Abstract
Malassezia are abundant, lipid-dependent, commensal yeasts in the skin microbiome that also have a pathogenic lifestyle associated with several common skin disorders. Malassezia genomes encode myriad lipases and proteases thought to mediate lipid utilization and pathogenesis. In this issue of the Journal of Investigative Dermatology, Li and colleagues report the biochemical characterization of a unique secreted aspartyl protease produced by Malassezia globosa, MgSAP1, and demonstrate its active role in hindering biofilm formation of the bacterium Staphylococcus aureus. Because biofilms are an established virulence attribute of S. aureus, this study reveals a potential benefit to the host of the fungal aspartyl protease MgSAP1 and opens the door for investigation of the roles of such molecules in microbial interactions and their possible effects on the host.
Pullquote
A secreted aspartyl protease from Malassezia globosa exhibits unexpected hydrolytic activity that acts on Staphylococcus aureus biofilms.
Fungi of the genus Malassezia
The skin of humans and animals is colonized by numerous microorganisms that constitute the skin microbiome. These include commensal bacteria and fungi that establish a non-pathogenic interaction with the host, but in certain circumstances, such as immune conditions within the host, or intrinsic microbial imbalance, some commensals can become pathogenic or beneficial. The study by Li and colleagues in this issue of the Journal of Investigative Dermatology (Li et al., 2017) reports a previously unknown beneficial role for a secreted aspartyl protease produced by the fungus Malassezia globosa.
Malassezia species are the most abundant fungal skin inhabitant of humans (Findley et al., 2013). This genus includes a monophyletic group of yeasts within the basidiomycetes that is closely related to the smut plant pathogen Ustilago maydis and more distantly related to skin-infecting fungal species, such as Candida albicans and the dermatophytes. The unique Malassezia genus currently includes 17 species, of which M. globosa, M. restricta, and M. sympodialis are most commonly associated with humans (Sparber and Leibund Gut-Landmann, 2017). A common feature is the inability of Malassezia to synthesize lipids due to the loss of the fatty acid synthase gene, and as a consequence these species mainly colonize sebaceous skin. Comprehensive molecular and genetic research on Malassezia has highlighted unique features compared with other fungi, such as a reduction in genome size (as small as ~7 to ~9 Mbp) with extensive loss of genes encoding hydrolases and other enzymes involved in carbohydrate metabolism, and concomitant expansion of genes encoding secreted lipases and proteases thought to mediate lipid utilization and play roles in pathogenesis (Wu et al., 2015).
Is Malassezia a beneficial skin inhabitant?
Although Malassezia are commensal inhabitants of the skin, they are associated with several skin disorders, including pityriasis versicolor, dandruff, seborrheic dermatitis, atopic dermatitis (AD), and folliculitis (Saunders et al., 2012). It is presumed that Malassezia strains associated with these clinical conditions are derived from normal commensal strains, but this has not been experimentally proven. As a result, the pathophysiology of Malassezia and the mechanisms that govern its shift from commensal to pathogen are poorly understood.
It is reasonable to hypothesize that the immune system has adapted to the commensal nature of Malassezia and thus tolerates its presence on the skin, but the immune system can also recognize the fungus as a pathogen and respond. Malassezia can be recognized by the host, either directly through membrane bound pattern recognition receptors or indirectly through production of inflammatory metabolites released by hydrolysis of sebum by fungal lipases and proteases. These metabolites are thought to be responsible for clinical dandruff and seborrheic dermatitis (White et al., 2014).
Another way in which Malassezia is known to interact with the immune system is through the production of specific allergens. These include a set of nine conserved and four unknown proteins identified in the M. sympodialis genome (Gioti et al., 2013). Approximately half of adult patients with AD have allergen-specific IgE and T-cell reactivity to Malassezia, which are rarely observed in respiratory allergies, suggesting a host-microbe interaction related to the skin environment in AD (White et al., 2014). A clinical hallmark of AD is dry, itchy skin with increased permeability and water loss. There is a distinct reciprocal expression pattern of induced inflammatory genes and repressed lipid metabolism genes (Sääf et al., 2008). This results in an inhospitable environment for the lipid-dependent Malassezia, and there is an increased pH level on AD skin that stimulates the yeast to release more allergens compared to culture at normal skin pH (Selander et al., 2006).
Yet another way for Malassezia to communicate with the host is through the release of extracellular nanosized vesicles, designated MalaEx, that carry allergens and can induce inflammatory cytokine responses (Gehrmann et al., 2011). MalaEx, which contain small RNAs as a cargo (Rayner et al., 2017), have the potential—like other fungal extracellular vesicles—to deliver functional messenger RNAs and microRNA-like RNAs to recipient host cells, thereby interfering with the host RNAi machinery to silence host immune genes and cause infection (Weiberg et al., 2013).
