Highlights
-
•
Film significantly lowered the patient-reported symptoms of the skin two weeks following RT.
-
•
Almost all patients treated after mastectomy would prefer film on their entire chest wall.
-
•
A significantly lower severity of radiodermatitis with film vs standard care was present after mastectomy.
-
•
Almost all breast cancer patients would prefer film as a standard offer during radiotherapy.
Keywords: Breast cancer, PRO, Radiotherapy, Mepitel film, Radiodermatitis
Abstract
Background and Purpose
Radiodermatitis is a well-known toxicity of radiotherapy and barrier film has been shown to reduce the severity of radiodermatitis. We have validated prior findings in a Danish cohort, using a similar barrier film and patient reported outcomes.
Materials and Methods
101 Danish breast cancer patients were included at three radiotherapy centres. Based on randomization either the lateral or medial part of their chest was covered by Mepitel film; making the patients their own control. The primary endpoint was patient reported symptoms and experience. A secondary endpoint was radiotherapy staff evaluation of dermatitis.
Results
Within the skin area covered by film, the patients reported a statistical significant lower level of pain (p < .001), itching (p = 0.005), burning sensation (p = 0.005) as well as edema (p = 0.017) and reduced sensitivity (p < .001). Most patients (76%) would have preferred film on the entire treatment area (p < 0.001) and Mepitel Film as a standard treatment option (84%) (p < 0.001). Patients treated after mastectomy had a significantly lower severity of radiation-induced dermatitis with film at the end of RT compared to standard care (p = 0.005). However, in the blinded staff evaluation, no significant differences were found at follow-up.
Conclusions
Patients reported reduced symptoms from the skin with Mepitel Film and the majority would have preferred film as a standard offer to cover their entire treatment area. Especially women treated after mastectomy had a significantly lower level of radiodermatitis and preferred the film over standard care.
Introduction
Radiodermatitis is a common acute normal tissue response to radiotherapy and the radiosensitivity of the skin is influenced by patient- and treatment related factors as well as physical dose factors [1]. It occurs during or shortly after completion of treatment due to the integumentary system response to exposure of ionizing radiation, which depletes stem cells from the basal layer of the epidermis [2]. Experienced pain and discomfort due to radiodermatitis makes it important to prevent and manage skin reactions [3]. In the past 10 years several studies have investigated preventive strategies with steroidal or non-steroidal topical treatments, oral systemic therapy, light emitting diode treatment (LED) and barrier film [3], [4], [5], [6], [7], [8].
The use of a Safetac technology-based film (Mepitel Film, Mölnlycke Healthcare) to protect the skin during the course of radiotherapy was investigated in 2014. Unlike other semi-permeable dressings, the film can be used from the first day of radiotherapy, potentially stays on for weeks and the transparency allows skin reactions to be assessed without removing the film [9]. Herst et al. succeeded in reducing their rate of moist desquamation from 26% when treated with aqueous cream compared to 0% when treated with Mepitel Film (n = 78) [6], [10]. A case study with 3 cases also reported improved patient experience and reductions of severe skin reactions [5].
CT-based treatment planning with IMRT (Intensity-Modulated Radiation Therapy) has resulted in more homogenous dose distributions [4], [11]. Results from the Danish Breast Cancer Group (DBCG) HYPO protocol showed on that grade 2 dermatitis (on the scale of the Radiation Therapy Oncology Group (RTOG-scale)) in week 5 of radiotherapy was 24% when treated with 15 fractions (40 Gray) and 67% when treated with 25 fractions (50 Gray) compared to 64% in the Herst population (46% <45 Gy) [6], [12]. Few studies have used patient-rated measures as a primary endpoint [13].
The primary endpoint of this Danish study was to investigate patient-reported symptoms related to radiodermatitis and to examine patient preferences using Mepitel Film during their treatment course compared to standard skin care. A secondary outcome was to validate the study of Herst in a Danish cohort with a lower incidence of severe radiodermatitis compared to the Herst et al population.
Materials and methods
Study design
The study was a multicentre trial with participation of three Danish hospitals: Aarhus University Hospital (Herning site), Vejle Hospital, and Odense University Hospital.
