hamblin 2016). At present, chitin degradation and chitinases are playing an important role in a wide variety of biological and biotechnological processes, ranging from the exploitation and environmental clean-up of chitinous wastes to plant defence systems and biological control.
Studies on characterisations and activity of extracellular chitinase from Trichoderma species had been started long time ago such as T. harzianum (Ulhoa and Peberdy 1991; El-Katatny et al. 2000, 2001; Sandhya et al. 2004), Trichoderma sp. (Lima et al. 1999), T. virens (Baek et al. 1999). However, studies in T. asperellum have only recently begun and only focused on chitinase coding genes, its characterisation and activity (Loc et al. 2011, 2013; Kumar et al. 2012a; Nadarajah et al. 2014; Asad et al. 2015; Wu et al. 2017; Pandian et al. 2018; Liu et al. 2019, etc.).
Many studies have shown Colletotrichum is a large genus of Ascomycete fungi, containing species that cause serious anthracnose diseases on a wide range of economic value crops such as mango (Rivera-Vargas et al. 2006; Ismail et al. 2015; Mo et al. 2018) and chilli (Than et al. 2008; Oo and Oh 2016; Saxena et al. 2016). S. rolfsii fungus was also reported that is the cause of serious stem and pod rot disease in peanut production in many peanut-growing regions (Mehan et al. 1994; Okabe and Matsumoto 2000; Cilliers et al. 2003; Jogi et al. 2016). Meanwhile, peanut, mango and chilli are three of agricultural crops of high economic value in Vietnam. The present work, therefore, purposed to evaluate the antifungal activity of extracellular chitinase from T. asperellum PQ34 strain on crop plant (peanut) and fruits (mango and chilli) for potential applications in agriculture and post-harvest storage.
2. Materials and methods
2.1. Fungal strains and experimental materials
T. asperellum PQ34 strain was isolated from the surface soil layer (15–20 cm in depth) under chilli cultivation in Thua Thien Hue province, Vietnam, which exhibited high chitinolytic secretion in the assay of colloidal chitin hydrolytic activity on an agar plate. Species identification for Trichoderma was done by PCR amplification of ITS (internal transcribed spacer) region from the fungal genome with ITS1 and ITS4 primers, and then the nucleotide sequence of the amplicon was used to search BLAST in Genbank database (Loc et al. 2011). The strain was stored at −70°C as 40% glycerol stock.
Disease fungal strains (S. rolfsii and Colletotrichum sp.) were supplied by the Department of Plant Protection, Hue University of Agriculture and Forestry, Hue University, Vietnam.
Healthy mango and chilli fruits, devoid of freckles (0 FPA, freckles per cm2 area), and peanut seeds (large and plump seed, bright seed coat, no scratches and over 90% germination rate) were used for research.
Fungicides were not applied at any stage of fruit development.
2.2. Fungal culture and chitinase production
Two millilitres of spore suspension (approx. 106 spores/mL) of T. asperellum PQ34 was cultured in 100 mL of Czapek-Dox medium supplemented with 10% glucose at 28°C on a shaker with a speed of 180 rpm for 96 h. Mycelia was harvested and rinsed with 2% MgCl2, and then with sterile distilled water for three times.
Mycelia was subcultured in Czapek-Dox medium without glucose but supplemented with 1% (w/v) colloidal chitin that prepared from native chitin (Sigma-Aldrich) as a carbon source (Shanmugaiah et al. 2008). The culture was incubated at 28°C on a shaker with a speed of 180 rpm. The broth was recovered after 96 h of culture, then filtered with Whatman filter paper (No. 1) and used as a crude enzyme (Harighi et al. 2007). For partial purification, the crude enzyme was precipitated by ammonium sulphate (70% saturation) at 4°C for 2 h, centrifuged at 15,000 rpm at 4°C for 10 min. The pellet was resuspended in 0.1 M sodium acetate (pH 5) and dialysed with MWCO of 12 kDa overnight against 0.05 M sodium acetate (pH 5). Enzyme after partial purification has been used for further studies (Loc et al. 2011).
2.3. Chitinase activity assay
Chitinase activity was determined by spectrophotometric method according to Tsujibo et al. (1998) with para-nitrophenyl-β-N-acetyl glucosaminide (pNp-GlcNAc) as a substrate. Seventy microlitres of partially purified enzyme was added to 140 µL of 2.5 mM pNp-GlcNAc that dissolved in 50 mM acetate buffer (pH 6) and incubated at 50°C for 10 min. Hydrolysis was then stopped using 1.4 mL of 0.2 M sodium carbonate. Absorbance was measured at a wavelength of 420 nm. Chitinase activity was defined as the amount of enzyme released 1 µmol p-nitrophenol from pNp-GlcNAc during 1 min. p-nitrophenol (Sigma-Aldrich) was used as a standard to determine chitinase activity with an estimated regression equation of y = 0.673x (R2 = 0.988), where y is p-nitrophenol content and x is absorbance at 420 nm.
2.4. Characterisation of chitinase
The optimal temperature and pH for enzyme activity were investigated in a range of 25–70°C and 4–10, respectively. Buffers for optimal pH determination were 20 mM citrate buffer (pH 4–6), 20 mM phosphate buffer (pH 7–8), and 20 mM glycine-NaOH buffer (pH 9–10). The thermal and pH stability of enzyme were studied by incubating enzyme for 30 min at temperatures of 25–70°C and pH of 4–10 without substrate, enzyme solution was then immediately cooled to 4°C. Effect of metal ions and some surfactants on enzyme activity were tested by incubating enzyme at appropriate temperature and pH for 30 min with 5 mM metal ion (Na+, Al3+, Fe2+, Mg2+, Cu2+, Co2+, Ca2+, Zn2+, Mn2+, or Fe3+) or surfactants such as 1% SDS (sodium dodecyl sulphate), 1 mM EDTA (ethylenediaminetetraacetic acid), 1 M urea, 5% DMSO (dimethyl sulphoxide) or 1% Triton X-100. Relative activity of chitinase was determined as described in “chitinase activity assay” section but at optimal temperature and pH. The boiling enzyme solution was used as the blank.
