ABSTRACT
In eusocial ants, aggressive behaviors require the ability to discriminate between chemical signatures such as cuticular hydrocarbons that distinguish nestmate friends from non-nestmate foes. It has been suggested that a mismatch between a chemical signature (label) and the internal, neuronal representation of the colony odor (template) leads to aggression between non-nestmates. Moreover, a definitive demonstration that odorant receptors are responsible for the processing of the chemical signals that regulate nestmate recognition has thus far been lacking. To address these issues, we have developed an aggression-based bioassay incorporating highly selective modulators that target odorant receptor functionality to characterize their role in nestmate recognition in the formicine ant Camponotus floridanus. Electrophysiological studies were used to show that exposure to either a volatilized antagonist or an agonist eliminated or dramatically altered signaling, respectively. Administration of these compounds to adult workers significantly reduced aggression between non-nestmates without altering aggression levels between nestmates. These studies provide direct evidence that odorant receptors are indeed necessary and sufficient for mediating aggression towards non-nestmates. Furthermore, our observations support a hypothesis in which rejection of non-nestmates depends on the precise decoding of chemical signatures present on non-nestmates as opposed to the absence of any information or the active acceptance of familiar signatures.
KEY WORDS: Social behavior, Odorant receptors, Orco, Aggression, Olfaction
Summary: Broad inhibition as well as activation of peripheral odorant receptor signaling decreases aggression between non-nestmate ants consistent with a ‘lock-and-key’ model that requires OR-based detection of unambiguous non-nestmate chemical labels.
INTRODUCTION
Aggression comprises a range of important social interactions with implications for individual behavior as well as the collective integrity of animal societies. While hostile behaviors can be observed throughout the Metazoa (Blanchard and Blanchard, 1977; Hölldobler and Wilson, 1990; Ayre and Grosberg, 1995; Mitani et al., 2010; Scheel et al., 2016), recently established experimentally tractable eusocial insect models present an opportunity to investigate the mechanistic basis of aggression within a social context. In this regard, ants provide a compelling model for the study of aggression and its triggering mechanisms. Ant colonial lifestyles and reproductive hierarchies are maintained by aggressive social interactions that are modulated by their ability to detect, discriminate between and respond to a large array of chemical cues (Hölldobler and Wilson, 1990; Morel et al., 1988; Endler et al., 2004; Moore and Liebig, 2010). Moreover, recent studies (Yan et al., 2017; Trible et al., 2017) have demonstrated the value of applying novel genetic and molecular techniques that have restricted availability in the study of humans and other social primates.
The formicine ant Camponotus floridanus lives in colonies that are founded by a single reproductive queen (Hölldobler and Wilson, 1990; Gadau et al., 1996). Workers nurse the queen's offspring, forage for food and defend nest and territory from non-nestmates (nNMs) (Hölldobler and Wilson, 1990). Although individual workers contribute to broader colony-level phenotypes, the integrity of social behaviors depends on the collective actions of the colony (Gordon, 2015). Among these social behaviors, nestmate (NM) recognition is especially important for establishing and maintaining discrete societal boundaries for C. floridanus and many other species of ant (Hölldobler and Wilson, 1990). NM recognition is a dynamic behavior that has been suggested to occur when an individual ant compares chemically encoded ‘labels’ that it encounters with potentially multiple neurally encoded ‘templates’ that represent its own particular global colony chemosensory signature, whereby a mismatch between a foreign label and the recognition templates leads to aggression between nNMs (Neupert et al., 2018; Vander Meer and Morel, 1998; Obin and Vandermeer, 1989). The foreign label is derived, at least in part, from subtle variations in the profile of cuticular hydrocarbons (CHCs) that distinguish nNMs from NMs (Morel et al., 1988; Guerrieri et al., 2009; Neupert et al., 2018).
Early genetic models provided a framework for understanding the criteria required to assess colony membership status when comparing the recognition template with a respective label (Crozier and Dix, 1979). These have been broadly organized into two categories: the gestalt model, in which label sharing between individuals yields a distinct template based on a blend; and individualistic models, which include requiring the exact matching of the label to the template (‘genotype matching’), rejection of any labels containing cues not found in the template (‘foreign-label rejection’), and the acceptance of labels that overlap with the template (‘habituated-label acceptance’). Similarly, there have been efforts to elucidate the rules governing template–label matching within a phenotypic context (Guerrieri et al., 2009; Neupert et al., 2018; Sherman et al., 1997). These models suggest that ants discriminate between friends and foes based on the presence and/or absence of NM (‘desirable’) cues or nNM (‘undesirable’) cues. While it was initially proposed that ants accept individuals if they possess desirable cues (D-present) or if they lack undesirable cues (U-absent) to the exclusion of all others (Sherman et al., 1997), more recent evidence suggests that ants actively detect foes but not friends through the detection of nNM odor cues (simple U-present model) (Guerrieri et al., 2009). Importantly, however, discrimination may also occur when critical components of the CHC profile are missing (Neupert et al., 2018). These studies suggest that multiple templates are used to assess different labels, and that the importance of a given component of the label varies.
While the importance of CHCs in mediating NM recognition among ants is well established, several alternative hypotheses have been proposed for the neuronal and molecular mechanisms allowing ants to distinguish friends from foes (Ozaki et al., 2005; Guerrieri et al., 2009; Brandstaetter et al., 2011; Neupert et al., 2018; Brandstaetter and Kleineidam, 2011; Sherman et al., 1997; Crozier and Dix, 1979). In all of these models, CHCs and other semiochemicals are initially detected by the peripheral olfactory sensory system, which relies on three major classes of peripheral chemosensory receptors: odorant receptors (ORs), gustatory receptors and ionotropic receptors. Insect ORs are expressed in olfactory receptor neurons (ORNs) housed within sensilla on the antennae (reviewed in Suh et al., 2014), where they function as heteromeric complexes consisting of an obligate and conserved OR co-receptor (Orco) and at least one ‘tuning’ OR that determines odorant (ligand) specificity (Zhou et al., 2012; Larsson et al., 2004; Benton et al., 2006; Sato et al., 2008; Wicher et al., 2008; Jones et al., 2011; Pask et al., 2011). Several studies have revealed a large expansion of the OR gene family in ants and other eusocial insects (Zhou et al., 2012; Zhou et al., 2015; Smith et al., 2011b; Robertson and Wanner, 2006; Bonasio et al., 2010; Wurm et al., 2011; Oxley et al., 2014; Werren et al., 2010; Smith et al., 2011a). Members of this chemoreceptor family detect socially relevant chemical cues such as CHCs (Slone et al., 2017; Pask et al., 2017).
Despite the long-held appreciation for the role of CHCs and other chemical cues in mediating NM recognition and social behaviors in ants, little is known about the specific molecular components of olfactory signal transduction that are active in regulating NM recognition and the triggering of aggression toward nNMs. Electrophysiological studies of Camponotus japonicus first suggested that a dedicated multiporous NM recognition sensilla exhibited an all-or-none response to nNM CHC blends but, importantly, did not respond to NM CHC blends – thus leading to a model in which ants are desensitized and ultimately anosmic to their own odor cues (Ozaki et al., 2005). In contrast, recent studies using both antennal electrophysiology and antennal lobe calcium imaging in the related ant species C. floridanus demonstrate these ants are capable of detecting both nNM and NM odors (Brandstaetter et al., 2011; Brandstaetter and Kleineidam, 2011; Sharma et al., 2015). It has been proposed that these seemingly contradictory findings support a model in which two sensilla subtypes – one broadly tuned to hydrocarbons and the other tuned to specific hydrocarbons – facilitate habituation to different labels (Bos and D'Ettorre, 2012).
