Highlights
-
•
Prostatic calculi are a frequent radiological finding and may aid prostate IGRT.
-
•
Incidence of prostatic calculi in a population of radiotherapy patients is reported.
-
•
Significant proportion of patients have calculi detectable on radiotherapy images.
-
•
Prostatic calculi may reduce the need for surgically implanted markers.
Keywords: Prostate, Calculi, Incidence, IGRT
Abstract
Purpose
This study aims to quantify the incidence and distribution of prostatic calculi in a population of prostate radiotherapy patients and assess their potential role in prostate image guided radiotherapy (IGRT).
Methods & materials
A retrospective analysis of trans-rectal ultrasound (TRUS), computed tomography (CT) planning and treatment verification cone beam CT (CBCT) scans from radical prostate radiotherapy patients (external beam and brachytherapy) between 2012 and 2014 was undertaken by a single experienced observer. An internationally validated schema from the Prostate Imaging Reporting and Data system (PIRADS) was used to map the location of calculi. The association of calculi with patient and disease characteristics was explored. Data was analysed using SPSS (IBM version 22.0) using descriptive statistical methods and logistic binary regression analysis.
Results
389 scan sets from 254 patients were included in the analysis. The overall incidence of calculi was 85% (n = 218) of which 79% (n = 201) were intra-prostatic calculi. The mean number of intra-prostatic calculi was 2 (range 1–10) and the mean size of calculi was 3.7 mm (range 0.5–15 mm). Calculi were most frequently observed in the posterior of the mid-gland (PI-RADs 3p, 9p) and posterior of the apex (PI-RADs 5p, 11p). 99% (n = 135) of CT planning scans with a corresponding CBCT had calculi in the same PIRADs location and all calculi were visible at the last fraction. There was no statistically significant association of calculi and N stage, M stage or Gleason score.
Conclusions
A significant proportion of prostate radiotherapy patients have prostatic calculi detectable on pre radiotherapy imaging. Calculi observed on CT were also detectable on CBCT in 99% of cases and remain visible at the end of treatment. These findings add to the growing evidence base supporting the potential of calculi as an alternative to fiducial markers to aid prostate IGRT.
Introduction & background
Prostate cancer (PCa) is the most common cancer in men in the UK and approximately half of patients diagnosed will receive radiotherapy alone or in conjunction with surgery or hormone therapy as part of their disease management [1]. Despite the rapid evolution of imaged guided radiotherapy (IGRT), verification of soft tissue targets like the prostate gland, remains challenging and image analysis is prone to uncertainty and inter-observer variability [2], [3]. IGRT for PCa frequently employs surgically implanted fiducial markers (FMs) as an aid to target verification. While they are not used universally they are an evidence based approach [4] and are recommended in a current clinical trial comparing surgery and stereotactic ablative radiotherapy (SABR) for PCa (PACE trial ISRCTN1762721).
FM insertion requires a surgical procedure prior to radiotherapy planning. A suitable non-invasive alternative to FMs for prostate IGRT would eliminate the need for this surgical intervention, the risk of infection, bleeding and also the associated costs and resources required.
Prostate calcifications, hereafter referred to as calculi, are small round, ovoid or irregular structures which can develop in the prostate gland and are frequently observed in prostatectomy specimens [5]. They are often asymptomatic and their role in prostatitis, benign prostatic hyperplasia, lower urinary tract symptoms and prostate cancer (PCa) is unclear. A recent paper by Venyo et al. [6] provides a review of the literature in relation to calculi.
Calculi in IGRT
There is a growing body of evidence suggesting that radiologically visible calculi could replace FMs as an aid to image analysis in IGRT. Calculi visible on CBCT have been investigated and proposed [7], [8], [9], while others have used them as valid reference points in the assessment of image registration methods [10], [11], [12].
Harnessing calculi as IGRT markers may have significant benefits for individual patients in terms of eliminating an invasive procedure and risk of infection. It may also have a significant impact on health budgets depending on how many patients present with calculi.
The reported incidence of calculi varies widely depending on the method of detection. A study by Suh et al detected calculi in almost 90% [5] of prostatectomy specimens. In contrast, the incidence is reported as 35% [7] of prostate radiotherapy patients based on the review of 131 cone beam computed tomography (CBCT) images [7].