2.5. In vitro antifungal activity of chitinase
The antifungal activity of chitinase was determined using an assay based upon inhibition of hyphal growth of the phytopathogenic fungi as S. rolfsii and Colletotrichum sp., all of which have chitin in their cell wall. Petri dish (Φ = 6 cm) containing 1/2 potato dextrose (PD) broth (potato dextrose agar [PDA] without agar) was supplemented with 10–60 U/mL of enzyme and 10 µL of fungal spore suspension (approx. 106 spores/mL). The culture was incubated at 28°C from 36 to 48 h to evaluate the growth of fungus. Mycelium cells were harvested by centrifugation at 4000 rpm for 5 min and rinsed with distilled water to determine fresh biomass, followed by drying at 65°C to a constant weight to determine dry biomass.
2.6. Antifungal activity of chitinase in peanut
Healthy peanut (Arachis hypogaea) seeds were peeled and treated with 1% sodium hypochlorite for 1 min, then rinsed with sterile distilled water and soaked with enzyme (10–60 U/mL) for 1 h. Finally, the seeds were sown in soil pots that have been inoculated artificially before with S. rolfsii, 15 seeds for each treatment, 5 days after sowing the seeds will be germinated. The samples before and after germination were gently taken by hand to observe the appearance of mycelium and count the number of infected samples. The rate of fungal infection (FI) of seeds was evaluated before and after germination.
where DB: FI before germination, NS: number of fungal-infected seeds, TS: total seeds.
where DA: FI after germination, NP: number of fungal-infected plants, TS: total plants.
The area under the disease progress curve (AUDPC) was determined by the method of Campbell and Madden (1990).
2.7. Antifungal activity of chitinase in mango and chilli fruits
Healthy mango and chilli fruits were collected and rinsed with tap water; they were then treated with 70% ethanol and rinsed again with sterile distilled water.
Fungal prevention. Mango and chilli fruits were sprayed with chitinase (10–60 U/mL) to wet, then allow to dry naturally and innoculate artificially with approx. 105 spores of Colletotrichum sp./lesion, 10 lesions/fruit (mango) or 1 lesion/fruit (chilli). Fruits were stored in boxes at room temperature (26 ± 2°C). Diameters of the anthracnose lesions (cm) were measured daily to evaluate the level of disease progression.
Fungal treatment. Mango and chilli fruits were inoculated artificially before with Colletotrichum sp. as described above; after the appearance of the anthracnose lesions on fruits (2–4 days), they will be treated with chitinase from 10 to 60 U/mL. Fruits were stored in boxes at room temperature (26 ± 2°C). Diameters of the anthracnose lesions (cm) were also measured daily to evaluate the level of disease progression.
2.8. Statistical analysis
The experiments were conducted with three replications per treatment (n ≥ 30). The data are represented as the mean of the repeats; the means were compared using one-way ANOVA (Duncan’s test at p = 0.05).
3. Results
3.1. Chitinase production from T. asperellum PQ34
Data in Figure 1 show a time course of extracellular chitinase production from T. asperellum PQ34 during 132 h. Samples were taken periodically for enzyme activity assay. Chitinase activity was present in medium after 24 h of culture at 28°C on a shaker with a speed of 180 rpm and increased continuously with time. The partial purity enzyme reached a maximum total activity of approx. 22 U/mL after 96 h; that is, approx. twofold higher than the crude enzyme.
Figure 1.
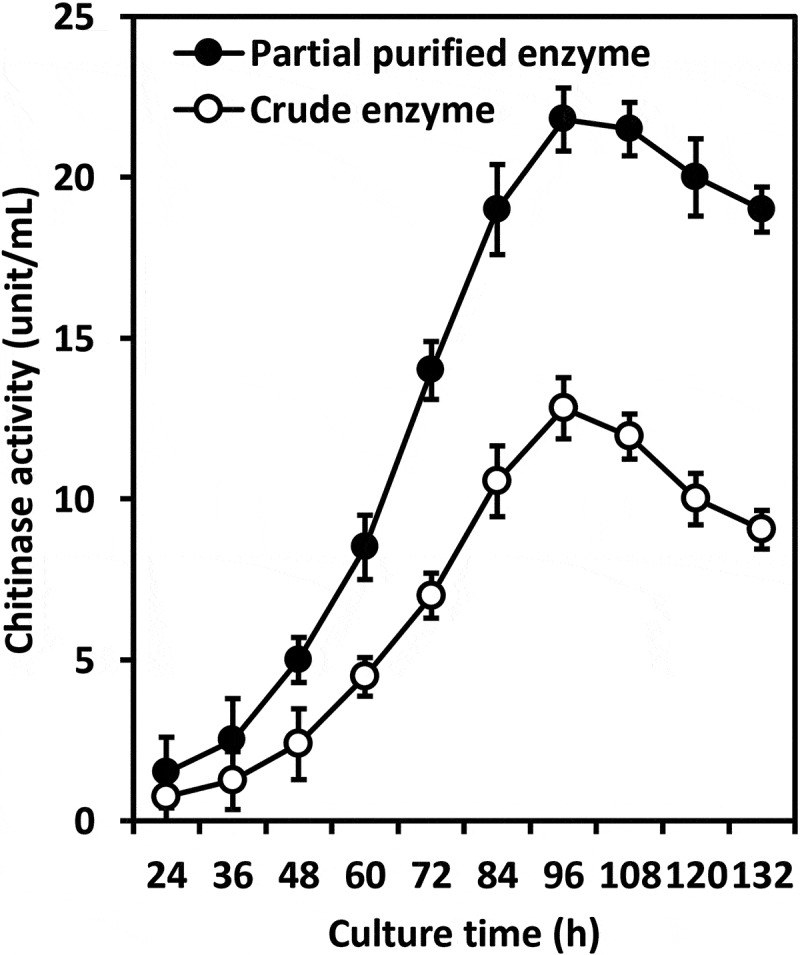
Time course of extracellular chitinase production from T. asperellum PQ34.
3.2. Characterisations of chitinase
The study showed that chitinase from T. asperellum PQ34 exhibited the optimal temperature and pH of 40°C and 7 with the relative activity of 110% (at 40°C and pH 6) and 150% (at 40°C and pH 7), respectively. The thermal and pH stability of enzyme ranged from 25–50°C and 4–10 with the relative activity of about 90% and from 80% to 90%, respectively (Figures 2 and 3).
Figure 2.