The paucity of data in this regard may be attributed, at least in part, to the challenges of targeted molecular approaches currently available in the study of hymenopteran insects. The development of these techniques represents an important step towards understanding the function and evolution of the molecular mechanisms involved in complex social behaviors such as NM recognition, with the potential to shed light on longstanding questions within the field of social insect biology. To begin to address this, a series of behavioral, physiological and gene knockout studies were carried out to characterize the relationship between ant ORs and CHCs as well as other biologically salient chemical cues. These studies demonstrated that CHCs and other general odorants were broadly detected across the various OR subclades while CRISPR-mediated gene knockout of orco resulted in alterations of both solitary and social behaviors as well as profound neuroanatomical disruptions in the antennal lobe (Slone et al., 2017; Pask et al., 2017; Yan et al., 2017; Trible et al., 2017). Taken together, these studies suggest that ORs play a critical role not only in a diversity of behaviors but also, importantly, in ant neural development.
We now extend these studies by employing a set of highly specific Orco allosteric modulators to examine the role of OR signaling in mediating NM recognition. The first member of this unique class of pharmacological agents (known as VUAA-class actives) was identified through high-throughput screening for small molecule modulators of mosquito Orco/OR complexes expressed in HEK293 cells (Jones et al., 2011; Pask et al., 2011; Rinker et al., 2012). In subsequent studies that revealed extraordinarily narrow structure–activity relationships, several additional VUAA-class actives were identified and characterized that now comprise several-more potent agonists (including VUAA4 used here), a non-competitive antagonist (VUANT1, used here) as well as an inactive structural analog (VUAA0, used here) (Jones et al., 2011, 2012; Rinker et al., 2012; Taylor et al., 2012; Romaine et al., 2014). The selective potency of these modulators against Orco targets in both volatile and non-volatile forms is conserved across a wide range of insect orders (Jones et al., 2012; Tsitoura and Iatrou, 2016; Tsitoura et al., 2015; Hansen et al., 2014; Sharma et al., 2015). Indeed, VUAA–Orco interactions have recently been directly confirmed by cryo-electron microscopy studies characterizing the structure of an Orco tetramer from the parasitic fig wasp Apocrypta bakeri (Butterwick et al., 2018). Importantly, single-sensillum recordings of the female-specific basiconic sensilla in C. floridanus have demonstrated the potency of at least one of these VUAA-class actives, such that exposure to VUANT1 significantly reduced olfactory responses to both a blend of hydrocarbons and cuticle extract (Sharma et al., 2015).
The use of these unique and highly specific chemical tools allowed us to selectively target Orco and therefore the functionality of all OR/Orco complexes to examine NM recognition with altered OR signaling in wild-type adult C. floridanus workers. This was an essential aspect of our approach in light of the broad neuroanatomical alterations that have recently been observed in the development of the antennal lobes of Orco mutants in two ant species (Trible et al., 2017; Yan et al., 2017), which are reasonably likely to impact olfactory processing and behavior. Indeed, the use of volatile Orco modulators represents a novel and requisite approach for disrupting OR functionality in insects such as ants that require alternatives to CRISPR-mediated targeting of pleiotropic genes such as orco (Trible et al., 2017; Yan et al., 2017). Here, we report studies that specifically address the odor coding of NM recognition by utilizing a novel volatilization paradigm. In this manner, we were able to directly test the hypotheses that aggression is triggered by the active detection and decoding of discrete chemosensory stimuli and more specifically that the functionality of the OR/Orco ion channel complex is necessary for NM recognition.
MATERIALS AND METHODS
Ant husbandry
Nine distinct laboratory colonies of Camponotus floridanus (Buckley 1866) originating from field collections were generously supplied by Dr J. Liebig (Arizona State University) from the Long Key (D242) and Sugarloaf Key (D601), and Dr S. Berger (University of Pennsylvania) from the Fiesta Key (C6, K17, K19, K28, K31, K34 and K39) in South Florida, USA. All colonies were independently maintained at 25°C and ambient humidity (approximately 70%), with a 12 h light:12 h dark photoperiod. Each colony was provided with Bhatkar diet, crickets, 10% sucrose solution, and distilled water 3 times per week. Adult minor workers were used for all experiments and were sampled from throughout the colonies.
Ablation aggression bioassay
Tests were conducted during the Zeitgeber time (ZT) diel light cycle between ZT2 and ZT12 at ambient room temperature and humidity and performed using a six-well culture plate with polytetrafluoroethylene-coated well walls (DuPont®). Individual wells of the six-well culture plate served as distinct bioassay arenas for behavioral trials (Fig. S1). In preparation for experiments, each well (9.6 cm2) of the six-well culture plate was fitted with a removable plastic divider that partitioned the well into two halves. The six-well culture plate and dividers were sterilized using ethanol, air dried, and positioned on top of a light box. Each individual bioassay well utilized two adult minor ants that were selected from either the same home colony (NMs) or two distinct colonies (nNMs). All ants were handled wearing gloves and using sterile, soft-tipped metal forceps, and were subsequently discarded in the freezer (−20°C) after each bioassay to ensure each ant was used only once.
Subject ants were briefly anesthetized with CO2 before removing their antennal flagella via an incision across the distal portion of the scape using a clean, unused razor blade. Bilaterally ablated ants had both flagella removed while unilaterally ablated ants had only a single (right or left, randomly selected) flagellum removed. Sham-treated ants were anesthetized with CO2, and the razor was gently touched to the antennae without damaging any structures. Subsequent to ablation (or sham) treatment, ants were allowed to recover along with similarly treated NMs for at least 2 h prior to testing.
Prior to bioassays, two ants (NMs or nNMs) were placed into each well arena, one in either half, and allowed 10 min to acclimate to handling. To document normal ant behavior within each well arena, mobility was recorded using a digital high-definition camera (Panasonic® HC-V750) for 3 min (detailed below). The plastic divider within each well arena was subsequently removed and all ant interactions were again recorded for 3 min. The order in which the treatments were conducted as well as the colony the ants were selected from for any given trial were randomized using RANDOM.ORG (Randomness and Integrity Services Ltd; https://www.random.org/).
Electroantennography
Electroantennograms (EAGs) were performed using an IDAC-232 controller (Ockenfels Syntech GmbH, Buchenbach, Germany) linked to a Windows XP computer running EAG2000 software (Ockenfels Syntech GmbH). A set of 12×75 mm test tubes placed atop a heat block set at 260°C containing 0.025 g of the respective treatment compound (VUAA0, VUANT1 or VUAA4) or an empty tube (blank control) were connected to a Syntech CS-05 stimulus flow controller (flow rate of 1.5 cm3 s−1; Ockenfels Syntech GmbH). Using this setup, both the constant background airflow and the 500 ms pulse of stimulus compound contained volatilized VU-class compounds or heated air (in the case of the blank control).