All patients had either the lateral or medial part of the treatment area covered by film based on a randomization; making the patients their own control. The randomization procedure was conducted by assigned radiotherapists (RTTs) at each hospital in the online system RedCap (Research Electronic Data Capture) provided by Odense Patient Data Explorative Network (OPEN). A block randomization was conducted stratifying for hospital to balance at each institution the number of patients having film applied at the medial or lateral part of the chest. The last follow-up was the 27th of April 2016.
Ethics
The study was approved by the Danish Data Protection Agency (no. 2008-58-0035) and the Regional Committees on Health Research Ethics for Southern Denmark (project-ID S-20150112). Oral and written informed consent was obtained before randomization.
Questionnaires
A questionnaire was developed as no available instruments covered all the preferred items for patient reported outcomes and experiences. The questionnaire consisted of four sections – two with patient-reported outcome measures (PROM) regarding skin symptoms, one section with patient-reported experience measures (PREM) related to the use and preference of the barrier film and finally a section for staff evaluation of dermatitis.
The PROM-questionnaire was developed guided by existing validated questionnaires [14], [15]. The PREM-questionnaire consisted of seven questions regarding comfort and preferences and both questionnaires used a 4-Point Likert Scale. The questionnaires were paper-based and cognitively validated by 3 randomly chosen patients. The patients answered the questionnaires on the day of their final radiotherapy treatment and a similar one at the two-week follow-up.
Blinded skin grading
Grade of radiodermatitis on both sides of the chest was assessed mutually by two RTTs at the final treatment and at the two-week follow-up. The RTTs were not involved in the treatment course of the patients and had no knowledge of the randomized treatment. To perform a blinded grading the film was removed the day before the final treatment. Patient blinding was not possible since the film was visible to the patient.
For skin grading, the RTOG/EORTC scale (grade 0–4) was used as it was the scale used for skin assessment in the departments involved in the study [16].
Participants
All women referred to postoperative adjuvant radiotherapy for breast cancer from October 1st 2015 to February 29th 2016 at the three departments were offered participation. The exclusion criteria were lack of compliance, not understanding Danish or inclusion in the ongoing Danish HYPO PBI protocol. Also, the women had to consent to a two-week follow-up.
Statistics
Patient characteristics were summarized separately for women with breast irradiation and chest wall irradiation. Differences in the distribution of the variables between these two groups were compared by Pearson x2 test, unpaired t-test and Wilcoxon rank-sum test and a significance level of the estimated P-values was chosen to be 0.05.
Descriptive statistics were made on patient-reported symptoms and they were compared using Wilcoxon signed-rank test (paired analyses). Subgroup analyses were performed of the distribution of severe pain and sensitivity according to type of surgery, total dose and adjuvant chemotherapy.
Patient-reported experiences were dichotomized and binomial tests were used to test the distribution of the observed positive and negative difference from the binomial distribution. Descriptive statistics and Wilcoxon signed-rank test were used to investigate the differences in radiodermatitis with or without Mepitel Film. The data was analyzed using Stata14.
Radiotherapy
Danish breast cancer patients are treated according to DBCG guidelines with loco-regional radiotherapy on linear accelerators using CT-based dose-planning and intensity modulated radiotherapy techniques (IMRT). Dose planning aimed at covering the residual breast/chest wall and regional lymph nodes with 95% and 90% of the prescribed dose, respectively. Regarding hotspots, the volume receiving more than 105% should be kept below 2% of the breast volume. For mastectomies the corresponding dose level was 107%. Absolute maximum dose should be 108% and 110% for hypo and normo fractionated RT, respectively.
Since March 2014 hypo-fractionated breast radiotherapy based on 40 Gy/15 fractions in 3 weeks has been standard therapy in Denmark to patients with an indication for breast only radiotherapy [17].
Information and application of film
Guidelines for digital data reporting, film application instructions and patient information were created to homogenize the management of Mepitel Film at the three departments. Trained RTTs managed the change of film every 1–2 week or more frequent if necessary.
At the first treatment fraction, the film was applied in treatment position to ensure that the shape of the breast could be replicated. The chest was divided into a medial and lateral side and according to the randomization result the film was applied in a cranial-caudal position starting 2 cm below the inframammary fold. For mastectomies this was measured according to the opposite breast.