The purpose of this study was to establish the radiological incidence and location of calculi in a contemporary population of prostate radiotherapy patients. The association of calculi with patient and disease characteristics was also explored.
Methods
Image review
Two hundred and fifty four PCa patients treated with radical radiotherapy to the prostate or prostate and pelvis (external beam and/or brachytherapy) between 2012 and 2014 were included in this study. An experienced single-observer analysed available trans-rectal ultrasound (TRUS) brachytherapy volume study scans, radiotherapy planning CT scans and treatment verification CBCT scans for the presence of calculi. To assess TRUS as a modality for the detection of PC, we included brachytherapy TRUS images since TRUS biopsy scans are not routinely archived at our institution and therefore not available for retrospective evaluation. TRUS scans were assessed using Variseed v8.0.2 (Varian medical Systems). CT planning scans acquired at 2.5 mm slices were reviewed in Centricity Web v3.0.10 (GE Medical Systems). CBCTs of patients with calculi identified on planning CT were reviewed to determine if CBCT detection of calculi is comparable to that detected by CT. CBCTs were acquired on a Varian Truebeam using standard pelvis mode (kVp 125, mA 80, reconstructed to 2 mm slices). CBCTs were assessed in Aria Off-Line review using pelvic windowing.
Data collection
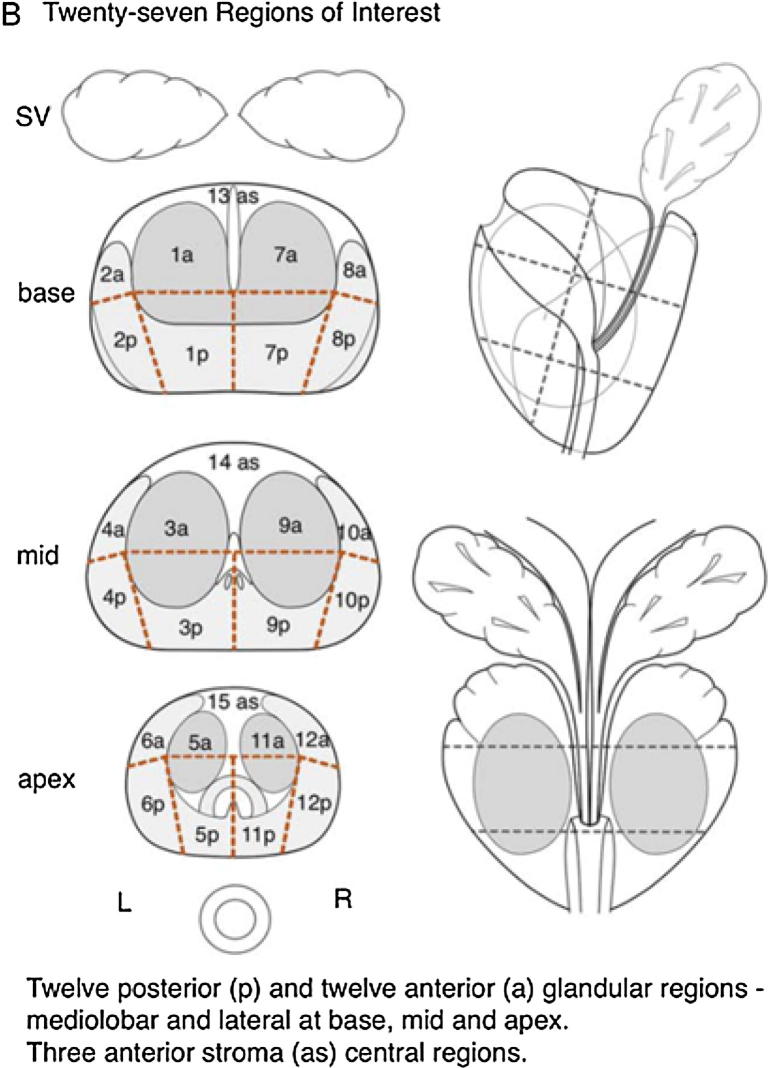
A study pro-forma was designed for the purpose of data collection. This incorporated a diagrammatic schema from the Prostate Imaging and Reporting Data system (PI-RADs™ Version 1.0) (see Fig. 1). This schematically divides the prostate into 27 sections and was used to map the distribution of calculi within the prostate gland. Calculi were recorded when 1 or more were easily identified within the prostate gland (intra-prostatic) or extra-prostatic but close to the prostatic capsule and visible in the 3 cardinal planes. The maximum axial dimension of the largest calculi was measured using the software in each of the aforementioned applications.