Subject ants were placed in a 20 µl disposable pipette tip that was modified such that the tip opening was sufficiently wide to allow the unimpeded exposure of the head and antennae. To prevent movement of the preparation, which might reduce the signal-to-noise of the recordings, the head and mandibles of the ant were restricted with wax. Borosilicate glass capillaries (FIL, o.d. 1.0 mm, World Precision Instruments, Inc.) were customized for EAGs on a P-2000 laser micro-pipette puller (Sutter Instruments), backfilled with 10−1 mol l−1 KCl and 0.05% PVP buffer and placed over tungsten electrodes. A 30-gauge needle was used to puncture the right eye to allow for insertion of the reference electrode. The recording electrode was placed over the distal tip of the left antenna. Decane (C10; CAS: 124-18-5, Sigma-Aldrich) was serially diluted in hexane (0.1 , 1, 10, 20 and 200 µg µl−1). An odor cartridge was filled with 10 µl of decane solution (or hexane alone as a solvent control) and a handheld butane torch (BernzOmatic, Worthington Industries, Columbus, OH, USA) was used to volatilize the decane compound by heating the odor cartridge for 1.5 s. 4-Methyl-3-heptanol (4M3H; CAS: 14979-39-6, Sigma-Aldrich) was serially diluted in paraffin oil (10−5, 10−4, 10−3 and 10−2 mol l−1). Serial concentrations were assayed sequentially starting with the lowest concentration and ending with the highest concentration. Decane responses were normalized to the hexane solvent control (set at 0) and 4M3H responses were normalized to the paraffin oil solvent control (set to 0) to account for changes in sensitivity and/or antennae degradation over time throughout the assay, and these values were used for subsequent data analysis.
Volatile Orco modulator aggression bioassay
To facilitate the administration of a continuous flow of air containing volatilized VUAA-class compounds (all custom synthesized as dry solids in-house at Vanderbilt University; Jones et al., 2011, 2012; Taylor et al., 2012; Romaine et al., 2014) into the aggression arena, bioassays were conducted in arenas consisting of modified square plastic boxes with a total area of 85 cm2 (Pioneer Plastics Inc.®) (Fig. S1). Mirroring the electroantennography, conditioned air (78% nitrogen, 21% oxygen) was delivered at a constant 34 kPa from a compressed source (Nashville Gas LLC) to the test arena at a flow rate of approximately 50 cm3 s−1. Air was controlled by a dual Y valve affixed to the compressed air tank and delivered through a 12×75 mm test tube atop a heat block set at 260°C, which contained 0.025 g of the respective treatment compound (VUAA0, VUANT1 or VUAA4) or an empty tube (blank control) via 18 gauge needles inserted into a rubber septum affixed to the top of the test tube. Air was cleared from the arena through a dedicated exhaust system. Trials were recorded using a digital high-definition camera and scored as described below. Although two plastic tubes were affixed to the arena during the volatilization aggression bioassays, only a single tube was actively delivering the test compound or heated air control (Fig. S2). In each assay, ants were acclimatized underneath 35 mm Petri dish lids (prewashed with ethanol) for 10 min, after which the lids were removed (allowing the ants to interact), the airflow started and the ants' behavior recorded for the 3 min test period. All treatment compounds were randomized and coded independently such that the investigator was blind to the treatment identity. Furthermore, the sequential order in which the compounds were tested as well as the colony from which the ants were selected for any given trial were randomized using RANDOM.ORG.
Aggression bioassay scoring
Digital video recordings of all bioassays were viewed post hoc and aggression incidents manually scored for analyses. Trials in which ants did not interact (N=23), were disrupted physically during removal of the plastic barrier (N=5) or appeared injured/unconscious at trial onset (N=3) were discarded from further analyses along with their respective mobility controls in the case of the antennal ablation bioassays. These interactions were scored by three independent, blinded observers at 10 s intervals using a binary scale such that aggression either did or did not occur (a score of 1 or 0, respectively). Prior to scoring, each observer was trained to recognize ‘aggression’ as instances in which one or both ants were lunging, biting or dragging one another. Each 10 s time interval was scored as either containing an instance of aggression or not to establish the proportion of time the ants were engaged in aggressive behavior. An aggression index was calculated by dividing the number of observed acts of aggression by the total number of observed time intervals. The mean aggression index of each video recording across all three independent scores was used for subsequent statistical analysis.
Mobility control parameters
Mobility control videos were analyzed using an automated tracking software package (EthoVision® XT v8.5, Noldus Information Technology, Wageningen, The Netherlands) to calculate total distance traveled (cm), time spent moving (%) and the frequency of rotations (count). Time spent moving/not moving was calculated with thresholds of 0.30 cm s−1 (start velocity) and 0.20 cm s−1 (stop velocity) as determined by the EthoVision® XT software with an averaging interval of 1 sample. A single rotation was defined as a cumulative turn angle of 90 deg over a distance of 1.00 cm. Turns in the opposite direction of less than 45 deg were ignored. The sum of both clockwise and counterclockwise rotations was used to determine rotational frequency. Trials in which the subject ant was not found for at least 95% of the recording were discarded (N=15).
Mechanically evoked biting and mandible opening response (BMOR) bioassay
To determine whether disrupting Orco-mediated olfactory signaling broadly disrupted aggression in a non-social context, individual adult minor workers were briefly anesthetized with CO2 before being secured with wax in a modified 200 µl pipette tip such that the head and antennae were accessible. The ants were allowed to acclimate for 10 min before being exposed to a continuous flow of heated air alone or volatilized VU-class compounds as described above for the volatile Orco modulator aggression bioassays. A clean, ethanol-washed 3.61/0.4 g Von Frey hair filament (Baseline® Fold-Up™ Monofilaments item no. 12-1741, Fabrication Enterprises Inc., White Plains, NY, USA) was then gently brushed along the anterior portion of the ant's head from the ventral to the dorsal side 5 times. Aggression was scored by six independent, blinded observers on a binary scale such that biting or attempting to bite the filament or wide opening of the mandibles (i.e. the mandibles were opened beyond parallel) either did (score of 1) or did not (score of 0) occur during the duration of the trial. An aggression index was calculated by taking the average score across all observers and used for subsequent statistical analysis. Trials in which the ants did not recover from the CO2 treatment were discarded.
Statistical analysis
Statistical analyses were performed using GraphPad Prism v8.0.0 (GraphPad Software, Inc.). For the aggression bioassays, a two-way ANOVA was first performed followed by Holm–Šidák's multiple comparisons test to compare NM versus nNM aggression as well as aggression across antennal treatments. For the antennal ablation mobility controls as well as the BMOR bioassays, a Kruskal–Wallis test was performed followed by Dunn's correction for multiple comparisons. As the volatilization mobility controls had matched samples across different time points, a repeated measures two-way ANOVA with the Greenhouse–Geisser correction for violations of sphericity was performed. For the electroantennography, linear regression analysis was used to test whether the best-fit slope differed significantly from 0 (i.e. a straight line with no dose response). The response to the solvent control (i.e. no decane or 4M3H) was normalized to 0 mV; therefore, the Y-intercept was constrained to X=0, Y=0. The inclusion and exclusion criteria for all samples was pre-established. The number of replicates for each study was as follows: ablation aggression bioassays N=6–10; mobility controls (ablation) N=24–29; volatile Orco modulator aggression bioassays N=10–12; volatile Orco modulator BMOR bioassay N=10–11; mobility controls (volatilization) N=7–9; electroantennography N=5–6. Information regarding the statistical test performed and the results from these analyses are detailed in Table S1.