On the opposite side the skin was treated according to Danish National Clinical Guidelines. Daily washing and use of moisturizing lotion was recommended and for itching skin a lotion with glucocorticoids [18].
Mepitel Film has a clinically insignificant bolus effect of 0.12 mm as confirmed in the previous study by Herst et al. [6].
Results
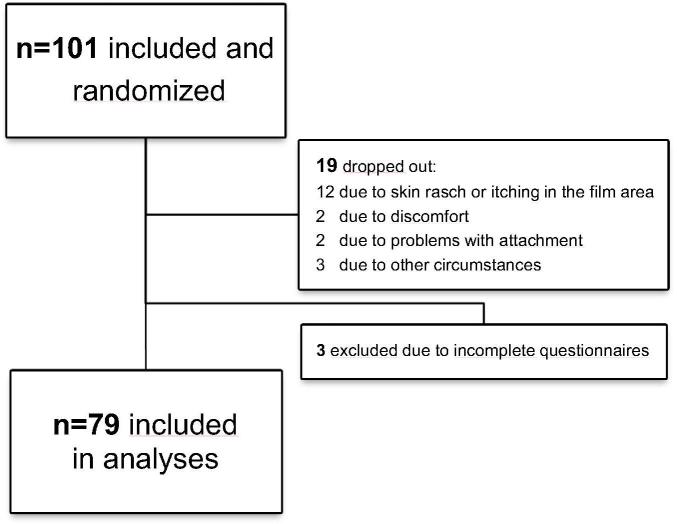
At the three centres 101 patients were included and randomized. 19 patients had the film removed and 3 patients had incomplete questionnaires and were excluded from analyses, leaving 79 evaluable patients (Fig. 1).
Fig. 1.
Flowchart of patients included in the Danish Mepitel Film Study 2015–2016.
The mean age of the patients included was 61.9. The majority of the patients (n = 63) were treated on the residual breast after lumpectomy and the other 16 patients on their chest wall. No significant differences in the distribution of age, BMI, smoking status or comorbidity were found (Table 1).
Table 1.
Patient characteristics of Danish breast cancer patients treated with Mepitel Film during radiotherapy (n = 79). The P-value measures whether the distributions for breast and chest wall are equal.
| Combined, n = 79 (%) | Breast, n = 63 (%) | Chest wall, n = 16 (%) | P-value | |
|---|---|---|---|---|
| Randomization | ||||
| Medial | 38 (48.1) | 32 (50.8) | 6 (37.5) | 0.342a |
| Lateral | 41 (51.9) | 31 (49.2) | 10 (62.5) | |
| Age, mean (range 31–82) | 61.9 | 61.8 | 62.0 | 0.957b |
| BMI, mean | 26.5 | 26.1 | 28.0 | 0.226b |
| Smoking status | ||||
| Never | 35 (44.3) | 28 (44.5) | 7 (43.7) | |
| Former | 28 (35.4) | 22 (34.9) | 6 (37.5) | 0.976a |
| Current | 16 (20.3) | 13 (20.6) | 3 (18.8) | |
| Comorbidity | ||||
| Yes | 26 (32.9) | 20 (31.8) | 6 (37.5) | 0.889a |
| No | 53 (67.1) | 43 (68.2) | 10 (62.5) | |
| Treatment types (1 missing) | ||||
| Sentinel node neg. or in situ | 49 | 49 | 0 | |
| Mastectomy <10 LN removed | 3 | 0 | 3 | |
| Mastectomy ≥10 LN removed | 13 | 0 | 13 | |
| Lumpectomy <10 LN removed | 4 | 4 | 0 | |
| Lumpectomy ≥10 LN removed | 9 | 9 | 0 | |
| Radiation therapy | ||||
| 40 Gy/15 fx | 59 (74.7) | 54 (85.7) | 5 (31.2) | <0.001a |
| 50 Gy/25 fx | 20 (25.3) | 9 (14.3) | 11 (68.8) | |
| Boost, yes | 6 (7.6) | 6 (9.7) | 0 | |
| 5,75 Gy (simultaneous integrated) | 1 | 1 | 0 | 0.195a |
| 10 Gy | 4 | 4 | 0 | |
| 16 Gy | 1 | 1 | 0 | |
| Bolus 5 mm | ||||
| Yes | 16 (20.3) | 0 (0) | 16 (100) | <0.001a |
| No | 63 (79.7) | 63 (100) | 0 (0) | |
| Volume of irradiated breast tissue (cm3), median (range) | 534 (0–1.746) | 636 (131–1746) | 226.5 (110–990) | <0.001c |
| Chemotherapy | ||||
| Yes | 42 (53) | 28 (44) | 14 (88) | 0.002a |
| No | 37 (47) | 35 (56) | 2 (12) | |
Differences in the distribution of the variables were compared by:
Pearson x2 test.