Fig. 1.
Prostate Imaging and Reporting Data System (PI-RADS). Reproduced from Dickinson et al., European Urology 59, 477–494, 2011 [13].
In addition to data on the presence, size and location of calculi, patient and disease characteristics were extracted and recorded from patients medical histories including patient age, stage, initial prostate specific antigen (iPSA) and Gleason score. Analysis of magnetic resonance images (MRI) was ruled out early in the study due to the inability to differentiate possible calculi from blood products on images acquired using standard MRI protocols at our institution.
Data analysis
Data was coded and analysed in SPSS (IBM version 22.0). Simple descriptive statistical methods were used to describe the incidence, size and distribution of calculi. Logistic binary regression analysis was used to explore associations of calculi with patient and disease characteristics.
Results
Study population
See Table 1.
Table 1.
Study Population.
| Study Participants | N = 254 |
|---|---|
| Age at diagnosis | |
| Median | 69 |
| (Range | 57–80) |
| Gleason Score | |
| 6 | N = 50 |
| 7 | N = 94 |
| 8 | N = 36 |
| 9 | N = 69 |
| 10 | N = 2 |
| Missing | N = 3 |
| Clinical Disease Stage | |
| T0-T1c | N = 28 |
| T2 | N = 79 |
| T3 | N = 132 |
| T4 | N = 8 |
| Missing | 7 |
Incidence, distribution and size of calculi
389 scan sets (73 TRUS, 180 CT planning, 136 CBCT) from 254 patients were included in the analysis.
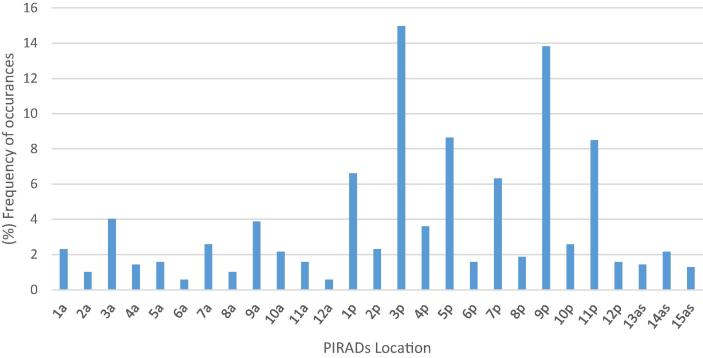
The overall incidence of patients with calculi (intra and extra-prostatic) recorded across all imaging modalities was 85% (n = 219). Limiting this to those patients presenting with intra-prostatic calculi (with or without extra-prostatic calculi) reduced this to 79% (n = 201). The mean number of intra-prostatic calculi observed within a patient was 2 (range 1–10). The mean size (maximum axial diameter) of calculi observed was 3.7 mm (range 0.5–15 mm). Calculi were most frequently observed in areas 3p and 9p (posterior portion of the mid-gland) and also 5p and 11p (post portion of the apex). Calculi were least often observed in 8a, 12a & 13as (anterior base and apex). The percentage incidence of total calculi recorded according to their PIRADs location is shown in Fig. 2.
Fig. 2.
The total number of intra-prostatic calculi recorded was 695. PIRADs location 3p and 9p (posterior mid gland) exhibited the highest frequency of PC of 14.9% and 13.8%, respectively.