While establishing the basis and the relationship between commensalism and pathogenicity is a recognized and active research field, Malassezia interactions with other microbes on the skin and their effects on the host are largely unexplored. Li and colleagues (Li et al., 2017) reported that a secreted aspartyl protease produced by M. globosa displays anti-biofilm properties against Staphylococcus aureus, which is responsible for skin and soft tissue infections and which frequently is associated with skin infections in AD. Previous genomic studies revealed a robust secretory repertoire for all species belonging to the genus Malassezia, consistent with the importance of these hydrolytic enzymes for Malassezia biology and pathophysiology (Wu et al., 2015, Zhu et al., 2017).
RNAseq analysis revealed that 2 of the 15 predicted aspartyl proteases of M. globosa, encoded by MGL_3328 and MGL_1932, were highly expressed in vitro during growth in both rich and minimal media. Through biochemical analyses, Li and colleagues (Li et al., 2017) characterized a specific aspartyl protease activity, revealing strong cleavage of specific substrates (RPKPYAvWM and RPKPVEvWR), increasing activity in the first 16 h of growth, and a pH optimum between 4 and 5. Mass spectrometry and trypsin in-gel digest analyses identified MGL_1932 as the major aspartyl protease in culture, and it was named MgSAP1 (from M. globosa Secreted Aspartyl Protease 1). Further analyses demonstrated that MgSAP1 has a cleavage preference adjacent to aromatic residues, in particular tryptophan, which distinguishes it from other known proteases.
Li and colleagues then aimed to establish if the secreted protease MgSAP1 was 1) produced in situ on human skin, 2) had any role in Malassezia biology and/or pathophysiology, and 3) could potentially mediate interactions with existing skin microflora. MgSAP1 was expressed on the faces of 17 of 18 healthy male and female volunteers, indicating its ubiquitous production. Among the skin microflora, S. aureus coexists in many sites with M. globosa (Oh et al., 2014) and produces protein A (SpA), which is essential for biofilm formation, a major S. aureus virulence factor (Archer et al., 2011). Because SpA is rich in lysine and aromatic residues, the authors hypothesized that it could be hydrolyzed by MgSAP1, thus impacting S. aureus pathogenicity. Strikingly, MgSPA1 rapidly degraded recombinant SpA in vitro and strongly reduced the in situ volume of S. aureus biofilm formation without affecting bacterial viability. A representation of MgSAP1-mediated biofilm destruction is shown in Figure 1.
Figure 1.
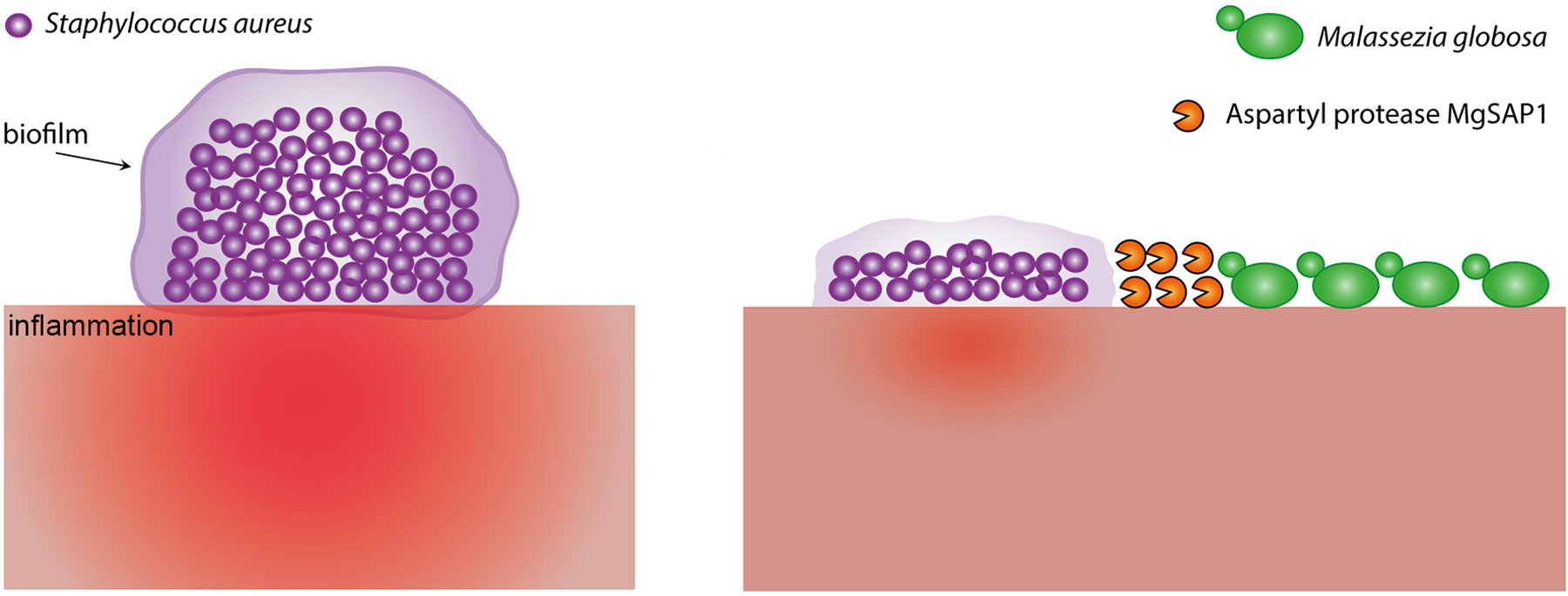
Staphylococcus aureus biofilms are inhibited by an aspartyl protease secreted by Malassezia globosa. On the left, a schematic view illustrates a biofilm formed on the skin by the bacterium S. aureus and the inflammation that is produced as a host response. On the right, formation of the biofilm is inhibited by the secreted aspartyl protease MgSAP1 of M. globosa, and as a consequence there is predicted reduced inflammation of the skin.