RESULTS
NM recognition requires antennal signaling
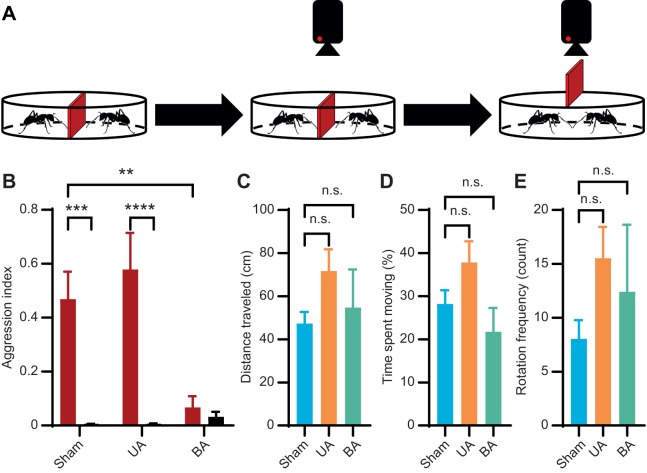
The initial phase of this study was to develop an olfactory-based NM recognition bioassay in which two ants – NMs from the same home colony or nNMs from two different colonies – were able to interact with one another after an acclimation period (Fig. 1A). To this end, we initially took a broad approach to assess the role of olfactory signaling in modulating NM/nNM aggression in the context of pairwise trials conducted using adult C. floridanus minor worker ants with either unilateral or bilateral antennal ablation. As it has long been established that antennal ablation decreases aggression between nNMs (Forel, 1928; Wang et al., 2016), these assays were undertaken to validate our experimental design. In these studies, both control C. floridanus workers (t=4.404, P=0.0001) and those that had undergone unilateral ablation (t=5.438, P<0.0001) were able to routinely discriminate nNMs from NMs and display only nNM aggression (two-way ANOVA with Holm–Šidák's multiple comparisons test) (Fig. 1B; Table S1). In contrast, ants with bilateral antennal ablation displayed a significant and indeed near-complete reduction in aggression against nNMs (t=3.384, P=0.003). These data are consistent with the widely reported ability of C. floridanus workers to robustly discriminate between nNMs and NMs and supports the hypothesis that their chemosensory apparatus is required to recognize and trigger aggression against nNMs (Hölldobler and Wilson, 1990; Morel et al., 1988; Leonhardt et al., 2007; Guerrieri et al., 2009; Ozaki et al., 2005; Brandstaetter et al., 2011; Slone et al., 2017; Pask et al., 2017; Neupert et al., 2018; Forel, 1928; Wang et al., 2016).
Fig. 1.
Aggression and mobility responses of adult minor workers following antennal ablation. Sham, control; UA, unilateral ablation; BA, bilateral ablation. (A) Schematic representation of the ablation bioassay depicting the acclimation period (left), mobility controls (center) and aggression bioassay (right). (B) Bilateral antennal ablation significantly reduced non-nestmate (nNM, red) aggression compared with the sham control. Black, nestmates (NMs). Two-way ANOVA with Holm–Šidák's multiple comparisons test (biological replicates: sham NMs N=9, sham nNMs N=10, UA NMs N=10, UA nNMs N=9, BA NMs N=6, BA nNMs N=6). (C–E) There was no significant difference in mobility between the sham control and the ablation treatments, as assessed by distance traveled (C), time spent moving (D) and rotation frequency (E). Kruskal–Wallis test (biological replicates: sham and UA N=29, BA N=24). Bars display mean; error bars display s.e.m. Asterisks indicate significance: **P<0.01, ***P<0.001, ****P<0.0001. n.s., not significant.
To further control for potentially confounding variables – including the outright death or incapacitation of the ants due to the damage sustained from the ablations – we measured a number of other behavioral indicators including total distance traveled, percentage of time spent moving/not moving and the frequency of rotations using an automated tracking program (see Materials and Methods). Here, the activity of a single ant was recorded for 3 min immediately following the 10 min acclimation period and preceding the ablation aggression bioassays (Fig. 1A). These assays revealed no significant difference between the sham control and the ablation treatments (Fig. 1C–E; Table S1). Treated ants were able to recover from the injury and retain fundamental aspects of mobility, and unilaterally ablated workers kept the ability to discriminate between NMs and nNMs. This suggests that the decrease in aggression was likely due to the absence of antennae-mediated signaling as opposed to confounding variables introduced by the ablation treatment. However, as the removal of the antennae disrupts a broad range of both mechanoreceptors and chemoreceptors (Nakanishi et al., 2009), a more targeted approach was required to assess the specific function of OR-dependent chemoreceptor signaling in this context.
NM recognition is an active, OR-dependent process
In order to further examine this process within the narrow context of assessing the role of ORs in NM recognition and aggression, we adapted our bioassay to incorporate the sustained volatile administration of a set of highly specific Orco allosteric modulators (Fig. 2A; Fig. S1).
Fig. 2.
Electrophysiological responses of adult minor workers to decane or 4M3H under different background airflow conditions. (A) Schematic diagram of the electroantennogram apparatus. (B,C) Best-fit lines derived from the solvent (hexane or paraffin oil) normalized responses to serial concentrations of decane (D–G) or 4M3H (H–K), respectively, for blank (control, heated air alone), VUAA0 (inert chemical analog control), VUANT1 (Orco antagonist) and VUAA4 (Orco agonist) backgrounds. The slope of the best-fit line for blank, VUAA0 and VUAA4 for both decane and 4M3H was significantly different from 0. Linear regression (biological replicates: decane blank N=5, VUAA0 N=5, VUANT1 N=6, VUAA4 N=5; 4M3H blank N=6, VUAA0 N=6, VUANT1 N=5, VUAA4 N=5; see Table S1). Points display mean; error bars display s.e.m.
While the use of certain VUAA-class actives has already been shown to disrupt OR-mediated detection of a blend of hydrocarbons (C7–C40) and cuticle extract (Sharma et al., 2015), we sought to validate the efficacy of these compounds in the context of our experimental setup where they were delivered within a constant background airflow to our aggression bioassay arena. We performed EAGs to assess whole-antennal responses to several concentrations of the hydrocarbon decane (C10) as well as 4M3H in adult workers exposed to heated air (blank control) or volatilized compound (Fig. 2A). These two compounds were chosen because they elicited strong responses in the basiconic sensilla, which presumably contains many OR-expressing neurons (Sharma et al., 2015; McKenzie et al., 2016).
For decane, we observed similar dose-dependent responses with our blank control and VUAA0 treatment (Fig. 2B,D,E). Indeed, linear regression analysis revealed that the slope of the blank control (F1,24=11.39, P=0.0025) and that of VUAA0 (F1,24=25.31, P<0.0001) were significantly different from 0 (i.e. a flat line) (Fig. 2B; Table S1). Consistent with expectations, the slope of VUANT1 was not significantly different from 0 (Fig. 2B,F; Table S1), suggesting that exposure to this compound completely eliminated dose-dependent detection of decane. Volatile administration of VUAA4 also disrupted hydrocarbon detection; however, it did not eliminate OR signaling. Rather, it displayed a muted and partially dose-dependent response with seemingly static, yet low, responsiveness at higher concentrations (Fig. 2B,G). These effects are likely the result of broad ORN desensitization after prolonged exposure to this potent Orco agonist. Nevertheless, the slope of VUAA4 was significantly different from 0 (F1,24=0.0320, P=0.032) (Fig. 2B; Table S1), suggesting that dose-dependent hydrocarbon detection and ORN firing still occur albeit not in the same manner as the controls. With regard to 4M3H, we again observed similar dose-dependent responses in the blank control (F1,23=22.58, P<0.0001) and VUAA0 (F1,23=42.11, P<0.0001), and these responses were eliminated in the VUANT1 treatment (Fig. 2C,H–J). Responses to VUAA4, however, were substantially increased (Fig. 2C,K). These elevated responses are consistent with the expected role of VUAA4 as an Orco agonist. These observations highlight the profound effects that acute volatile administration of VUAA4 has on olfactory signaling. Taken together, these data foster the view that ambiguous/altered odor coding results from a combination of both cryptic activation and desensitization of ORNs. Furthermore, responses to odorants were not completely eliminated but nevertheless deviate from control responses. Altogether, these studies demonstrate that VUAA-class actives disrupt Orco-mediated olfactory signal transduction in ants.