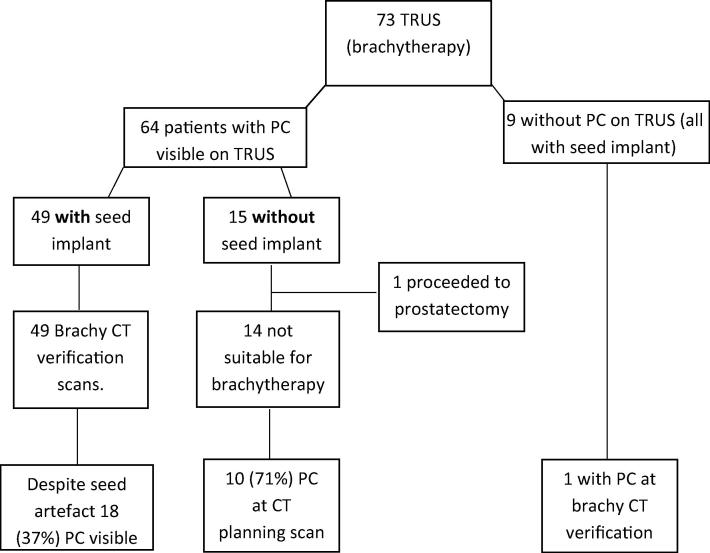
TRUS calculi and CT calculi (see Fig. 3)
Fig. 3.
Schematic to show TRUS PC and subsequent CT PC.
TRUS scans from 73 brachytherapy patients were analysed of which 64 patients (88%) had calculi visible. Of these 64, 49 had a seed implant and 15 had no seed implant. 1 of the patients without implant proceeded to prostatectomy. The remaining 14 patients, following initial referral for brachytherapy, were subsequently deemed unsuitable for implant due to larger than predicted prostate volume. These 14 had a subsequent CT scan for EBRT of which 10 (71%) had calculi in the same PIRADs location as detected on TRUS. The 49 patients with implant also had a corresponding CT but meaningful comparison was difficult due to significant seed artefact. Despite this 18 (37%) CT scans with seed implant had calculi discernible. Nine patients TRUS demonstrated no calculi. One of these 9 with seed implant presented with calculi on subsequent CT.
There were no corresponding CBCTs for comparison in the TRUS group of patients.
CT calculi & CBCT calculi
CT planning scans from 180 patients were analysed of which 154 (86%) had calculi detected. 136 of the 154 with calculi had a corresponding CBCT. 135/136 (99%) had calculi observed within the same PIRADs section. 1 patient in the CT group without a corresponding treatment verification CBCT had widespread pelvic calcification including multiple intra and extra prostatic calculi. These were visible on an anterior 2DkV image however not clearly seen on the lateral 2D kV image.
Calculi and disease characteristics
A binary logistic regression test was used in univariate and multivariate analysis to investigate the associations between different variables and the presence of calcifications as observed on CT. (See Table 2) The incidence of calculi was positively correlated with age, PSA and increasing T stage and negatively correlated with increasing N stage, but none were found to be statistically significant. Univariate analysis revealed that the odds ratio (OR) of having calcifications increase by a factor 1.2 per unit increase in Gleason score and decrease by 2% for increase in N stage. However, a step wise regression showed that after adjusting for N and M stages none of the investigated variables were significantly associated with the presence of calcifications.
Table 2.
Odds Ratio (OR) and 95% confidence interval (CI) and p-values for univariate and multivariate analysis of association of calculi and Gleason score, N and M staging.
| Univariate OR (95% CI) |
P | Multivariate OR (95% CI) |
P | |
|---|---|---|---|---|
| Gleason | 1.246 (1.026–1.513) | 0.027 | 1.205 (0.981–1.480) | 0.076 |
| N | 0.980 (0.962–0.998) | 0.029 | 0.991 (0.961–1.022) | 0.580 |
| M | 0.977 (0.962–1.001) | 0.066 | 0.988 (0.951–1.027) | 0.546 |
Discussion
To our knowledge this is the first study to report the radiologically detected incidence of prostatic calculi using an internationally validated anatomical mapping schema. The incidence of prostatic calculi in our sample of 254 radical prostate radiotherapy patients was 85%. Where present the average number of calculi per patient was 2 and the average size 3.7 mm. They were most frequently observed in the posterior of the apex and mid-gland and least frequently in the anterior base and apex.