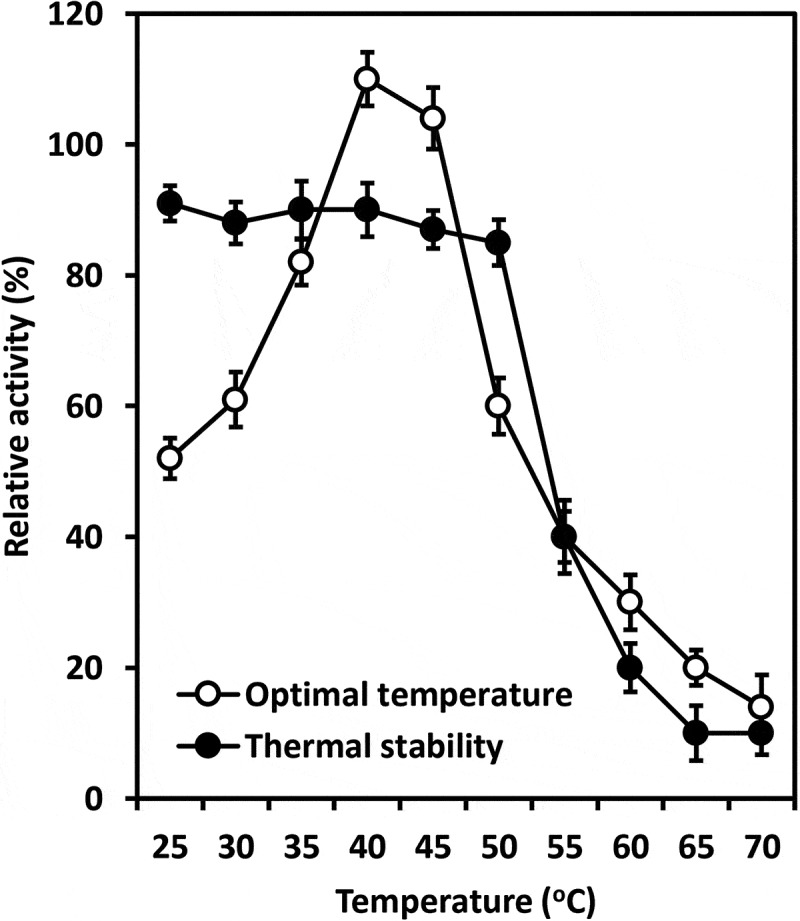
Optimal temperature and thermal stability of chitinase from T. asperellum PQ34 at pH 6.
Figure 3.
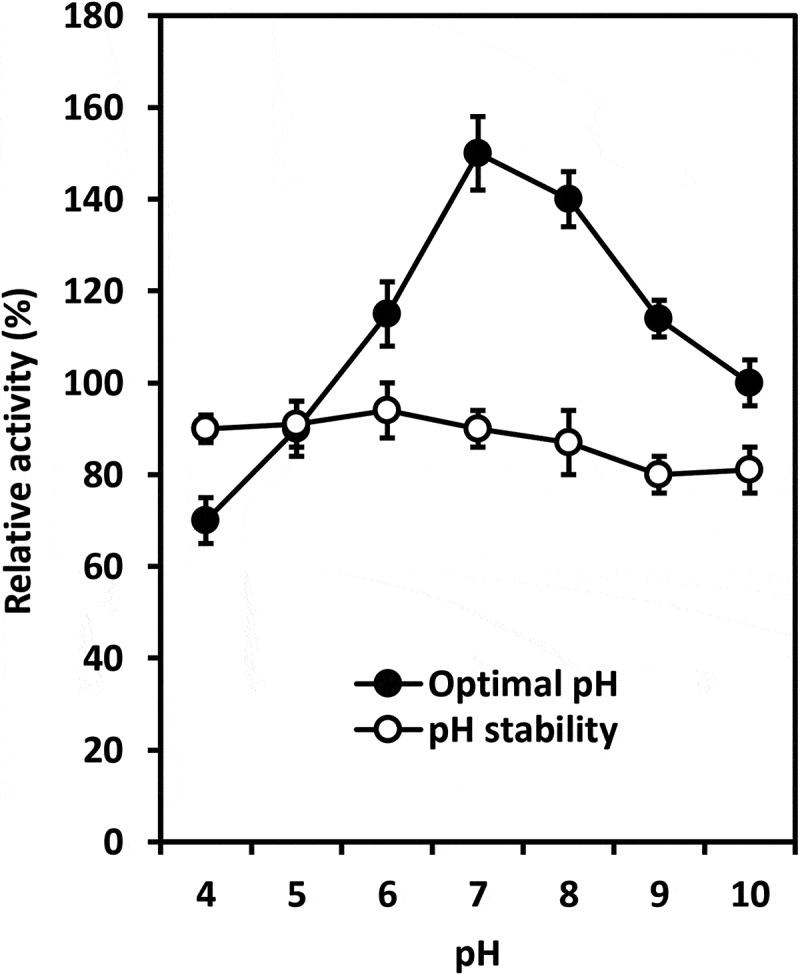
Optimal pH and pH stability of chitinase from T. asperellum PQ34 at 40°C.
As shown in Figure 4, the chitinase activity was significantly reduced by Zn2+ and SDS (about 31% and 22%, respectively), whereas it remained about 61–71% under the effect of Triton X-100, urea, DMSO and Mn2+. Except for Al3+, Fe3+, Fe2+ and Ca2+ that increased clearly chitinase activity (about 119–160%); ions such as Na+, Mg2+, Cu2+ and Co2+ had negligible effects on enzyme, relative activity ranged from 90% to 103%.
Figure 4.
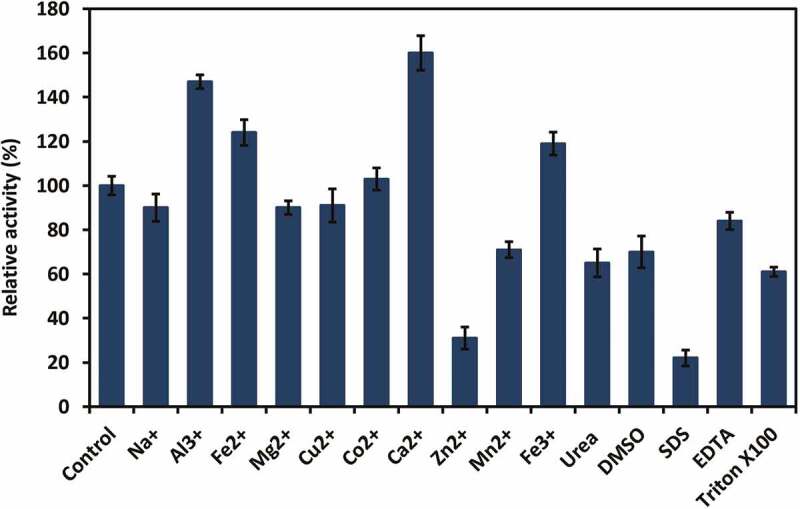
Effect of metal ions and surfactants on chitinase activity from T. asperellum PQ34.