Unpaired t-test.
Wilcoxon rank-sum test.
Patient-reported symptoms
Within the skin area covered by film, patients reported a statistically significantly lower level of pain (p < .001) and sensitivity of the skin (p < .001) as well as itching (p = 0.005), burning sensation (p = 0.005) and edema (p = 0.017) two weeks following RT (Table 2).
Table 2.
Patient-reported symptoms from the skin with Mepitel film opposed to standard care 14 days after RT. The P-value measures whether the distributions for the Mepitel and standard of care area are equal. Number of evaluable pairs are indicated after symptom name.
| Mepitel film Numbers of reported skin symptoms |
Standard care Numbers of reported skin symptoms |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Not at all | A little | Quite a bit | Very much | Not at all | A little | Quite a bit | Very much | P-value* | |
| Tenderness, discomfort or pain (n = 78) | 39 | 32 | 7 | 0 | 18 | 40 | 16 | 4 | <0.001 |
| Itching (n = 77) | 36 | 30 | 9 | 2 | 20 | 41 | 11 | 5 | 0.005 |
| Burning sensation (n = 78) | 52 | 18 | 7 | 1 | 37 | 28 | 10 | 3 | 0.005 |
| Edema (n = 78) | 55 | 20 | 3 | 0 | 45 | 27 | 6 | 0 | 0.017 |
| Sensitive skin (n = 78) | 44 | 28 | 5 | 1 | 27 | 36 | 11 | 4 | <0.001 |
| Flaky skin (n = 77) | 54 | 17 | 6 | 0 | 49 | 17 | 9 | 2 | 0.066 |
| Effect on work/daily activities (n = 77) | 66 | 7 | 4 | 0 | 62 | 11 | 4 | 0 | 0.253 |
P-values are calculated using Wilcoxon signed-rank test (paired analyses).
The proportion of severe pain or sensitivity of the skin with or without film was assessed to search for subgroups clearly having a benefit of the barrier film. For the mastectomies, severe sensitivity of the skin treated with standard care was experienced by more than half of the women (53%), but no severe sensitivity was reported in the skin area covered by film (Table 3).
Table 3.
Subgroup analyses of the proportion of patients who 14 days after RT reports severe skin pain or sensitivity related to the film and non-film area. The subgroups are type of surgery, radiation dose, and administration of chemotherapy. E.g. within the group of patients having chemotherapy 34% of the patients reported severe pain from the standard of care area while 12% reported severe pain from the Mepitel covered area.
| Severe pain of the skin |
Severe sensitivity of the skin |
|||
|---|---|---|---|---|
| Standard care (%) | Mepitel film (%) | Standard care (%) | Mepitel film (%) | |
| Type of surgery | ||||
| Mastectomy (n = 16) | 50 | 13 | 53 | 0 |
| Lumpectomy (n = 63) | 19 | 8 | 11 | 10 |
| Radiation dose | ||||
| 40 Gy (n = 59) | 21 | 5 | 12 | 7 |
| 50 Gy (n = 20) | 40 | 20 | 42 | 11 |
| Chemotherapy | ||||
| Yes (n = 42) | 34 | 12 | 24 | 10 |
| No (n = 37) | 16 | 5 | 14 | 5 |
Patient-reported experience
One of the primary endpoints of the study was whether patients based on their personal experience with radiodermatitis and Mepitel Film, would prefer Mepitel film as a standard offer. This was the case among 84% of the patients. 94% of the patients with a mastectomy would have preferred barrier film on their entire chest wall compared to 71% of patients with a lumpectomy. The majority of those treated with a total treatment dose of 50 Gy (85%) preferred full film coverage but also 72% of those treated with 40 Gy (Table 4).