These results are in-line with Suh et al. [5] who report the incidence to be 88.6% (264/298) of prostatectomy specimens. The incidence in our sample is considerably higher than the 35% reported by Zeng et al. [7] from a review of 131 prostate radiotherapy CT planning scans. One possible reason for the difference might be that we did not pre-define calculi size criteria. Zeng et al. [7] set a size criteria of >2 mm in diameter for a calculi to be recorded. This is reasonable particularly as they used low dose CBCT, but may have eliminated a significant number of smaller yet visible calculi. More recently Hama et al. [14] report the highest incidence from a radiological study of 93% of 30 patients detected on kVCT. They defined calculi criteria based on at least 2 pixels equating to 1.9 mm2. In our study we did not predefine criteria adopting the pragmatic approach that if a calculi could be visualised then it could also be contoured and therefore potentially used to aid IGRT. In our study 135 out of 136 planning CT scans with calculi had corresponding calculi on CBCT. The calculi not subsequently detected on CBCT had a maximum diameter of 1.5 mm axially. In total there were 39 calculi detected measuring 2 mm or less. One sub millimetre calculi was visible on the anterior view of the CBCT but not on the lateral view. These observations suggest that factors other than size affect radiological calculi detection. Such factors may include, imaging parameters, day to day variations in CBCT quality, image artefact, patient specific variations such as rectal gas or patient size, and variations in the density and radio-opacity of individual calculi. Notably, calculi were present at the beginning and end of treatment demonstrating that, at least for the duration of treatment, their presence appeared unaffected by radiation.
One of the largest and most recent studies of calculi was reported by Park et al. [15] who found that 76.6% of TRUS scans from 606 patients demonstrated calculi supporting the idea that they are a very common phenomenon.
The role of IGRT in ensuring adequate dose coverage of the prostate and avoidance of the rectal wall is well documented [16], [17], [18], [19]. In this study calculi were most frequently observed in the posterior portion of the gland. This aligns with one of the earliest reports on prostatic calculi [20]. These calculi may have a particular relevance for prostate IGRT providing a marker at the prostate/rectal interface where planning margins are often reduced to minimise rectal toxicity. Location may prove to be even more significant if proven to be linked to PCa or even foci of PCa within the prostate as suggested by Smolski et al. [21]. In this scenario the calculi might actually become the target, or surrogate, for an intra-prostatic boost.
There are a number of limitations inherent in our study. TRUS biopsy images are not routinely acquired at our institution therefore this analysis was limited to the use of brachytherapy images. 18 out of 49 patients with seed implant in our patient cohort had calculi visible on CT. There may have been more that were obscured by seed artefact therefore it’s not possible to draw any sound conclusion on the correlation of TRUS calculi with CT calculi from this group. However, 71% of patients with calculi on TRUS had corresponding calculi on CT based on the 14 patients without implant, indicating that even in this small sample TRUS is not reliable for predicting calculi on CT. Another weakness is of course that this was a retrospective analysis and therefore selection bias cannot be completely ruled out.
While not the primary focus of this study, the authors believed it pertinent to provide a brief analysis of calculi in relation to any associations with the basic clinical patient data collected. Reports exist that calculi may be associated with increasing age, disease stage, Gleason score or other characteristics [5], [22], [23]. Suh et al. [5] reported a linear correlation of calculi incidence and age. In this study also calculi incidence is associated with increasing age however this was not statistically significant. (p = 0.08) Our data suggested that the presence of calculi were more likely with increasing Gleason score and less likely with nodal stage. However, on multivariate analysis neither association was statistically significant.
Issues for clinical implementation of prostate calculi guided IGRT
The correlation of calculi detectable on pre-treatment CT planning scans and subsequently on CBCT scans is important in order to establish that calculi can be detected by both imaging modalities. In our sample this was found to be the case in 99% of patients. In a centre, such as ours, where FMs are not routinely employed, calculi could be used for prostate IGRT by simply identifying them at CT planning. If they are then also visible on CBCT, which our data suggest they are, then they may be used to enhance existing prostate IGRT protocols as a recognised reference structure for image analysis. Should the case occurs that calculi are not visible on CBCT then standard bony anatomy and soft tissue registrations may be the default.