3.3. In vitro antifungal activity of chitinase
Results in Table 1 show that chitinase strongly inhibited the growth of Colletotrichum sp. and S. rolfsii. Fresh biomass of Colletotrichum sp. and S. rolfsii significantly reduced approximately 89% (~0.08 g vs 0.72 g) and 27% (~0.9 g vs 1.2 g) for 10 U/mL chitinase treatment, respectively. When treated with 60 U/mL chitinase, growths of Colletotrichum sp. and S. rolfsii were drastically inhibited, approximately 95% (~0.4 g vs 0.72 g) and 97% (~0.4 g vs 1.2 g), respectively. Figure 5 illustrates the effect of chitinase from 10 to 60 U/mL on the growth of S. rolfsii.
Table 1.
In vitro antifungal activity of chitinase from T. asperellum PQ34.
| Chitinase (U/mL) |
Colletotrichum sp. |
S. rolfsii |
||
|---|---|---|---|---|
| FB (g) | DB (g) | FB (g) | DB (g) | |
| 10 | 0.080b | 0.008b | 0.873ab | 0.007ab |
| 20 | 0.067b | 0.006b | 0.504bc | 0.005b |
| 40 | 0.049b | 0.005b | 0.272cd | 0.003c |
| 60 | 0.037b | 0.003b | 0.039d | 0.001d |
| Control | 0.720a | 0.015a | 1.201a | 0.009a |
Different letters in a column show significant statistical differences with Duncan’s test (p < 0.05). This note is also used for all tables. FB: fresh biomass, DB: dry biomass.
Figure 5.
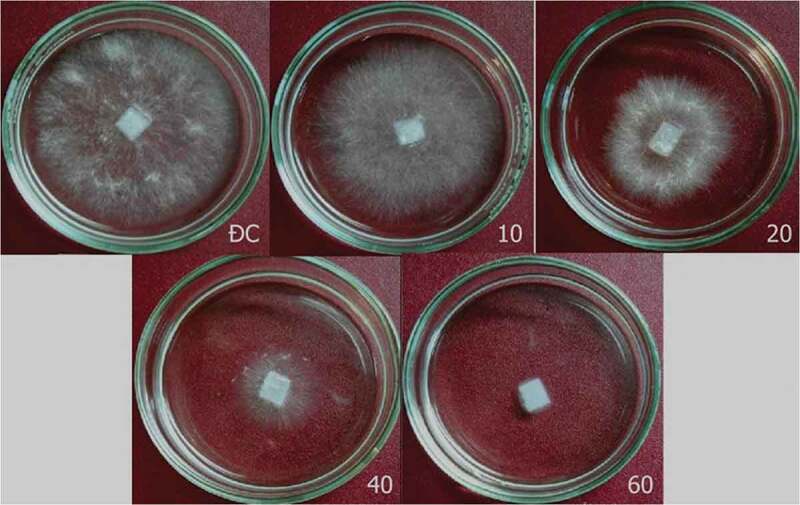
Effect of chitinase from T. asperellum PQ34 on in vitro growth of S. rolfsii. ĐC: control without chitinase, 10–60: chitinase from 10 to 60 U/mL.
3.4. Antifungal activity of chitinase in peanut
T. asperellum PQ34 chitinase significantly inhibited the growth of S. rolfsii in peanuts. Pre-treated seeds with chitinase (10–60 U/mL) had a very low rate of fungal infection compared to control, before and 30 days after germination, respectively, 2.22–8.89% and 2.22–7.32% (control: 13.33–17.95%). Especially, chitinase from 20 to 60 U/mL drastically reduced the fungal-infected rate in plants (2.22–4.6%) (Figure 6). The AUDPC also reached the lowest in chitinase treatments from 20 to 60 U/mL compared to control, 16.67–34.52 vs 185.9. The antifungal activity of chitinase from 20 to 60 U/mL was not significantly different with p> 0.05 (Table 2).
Figure 6.

Effect of chitinase from T. asperellum PQ34 on the growth of S. rolfsii in peanuts. ĐC: control without chitinase, 10 and 20: chitinase of 10 and 20 U/mL, respectively.
Table 2.
Antifungal activity of chitinase against S. rolfsii in peanuts.
| Chitinase (U/mL) | Percentage of fungal-infected plants |
|||
|---|---|---|---|---|
| Before germination | After germination |
|||
| 15 days | 30 days | AUDPC | ||
| 10 | 8.89a | 4.76ab | 7.32ab | 102.56ab |
| 20 | 2.22b | 0.00b | 2.38b | 17.86b |
| 40 | 2.22b | 0.00b | 2.22b | 16.67b |
| 60 | 2.22b | 0.00b | 4.60b | 34.52b |
| Control | 13.33a | 5.13a | 17.95a | 185.90a |
3.5. Anthracnose prevention on mango and chilli fruit
Overall, T. asperellum PQ34 chitinase is capable of preventing the development of anthracnose on both mango and chilli. After 96 h of chitinase treatment, there were signs of fungal infection but at different levels on mango fruits (Figure 7(a)). In the control group, the lesion appeared after 48 h of infection, whereas lesions only began to appear in enzyme treatments after 96 h of inoculation. The level of disease progression in treated groups was also slower than the control; the lowest AUDPC index belongs to the treatment of 40 U/mL chitinase (Table 3). These results showed that chitinase could prevent anthracnose that caused by Colletotrichum sp. on mango up to 72 h after treatment.
Figure 7.