Table 4.
Patient-reported experience with the use of Mepitel Film 14 days after RT (n = 79).
| % | P-valuea | |
|---|---|---|
| Mepitel Film was comfortable on the skin | 92.4 | <0.001 |
| Mepitel Film was easy to handle | 93.6 | <0.001 |
| Mepitel Film was curling at the edges | 94.9 | <0.001 |
| It made a difference for me, wearing the film | 77.0 | <0.001 |
| I would not have wanted to be treated without the film (mastectomies 100%) | 75.0 | <0.001 |
| I would have preferred Mepitel Film covering my entire breast | 76.0 | <0.001 |
| Mepitel Film should be a standard offer for all women undergoing breast irradiation | 83.5 | <0.001 |
Binomial tests that test the null hypothesis that the two outcomes (yes/no) occur with equal probability.
None of the patients with a mastectomy, who completed the radiotherapy course with Mepitel Film on half of their chest (n = 16) would have wanted to be treated without the film compared to 30% of the patients with a lumpectomy (p = 0.018) (Table 4).
Observer-rated radiodermatitis
Overall, there was no significant difference (p = 0.100) in radiodermatitis with or without film evaluated on the last day of radiotherapy also after a lumpectomy (p = 0.835) and those treated with a total dose of 40 Gy (p = 0.637). There was, however, a significant difference in skin toxicity at the last day of radiotherapy among patients after a mastectomy (p = 0.005) and also in the group of patients treated with a total dose of 50 Gy (p = 0.002) (Table 5).
Table 5.
Blinded gradings of radiodermatitis with or without barrier film (RTOG-scale). The P-value measures whether the distributions for the Mepitel and standard of care area are equal.
| 3 missing gradings | Grade 0 | Grade 1 | Grade 2 | Grade 3 | P-value* |
|---|---|---|---|---|---|
| Last day of RT (n = 76) | |||||
| Mepitel film | 24 (31%) | 47 (62%) | 5 (7%) | 0 | 0.100 |
| Standard care | 21 (28%) | 44 (58%) | 10 (13%) | 1 (1%) | |
| 14 days after RT (n = 79) | |||||
| Mepitel film | 20 (25%) | 53 (67%) | 6 (8%) | 0 | 0.493 |
| Standard care | 20 (25%) | 49 (62%) | 10 (13%) | 0 | |
P-values are calculated using Wilcoxon signed-rank test (paired analyses).
Fourteen days after radiotherapy the difference in radiodermatitis was, likewise, overall non-significant (p = 0.493) also when stratified for lumpectomy (p = 0.549), mastectomy (p = 0.739) and total dose 40 Gy (p = 0.835) as well as 50 Gy (p = 0.366) (Table 5).
Film application and patient dropout
For various reasons 19 patients (18.8%) dropped out of the study (Fig. 1). Only two patients dropped out due to problems handling the film, two patients wanted the film removed, three films were removed for treatment-related reasons and twelve due to itching, skin rasch with erythema, dry and flaky skin and due to spots/pustules in the film area (n = 3). Assuming all twelve patients had reported one symptom level worse on the film side compared to standard of care the result from skin pain would still be statistically significant with a P-value of 0.009 (data not shown).
The majority used a maximum of 4 barrier films during their treatment (83%) and one-third of the patients with 15 fractions used 2 films opposed to 3 films when treated with 25 fractions (range 1–9). One film (15 × 20 cm) was enough to cover half of the chest.
Discussion
The use of Mepitel Film in the current study decreased the patient symptom burden significantly two weeks following RT especially the patients treated on their chest wall had no severe sensitivity from the skin area where the film was applied (53% with standard care). Although the majority would have preferred film as a standard offer used on the entire treatment area, there was no overall statically significant difference in observer-rated radiodermatitis two weeks after RT. However, those treated after a mastectomy especially preferred film on their entire treatment area, the difference in radiodermatitis also being significant at the last day of radiotherapy.