For those centres where prostate FMs are standard the ability to correlate TRUS detection of calculi with pre-treatment CT and CBCT is key. In this scenario it would be necessary to identify with a high degree of success patients whose calculi could substitute FMs. TRUS biopsy may present an opportunity to assess calculi status. However TRUS detection of calculi not then detectable on CT, as demonstrated in 4 out of 14 patients in this study for example, may result in a patient receiving non-marker based treatment or a introduce a delay in treatment starting to facilitate FM insertion. Ideally calculi would be detected at MRI staging. This may be possible in the future as multi-parametric MR (mpMRI) becomes more widely practiced. Susceptibility weighted imaging (SWI) is one example where mpMRI may assist in the diagnosis of PCa and has been shown to be as effective as CT in detecting calculi [24].
In addition to the timely detection of calculi, the number of calculi is also relevant to consider if they are to replace FMs. Two or three FMs, depending on length and separation, have been suggested as adequate to assess the position of the prostate [25]. Other factors are relevant in relation to this such as the imaging modality used and as previously mentioned, the location. Three markers may be preferable when using 2D imaging but when used in conjunction with 3D imaging, fewer markers and therefore calculi may provide a viable reference point. Some evidence exists suggesting that the existence of 1 or more calculi improves the accuracy of prostate CT/CBCT image registrations [11], however to date a direct comparison of calculi with FMs has not been reported.
As stereotactic radiotherapy for prostate cancer becomes more widely available, so too the need for ensuring accuracy in treatment delivery with currently available imaging technology. It is likely the use of FMs will increase and this will have a real impact on local health budgets. Calculi present a potential non-surgical alternative to FMs in a significant proportion of patients. Despite the incidence of calculi and the growing evidence base for their role in IGRT, more robust comparison directly with FMs in larger cohorts of patients is required.
Conclusions and future work
The PIRADs schema proved an invaluable tool, providing a consistent and reproducible way to report the location of calculi. The incidence of calculi was positively correlated with age, PSA and increasing T stage and negatively correlated with increasing N stage, but none were found to be statistically significant. Correlation of TRUS calculi and CT calculi was not completely reliable.
In this study, a significant proportion (85%) of patients with PCa have calculi detectable on pre-radiotherapy imaging and 99% of those detected on CT were subsequently detected on CBCT and remained visible at the end of a course if radiotherapy. This supports the use of calculi as a possible alternative to FMs for prostate IGRT.
A prospective clinical trial is underway at our institution evaluating calculi for IGRT image analysis and the clinical feasibility of such an approach for localised prostate cancer patients. All patients within the trial have FMs implanted and receive daily CBCT FM marker guided treatment. A comprehensive analysis of daily CBCTs, treatment set-up accuracy and planning margins based on data from FMs and calculi, were present, will be directly compared within each patient. The study will incorporate PI-RADs™ Version 2.0 [26] and a prospective radiological analysis of prostatic calculi in TRUS, CT and CBCT.
Conflict of interest declaration
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Contributor Information
A.G.M. O'Neill, Email: aoneill533@qub.ac.uk.
S.O. Osman, Email: S.Osman@qub.ac.uk.
S. Jain, Email: s.jain@qub.ac.uk.
A.R. Hounsell, Email: a.hounsell@qub.ac.uk.
J.M. O'Sullivan, Email: joe.osullivan@qub.ac.uk.
References
- 1.Ferlay J., Steliarova-Foucher E., Lortet-Tieulent J., Rosso S., Coebergh J.W.W., Comber H. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Barney B.M., Lee R.J., Handrahan D., Welsh K.T., Cook J.T., Sause W.T. Image-guided radiotherapy (IGRT) for prostate cancer comparing kV imaging of fiducial markers with cone beam computed tomography (CBCT) Int J Radiat Oncol Biol Phys. 2011;80(1):301–305. doi: 10.1016/j.ijrobp.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Moseley D.J., White E.A., Wiltshire K.L., Rosewall T., Sharpe M.B., Siewerdsen J.H. Comparison of localization performance with implanted fiducial markers and cone-beam computed tomography for on-line image-guided radiotherapy of the prostate. Int J Radiat Oncol Biol Phys [Internet] 2007;67(3):942–945. doi: 10.1016/j.ijrobp.2006.10.039. http://www.sciencedirect.com/science/article/pii/S0360301606033633 [cited 2014 Nov 17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Neill A.G.M., Jain S., Hounsell A.R., O’Sullivan J.M. Fiducial marker guided prostate radiotherapy: a review. Br J Radiol [Internet] 2016;89(1068):20160296. doi: 10.1259/bjr.20160296. [cited 2017 Apr 5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suh J.H., Gardner J.M., Kee K.H., Shen S., Ayala A.G., Ro J.Y. Calcifications in prostate and ejaculatory system: a study on 298 consecutive whole mount sections of prostate from radical prostatectomy or cystoprostatectomy specimens. Ann Diagn Pathol [Internet] 2008;12(3):165–170. doi: 10.1016/j.anndiagpath.2007.07.001. http://www.sciencedirect.com/science/article/pii/S1092913407001189 [cited 2015 Jan 22] [DOI] [PubMed] [Google Scholar]
- 6.Venyo AK-G. Prostatic calculi: a review of the literature. 2012 [cited 2015 Jan 28]. https://www.webmedcentral.com/article_view/3463.