Using this newly established volatilization paradigm, we then sought to determine the precise role of OR signaling in mediating aggression towards nNMs. Ants taken from across nine independent colonies exposed to either Orco modulator (VUANT1 or VUAA4) displayed a significant reduction, and indeed a near-complete elimination, of aggression towards nNMs (two-way ANOVA with Holm−Šidák's multiple comparisons test; VUANT1: t=2.372, P=0.0399; VUAA4: t=3.466, P=0.0026) (Fig. 3A; Table S1). Importantly, ants treated with either the Orco agonist or the Orco antagonist displayed no significant difference in their responses to NMs. This lack of misdirected aggression toward NMs as well as the failure to correctly attack nNMs in ants treated with these highly selective Orco/OR modulators demonstrates that, in C. floridanus, aggression is specifically mediated by the OR-dependent detection of specific and unambiguous odor cue signatures from nNM foes rather than the general absence or incorrect processing of familiar signatures of NM friends.
Fig. 3.
Aggression and mobility responses of adult minor workers during exposure to volatilization treatments. (A) Disrupting Orco-mediated olfactory signal transduction significantly reduced aggression towards nNMs (red). Black, NMs. Two-way ANOVA with Holm–Šidák's multiple comparisons test (biological replicates: blank NMs N=10, nNMs N=12; VUAA0 NMs N=10, nNMs N=11; VUANT1 NMs N=12, nNMs N=10; VUAA4 NMs N=10, nNMs N=12). (B–D) There was no significant interaction between treatments across the mobility parameters tested (acclimation, blue; treatment, yellow; recovery, green). RM two-way ANOVA with Greenhouse–Geisser epsilon (biological replicates: blank N=8, VUAA0 N=8, VUANT1 N=7, VUAA4 N=9). Bars display mean; error bars display s.e.m. Asterisks indicate significance: *P<0.05, **P<0.01, ***P<0.001. n.s., not significant.
Furthermore, in order to assess whether the disruption of OR signaling reduced aggression within the narrow social context of NM recognition or alternatively acted to broadly inhibit aggressive behaviors, we conducted parallel bioassays that utilized mechanical rather than chemical stimuli to evoke aggression. Using a modified aggression bioassay based on previous methods (see Guerrieri and D'Ettorre, 2008; Gospocic et al., 2017), individual ants were challenged with a chemically neutral mechanical stimulus (i.e. a clean Von Frey filament) and subsequently scored for biting responses as well as wide opening of the mandibles as indicators of aggression. Importantly, as there was no significant difference in aggression among the various treatment groups (Fig. S2), we could conclude that disrupting Orco-mediated olfactory signaling did not generally inhibit aggressive responses in C. floridanus but instead specifically impacted workers' ability to discriminate NMs from nNMs and aggressively respond to the latter.
In order to further control for potentially confounding variables in response to these volatilization treatments, the activity of a single ant was recorded immediately following a 10 min acclimation period. These trials consisted of a continuous 9 min bioassay separated into three 3 min segments. During the first segment, the ants were exposed to a continuous flow of untreated air (‘acclimation’); for the second segment, the ants were exposed to a continuous flow of volatilized VUAA-class active or untreated air in the case of the blank control using the same parameters established for the volatilization aggression bioassay (‘treatment’); and during the third segment, the ants were again exposed to a continuous flow of untreated air (‘recovery’). A Y-junction connected to the compressed air tank alternated between the empty test tube during the acclimation and recovery phases and the treatment or blank tube during the treatment phase. An examination of overall mobility parameters revealed no significant interaction effect when comparing control ants and ants treated with either the Orco agonist or Orco antagonist before, during or after exposure to each treatment (Fig. 3B–D; Table S1).
DISCUSSION
In ants and other eusocial insects, NM recognition depends on the ability to discriminate between self (NMs) and non-self (nNMs) (reviewed in Sturgis and Gordon, 2012). While it is clear that these aggressive responses are mediated by the detection of chemical cues on the cuticle (Morel et al., 1988; Guerrieri et al., 2009; Leonhardt et al., 2007; Neupert et al., 2018), the precise molecular mechanisms responsible for the detection and coding of that information within the olfactory system have remained ambiguous. Previous studies have demonstrated that the antennae are required for eliciting aggressive behaviors towards nNMs (Wang et al., 2016; Forel, 1928). Therefore, we took a conservative approach to validate our aggression bioassay within the context of antennal ablations (Fig. 1). Once established, this experimental paradigm was further adapted to accommodate the sustained volatile administration of VUAA-class Orco modulators to test the hypothesis that NM recognition in adult C. floridanus workers is solely dependent upon OR-based olfactory signaling as well as facilitate the characterization of odor coding in this process. Because of the broad developmental defects that result from the loss of Orco in other ant systems (Trible et al., 2017; Yan et al., 2017), these pharmacological tools provide a unique opportunity to acutely examine the role of OR-based signaling in a wild-type adult nervous system. At the same time, in light of the obligate co-localization of Orco together with tuning ORs in every insect ORN (Larsson et al., 2004; Jones et al., 2005; Taylor et al., 2012), exposure to Orco modulators is expected to have profound and widespread effects.
As previously observed in other contexts (Sharma et al., 2015), treatment with the VUANT1 antagonist effectively silenced all Orco/OR complexes and prevented the generation of any interpretable signal (Fig. 2). In the case of the VUAA4 Orco agonist, activation of all Orco/OR complexes led to either the activation of ORNs or a broad desensitization resulting in disrupted signaling (Fig. 2) that we postulate effectively generates an uninterpretable or ‘confused’ coding signal. In either case, the lack of any odor signal or the presence of imprecise odor cues that are expected after treatment with an Orco antagonist or agonist, respectively, were both equally insufficient to elicit aggression between nNMs (Fig. 3).