A recent review of studies using of a thicker dressing, Mepilex Lite, participants scored lower levels of pain, discomfort, itchiness and burning [9]. Herst found with Mepitel Film decreasing levels of pain, itching and burning skin reported by the patients in the patient component of the RISRAS-scale. However, the patient-reported symptoms were not clearly specified and the level of sensitivity or edema was not disclosed [6]. The patient reported outcomes in the studies were, however, biased as the patients were not blinded for skin treatment with or without film like in the current study.
In the study by Herst the majority preferred the film over standard care and almost all of the patients reported that the film was comfortable to wear as well as easy to handle. However, 23% (n = 18) of the patients did not answer the questionnaire related to advantages an d disadvantages of Mepitel Film [6]. In the current study 3 out of 4 patients would have liked the film over standard care and all of the mastectomies preferred the barrier film (n = 16) with a complete response rate on patient experience (n = 79).
The trial endpoint of this study was significant as 84% (n = 66) based on their personal experience with radiodermatitis and Mepitel Film would prefer Mepitel Film as a standard offer. This was similar to the findings by Herst, where the majority of the patients answering the exit questionnaires (n = 55) preferred Film to cream [6].
Overall, the blinded skin grading (RTOG) in the current study indicated a less severe grade of radiodermatitis where the skin had been covered by Mepitel Film. However, there is a major difference in skin reactions when using standard care in the Danish cohort opposed to the results by Herst et al. [6]. The current Danish study found 14% grade 2–3 dermatitis with standard care opposed to 72% in the New Zealand study. However, with Mepitel Film the studies both observed 8% having grade 2 dermatitis. Part of the explanation might be that the proportion of patients treated with a prescription dose ≥50 Gy was 50% in the study by Herst (n = 39) compared to 25% (n = 20) in the current Danish study [10].
In the current Danish study up to 28% had no skin reaction (grade 0) when treated with standard care opposed to 0% in the study by Herst. However, 56% of the Herst population had no skin reaction with Mepitel Film, which was not the case in the current study with 25–31% having grade 0 skin reaction with the barrier film.
There are some limitations in our study. The dropouts were not included in the final analyses since the self-reported questionnaires were not available for these patients. Dropout of twelve patients due to skin rash could be problematic for the conclusion of the article. However, the dropout rate was very different among the centers – ranging from 9% to half the patients. This indicates a bias, one center being more inclined to remove the film; an observation confirmed by that center. As earlier mentioned, had all twelve patients reported one symptom level worse on the film side compared to standard of care skin pain would still be statistically significant indicating that the dropout do not impact the main conclusion of the study.
Also, standard skin care in the three participating centres in the current study varied between centres. All departments recommended washing and the use of an unscented moisturizing lotion according to the national guidelines, but some departments supplied a specific lotion for the patients while others asked the patients to buy their own.
The strength of this study was the blinding of the RTTs. In the review of semi-permeable dressings neither the research RTT nor the patients were blinded in six RCTs [9]. However, in order to have a blinded grading of radiodermatitis in the current study Mepitel Film had to be removed the day before the last RT treatment. The film therefore did not provide protection against friction between the patient skin and cloths the following 14 days, which may have decreased the reported difference between area covered by film and area of standard care.
Conclusion
Patients reported reduced symptoms of the skin in the chest side covered by Mepitel Film. The majority would have preferred the film covering their entire treatment area, especially patients treated on their chest wall. Based on their own experience the patients recommend that the use of Mepitel Film should be a standard offer to other patients irradiated for breast cancer. Patients treated after a mastectomy had a significantly lower severity of radiation-induced dermatitis with film at the end of RT, but no significant differences were found at follow-up.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
The authors wish to thank the departments of radiotherapy in Vejle, Herning and Odense for their work with collecting data during the study period and OPEN, Odense Patient data Explorative Network, Odense University Hospital, Odense, Denmark.