- 7.Zeng G.G., McGowan T.S., Larsen T.M., Bruce L.M., Moran N.K., Tsao J.R. Calcifications are potential surrogates for prostate localization in image-guided radiotherapy. Int J Radiat Oncol Biol Phys [Internet] 2008;72(4):963–966. doi: 10.1016/j.ijrobp.2008.07.021. http://www.redjournal.org/article/S0360301608031040/fulltext [cited 2015 Jan 28] [DOI] [PubMed] [Google Scholar]
- 8.Hanna S.A., Neves-Junior W.F.P., Marta G.N., Haddad C.M.K., da Silva J.L.F. Role of intra- or periprostatic calcifications in image-guided radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys [Internet] 2012;82(3):1208–1216. doi: 10.1016/j.ijrobp.2011.03.059. http://www.ncbi.nlm.nih.gov/pubmed/21640492 [cited 2015 Jan 28] [DOI] [PubMed] [Google Scholar]
- 9.Sbai A., Thariat J., Tachfouti N., Pan Q., Lagrange J.-L. Intraprostatic calcifications as natural fiducial markers in image-guided radiotherapy for prostate cancer. Cancer Radiother [Internet] 2014;18(8):740–744. doi: 10.1016/j.canrad.2014.07.161. http://www.sciencedirect.com/science/article/pii/S1278321814003539 [cited 2015 Feb 4] [DOI] [PubMed] [Google Scholar]
- 10.Kim J., Kumar S., Liu C., Zhong H., Pradhan D., Shah M. . My IOPscience A novel approach for establishing benchmark CBCT/CT deformable image registrations in prostate. Phys Med Biol. 2013;8077(22) doi: 10.1088/0031-9155/58/22/8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J., Hammoud R.A., Pradhan D.E., Zhhong H.U., Jin R., Movsas B.E. Prostate localization on daily cone-beam computed tomography images: accuracy assessment of similarity metrics. Int J Radiat Oncol Biol Phys [Internet] 2010;77(4):1257–1265. doi: 10.1016/j.ijrobp.2009.09.068. [cited 2014 Nov 25] [DOI] [PubMed] [Google Scholar]
- 12.Smitsmans M.H.P., de Bois J., Sonke J.-J., Betgen A., Zijp L.J., Jaffray D.A. Automatic prostate localization on cone-beam CT scans for high precision image-guided radiotherapy. Int J Radiat Oncol Biol Phys [Internet] 2005;63(4):975–984. doi: 10.1016/j.ijrobp.2005.07.973. http://www.sciencedirect.com/science/article/pii/S0360301605022686 [cited 2014 Nov 17] [DOI] [PubMed] [Google Scholar]
- 13.Dickinson L., Ahmed H.U., Allen C., Barentsz J.O., Carey B., Futterer J.J. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol [Internet] Eur Assoc Urol. 2011;59(4):477–494. doi: 10.1016/j.eururo.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Hama Y. Detection of prostate calcification with megavoltage helical CT. Acad Radiol [Internet] 2014;21(5):565–568. doi: 10.1016/j.acra.2014.01.006. http://www.sciencedirect.com/science/article/pii/S1076633214000099 [cited 2015 Jan 22] [DOI] [PubMed] [Google Scholar]
- 15.Park B., Choo A. The burden of prostatic calculi is more important than the presence. Asian J Androl. 2016;18:1–4. doi: 10.4103/1008-682X.181193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zelefsky M.J., Crean D., Mageras G.S., Lyass O., Happersett L., Clifton Ling C. Quantification and predictors of prostate position variability in 50 patients evaluated with multiple CT scans during conformal radiotherapy. Radiother Oncol [Internet] 1999;50(2):225–234. doi: 10.1016/s0167-8140(99)00011-0. [cited 2015 Jan 24] [DOI] [PubMed] [Google Scholar]