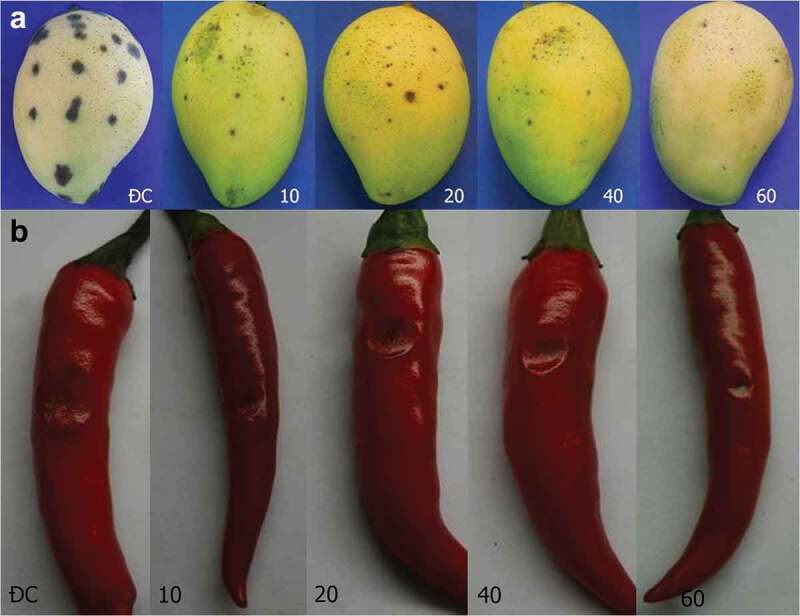
Lesions on mango (a) and chilli (b) fruits after 96 h of Colletotrichum sp. infection. ĐC: control without chitinase. 10–60: fruits were pre-treated with chitinase from 10 to 60 U/mL.
Table 3.
Inhibition effect of chitinase to Colletotrichum sp. on mango fruit.
| Diameter of brown spot (cm) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chitinase (U/mL) |
0 h | 24 h | 48 h | 72 h | 96 h | 120 h | 144 h | 168 h | 192 h | AUDPC |
| 10 | - | - | - | 0.1 | 0.2 | 0.32 | 0.41 | 0.69 | 0.84 | 46.56b |
| 20 | - | - | - | - | 0.12 | 0.22 | 0.34 | 0.54 | 0.60 | 35.16c |
| 40 | - | - | - | - | 0.11 | 0.19 | 0.27 | 0.36 | 0.46 | 26.52d |
| 60 | - | - | - | - | 0.10 | 0.16 | 0.29 | 0.49 | 0.70 | 31.16cd |
| Control | - | - | 0.1 | 0.15 | 0.35 | 0.47 | 0.56 | 0.76 | 0.91 | 58.80a |
The chitinase treatments for chilli also obtained similar results in mango; the effective of fungal prevention was in about 72 h. The lesions appeared in the control group at 72 h, and after 144 h, they spread, stuck together and rotted. Treatment of chitinase from 10 to 60 U/mL was only effective before 96 h (Figure 7(b) and Table 4). The lowest AUDPC also belongs to the 40 U/mL treatment similar to mango.
Table 4.
Inhibition effect of chitinase to Colletotrichum sp. on chilli fruit.
| Diameter of rotten spot (cm) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chitinase (U/mL) | 0 h | 24 h | 48 h | 72 h | 96 h | 120 h | 144 h | 168 h | 192 h | AUDPC |
| 10 | - | - | - | - | 0.2 | 0.35 | 0.65 | 1.05 | 2.05 | 76.20ab |
| 20 | - | - | - | - | 0.15 | 0.30 | 0.50 | 0.80 | 1.90 | 63.00bc |
| 40 | - | - | - | - | 0.1 | 0.20 | 0.30 | 0.80 | 1.55 | 51.00c |
| 60 | - | - | - | - | 0.1 | 0.15 | 0.40 | 0.80 | 1.55 | 52.20c |
| Control | - | - | - | 0.1 | 0.17 | 0.50 | 0.95 | 1.35 | 2.25 | 96.30a |
3.6. Anthracnose treatment in mango and chilli fruit
The higher activity of chitinase significantly inhibited the growth of fungus on mango. After 96 h of treatment with chitinase from 40 to 60 U/mL, the diameters of lesions on mango fruits were only in the range of 0.88–0.90 cm (control: 1.67 cm). The progression of AUPDC was only half that of the control, 41.16 vs 81.84 (Figure 8(a) and Table 5).
Figure 8.
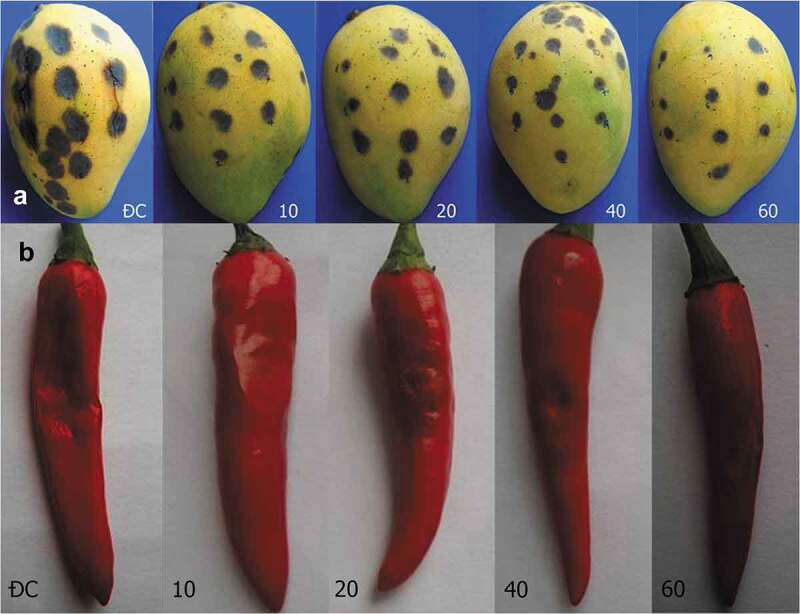
Treatment of anthracnose for mango (a) and chilli (b) fruits after 168 h. ĐC: control with chitinase. 10–60: fruits were treated with chitinase from 10 to 60 U/mL.
Table 5.