References
- 1.Barnett G.C., Kerns S.L., Noble D.J., Dunning A.M., West C.M., Burnet N.G. Incorporating genetic biomarkers into predictive models of normal tissue toxicity. Clin Oncol (R Coll Radiol) 2015;27(10):579–587. doi: 10.1016/j.clon.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Feight D., Baney T., Bruce S., McQuestion M. Putting evidence into practice. Clin J Oncol Nurs. 2011;15(5):481–492. doi: 10.1188/11.CJON.481-492. [DOI] [PubMed] [Google Scholar]
- 3.Chan R.J., Webster J., Chung B., Marquart L., Ahmed M., Garantziotis S. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2014;14:53. doi: 10.1186/1471-2407-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McQuestion M. Evidence-based skin care management in radiation therapy: clinical update. Semin Oncol Nurs. 2011;27(2):e1–e17. doi: 10.1016/j.soncn.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Morgan K. Radiotherapy-induced skin reactions: prevention and cure. Br J Nurs. 2014;23(16):s6–s32. doi: 10.12968/bjon.2014.23.Sup16.S24. S24. [DOI] [PubMed] [Google Scholar]
- 6.Herst P.M., Bennett N.C., Sutherland A.E., Peszynski R.I., Paterson D.B., Jasperse M.L. Prophylactic use of Mepitel Film prevents radiation-induced moist desquamation in an intra-patient randomised controlled clinical trial of 78 breast cancer patients. Radiother Oncol. 2014;110(1):137–143. doi: 10.1016/j.radonc.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Ulff E., Maroti M., Serup J., Nilsson M., Falkmer U. Prophylactic treatment with a potent corticosteroid cream ameliorates radiodermatitis, independent of radiation schedule: a randomized double blinded study. Radiother Oncol. 2017;122(1):50–53. doi: 10.1016/j.radonc.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Graham P., Browne L., Capp A., Fox C., Graham J., Hollis J. Randomized, paired comparison of No-Sting Barrier Film versus sorbolene cream (10% glycerine) skin care during postmastectomy irradiation. Int J Radiat Oncol Biol Phys. 2004;58(1):241–246. doi: 10.1016/s0360-3016(03)01431-7. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Castro M., Martin-Gil B., Pena-Garcia I., Lopez-Vallecillo M., Garcia-Puig M.E. Effectiveness of semi-permeable dressings to treat radiation-induced skin reactions. A systematic review. Eur J Cancer Care (Engl) 2017 doi: 10.1111/ecc.12685. [DOI] [PubMed] [Google Scholar]
- 10.Herst P.M. Protecting the radiation-damaged skin from friction: a mini review. J Med Radiat Sci. 2014;61(2):119–125. doi: 10.1002/jmrs.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haviland J.S., Owen J.R., Dewar J.A., Agrawal R.K., Barrett J., Barrett-Lee P.J. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 12.Haislund B., Bang T., Ellegaard M.B., Offersen B. po-0954 Acute morbidity in patients with early breast cancer in adjuvant radiotherapy in the DBCG hypo and DBCG PBI protocols. Radiother Oncol. 2012;103:S375–S376. [Google Scholar]
- 13.Schnur J.B., Love B., Scheckner B.L., Green S., Wernicke A.G., Montgomery G.H. A systematic review of patient-rated measures of radiodermatitis in breast cancer radiotherapy. Am J Clin Oncol. 2011;34(5):529–536. doi: 10.1097/COC.0b013e3181e84b36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noble-Adams R. Radiation-induced skin reactions 3 evaluating the RISRAS. Brit J Nurs (Mark Allen Publishing) 1999;8(19):1305–1312. doi: 10.12968/bjon.1999.8.19.1305. [DOI] [PubMed] [Google Scholar]
- 15.Sprangers M.A., Groenvold M., Arraras J.I., Franklin J., te Velde A., Muller M. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol: Off J Am Soc Clin Oncol. 1996;14(10):2756–2768. doi: 10.1200/JCO.1996.14.10.2756. [DOI] [PubMed] [Google Scholar]
- 16.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 17.DBCG. Danish Breast Cancer Cooperative Group. Available from: <http://www.dbcg.dk/>.
- 18.Clearinghouse CfKR-. Forebyggelse og behandling af akutte hudreaktioner til patienter med kræft, der modtager ekstern strålebehandling for deres kræftsygdom 2016 (updated January 29 2016; cited 2013 July 29). Available from: <http://www.cfkr.dk/media/353352/Forebyggelse%20og%20behandling%20af%20akutte%20hudreaktioner%20hos%20kr%C3%A6ftpatienter,%20der%20modtager%20ekstern%20str%C3%A5lebehandling%20for%20deres%20kr%C3%A6ftsygdom..pdf>.