This study raises questions about the specific roles of Malassezia proteases, as their common in situ-expression has been correlated with their involvement in pathogenesis (White et al., 2014). This predicted function as virulence factors is likely based on the role of analogous proteases in more well-studied fungi that can live on the skin such as the dermatophytes and C. albicans. In fact, their genome comparisons reveal a significant expansion of genes predicted to encode proteases in Malassezia, dermatophytes, and C. albicans, suggesting an important role of proteases for evolution and adaptation on the skin. In C. albicans, secreted aspartyl proteases have long been recognized as virulence factors that act by promoting adhesion to, invasion of, and damage to epithelial cells and tissues and by inducing the secretion of pro-inflammatory cytokines independently from their proteolytic activity (Pietrella et al., 2010). With respect to dermatophytes, these fungi secrete an abundance of proteases for multiple functions, such as adherence on the skin, keratin digestion for penetration, and modulation of cell metabolism and the host immune system (de Hoog et al., 2017, White et al., 2014).
The study by Li and colleagues (Li et al., 2017) represents a paradigmatic example of a fungal-bacterial interaction that is mediated by a secreted molecule and that potentially results in a beneficial effect for the host. Fungal and bacterial interactions are common, although they are understudied and technically challenging to elucidate. However, there is an increasing awareness of their biological and pathophysiological importance (Peleg et al., 2010). As an example, Graham and colleagues recently described an interesting interaction between the bacterium Enterococcus faecalis and C. albicans. These two microbes occupy overlapping niches in the mammalian microbiome and display antagonistic activity resulting in reduced virulence for both microbes. In C. albicans, this reduced virulence was attributed to the reduction of hyphal development and biofilm formation due to the activity of the secreted E. faecalis bacteriocin, EntV (Graham et al., 2017).
Conclusions
In conclusion, the study of Li and colleagues (Li et al., 2017) reveals that the commensal and sometimes pathogenic fungus M. globosa produces an aspartyl protease, unique in its evolutionary trajectory and substrate specificity, which impacts a recognized virulence attribute of a commensal and pathogenic bacteria, thus potentially resulting in unexpected benefits for the host. This finding underscores the importance of in vivo studies to determine the function of unknown proteins during microbial interactions, and the beneficial or detrimental impact that they might have on the host. For example, whether the characterized MgSAP1 protease also plays a role in Malassezia pathogenesis as described for other aspartyl proteases is still unknown. There is a crucial need to develop and establish reliable host-pathogen models to study how Malassezia interacts with both cells in the skin and different immune cells, and with other microbes that live on the skin, as well as the induced immune responses in robust mouse and humanized-mouse models of infection. This, coupled with the recent development of tools for targeted and insertional mutagenesis (Celis et al., 2017, Ianiri et al., 2016), will help to characterize the mechanisms that establish the commensal, pathogenic, and beneficial lifestyles of Malassezia species on the skin.