The observation that an Orco antagonist decreases aggression between nNMs is broadly consistent with a simple U-present rejection model and supports the view that ants are not actively recognizing friends (Guerrieri et al., 2009; van Zweden and D'Ettorre, 2010). However, the finding that an Orco agonist, which would be expected to generate a signal different from that of the endogenous template, would also decrease aggression between nNMs rather than increase aggression between NMs suggests that the simple presence of foreign yet imprecise cues is also insufficient to elicit aggression. These studies therefore support a model in which an unambiguous triggering stimulus must be precisely detected in order to evoke aggression. We propose that the recognition mechanism in C. floridanus occurs via a lock-and-key mechanism whereby the specific parameters of the foreign chemical label key, defined by the combinatorial presence and/or absence of salient odor cues, must be precisely decoded by an OR-mediated lock (Fig. 4). Under the assumption that a precise nNM label is compared with a neuronal template (of which many may exist), we conclude that ants may identify nNMs in two different ways which are not necessarily mutually exclusive. (1) As with previous models, unfamiliar nNM labels are compared with a familiar NM template and the dissimilarity between the two leads to aggression (Neupert et al., 2018; Vander Meer and Morel, 1998; Obin and Vandermeer, 1989). However, given that neither VUANT1 nor VUAA4 elicited aggression, this dissimilarity must be constrained in some way with bounded thresholds wherein the label must be sufficiently different from the template but not so different as to be ambiguous. (2) If unfamiliar nNM labels are compared with intruder templates that represent odor profiles which should be rejected from the colony and a certain level of precision between the label and template is required to elicit aggression, then we would similarly expect both VUANT1 and VUAA4 to decrease aggression between nNMs.
Fig. 4.

Lock-and-key model of nNM recognition and aggression. The triggering stimuli, represented by the teeth on a key, must be precisely detected by the OR tumblers in the lock. OR-dependent recognition of nNM cues leads to aggression against foes (green open lock); however, blocking OR-dependent recognition of NM/nNM cues does not lead to aggression, nor does the presence of an ambiguous chemical cue (closed red locks).
Furthermore, these data suggest that, when faced with some level of uncertainty, C. floridanus workers default towards acceptance rather than rejection. Over and above the benefits of conserving energy by avoiding potentially unnecessary aggression, for ants that spend the majority of their life cycle within colonies where they are more likely to encounter NMs than nNMs, this strategy may also reduce acceptance errors and therefore increase overall colony fitness (Reeve, 1989). It will be interesting to determine whether similar processes occur across worker behavioral task groups that may spend more time outside the nest (i.e. scouts and foragers) or whether different recognition methods have evolved across castes and/or species.
Our data definitively demonstrate that Orco/OR-mediated signaling is necessary for mediating aggression towards nNMs in C. floridanus and moreover excludes the sufficiency of other signaling pathways and sensory modalities in this context. These results are consistent with previous literature suggesting that aggression-mediated NM recognition may be more appropriately described as nNM recognition (Guerrieri et al., 2009; van Zweden and D'Ettorre, 2010). While the roles of individual ant ORs or specific subsets of ORs in nNM recognition remain to be elucidated, the combinatorial interactions among specialized ORs (Slone et al., 2017; Pask et al., 2017), the plasticity of the neuronal templates (Neupert et al., 2018; Leonhardt et al., 2007), the similarly diverse and plastic labels (Vander Meer et al., 1989; Wagner et al., 1998; Kaib et al., 2000; Nascimento et al., 2013), and the observation that repeated stimulation with colony odors produced variable response patterns in the antennal lobe (Brandstaetter et al., 2011) are likely to make those studies extremely challenging. Nevertheless, the demonstration that precise and unambiguous OR-based coding is necessary for ants to distinguish foe from friend represents a significant advance to link the longstanding interest in social insect behavior with more recent studies detailing the evolutionary complexity of the insect olfactory system (Hölldobler and Wilson, 1990; Zhou et al., 2012, 2015).
Supplementary Material
Acknowledgements
We thank the laboratories of our colleagues Dr J. Liebig (Arizona State University) and Dr S. L. Berger (University of Pennsylvania) for ant collections. We also thank Drs Berger, R. Bonasio, P. Abbot, A. Carr and H. W. Honegger for comments on the manuscript. We lastly thank Drs H. W. Honegger, J. Slone and R. Jason Pitts and Mr Zi Ye along with other members of the Zwiebel lab for suggestions throughout the course of this work, Dr S. Ochieng for ant rearing and technical help, and Dr A. M. McAinsh for editorial assistance.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: S.T.F., A.R., L.J.Z.; Methodology: S.T.F., K.Y.P., A.R., I.B., L.J.Z.; Validation: S.T.F., L.J.Z.; Formal analysis: S.T.F.; Investigation: S.T.F., K.Y.P., A.R., I.B.; Resources: L.J.Z.; Data curation: S.T.F., L.J.Z.; Writing - original draft: S.T.F., L.J.Z.; Writing - review & editing: S.T.F., K.Y.P., A.R., I.B., L.J.Z.; Visualization: S.T.F.; Supervision: S.T.F., L.J.Z.; Project administration: S.T.F., L.J.Z.; Funding acquisition: L.J.Z.
Funding
This work was supported by a grant from the National Institutes of Health (NIGMS/RO1GM128336) to L.J.Z. and with endowment funding from Vanderbilt University. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jeb.biologists.org/lookup/doi/10.1242/jeb.215400.supplemental
References
- Ayre D. J. and Grosberg R. K. (1995). Aggression, habituation, and clonal coexistence in the sea anemone Anthopleura elegantissima. Am. Nat. 146, 427-453. 10.1086/285808 [DOI] [Google Scholar]
- Benton R., Sachse S., Michnick S. W. and Vosshall L. B. (2006). Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 4, e20 10.1371/journal.pbio.0040020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard R. J. and Blanchard D. C. (1977). Aggressive behavior in the rat. Behav. Biol. 21, 197-224. 10.1016/S0091-6773(77)90308-X [DOI] [PubMed] [Google Scholar]
- Bonasio R., Zhang G., Ye C., Mutti N. S., Fang X., Qin N., Donahue G., Yang P., Li Q., Li C. et al. (2010). Genomic comparison of the ants Camponotus floridanus and Harpegnathos saltator. Science 329, 1068-1071. 10.1126/science.1192428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos N. and D'Ettorre P. (2012). Recognition of social identity in ants. Front. Psychol. 3, 83 10.3389/fpsyg.2012.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstaetter A. S. and Kleineidam C. J. (2011). Distributed representation of social odors indicates parallel processing in the antennal lobe of ants. J. Neurophysiol. 106, 2437-2449. 10.1152/jn.01106.2010 [DOI] [PubMed] [Google Scholar]