The effect of chitinase on the progression of lesions on mango.
| Diameters of lesions (cm) |
||||||
|---|---|---|---|---|---|---|
| After treatment |
||||||
| Chitinase (U/mL) | Before treatment | 24 h | 48 h | 72 h | 96 h | AUDPC |
| 10 | 0.11 | 0.23 | 0.50 | 0.84 | 1.24 | 53.88b |
| 20 | 0.10 | 0.28 | 0.47 | 0.78 | 1.13 | 51.48b |
| 40 | 0.11 | 0.22 | 0.38 | 0.62 | 0.88 | 41.16c |
| 60 | 0.10 | 0.16 | 0.37 | 0.59 | 0.90 | 38.88c |
| Control | 0.18 | 0.46 | 0.81 | 1.21 | 1.67 | 81.84a |
Similar to mango fruit, the development of fungal pathogens on chilli was partially inhibited by chitinase. However, the growth rate of disease fungi on chilli fruit was faster, and the diameters of the lesions after 96 h of chitinase treatments from 40 to 60 U/mL were 1.35–1.45 cm (control: 2.85 cm). AUPDC of 40–60 U/mL chitinase treatment was only half that of the control, 66.6–70.2 vs 143.4 (Figure 8(b) and Table 6).
Table 6.
The effect of chitinase on the progression of lesions on chilli.
| Diameters of lesions (cm) |
||||||
|---|---|---|---|---|---|---|
| After treatment |
||||||
| Chitinase (U/mL) | Before treatment | 24 h | 48 h | 72 h | 96 h | AUDPC |
| 10 | 0.15 | 0.50 | 0.95 | 1.50 | 1.80 | 95.40b |
| 20 | 0.13 | 0.60 | 0.90 | 1.35 | 1.65 | 91.20bc |
| 40 | 0.10 | 0.35 | 0.65 | 1.00 | 1.45 | 66.60c |
| 60 | 0.10 | 0.40 | 0.75 | 1.05 | 1.35 | 70.20bc |
| Control | 0.12 | 0.80 | 1.35 | 2.25 | 2.85 | 143.40a |
4. Discussion
Sandhya et al. (2004) fermented T. harzianum TUBF 966 strain at 30°C for 96 h and obtained extracellular chitinase with a maximum activity of 14.7 U/mL. Asad et al (2105) found a peak of chitinolytic activity (173 U/mL) by T. asperellum after 96 h of culture at 25°C which was the highest among all the isolates, whereas that of T. harzianum was 117 U/mL after 72 h of culture at the same temperature. In which, one unit of the activity was defined as the amount of enzyme that to produce 1 mmol of the product per mg of the protein per hour. A T. harzianum strain from the study of Urbina-Salazar et al. (2018) also gave the chitinase yield of 261.5 mU/L per day. Similar to the T. asperellum strain in the study of Asad et al. (2015), T. asperellum PQ34 also required an incubation period of 96 h but at a higher temperature of 28°C to produce the chitinase with a maximum activity (about 22 U/mL). The suitable incubation period or temperature of mentioned T. harzianum strains are different from T. asperellum PQ34.
In general, the characteristics of chitinases from Trichoderma species are relatively different. Chitinase from T. asperellum PQ34 strain had the optimal temperature and pH are 40°C and 7, the thermal and pH stability ranged from 25–50°C and 4–10, respectively. Meanwhile, chitinase from T. harzianum Rifai (T24) strain was stable at 30°C and it is rapidly inactivated at 60°C (El-Katatny et al. 2001). The optimal temperature and pH for chitinolytic activity of T. viride were 50°C and 5, respectively. The chitinase produced by this fungal strain was stable at temperatures of 40–50°C and pH 6–7 (Ekundayo et al. 2016). According to Ulhoa and Peberdy (1992), of the metal ions tested, only Zn2+ had a significant inhibitory effect on chitinase from T. hazianum 39.1 strain, and its relative activity was about 43%. No significant loss of enzyme activity was observed after incubation with the EDTA and other metal ions as Ca2+, Cu2+, Fe2+, K+, Mg2+, Mn2+ and Na+; the relative activity ranged from 78% to 99%. Ekundayo et al. (2016) found the relative activity of chitinase from T. viride was reduced when EDTA and MnCl2 were used. This enzyme was most sensitive to EDTA followed by MnCl2 and it achieved the maximum activity when CaCl2 was used. The effects of metal ions and surfactants on the chitinase activity from T. asperellum PQ34 strain were relatively different to chitinase from various Trichoderma species. Chitinase activity from PQ34 strain was significantly reduced by Zn2+ and SDS, whereas it remained about 61–71% under the effect of Triton X-100, urea, DMSO and Mn2+. Except for Al3+, Fe3+, Fe2+ and Ca2+ that increased clearly chitinase activity; ions such as Na+, Mg2+, Cu2+ and Co2+ had negligible effects on enzyme activity.
Lima et al. (1999) showed the endochitinase (CHIT 46) from Trichoderma sp. drastically affected the cell walls of S. rolfsii and Rhizoctonia solani. According to El-Katatny et al. (2000), chitinase of T. harzianum has significantly inhibited the growth of S. rolfsii (up to 61.8%). Kumar et al. (2012b) also found the antagonistic activity of chitinase from Trichoderma spp. against root rot and foliar pathogens. Pacheco et al. (2016) evaluated the efficacy of six Trichoderma isolates to control sclerotium wilt (S. rolfsii) of common bean (Phaseolus vulgaris) and found that three of them (T. hazianum, T. longibrachiatum and T. reesei) were more efficient in reducing sclerotial germination. Marques et al. (2018) isolated two strains CEN1245 and CEN1274, both belonging to the species T. brevicompactum, exhibiting a broad spectrum against S. rolfsii, C. gloesporioides, and the other disease fungi. De la Cruz-quiroz et al. (2018) studied in vitro antagonistic activity in dual culture detected that T. asperellum (T2-32) strain displayed the highest capacity of inhibition against Colletotrichum gloeosporioides up to 22.5%. However, it had no effect on the spores of Colletotricum isolates. Our study showed that chitinase at 60 U/mL inhibited nearly completely in vitro growth of Colletotrichum sp. and Sclerotium rolfsii. In peanut plants, 20 U/mL of chitinase significantly reduced the incidence of S. rolfsii infection. The fungal infection incidence of seedsbefore germination and 30 days after germination was only 2.22% and 2.38%, while the control was 13.33% and 17.95%. Besides, this enzyme can also prevent anthracnose that is caused by Colletotrichum sp. on both mango and chilli fruits up to 72 h after enzyme pre-treatment at 40 U/mL. In mango and chilli fruits infected with anthracnose, 40 U/mL dose of chitinase inhibited growth of fungi after 96 h of treatment.