- 17.Padhani A.R., Khoo V.S., Suckling J., Husband J.E., Leach M.O., Dearnaley D.P. Evaluating the effect of rectal distension and rectal movement on prostate gland position using cine MRI. Int J Radiat Oncol [Internet] 1999;44(3):525–533. doi: 10.1016/s0360-3016(99)00040-1. http://www.sciencedirect.com/science/article/pii/S0360301699000401 [cited 2015 Mar 6] [DOI] [PubMed] [Google Scholar]
- 18.Park S.S., Yan D., McGrath S., Dilworth J.T., Liang J., Ye H. Adaptive image-guided radiotherapy (IGRT) eliminates the risk of biochemical failure caused by the bias of rectal distension in prostate cancer treatment planning: clinical evidence. Int J Radiat Oncol [Internet] 2012;83(3):947–952. doi: 10.1016/j.ijrobp.2011.08.025. http://linkinghub.elsevier.com/retrieve/pii/S0360301611031816 [cited 2017 Jun 27] [DOI] [PubMed] [Google Scholar]
- 19.Zelefsky M.J., Kollmeier M., Cox B., Fidaleo A., Sperling D., Pei X. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys [Internet] 2012;84(1):125–129. doi: 10.1016/j.ijrobp.2011.11.047. http://www.sciencedirect.com/science/article/pii/S0360301611036042 [cited 2014 Nov 25] [DOI] [PubMed] [Google Scholar]
- 20.Huggins Charles, Bear Richard S. Course of prostatic ducts and anatomy: chemical and X-ray diffraction analysis of prostatic calculi. J Urol. 1944;51(37) [Google Scholar]
- 21.Smolski M., Turo R., Whiteside S., Bromage S., Collins G.N. Prevalence of prostatic calcification subtypes and association with prostate cancer. Urology [Internet] 2015;85(1):178–181. doi: 10.1016/j.urology.2014.09.026. http://www.sciencedirect.com/science/article/pii/S0090429514010474 [cited 2016 Mar 15] [DOI] [PubMed] [Google Scholar]
- 22.Shoskes D.A., Lee C.-T., Murphy D., Kefer J., Wood H.M. Incidence and significance of prostatic stones in men with chronic prostatitis/chronic pelvic pain syndrome. Urology [Internet] 2007;70(2):235–238. doi: 10.1016/j.urology.2007.04.008. http://www.sciencedirect.com/science/article/pii/S0090429507004943 [cited 2014 Nov 25] [DOI] [PubMed] [Google Scholar]
- 23.Hwang E., Choi H., Im C., Jung S., Kim S., Kang T. Prostate calculi in cancer and BPH in a cohort of Korean men: presence of calculi did not correlate with cancer risk. Asian J Androl [Internet] 2009;2:215–220. doi: 10.1038/aja.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai Y., Wang M., Han Y., Dou S., Lin Q., Guo Y. Susceptibility weighted imaging: a new tool in the diagnosis of prostate cancer and detection of prostatic calcification. 2013;8:1. doi: 10.1371/journal.pone.0053237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Boer J., de Bois J., van Herk M., Sonke J.-J. Influence of the number of elongated fiducial markers on the localization accuracy of the prostate. Phys Med Biol. 2012;57:6211–6226. doi: 10.1088/0031-9155/57/19/6211. [DOI] [PubMed] [Google Scholar]
- 26.Weinreb J.C., Barentsz J.O., Choyke P.L., Cornud F., Haider M.A., Macura K.J. PI-RADS prostate imaging? Reporting and data system: 2015, version 2. Eur Urol [Internet] 2016;69(1):16–40. doi: 10.1016/j.eururo.2015.08.052. http://linkinghub.elsevier.com/retrieve/pii/S0302283815008489 [cited 2017 Jun 23] [DOI] [PMC free article] [PubMed] [Google Scholar]