- Brandstaetter A., Rössler W. and Kleineidam C. J. (2011). Friends and foes from an ant brain's point of view – neuronal correlates of colony odors in a social insect. PLoS ONE 6, e21383-e21392. 10.1371/journal.pone.0021383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterwick J. A., del Mármol J., Kim K. H., Kahlson M. A., Rogow J. A., Walz T. and Ruta V. (2018). Cryo-EM structure of the insect olfactory receptor Orco. Nature 560, 447-452. 10.1038/s41586-018-0420-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier R. H. and Dix M. W. (1979). Analysis of two genetic models for the innate components of colony odor in social Hymenoptera. Behav. Ecol. Sociobiol. 4, 217-224. 10.1007/BF00297645 [DOI] [Google Scholar]
- Endler A., Liebig J., Schmitt T., Parker J. E., Jones G. R., Schreier P. and Hölldobler B. (2004). Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc. Natl. Acad. Sci. USA 101, 2945-2950. 10.1073/pnas.0308447101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forel A. (1928). The Social World of the Ants Compared with that of Man. London and New York: G.P. Putnam's Sons, Ltd. [Google Scholar]
- Gadau J., Heinze J., Hölldobler B. and Schmid M. (1996). Population and colony structure of the carpenter ant Camponotus floridanus. Mol. Ecol. 5, 785-792. 10.1111/j.1365-294X.1996.tb00374.x [DOI] [PubMed] [Google Scholar]
- Gordon D. M. (2015). From division of labor to the collective behavior of social insects. Behav. Ecol. Sociobiol. 70, 1101-1108. 10.1007/s00265-015-2045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospocic J., Shields E. J., Glastad K. M., Lin Y., Penick C. A., Yan H., Mikheyev A. S., Linksvayer T. A., Garcia B. A., Berger S. L. et al. (2017). The neuropeptide Corazonin controls social behavior and caste identity in ants. Cell 170, 748-759.e12. 10.1016/j.cell.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri F. J. and D'Ettorre P. (2008). The mandible opening response: quantifying aggression elicited by chemical cues in ants. J. Exp. Biol. 211, 1109-1113. 10.1242/jeb.008508 [DOI] [PubMed] [Google Scholar]
- Guerrieri F. J., Nehring V., Jørgensen C. G., Nielsen J., Galizia C. G. and D'Ettorre P. (2009). Ants recognize foes and not friends. Proc. R. Soc. B Biol. Sci. 276, 2461-2468. 10.1098/rspb.2008.1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen I. A., Rodriguez S. D., Drake L. L., Price D. P., Blakely B. N., Hammond J. I., Tsujimoto H., Monroy E. Y., Maio W. A. and Romero A. (2014). The odorant receptor co-receptor from the bed bug, Cimex lectularius L. PLoS ONE 9, e113692 10.1371/journal.pone.0113692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölldobler B. and Wilson E. O. (1990). The Ants. Cambridge, MA: Belknap Press of Harvard Univ Press. [Google Scholar]
- Jones W. D., Nguyen T.-A. T., Kloss B., Lee K. J. and Vosshall L. B. (2005). Functional conservation of an insect odorant receptor gene across 250 million years of evolution. Curr. Biol. 15, R119-R121. 10.1016/j.cub.2005.02.007 [DOI] [PubMed] [Google Scholar]
- Jones P. L., Pask G. M., Rinker D. C. and Zwiebel L. J. (2011). Functional agonism of insect odorant receptor ion channels. Proc. Natl. Acad. Sci. USA 108, 8821-8825. 10.1073/pnas.1102425108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. L., Pask G. M., Romaine I. M., Taylor R. W., Reid P. R., Waterson A. G., Sulikowski G. A. and Zwiebel L. J. (2012). Allosteric antagonism of insect odorant receptor ion channels. PLoS ONE 7, e30304 10.1371/journal.pone.0030304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaib M., Eisermann B., Schoeters E., Billen J., Franke S. and Francke W. (2000). Task-related variation of postpharyngeal and cuticular hydrocarbon compositions in the ant Myrmicaria eumenoides. J. Comp. Physiol. A 186, 939-948. 10.1007/s003590000146 [DOI] [PubMed] [Google Scholar]
- Larsson M. C., Domingos A. I., Jones W. D., Chiappe M. E., Amrein H. and Vosshall L. B. (2004). Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703-714. 10.1016/j.neuron.2004.08.019 [DOI] [PubMed] [Google Scholar]
- Leonhardt S. D., Brandstaetter A. S. and Kleineidam C. J. (2007). Reformation process of the neuronal template for nestmate-recognition cues in the carpenter ant Camponotus floridanus. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 193, 993-1000. 10.1007/s00359-007-0252-8 [DOI] [PubMed] [Google Scholar]
- Mckenzie S. K., Fetter-Pruneda I., Ruta V. and Kronauer D. J. C. (2016). Transcriptomics and neuroanatomy of the clonal raider ant implicate an expanded clade of odorant receptors in chemical communication. Proc. Natl. Acad. Sci. USA 113, 14091-14096. 10.1073/pnas.1610800113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani J. C., Watts D. P. and Amsler S. J. (2010). Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr. Biol. 20, R507-R508. 10.1016/j.cub.2010.04.021 [DOI] [PubMed] [Google Scholar]
- Moore D. and Liebig J. (2010). Mechanisms of social regulation change across colony development in an ant. BMC Evol. Biol. 10, 328 10.1186/1471-2148-10-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel L., Vander Meer R. K. and Lavine B. K. (1988). Ontogeny of nestmate recognition cues in the red carpenter ant (Camponotus Floridanus) - behavioral and chemical evidence for the role of age and social experience. Behav. Ecol. Sociobiol. 22, 175-183. 10.1007/BF00300567 [DOI] [Google Scholar]
- Nakanishi A., Nishino H., Watanabe H., Yokohari F. and Nishikawa M. (2009). Sex-specific antennal sensory system in the ant Camponotus japonicus: structure and distribution of sensilla on the flagellum. Cell Tissue Res. 338, 79-97. 10.1007/s00441-009-0863-1 [DOI] [PubMed] [Google Scholar]
- Nascimento F. S., Tannure-Nascimento I. C., Dantas J. O., Turatti I. C. and Lopes N. P. (2013). Task-related variation of cuticular hydrocarbon profiles affect nestmate recognition in the giant ant Dinoponera quadriceps. J. Insect Behav. 26, 212-222. 10.1007/s10905-012-9353-5 [DOI] [Google Scholar]
- Neupert S., Hornung M., Millar J. G. and Kleineidam C. J. (2018). Learning distinct chemical labels of nestmates in ants. Front. Behav. Neurosci. 12, 191 10.3389/fnbeh.2018.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obin M. S. and Vander Meer R. K. (1989). Mechanism of template-label matching in fire ant, Solenopsis invicta buren, nestmate recognition. Anim. Behav. 38, 430-435. 10.1016/S0003-3472(89)80036-3 [DOI] [Google Scholar]
- Oxley P. R., Ji L., Fetter-Pruneda I., Mckenzie S. K., Li C., Hu H., Zhang G. and Kronauer D. J. C. (2014). The genome of the clonal raider ant Cerapachys biroi. Curr. Biol. 24, 451-458. 10.1016/j.cub.2014.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki M., Wada-Katsumata A., Fujikawa K., Iwasaki M., Yokohari F., Satoji Y., Nisimura T. and Yamaoka R. (2005). Ant nestmate and nonnestmate discrimination by a chemosensory sensillum. Science 309, 311-314. 10.1126/science.1105244 [DOI] [PubMed] [Google Scholar]
- Pask G. M., Jones P. L., Rützler M., Rinker D. C. and Zwiebel L. J. (2011). Heteromeric Anopheline odorant receptors exhibit distinct channel properties. PLoS ONE 6, e28774 10.1371/journal.pone.0028774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pask G. M., Slone J. D., Millar J. G., Das P., Moreira J. A., Zhou X., Bello J., Berger S. L., Bonasio R., Desplan C. et al. (2017). Specialized odorant receptors in social insects that detect cuticular hydrocarbon cues and candidate pheromones. Nat. Commun. 8, 297 10.1038/s41467-017-00099-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve H. K. (1989). The evolution of conspecific acceptance thresholds. Am. Nat. 133, 407-435. 10.1086/284926 [DOI] [Google Scholar]