In addition to enzymes such as chitinase, glucanase and protease, some reports showed that Trichoderma species also secreted trichodermin, an antibiotic that has antifungal activity, into medium during fermentation, such as T. reesei (Watts et al. 1988), T. brevicompactum (Shentu et al. 2014), T. koningiopsis (Leylaie and Zafari 2018). In this work, we have not investigated the production of trichodermin in T. asperellum PQ34. Chitinase was partially purified by ammonium sulphate precipitation which can still contain trichodermin; therefore, antifungal activity may not be only of the enzyme.
In conclusion, the characteristics and antifungal activity of chitinase from Trichoderma species are relatively different. Chitinase of T. asperellum PQ34 can inhibit the growth of two strains of S. rolfsii and Colletotrichum sp. has opened up the potential for environmental friendly fungicides later on.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Asad SA, Tabassum A, Hameed A, Hassan FU, Afzal A, Khan SA, Ahmed R, Shahzad M.. 2015. Determination of lytic enzyme activities of indigenous Trichoderma isolates from Pakistan. Braz J Microbiol. 46:1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek JM, Howell CR, Kenerley CM.. 1999. The role of an extracellular chitinase from Trichoderma virens Gv29-8 in the biocontrol of Rhizoctonia solani. Curr Genet. 35:41–50. [DOI] [PubMed] [Google Scholar]
- Baiyee B, Ito SI, Sunpapao A. 2019a. Trichoderma asperellum T1 mediated antifungal activity and induced defense response against leaf spot fungi in lettuce (Lactuca sativa L.). Physiol Mol Plant Pathol. 106:96–101. [Google Scholar]
- Baiyee B, Pornsuriya C, Ito SI, Sunpapao A. 2019b. Trichoderma spirale T76-1 displays biocontrol activity against leaf spot on lettuce (Lactuca sativa L.) caused by Corynespora cassiicola or Curvularia aeria. Biol Control. 129:195–200. [Google Scholar]
- Campbell CL, Madden LV. 1990. Introduction to plant disease epidemiology. 1st ed. New York (USA): John Wiley and Sons. [Google Scholar]
- Cilliers AJ, Pretorius ZA, Van Wyk PS. 2003. Integrated control of Sclerotium rolfsii on groundnut in South Africa. J Phytopathol. 151:249–258. [Google Scholar]
- De la Cruz-quiroz R, Roussos S, Rodríguez-Herrera R, Hernandez-Castillo D, Aguilar CN. 2018. Growth inhibition of Colletotrichum gloeosporioides and Phytophthora capsici by native Mexican Trichoderma strains. Karbala Int J Mod Sci. 4:237–243. [Google Scholar]
- Ekundayo EA, Ekundayo FO, Bamidele F. 2016. Production, partial purification and optimization of a chitinase produced from Trichoderma viride, an isolate of maize cob. Mycosphere. 7:786–793. [Google Scholar]
- Elieh-Ali-Komi D, Hamblin MR. 2016. Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int J Adv Res. 4:411–427. [PMC free article] [PubMed] [Google Scholar]
- El-Katatny MH, Gudelj M, Robra KH, Elnaghy MA, Gübitz GM. 2001. Characterization of a chitinase and an endo-beta-1,3-glucanase from Trichoderma harzianum rifai T24 involved in control of the phytopathogen Sclerotium rolfsii. Appl Microbiol Biotechnol. 56:137–143. [DOI] [PubMed] [Google Scholar]
- El-Katatny MH, Somitsch W, Robra KH, El-Katatny MS, Gübitz GM. 2000. Production of chitinase and β-1,3-glucanase by Trichoderma harzianum for control of the phytopathogenic fungus Sclerotium rolfsii. Food Technol Biotechnol. 38:173–180. [Google Scholar]
- Hamid R, Khan MA, Ahmad M, Ahmad MM, Abdin MZ, Musarrat J, Javed S. 2013. Chitinases: an update. J Pharm Bioallied Sci. 5:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harighi MJ, Zamani MR, Motallebi M. 2007. Evaluation of antifungal activity of purified chitinase 42 from Trichoderma atroviride PTCC5220. Biotechnology. 6:28–33. [Google Scholar]
- Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. 2004. Trichoderma species-opportunistic, avirulent plant symbionts. Nat Rev. 2:43–56. [DOI] [PubMed] [Google Scholar]
- Ismail AM, Cirvilleri G, Yaseen T, Epifani F, Perrone G, Polizzi G. 2015. Characterisation of Colletotrichum species causing anthracnose disease of mango in Italy. J Plant Pathol. 97:167–171. [Google Scholar]
- Jogi A, Kerry JW, Brenneman TB, Leebens-Mack JH, Gold SE. 2016. Identification of genes differentially expressed during early interactions between the stem rot fungus (Sclerotium rolfsii) and peanut (Arachis hypogaea) cultivars with increasing disease resistance levels. Microbiol Res. 184:1–12. [DOI] [PubMed] [Google Scholar]
- Kramer KJ, Muthukrishnan S. 1997. Insect chitinases: molecular biology and potential use as biopesticides. Insect Biochem Mol Biol. 27:887–900. [DOI] [PubMed] [Google Scholar]
- Kumar DP, Singh RK, Anupama PD, Solanki MK, Kumar S, Srivastava AK, Singhal PK, Arora DK. 2012a. Studies on exo-chitinase production from Trichoderma asperellum UTP-16 and its characterization. Indian J Microbiol. 52:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K, Amaresan N, Bhagat S, Madhuri K, Srivastava RC. 2012b. Isolation and characterization of Trichoderma spp. for antagonistic activity against root rot and foliar pathogens. Indian J Microbiol. 52:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leylaie S, Zafari D. 2018. Antiproliferative and antimicrobial activities of secondary metabolites and phylogenetic study of endophytic Trichoderma species from Vinca plants. Front Microbiol. 9:1484. doi: 10.3389/fmicb.2018.01484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima LHC, De Marco JL, Ulhoa CJ, Felix CR. 1999. Synthesis of a Trichoderma chitinase which affects the Sclerotium rolfsii and Rhizoctonia solani cell walls. Folia Microbiol. 44:45–49. [DOI] [PubMed] [Google Scholar]