References
- Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME. Staphylococcus aureus biofilms: Properties, regulation and roles in human disease. Virulence 2011;2(5):445–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis AM, Vos AM, Triana S, Medina CA, Escobar N, Restrepo S, et al. Highly efficient transformation system for Malassezia furfur and Malassezia pachydermatis using Agrobacterium tumefaciens-mediated transformation. J Microbiol Methods 2017;134:1–6. [DOI] [PubMed] [Google Scholar]
- de Hoog S, Monod M, Dawson T, Boekhout T, Mayser P, Gräser Y. Skin fungi from colonization to infection. Microbiol Spectr 2017;5(4):FUNK-0049–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013;498(7454):367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann U, Qazi KR, Johansson C, Hultenby K, Karlsson M, Lundeberg L, et al. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses--Novel mechanisms for host-microbe interactions in atopic eczema. PLoS One 2011;6(7):e21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioti A, Nystedt B, Li W, Xu J, Andersson A, Averette AF, et al. Genomic insights into the atopic eczema-associated skin commensal yeast Malassezia sympodialis. mBio 2013;4(1):e00572–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CE, Cruz MR, Garsin DA, Lorenz MC. Enterococcus faecalis bacteriocin EntV inhibits hyphal morphogenesis, biofilm formation, and virulence of Candida albicans. Proc Natl Acad Sci U S A 2017;114(17):4507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianiri G, Averette AF, Kingsbury JM, Heitman J, Idnurm A. Gene function analysis in the ubiquitous human commensal and pathogen Malassezia genus. mBio 2016;7(6):e01853–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Goh B, Teh WK, Jiang Z, Goh JPZ, Goh A, et al. Skin commensal Malassezia globosa secreted protease attenuates Staphylococcus aureus biofilm formation. J Investig Dermatol 2017;S0022–202X(17)33276–1. [DOI] [PubMed] [Google Scholar]
- Oh J, Byrd AL, Deming C, Conlan S, Kong HH, Segre JA. Biogeography and individuality shape function in the human skin metagenome. Nature 2014;514(7520):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg AY, Hogan DA, Mylonakis E. Medically important bacterial-fungal interactions. Nat Rev Microbiol 2010;8(5):340–9. [DOI] [PubMed] [Google Scholar]
- Pietrella D, Rachini A, Pandey N, Schild L, Netea M, Bistoni F, et al. The inflammatory response induced by aspartic proteases of Candida albicans is independent of proteolytic activity. Infect Immun 2010;78(11):4754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner S, Bruhn S, Vallhov H, Andersson A, Billmyre RB, Scheynius A. Identification of small RNAs in extracellular vesicles from the commensal yeast Malassezia sympodialis. Sci Rep 2017;7:39742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sääf AM, Tengvall-Linder M, Chang HY, Adler AS, Wahlgren C-F, Scheynius A, et al. Global expression profiling in atopic eczema reveals reciprocal expression of inflammatory and lipid genes. PLoS One 2008;3(12):e4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders CW, Scheynius A, Heitman J. Malassezia fungi are specialized to live on skin and associated with dandruff, eczema, and other skin diseases. PLoS Pathogens 2012;8(6):e1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander C, Zargari A, Mollby R, Rasool O, Scheynius A. Higher pH level, corresponding to that on the skin of patients with atopic eczema, stimulates the release of Malassezia sympodialis allergens. Allergy 2006;61(8):1002–8. [DOI] [PubMed] [Google Scholar]
- Sparber F, LeibundGut-Landmann S. Host responses to Malassezia spp. in the mammalian skin. Front Immunol 2017;8:1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiberg A, Wang M, Lin FM, Zhao H, Zhang Z, Kaloshian I, et al. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 2013;342(6154):118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TC, Findley K, Dawson TL Jr., Scheynius A, Boekhout T, Cuomo CA, et al. Fungi on the skin: Dermatophytes and Malassezia. Cold Spring Harb Perspect Med 2014;4(8):a019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Zhao H, Li C, Rajapakse MP, Wong WC, Xu J, et al. Genus-wide comparative genomics of Malassezia delineates its phylogeny, physiology, and niche adaptation on human skin. PLoS Genetics 2015;11(11):e1005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Engstrom PG, Tellgren-Roth C, Baudo CD, Kennell JC, Sun S, et al. Proteogenomics produces comprehensive and highly accurate protein-coding gene annotation in a complete genome assembly of Malassezia sympodialis. Nucleic Acids Res 2017;45(5):2629–43. [DOI] [PMC free article] [PubMed] [Google Scholar]