- Rinker D. C., Jones P. L., Pitts R. J., Rutzler M., Camp G., Sun L. J., Xu P. X., Dorset D. C., Weaver D. and Zwiebel L. J. (2012). Novel high-throughput screens of Anopheles gambiae odorant receptors reveal candidate behaviour-modifying chemicals for mosquitoes. Physiol. Entomol. 37, 33-41. 10.1111/j.1365-3032.2011.00821.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. M. and Wanner K. W. (2006). The chemoreceptor superfamily in the honey bee, Apis mellifera: expansion of the odorant, but not gustatory, receptor family. Genome Res. 16, 1395-1403. 10.1101/gr.5057506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaine I. M., Taylor R. W., Saidu S. P., Kim K., Sulikowski G. A., Zwiebel L. J. and Waterson A. G. (2014). Narrow SAR in odorant sensing Orco receptor agonists. Bioorg. Med. Chem. Lett. 24, 2613-2616. 10.1016/j.bmcl.2014.04.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Pellegrino M., Nakagawa T., Vosshall L. B. and Touhara K. (2008). Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452, 1002-1006. 10.1038/nature06850 [DOI] [PubMed] [Google Scholar]
- Scheel D., Godfrey-Smith P. and Lawrence M. (2016). Signal use by octopuses in agonistic interactions. Curr. Biol. 26, 377-382. 10.1016/j.cub.2015.12.033 [DOI] [PubMed] [Google Scholar]
- Sharma K. R., Enzmann B. L., Schmidt Y., Moore D., Jones G. R., Parker J., Berger S. L., Reinberg D., Zwiebel L. J., Breit B. et al. (2015). Cuticular hydrocarbon pheromones for social behavior and their coding in the ant antenna. Cell Rep. 12, 1261-1271. 10.1016/j.celrep.2015.07.031 [DOI] [PubMed] [Google Scholar]
- Sherman P. W., Reeve H. K. and Pfennig D. W. (1997). Recognition systems. In Behavioural Ecology: An Evolutionary Approach (ed. Krebs J. R. and Davies N. B.), pp. 69-96. Oxford, UK: Wiley-Blackwell. [Google Scholar]
- Slone J. D., Pask G. M., Ferguson S. T., Millar J. G., Berger S. L., Reinberg D., Liebig J., Ray A. and Zwiebel L. J. (2017). Functional characterization of odorant receptors in the ponerine ant, Harpegnathos saltator. Proc. Natl. Acad. Sci. USA 114, 8586-8591. 10.1073/pnas.1704647114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. D., Zimin A., Holt C., Abouheif E., Benton R., Cash E., Croset V., Currie C. R., Elhaik E., Elsik C. G. et al. (2011a). Draft genome of the globally widespread and invasive Argentine ant (Linepithema humile). Proc. Natl. Acad. Sci. USA 108, 5673-5678. 10.1073/pnas.1008617108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. R., Smith C. D., Robertson H. M., Helmkampf M., Zimin A., Yandell M., Holt C., Hu H., Abouheif E., Benton R. et al. (2011b). Draft genome of the red harvester ant Pogonomyrmex barbatus. Proc. Natl Acad. Sci. USA, 1-6. 10.1073/pnas.1007901108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgis S. J. and Gordon D. M. (2012). Nestmate recognition in ants (Hymenoptera: Formicidae): a review. Myrmecol. News 16, 101-110. [Google Scholar]
- Suh E., Bohbot J. D. and Zwiebel L. J. (2014). Peripheral olfactory signaling in insects. Curr. Opin. Insect Sci. 6, 86-92. 10.1016/j.cois.2014.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. W., Romaine I. M., Liu C., Murthi P., Jones P. L., Waterson A. G., Sulikowski G. A. and Zwiebel L. J. (2012). Structure-activity relationship of a broad-spectrum insect odorant receptor agonist. ACS Chem. Biol. 7, 1647-1652. 10.1021/cb300331z [DOI] [PubMed] [Google Scholar]
- Trible W., Olivos-Cisneros L., McKenzie S. K., Saragosti J., Chang N.-C., Matthews B. J., Oxley P. R. and Kronauer D. J. C. (2017). Orco mutagenesis causes loss of antennal lobe glomeruli and impaired social behavior in ants. Cell 170, 727-735.e10. 10.1016/j.cell.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitoura P. and Iatrou K. (2016). Positive allosteric modulation of insect olfactory receptor function by ORco agonists. Front Cell Neurosci 10, 275 10.3389/fncel.2016.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitoura P., Koussis K. and Iatrou K. (2015). Inhibition of Anopheles gambiae odorant receptor function by mosquito repellents. J. Biol. Chem. 290, 7961-7972. 10.1074/jbc.M114.632299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zweden J. S. and D'Ettorre P. (2010). Nestmate recognition in social insects and the role of hydrocarbons. In Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology (ed. Blonquist G. J. and Bagneres A. G.), pp. 222-243. Cambridge University Press. [Google Scholar]
- vander Meer R. and Morel L. (1998). Nestmate recognition in ants. In Pheromone Communication in Social Insects (ed. vander Meer R., Breed M. D., Winston M. L. and Espelie K. E.), pp. 79-103. Boulder, CO: Westview Press. [Google Scholar]
- vander Meer R. K., Saliwanchik D. and Lavine B. (1989). Temporal changes in colony cuticular hydrocarbon patterns of Solenopsis invicta: implications for nestmate recognition. J. Chem. Ecol. 15, 2115-2125. 10.1007/BF01207442 [DOI] [PubMed] [Google Scholar]
- Wagner D., Brown M. J. F., Broun P., Cuevas W., Moses L. E., Chao D. L. and Gordon D. M. (1998). Task-related differences in the cuticular hydrocarbon composition of harvester ants, Pogonomyrmex barbatus. J. Chem. Ecol. 24, 2021-2037. 10.1023/A:1020781508889 [DOI] [Google Scholar]
- Wang Q., Goodger J. Q. D., Woodrow I. E. and Elgar M. A. (2016). Location-specific cuticular hydrocarbon signals in a social insect. Proc. Biol. Sci. 283, 20160310 10.1098/rspb.2016.0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren J. H., Richards S., Desjardins C. A., Niehuis O., Gadau J., Colbourne J. K., Nasonia Genome Working Group, Werren J. H., Richards S., Desjardins C. A., Niehuis O., et al. (2010). Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science 327, 343-348. 10.1126/science.1178028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher D., Schäfer R., Bauernfeind R., Stensmyr M. C., Heller R., Heinemann S. H. and Hansson B. S. (2008). Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 452, 1007-1011. 10.1038/nature06861 [DOI] [PubMed] [Google Scholar]
- Wurm Y., Wang J., Riba-Grognuz O., Corona M., Nygaard S., Hunt B. G., Ingram K. K., Falquet L., Nipitwattanaphon M., Gotzek D. et al. (2011). The genome of the fire ant Solenopsis invicta. Proc. Natl. Acad. Sci. USA 108, 5679-5684. 10.1073/pnas.1009690108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Opachaloemphan C., Mancini G., Yang H., Gallitto M., Mlejnek J., Leibholz A., Haight K., Ghaninia M., Huo L. et al. (2017). An engineered orco mutation produces aberrant social behavior and defective neural development in ants. Cell 170, 736-747.e9. 10.1016/j.cell.2017.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Slone J. D., Rokas A., Berger S. L., Liebig J., Ray A., Reinberg D. and Zwiebel L. J. (2012). Phylogenetic and transcriptomic analysis of chemosensory receptors in a pair of divergent ant species reveals sex-specific signatures of odor coding. PLoS Genet. 8, e1002930 10.1371/journal.pgen.1002930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Rokas A., Berger S. L., Liebig J., Ray A. and Zwiebel L. J. (2015). Chemoreceptor evolution in hymenoptera and its implications for the evolution of eusociality. Genome Biol. Evol. 7, 2407-2416. 10.1093/gbe/evv149 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.