- Liu H, Cheng M, Zhao S, Lin C, Song J, Yang Q. 2019. ATP-binding cassette transporter regulates N,N0-diacetylchitobiose transportation and chitinase production in Trichoderma asperellum T4. Int J Mol Sci. 20:E2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loc NH, Hoa NTQ, Cuc PTK, Quang HT. 2013. Expression of chitinase (chi42) gene from Trichoderma asperellum in Saccharomyces cerevisiae. Ann Biol Res. 4:15–19. [Google Scholar]
- Loc NH, Quang HT, Hung NB, Huy ND, Phuong TTB, Ha TTT. 2011. Trichoderma asperellum Chi42 genes encode chitinase. Mycobiology. 39:182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques E, Martins I, de Mello SCM. 2018. Antifungal potential of crude extracts of Trichoderma spp. Biota Neotrop. 18:e20170418. [Google Scholar]
- Mehan VK, Mayee CD, McDonald D. 1994. Management of Sclerotium rolfsii‐caused stem and pod rots of groundnut-a critical review. Int J Pest Manage. 40:313–320. [Google Scholar]
- Mo J, Zhao G, Li Q, Solangi GS, Tang L, Guo T, Huang S, Hsiang T. 2018. Identification and characterization of Colletotrichum species associated with mango anthracnose in Guangxi, China. Plant Dis. 102:1283–1289. [DOI] [PubMed] [Google Scholar]
- Nadarajah K, Ali HZ, Omar NS. 2014. The isolation and characterization of an endochitinase gene from a Malaysian isolate of Trichoderma sp. Aust J Crop Sci. 8:711–721. [Google Scholar]
- Okabe I, Matsumoto N. 2000. Population structure of Sclerotium rolfsii in peanut fields. Mycoscience. 41:145–148. [Google Scholar]
- Oo MM, Oh SK. 2016. Chilli anthracnose (Colletotrichum spp.) disease and its management approach. Korean J Agric Sci. 43:153–162. [Google Scholar]
- Pacheco KR, Viscardi BSM, de Vasconcelos TMM, Moreira GAM, Do Vale HMM, Blum LEB. 2016. Efficacy of Trichoderma asperellum, T. harzianum, T. longibrachiatum and T. reesei against Sclerotium rolfsii. Biosci J. 32:412–421. [Google Scholar]
- Pandian RTP, Raja M, Sharma P. 2018. Characterization of three different chitinase genes from Trichoderma asperellum strain Ta13. Proc National Acad Sci India Sect B. 88:1661–1668. [Google Scholar]
- Rivera-Vargas LI, Lugo-Noel Y, McGovern RJ, Seijo T, Davis MJ. 2006. Occurrence and distribution of Colletotrichum spp. on mango (Mangifera indica L.) in Puerto Rico and Florida, USA. Plant Pathol J. 5:191–198. [Google Scholar]
- Samuels GJ. 1996. Trichoderma: a review of biology and systematics of the genus. Mycol Res. 100:923–935. [Google Scholar]
- Sandhya C, Adapa LK, Nampoothiri KM, Binod P, Szakacs G, Pandey A. 2004. Extracellular chitinase production by Trichoderma harzianum in submerged fermentation. J Basic Microbiol. 44:49–58. [DOI] [PubMed] [Google Scholar]
- Saxena A, Raghuwanshi R, Gupta VK, Singh HB. 2016. Chilli anthracnose: the epidemiology and management. Front Microbiol. 7:1527. doi: 10.3389/fmicb.2016.01527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugaiah V, Mathivanan N, Balasubramanian N, Manoharan PT. 2008. Optimization of cultural conditions for production of chitinase by Bacillus laterosporous MML2270 isolated from rice rhizosphere soil. Afr J Biotechnol. 7:2562–2568. [Google Scholar]
- Shentu X, Zhan X, Ma Z, Yu X, Zhang C. 2014. Antifungal activity of metabolites of the endophytic fungus Trichoderma brevicompactum from garlic. Braz J Microbiol. 45:248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JZ, Yang Q, Liu BD, Chen DF. 2005. Expression of the chitinase gene from Trichoderma aureoviride in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 69:39–43. [DOI] [PubMed] [Google Scholar]
- Than PP, Prihastuti H, Phoulivong S, Taylor PWJ, Hyde KD. 2008. Chilli anthracnose disease caused by Colletotrichum species. J Zhejiang Univ Sci B. 9:764–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujibo H, Hatano N, Mikami T, Hirasawa A, Miyamoto K, Inamori Y. 1998. A novel β-N-acetylglucosaminidase from Streptomyces thermoviolaceus OPC-520: gene cloning, expression, and assignment to family 3 of the glycosyl hydrolases. Appl Environ Microbiol. 64:2920–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulhoa CJ, Peberdy JF. 1991. Regulation of chitinase synthesis in Trichoderma harzianum. J Gen Microbiol. 137:2163–2169. [DOI] [PubMed] [Google Scholar]
- Ulhoa CJ, Peberdy JF. 1992. Purification and some properties of the extracellular chitinase produced by Trichoderma harzianum. Enzyme Microb Technol. 14:236–240. [Google Scholar]
- Urbina-Salazar A, Inca-Torres AR, Falcón-García G, Carbonero-Aguilar P, Rodríguez-Morgado B, Campo J, Parrado J, Bautista J. 2018. Chitinase production by Trichoderma harzianum grown on a chitin-rich mushroom by product formulated medium. Waste Biomass Valorization. doi: 10.1007/s12649-018-0328-4. [DOI] [Google Scholar]
- Watts R, Dahiya J, Chaudhary K, Tauro P. 1988. Isolation and characterization of a new antifungal metabolite of Trichoderma reesei. Plant Soil. 107:81–84. [Google Scholar]
- Wonglom P, Daengsuwan W, Ito SI, Sunpapao A. 2019. Biological control of Sclerotium fruit rot of snake fruit and stem rot of lettuce by Trichoderma sp. T76-12/2 and the mechanisms involved. Physiol Mol Plant Pathol. 107:1–7. [Google Scholar]
- Wu Q, Sun R, Ni M, Yu J, Li Y, Yu C, Dou K, Ren J, Chen J. 2017. Identification of a novel fungus, Trichoderma asperellum GDFS1009, and comprehensive evaluation of its biocontrol efficacy. PLoS One. 12:e0179957. [DOI] [PMC free article] [PubMed] [Google Scholar]


